Abstract
Lineage commitment in B lymphopoiesis remains poorly understood due to the inability to clearly define newly committed B lineage progenitors and their multipotential descendants. We examined the potential of three recently described progenitor populations in adult mouse bone marrow to differentiate into each hematopoietic lineage. The earliest of these, termed fraction (Fr.) A0, exhibited myeloid, erythroid, and B and T lymphoid progenitor activity and included individual cells with myeloid/B lymphoid potential. In sharp contrast, two later populations, termed Frs. A1 and A2 and characterized by surface B220 expression and transcription of the germline immunoglobulin heavy chain (IgH) locus, lacked progenitor activity for all hematopoietic lineages except B lymphocytes. These observations, together with single cell polymerase chain reaction analysis showing a lack of DHJH rearrangements in each population and experiments showing identical precursor potentials when these populations were derived from recombination activating gene (Rag)-1−/− and JH −/− mice, demonstrate that commitment to the B lymphoid lineage occurs before and independently of VHDHJH recombination.
Keywords: lineage restriction, B lymphopoiesis, progenitor, hematopoiesis, stem cell
In adult mice, B lymphocytes ultimately arise from pluripotent stem cells in the bone marrow (BM). Current models predict that the differentiation of multipotential BM progenitor cells into B lineage–committed precursors, incapable of giving rise to alternative hematopoietic lineages, requires the stage-specific activity of key gene products (1). However, despite intensive interest in early B cell development, elucidation of the molecular mechanisms underlying B lineage commitment has been hampered by the inability to clearly define the earliest B lineage–restricted progenitor cells and their immediate unrestricted precursors.
A large body of work has been devoted to defining multipotent hematopoietic progenitor populations. Pluripotent stem cells have been identified in BM (2) and fetal liver (3, 4), bipotential myeloid/B lymphoid progenitors have been identified in fetal liver (5), and lymphoid-restricted progenitors have been identified in adult thymus (6) and BM (7). Recently we described three novel cell populations in adult BM, termed fractions (Frs.) A0, A1, and A2 (8). Among these, Frs. A1 and A2 exhibit numerous characteristics consistent with their designation as precursors for pro-B cells or pre–pro-B cells (9). Thus, whereas expression of λ5 and recombination activating gene (Rag)-1/2 is restricted to Fr. A2 and more mature subsets, Frs. A1 and A2 each exhibit surface B220 expression, express transcripts for the germline IgH locus (μ0), and proliferate in short-term stromal cultures supplemented with IL-7 (8).
The developmental potential of pre–pro-B cells and the role VHDHJH recombination plays in the restriction of multipotential progenitor cells to the B lymphoid lineage have not been explored. Although cells containing IgH recombination events are often referred to as B lineage committed, the existence of natural and induced myelogenous tumor cells containing IgH rearrangements suggests that B lineage commitment can occur after such events (10–13). However, here we report that whereas the precursors of pre–pro-B cells in Fr. A0 exhibit progenitor activity for multiple hematopoietic lineages, pre–pro-B cells in Frs. A1 and A2 are B lineage restricted but lack DHJH rearrangements. This finding demonstrates that B lineage commitment occurs very early in B cell development, before λ5 expression and initiation of DHJH recombination and coincident with μ0 and surface B220 expression. Furthermore, identical results were obtained with the equivalent cell populations from mice incapable of initiating VHDHJH recombination, indicating that mechanisms controlling the differentiation of multipotential progenitors into B lineage– committed cells function independently of VHDHJH recombination.
Materials and Methods
Preparation of BM Cell Suspensions from Mice.
2–4-mo-old female BALB/c and C57BL/6 mice were obtained from the Institute for Cancer Research Laboratory Animal Facility. Female B6.Ly5.2 congenics were obtained from the National Cancer Institute animal facility in Frederick, MD. Rag-1−/− and JH −/− mice are maintained in our breeding colony. BM cell suspensions were made as described previously (9).
Flow Cytometric Analysis and Cell Sorting.
Four-color FACS® analysis and cell sorting of each B lineage cell population were described previously (8, 9, 14). Five-color FACS® analyses were performed by staining adult BM cells with Oregon Green (OG)- coupled anti-CD4 (GK1.5), biotin anti-B220 (RA3-6B2), allophycocyanin (APC) anti-AA4.1, and PE-Cy5 anti–heat-stable antigen (anti-HSA) (30F1). PE-coupled antibodies were anti– Sca-1, anti–IL-7Rα (A7R34), anti-CD3 (500-A2), anti-CD11b (Mac-1), anti–GR-1 (8C5), anti-CD19 (1D3), and anti-NK1.1 (PK136). APC anti-CD117 (c-kit, clone 2B8) was used in conjunction with OG–anti-CD4, PE anti-AA4.1, PE-Cy5 anti-HSA, and biotin anti-B220. Biotinylated antibodies were revealed with Texas Red Avidin, and HSA+ propidium iodide (PI)+ cells were excluded during data collection. Reagents were prepared in our laboratory except for FL anti-Ly5.1 (104) and APC anti–c-kit (2B8), which were purchased from PharMingen.
Stromal Cell Cultures.
Bulk cultures were established by sorting 104 cells onto S17 (5) stromal cells in 24-well flat-bottomed plates as described (9). Numbers of B220+ HSAint and CD11b+ HSAint cells were determined 4 or 8 d later by flow cytometry. Limiting dilution cultures were established by sorting 1–100 cells per well with an automated cell deposition unit directly into 96-well plates with preestablished S17 stromal cells in standard medium and 100 U/ml rIL-7. Wells containing cell growth were scored 14 d later, and the presence of B and myeloid lineage cells was assessed by staining cells with APC-B220, PE-CD11b, and FL-HSA.
Methylcellulose Cultures.
Methylcellulose medium containing IL-3, IL-6, stem cell factor (SCF), and erythropoietin was purchased from Stem Cell Technologies. Triplicate cultures were established by plating sorted cells according to the manufacturer's instructions. Each culture was scored for the number of granulocytic/macrophage (CFU-GM), erythroid (CFU-BFE), and mixed (CFU-GEMM) colonies 12 d later.
Reverse Transcription PCR.
RNA and cDNA from 105 sorted cells were prepared and amplified with gene-specific primers as described previously (14). Primers for β-actin and μ0 have been described (8, 14). Primers for myeloid-specific genes were as follows: lysozyme, 5′-CTCTGAAAAGGAATGGAATGGCTG, 3′-AAATCGAGGGAATGTGACCTCTCTC; c-fms, GAACGTGTTCATCTGTTCCCGTCC, 3′-TCCCCTTACCATGCCAAACTGTGG.
PCR, blotting, and hybridization with gene-specific riboprobes were performed as described (14). 10 μl aliquots were withdrawn at 18, 22, and 26 cycles (β-actin) or 22, 26, and 30 cycles for separate analysis to ensure that amplification was in the linear range.
Single-cell PCR for Detection of DHJH Rearrangements.
Detec tion of DHJH rearrangements and/or an IgH germline fragment from individual cells was accomplished by seminested PCR as described by Ehlich et al. (15). Cloning and nucleotide sequencing confirmed the identity of every PCR product.
Intrathymic Transfer.
Intrathymic transfers were performed as described by Wu et al. (6). 104 sorted cells from female C57BL/6 or JH −/− (both H-2b and Ly5.1+) mice were injected into the thymus of anesthetized female B6.Ly5.2 mice given 500 rads 18–24 h previously. Enumeration and characterization of donor-derived thymocytes were accomplished 14–28 d later by staining recipient thymocytes with FL anti-Ly5.1 (104) and combinations of PE or APC anti-CD3 (500-A2), PE anti-CD8 (Lyt2), and APC anti-CD4 (GK1.5).
Results
Isolation of Early B Lineage Progenitor Cells in Adult BM.
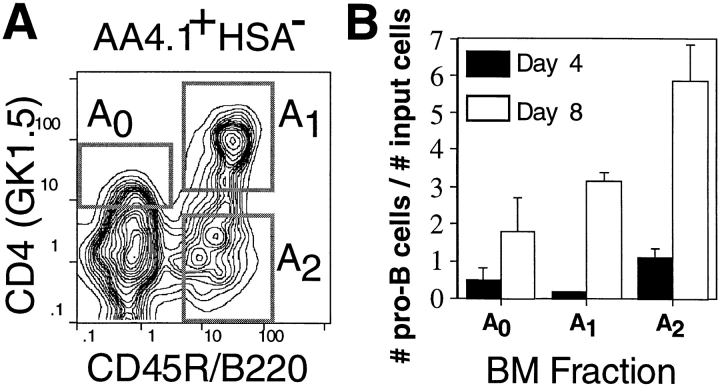
We previously described a flow cytometric system for the isolation of three early B lineage progenitor populations, termed Frs. A0, A1, and A2 (8). Each population constitutes 0.1% of all cells in adult BM, bears the surface phenotype AA4.1+ HSA−, and is further defined as B220− CD4low (A0), B220+ CD4+ (A1), or B220+ CD4− (A2) (Fig. 1 A). Each fraction contains cells with the capacity to differentiate into B220+ HSAint pro-B cells in stromal cultures (Fig. 1 B). Interestingly, these cultures also routinely contained CD11b+ B220− myeloid lineage cells (not shown), suggesting that these populations might also contain myeloid progenitors. However, because these bulk cultures often contain contaminating stem cells, we adopted a series of quantitative culture and adoptive transfer systems in conjunction with semiquantitative reverse transcription (RT)- PCR and five-color flow cytometry to more precisely define the ability of these populations to give rise to cell types other than B lymphocytes.
Figure 1.
Early B lineage precursor cell populations in adult BM. (A) Four-color immunofluorescence analyses reveal the three indicated populations. (B) 104 sorted cells from Fr. A0, A1, or A2 from adult BALB/c BM were cultured for 4 or 8 d on S17 stromal cells with 100 U/ml rIL-7, and the number of B220+ HSAint pro-B cells was determined by flow cytometry.
Cell Surface Phenotype of Early B Lineage Progenitor Populations.
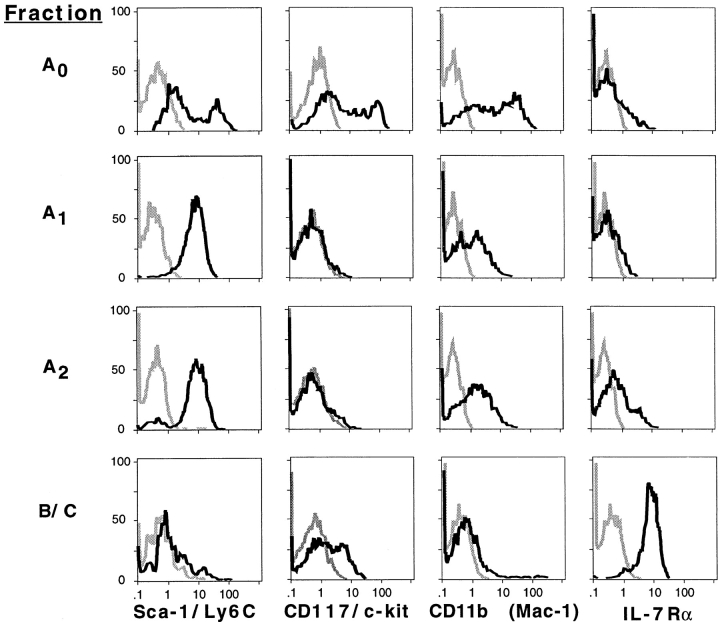
Five-color FACS® data for surface molecules associated with multilineage and lineage-restricted progenitor cells are presented in Fig. 2. Bimodal expression of surface molecules associated with multipotential progenitors such as Sca-1/Ly6C and CD117/c-kit and the CD11b surface antigen associated with the myeloid lineage was observed in Fr. A0, implying cellular heterogeneity within this population. In subsequent experiments, we determined that Fr. A0 can be further subdivided into two discrete Sca-1+ c-kit+ CD11b− and Sca-1− c-kit− CD11b+ populations (not shown).
Figure 2.
Surface molecule expression among defined B lineage subsets. Five-color (Frs. A0–A2) or four-color (Frs. B/C) immunofluorescence of adult BM cells. For Frs. A0–A2, cells were stained simultaneously with antibodies to CD4, B220, HSA, AA4.1, and the indicated surface molecule, and expression of the indicated surface molecule on the indicated population was determined as described in Materials and Methods. For Frs. B/C, cells were stained with antibodies to CD43, HSA, B220, and the indicated surface molecule, and expression was determined by gating on CD43low B220low HSAint cells. BM cells were from BALB/c mice except for Sca-1/Ly6C, for which C57BL/6 mice were used.
In contrast to Fr. A0, Frs. A1 and A2 each exhibited a uniform Sca-1low c-kit− CD11blow phenotype, whereas B220+ CD43+ HSAint pro-B cells, previously referred to as Frs. B and C (9), were Sca-1low CD11b− and bimodal for c-kit. Since Rolink and colleagues have used a CD19+ c-kit+ surface phenotype to define pro-B cells (16), we were surprised to find undetectable levels of c-kit among Frs. A1 and A2 and bimodal expression within Frs. B and C. Although this might be attributed to differences between particular antibodies, the c-kit antibody used (2B4) gave brighter staining among CD19+ BM cells compared with the alternative available clone, ACK4 (data not shown). That Frs. A1 and A2 are unique and separate B lineage progenitor populations relative to pro-B cells defined as CD19+ c-kit+ or B220 low CD43low HSAint is also evident from their lack of expression of CD19 (8). Frs. A0–A2 also exhibited low to undetectable staining for IL-7Rα relative to pro-B cells in Frs. B/C (Fig. 2). Finally, surface molecules associated with lineage-restricted progenitors such as CD3, TER-119, and Gr-1 were undetectable on all populations (data not shown).
The Extent of DHJH Recombination among Subsets of pre– pro-B Cells.
Previous experiments examining the degree of recombination at the IgH locus in various B lineage subsets suggest that DH-JH recombination occurs largely within the pro-B cell compartment (9, 14, 15). However, since these experiments were performed with a pre–pro-B cell population subsequently shown to be heterogeneous (8, 17), we adopted a PCR assay previously shown to detect DHJH rearrangements among individual cells (15) to rule out the possibility that DH-JH rearrangement initiates in Frs. A1/A2. Only cells with germline configuration of the IgH locus were detected in 68 and 71 cells from Frs. A0 and A1, respectively. Similarly, only 2 out of 49 cells from Fr. A2 in which a PCR product was detected contained a D-J rearrangement. By comparison, 25 out of 44 pro-B cells (Fr. B) in which a PCR product was detected displayed D-J rearrangements on at least 1 allele (not shown). Thus, these data confirm previous conclusions that DHJH rearrangements are initiated among pro-B cells.
Loss of Monocyte/Macrophage Progenitor Activity Occurs before and Independently of VDJ Recombination.
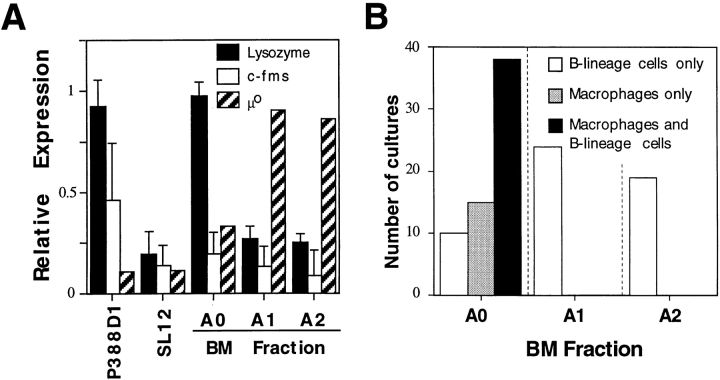
To examine the myeloid lineage potential in each subset, we first used semiquantitative RT-PCR for myeloid-specific genes. As shown in Fig. 3 A, low level expression of c-fms was observed in Frs. A0–A2 relative to the macrophage cell line P388D1. In contrast, relatively strong expression of lysozyme was observed in P388D1 and Fr. A0 but not Fr. A1 or A2. This expression pattern was the reverse of that observed for μ0, suggesting an absence of myeloid progenitor activity in Frs. A1 and A2.
Figure 3.
Myeloid lineage progenitor activity in Frs. A0, A1, and A2. (A) Expression of c-fms, lysozyme, and μ0 among Frs. A0, A1, and A2 was determined by RT-PCR, and expression levels were normalized to β-actin expression. Data are from three separate experiments. (B) Cultures established at limiting dilution using 1, 100, and 10 cells/well for Frs. A0, A1, and A2, respectively, were stained at day 14 for CD45R/B220 and CD11b expression. The numbers of wells containing exclusively B220+ CD11b− cells, B220− CD11b+ cells, or both are shown.
To directly assess myeloid progenitor activity, cells from each fraction were cultured at limiting dilution on S17 stromal cells under conditions previously shown to support the growth of monocyte/macrophage and B lymphoid progenitors (5). Interestingly, each fraction exhibited markedly different cloning potentials under these conditions: whereas limiting dilution was easily achieved by plating 1 cell/well for Fr. A0, 100- and 10-fold greater numbers of cells were necessary for Frs. A1 and A2, respectively (not shown). Regardless, 65% of wells seeded with single cells from Fr. A0 contained both B lineage and myeloid lineage progenitors as determined by the outgrowth of both B220+ CD11b− HSAint and B220− CD11b+ HSAint cells, demonstrating the presence of bipotential myeloid/B lymphoid progenitor cells within Fr. A0 (Fig. 3 B). In contrast, at limiting dilution 100% of cultures seeded with Fr. A1 or A2 contained only B lineage B220+ CD11b− HSAint cells, indicating an absence of myeloid progenitor activity within these populations. Likewise, when introduced into methylcellulose cultures optimized for myeloid and erythroid progenitors, Fr. A0 but not Fr. A1 or A2 gave rise to both macrophage/granulocyte and erythroid colonies, with greater numbers of macrophage/granulocyte colonies and fewer erythroid colonies relative to Lin− Sca-1+ pluripotent stem cells (Table I; reference 2). These data, together with the stromal cell cultures described in Fig. 3 B, indicate that loss of myeloid and erythroid precursor potential occurs before DH-JH rearrangements. Finally, we tested whether this process is independent of VHDHJH recombination using methylcellulose cultures initiated with cells from Rag-1−/− mice. The inability of cells within Frs. A1 and A2 to give rise to the myeloid and erythroid lineage was unaffected by the Rag-1 mutation, showing that loss of myeloid/erythroid precursor potential occurs independent of VHDHJH recombination (Table I).
Table I.
Capacity of Defined Precursor Populations from C57BL/6 and Rag-1− /− Mice to Differentiate into Myeloid and Erythroid Lineage Cells
| Mouse | Cells cultured | CFU-GM | CFU-BFE | CFU-GEMM | ||||
|---|---|---|---|---|---|---|---|---|
| C57BL/6 | Stem | 26.4 (5.0) | 6.80 (1.2) | 8.20 (1.5) | ||||
| A0 | 14.2 (1.4) | 1.70 (0.5) | 0.68 (0.15) | |||||
| A1 | 0 | 0 | 0 | |||||
| A2 | 0 | 0 | 0 | |||||
| Rag-1−/− | A0 | 15.1 (1.17) | 1.58 (0.15) | 0.89 (0.51) | ||||
| A1 | 0 | 0 | 0 | |||||
| A2 | 0 | 0 | 0 |
2,000 (stem, Lin− Sca-1+) or 4,000 (Frs. A0–A2) sorted cells were cultured in methylcellulose medium optimized for myeloid and erythroid cell types. After 12 d, cultures were scored for the number of granulocytic/macrophage (CFU-GM), erythroid (CFU-BFE), and mixed (CFU-GEMM) colonies. Means for triplicate cultures are shown with SDs in parentheses. Data are normalized for 1,000 input cells/culture.
Loss of NK and T Lymphoid Progenitor Activity Occurs before and Independently of VDJ Recombination.
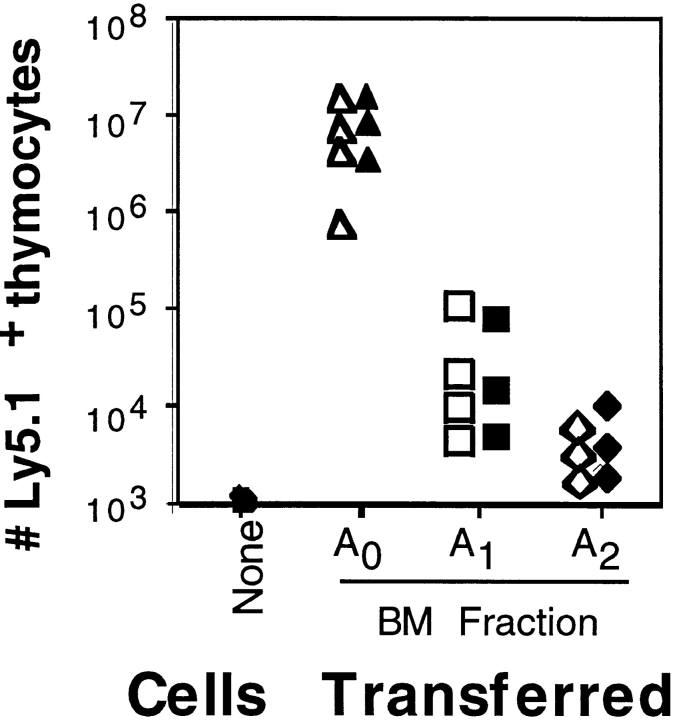
Since low surface expression of CD4 has been associated with a thymocyte precursor population in adult BM (18, 19) and with the earliest T lineage precursor cell in adult thymus (6), we reasoned that Frs. A0 and/or A1 might also contain T lineage progenitors. To determine T lineage progenitor activity within Frs. A0–A2 and to test whether IgH recombination influences B–T lymphoid lineage commitment, we performed intrathymic transfers with cells from C57BL/6 or JH −/− mice (both H-2b, Ly5.1) into B6.Ly5.2 recipients. As shown in Fig. 4, Fr. A0 exhibited clear thymic precursor activity whether taken from C57BL/6 or JH −/− mice. Separate experiments demonstrated similar thymic reconstitution potentials between Fr. A0 and early thymocyte pro-T cells (6), and thymi repopulated with Fr. A0 contained conventional CD4+CD8+CD3low double positives and CD3high single-positive thymocytes (not shown). In sharp contrast, no T lineage progenitor activity was observed when thymi were injected with equivalent numbers of cells from Fr. A1 or A2, regardless of whether these populations were taken from C57BL/6 or JH −/− mice (Fig. 4). Moreover, we were unable to amplify T lineage genes such as pre-Tα and Cβ using cDNA from Frs. A0–A2 (not shown). Finally, consistent with a lack of NK cell progenitors in Frs. A0–A2, these populations failed to proliferate in cultures supplemented with IL-2 compared with the BM NK cell progenitors described by Rolink et al. (17). Together, these data indicate that pre–pro-B cells, defined as Frs. A1 and A2, lack NK and T lymphoid as well as myeloid and erythroid lineage precursor cells, demonstrating that lineage restriction in developing B lineage progenitors occurs before and independently of VHDHJH recombination.
Figure 4.
T lymphocyte progenitor activity in Frs. A0, A1, and A2 from C57BL/6 or JH −/− mice. Intrathymic transfers of the indicated BM fraction 19 d after transfer. 104 sorted cells from C57BL/6 (open symbols) or JH −/− (filled symbols) (both Ly5.1+) mice were transferred into each irradiated B6.Ly5.2 recipient, and the number of Ly5.1+ thymocytes was determined.
Discussion
Although previous studies have defined multipotent progenitor populations in BM (2, 20–22), fetal liver (3–5, 23), and the thymus (6), until now the lineage potential of developing B lymphocyte progenitors has not been addressed. Here we show that the earliest B lineage progenitor populations, termed Frs. A1 and A2 and defined in part by μ0 expression but lack of DH-JH rearrangements, are composed largely if not entirely of B lineage–restricted precursors. This conclusion is supported by their homogeneous expression of multiple cell surface molecules (Fig. 2), their lack of expression of myeloid and T lymphoid–specific genes (Fig. 3 A, and data not shown), their inability to give rise to myeloid, erythroid, and non-B lymphoid lineage cells (Figs. 3 and 4), and their near 100% expression of an IgH transgene (8). Finally, we show that mechanisms controlling B lineage restriction do not require VHDHJH rearrangements, since commitment was unaffected in early B lineage progenitors taken from mice incapable of initiating this process.
Significantly, although others have shown that pro-B cells incapable of VHDHJH recombination can be driven to mature in vitro (24), such experiments did not test whether pro-B cells can give rise to alternative lineages or whether IgH rearrangements play a role in this process. Similarly, although mutations in transcription factors such as E12/E47 that result in impairment of DH-JH rearrangements have been said to affect B lineage commitment per se (25), whether these mutations affect B lineage commitment versus differentiation or survival of early B lineage progenitors remains to be determined. However, our data do not exclude a connection between mechanisms controlling B lineage commitment and other events associated with very early B lymphopoiesis such as alteration of chromatin structure at the IgH locus and initiation of μ0 expression.
The definition of B lineage progenitor populations lacking both DH-JH rearrangements and myeloid lineage potential also has implications for the origins of natural and induced myelogenous leukemias containing IgH rearrangements (10–13). While one interpretation of these observations was that these cells arose from bipotential myeloid/B lymphoid precursors after DH-JH rearrangement, an alternative explanation supported by our data is that such cells are the result of oncogene-induced lineage instability among pro-B cells. Similarly, cells within Fr. A1 share many characteristics with a novel lymphoid tumor derived from Eμ-myc × Eμ–bcl-2 transgenic mice (26). These tumor cells, like Fr. A1, are B220+ CD4+ HSA−, and express μ0 but lack IgH rearrangements. However, unlike Fr. A1, these tumors exhibit myeloid and B lymphoid progenitor activity. These observations, together with our data, suggest that coordinated expression of myc and bcl-2 within Fr. A1 disrupts lineage commitment in otherwise lineage-restricted pre–pro-B cells.
In conclusion, the differentiation of pluripotent stem cells into lineage-restricted progenitors for each hematopoietic lineage is a complex and highly controlled process. This complexity is reflected in part by the presence of multiple discrete cell populations with varying levels of multilineage progenitor activity, the existence of key transcriptional regulatory proteins such as Pax-5/BSAP, EBF, E12/47, and Ikaros, important for the differentiation or survival of unique progenitor populations (27), and potential differences in soluble factors important for multilineage and lineage-restricted cells (28). A reassessment of those studies vis a vis the data presented here, together with a description of the developmental relationship between pre–pro-B cells and the recently described common lymphoid progenitor population in adult BM (7), should eventually yield an understanding of the cellular and molecular interactions responsible for B lineage commitment.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI-26782, AI-40946, and CA-06972). D. Allman is supported by an award from the Arthritis Foundation.
Footnotes
The authors wish to acknowledge the expert technical assistance of Ms. Susan Shinton, and thank Dr. Avinash Bhandoola for instruction in intrathymic transfers. We also thank Drs. Michael Cancro, Dietmar Kappes, Jennifer Punt, and David Wiest for critical review of this manuscript.
References
- 1.Kee BL, Paige CJ. Murine B cell development: commitment and progression from multipotential progenitors to mature B lymphocytes. Int Rev Cytol. 1995;157:129–179. doi: 10.1016/s0074-7696(08)62158-0. [DOI] [PubMed] [Google Scholar]
- 2.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells [published erratum in 244(4908):1030] Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 3.McKearn JP, McCubrey J, Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc Natl Acad Sci USA. 1985;82:7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan CT, McKearn JP, Lemischka IR. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990;61:953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 5.Cumano A, Paige CJ, Iscove NN, Brady G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature. 1992;356:612–615. doi: 10.1038/356612a0. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Antica M, Johnson GR, Scollay R, Shortman K. Developmental potential of the earliest precursor cells from the adult mouse thymus. J Exp Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 8.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Principato M, Cleveland JL, Rapp UR, Holmes KL, Pierce JH, Morse HC, III, Klinken SP. Transformation of murine bone marrow cells with combined v-raf-v-myc oncogenes yields clonally related mature B cells and macrophages. Mol Cell Biol. 1990;10:3562–3568. doi: 10.1128/mcb.10.7.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seremetis SV, Pelicci PG, Tabilio A, Ubriaco A, Grignani F, Cuttner J, Winchester RJ, Knowles DM, II, Dalla-Favera R. High frequency of clonal immunoglobulin or T cell receptor gene rearrangements in acute myelogenous leukemia expressing terminal deoxyribonucleotidyltransferase. J Exp Med. 1987;165:1703–1712. doi: 10.1084/jem.165.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinken SP, Alexander WS, Adams JM. Hemopoietic lineage switch: v-raf oncogene converts Eμ-myc transgenic B cells into macrophages. Cell. 1988;53:857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- 13.Holmes KL, Pierce JH, Davidson WF, Morse HC., III Murine hematopoietic cells with pre-B or pre-B/ myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986;164:443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 16.ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 17.Rolink A, ten Boekel E, Melchers F, Fearon DT, Krop I, Andersson J. A subpopulation of B220+cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J Exp Med. 1996;183:187–194. doi: 10.1084/jem.183.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antica M, Wu L, Shortman K, Scollay R. Thymic stem cells in mouse bone marrow. Blood. 1994;84:111–117. [PubMed] [Google Scholar]
- 19.Chervenak R, Dempsey D, Soloff R, Wolcott RM, Jennings SR. The expression of CD4 by T cell precursors resident in both the thymus and the bone marrow. J Immunol. 1993;151:4486–4493. [PubMed] [Google Scholar]
- 20.Wineman JP, Gilmore GL, Gritzmacher C, Torbett BE, Muller-Sieburg CE. CD4 is expressed on murine pluripotent hematopoietic stem cells. Blood. 1992;80:1717–1724. [PubMed] [Google Scholar]
- 21.Onishi M, Nagayoshi K, Kitamura K, Hirai H, Takaku F, Nakauchi H. CD4dull+hematopoietic progenitor cells in murine bone marrow. Blood. 1993;81:3217–3225. [PubMed] [Google Scholar]
- 22.Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cumano A, Paige CJ. Enrichment and characterization of uncommitted B-cell precursors from fetal liver at day 12 of gestation. EMBO (Eur Mol Biol Organ) J. 1992;11:593–601. doi: 10.1002/j.1460-2075.1992.tb05091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 25.Bain G, Robanus EC, Maandag, te Riele HP, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 26.Strasser A, Elefanty AG, Harris AW, Cory S. Progenitor tumours from Eμ-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cell survival. EMBO (Eur Mol Biol Organ) J. 1996;15:3823–3834. [PMC free article] [PubMed] [Google Scholar]
- 27.Reya T, Grosschedl R. Transcriptional regulation of B-cell differentiation. Curr Opin Immunol. 1998;10:158–165. doi: 10.1016/s0952-7915(98)80244-6. [DOI] [PubMed] [Google Scholar]
- 28.Kee BL, Paige CJ. In vitro tracking of IL-7 responsiveness and gene expression during commitment of bipotent B-cell/macrophage progenitors. Curr Biol. 1996;6:1159–1169. doi: 10.1016/s0960-9822(02)70683-0. [DOI] [PubMed] [Google Scholar]