Abstract
It is widely accepted that cellular immune responses are induced by CD4+ T helper 1 (Th1) cells secreting interleukin (IL)-2 and interferon (IFN)-γ. Tumor immunity is often mediated by cytotoxic T lymphocytes (CTLs) whose activation is supported by Th1 cytokines. Since IL-4 directs Th2 development and has been shown to inhibit Th1-dominated responses, we assumed that IL-4–deficient (IL-4−/−) mice would develop vigorous CTL-mediated tumor immunity compared with IL-4–competent (IL-4+/+) mice. Surprisingly, IL-4−/− mice were severely impaired to develop tumor immunity to both a mammary adenocarcinoma line and a colon carcinoma line. The lack of tumor immunity in IL-4−/− mice was associated with reduced IFN-γ production, diminished levels of tumor-reactive serum IgG2a, and undetectable CTL activity, indicating a defective Th1 response in the absence of endogenous IL-4. Anti–IL-4 monoclonal antibody blocked tumor immunity in IL-4+/+ mice when administered at the time of immunization but not at the time of challenge. Additionally, tumor immunity could be induced in IL-4−/− mice, if IL-4 was provided by gene-modified cells together with immunizing tumor cells. These results demonstrate that tumor immunity requires IL-4 in the priming phase for the generation of effector cells rather than for their maintenance and exclude secondary, developmental defects in the “knockout” strain. Together, our results demonstrate a novel and previously unanticipated role of IL-4 for the generation of Th1-associated, CTL-mediated tumor immunity.
Keywords: tumor vaccination, interleukin 4, T cell immunity, interleukin 4–deficient mice
Most experimental tumors and probably many human tumors express antigens against which an immune response can be induced (1). In mice it has been shown clearly that such antigens can serve as rejection antigens, if the mice are immunized with tumor cells in an immunogenic, nontumorigenic form (2) or with tumor antigens themselves (3) and subsequently challenged with a tumorigenic dose of that tumor. Recent results from different groups have shown that tumor immunogenicity is defined operationally rather than absolutely and that it can be enhanced in various ways. Tumor immunity can be induced, at least in some models, by tumor cells engineered to express certain cytokines or the T cell costimulatory molecule B7 (4) or, more simply, by the use of adjuvants (5), repeated immunizations (6), or with larger numbers of tumor cells (7). The cellular requirements for induction of tumor immunity seem to be quite similar regardless of the type of immunization. Rejection of MHC class I+, class II− tumors (like the ones used in this study) often requires CD8+ CTLs whose activation (8), and in some models maintenance (3), depends on help by CD4+ T cells. To provide help, CD4+ T cells must first be activated, which requires tumor-derived antigens to be taken up, processed, and presented by an MHC class II+ APC. The fact that activation of CTLs specific to minor H antigens (9) or surrogate tumor antigens (10, 11) similarly depends on host APCs suggests that the same APC presents antigens to CD4+ and CD8+ T cells by MHC class II and class I molecules, respectively (12). The close proximity of CD4+ and CD8+ T cells recognizing antigens on the same APC would be compatible with the assumption that help by CD4+ to CD8+ T cells occurs by providing cytokines that are supposed to act on a short distance, e.g., in the same lymph node. However, CD4+ T cell help for CTL generation must not necessarily consist in cytokine supply. Recently, it has been demonstrated that signaling through CD40 on APCs can replace the requirement for CD4+ T cells during CTL generation (13–15).
CD4+ T cell lines can be divided into two subpopulations, Th1 and Th2, based on the profile of secreted cytokines (16). Th1 cells predominantly produce IL-2 and IFN-γ and are associated with cellular immune responses such as delayed hypersensitivity or differentiation of CD8+ T cells into CTLs (17). They also induce class switch in B cells to IgG2a. Th2 cells preferentially produce cytokines such as IL-4 and IL-10, and are associated with a humoral immune response, e.g., inducing class switch to IgE and IgG1. Th1 and Th2 cells have been implicated in different models of infectious disease or autoimmunity with disease susceptibility or protection, respectively (18). IFN-γ production by Th1 cells inhibits the maturation of Th2 cells (19). Conversely, IL-4 production by Th2 cells suppresses the generation of Th1 cells (20). It is important to note that the cross-inhibitory effects of IL-4 and IFN-γ have been demonstrated mainly in vitro or in the late phase of the immune response to chronic antigen exposure (18). The decision whether an immune response is directed to Th1 or Th2 seems to depend on a number of variables (e.g., nature of the antigen, amount of antigen, type of APC, local cytokine milieu, and mouse strain) (21); however, it appears to be made rapidly. Thus, antigen-specific IgG2a or IgG1 can be detected within 5 d of immunization in a Th1- or Th2-inducing fashion (22). The cytokine requirements for induction of the Th1 pathway in the early phase are not completely understood. However, a few reports have challenged the assumption of a simple inverse relationship between IL-4 and IFN-γ production in vivo. Transgenic animals constitutively overexpressing small amounts of IL-4 have elevated, not decreased, IFN-γ mRNA levels (23). In an in vitro rat model of activated T cell blasts, IL-4–stimulated IFN-γ synthesis early in the culture but inhibited the development of IFN-γ–producing T cells (24). Kamogawa et al. showed, in a transgenic mouse model that allowed ablation of IL-4 producing cells, that IFN-γ producing cells were also concomitantly deleted (25). The interpretation was that Th1 and Th2 cells were derived from a common precursor. The question whether early IL-4 expression is required for the generation of Th1 cells could not be addressed with these mice. Using BALB/c IL-4−/− mice along with IL-4 reconstitution experiments, we show that the absence of IL-4 in the priming phase results in disabled Th1 generation and CTL development and defective tumor immunity.
Materials and Methods
Mice.
BALB/c mice were purchased from Bomholtgaard Breeding & Research Centre, Denmark. IL-4-deficient mice (IL-4−/−) were generated by targeted disruption of the IL-4 gene in embryonic stem cells derived from BALB/c mice (26) and produced in a manner such that they can be considered IL-4–congenic to BALB/c. The deficiency of the IL-4 gene was confirmed by PCR of tail DNA as previously described (27). All mice used in the experiments were 6–12-wk-old and were sex matched. Heterozygous control littermates were also included in some vaccination experiments and showed no significant differences in tumor rejection compared with BALB/c mice that were commercially obtained (data not shown).
Cell Lines.
TS/A is a spontaneous mammary adenocarcinoma cell line (28) and CT-26 is an N-nitroso-N-methylurethane–induced colon carcinoma (29), both syngeneic to BALB/c. Both tumor cell lines express MHC class I but not MHC class II molecules (data not shown). NIH-3T3-IL-4 cells were generated by transfection of NIH-3T3 with an expression vector encoding the cDNA coding region of murine IL-4. 106 NIH-3T3-IL-4 cells produce 130 U/ml within 24 h of culture (data not shown). Biological activity of IL-4 was determined by a proliferation assay with the IL-4–dependent cell line CT.4S (30). 1 U of IL-4 was defined as the amount of IL-4 required to obtain half-maximal proliferation. YAC-1 is an MHC-deficient NK cell target cell line (31). All cell lines were cultured in RPMI-1640 with 10% FCS.
Tumor Vaccination Experiments.
For vaccination experiments, cells of the indicated lines were washed twice in Dulbecco's PBS, irradiated (100 Gy), and injected subcutaneously in a vol of 0.2 ml Dulbecco's PBS in the middle of the left flank. 2 wk after immunization, viable tumor cells were injected contralaterally. Mice bearing tumors of 1 cm in diameter were scored as tumor positive. Tumor incidence is expressed as percentage of tumor-free mice.
Serum Ig Detection and Analysis of Tumor-specific IgG2a.
Mice were immunized twice with 106 irradiated (100 Gy) CT-26 cells on days 0 and 21. Serum Ig levels before and 14 d after the second immunization were determined by ELISA. Relative serum levels of IgG1 and IgG2a were measured using the Ig isotyping kit according to the manufacturer's recommendation (PharMingen, Germany). For IgE detection, a pair of commercially available mAbs and the appropriate mouse IgE standard were used (all from PharMingen). For detection of tumor-reactive IgG2a, 106 CT-26 cells were incubated with sera (1:20 dilutions), rat anti– mouse IgG2a (PharMingen), and PE F(ab′)2 goat anti–rat Ig (Immunotech, France), followed by flow cytometric analysis using Coulter EPICS-XL (Coulter Electronics GmbH, Germany). Fold above background was determined by dividing the median fluorescence of a stained sample by that of cells treated identically but without serum.
Cellular Cytotoxicity.
For the generation of CT-26–specific CTLs, mice were immunized twice with 106 irradiated (100 Gy) CT-26 cells on days 0 and 21. 14 d after the second injection, single cell suspensions of splenocytes were prepared and red blood cells were removed by NH4Cl treatment. Splenocytes were cultured at 2 × 106/ml with irradiated (100 Gy) CT-26 cells at a responder/stimulator cell ratio of 30:1 in RPMI-1640 plus 10% FCS, penicillin/streptomycin, MEM, 2-ME (50 μM), and 50 U/ml IL-2 (Boehringer Mannheim, Germany). After 5 d of culture, responder cells were harvested, washed twice, and incubated with 51Cr (1 mCi/ml; NEN)-labeled CT-26 or YAC-1 cells at different E/T cell ratios. After an incubation period of 4.5 h, supernatants were assayed for radioactivity on a gamma counter (Top Count; Packard). The percentage of specific lysis was calculated as [(sample cpm − spontaneous cpm) / (maximal cpm − spontaneous cpm)] × 100%. Spontaneous release was <19%.
IFN-γ Detection.
For IFN-γ detection, mice were immunized as described above. Splenocytes of immunized mice were cultured in 24-well plates at 2 × 106/ml in the presence of Con A (2.5 μg/ml) for 3 d and supernatants were collected. CD8+ T cells were depleted by negative selection with anti-CD8a (53-6.7), followed by separation using magnetic beads coated with sheep anti–rat IgG (Dynal A.S., Norway). Depletion was checked by cytofluorimetric analysis using PE-conjugated anti-CD8a (53-6.7) (PharMingen). Culture supernatants were assayed for IFN-γ by ELISA using commercially available reagents (all from PharMingen). The detection limit was 30 pg/ml.
In Vivo Neutralization of IL-4.
Groups of IL-4+/+ mice were immunized with 5 × 105 irradiated (100 Gy) TS/A cells and challenged 14 d later with 105 of viable TS/A cells. Treatment with the IL-4–neutralizing mAb 11B11 was done by intraperitoneal injections of 4 mg in 0.2 ml every 2 d from day −2 to 6 of immunization or challenge, respectively. Animals of the control group received Dulbecco's PBS.
Results
Induction of Tumor Immunity Is Impaired in IL-4−/− Mice.
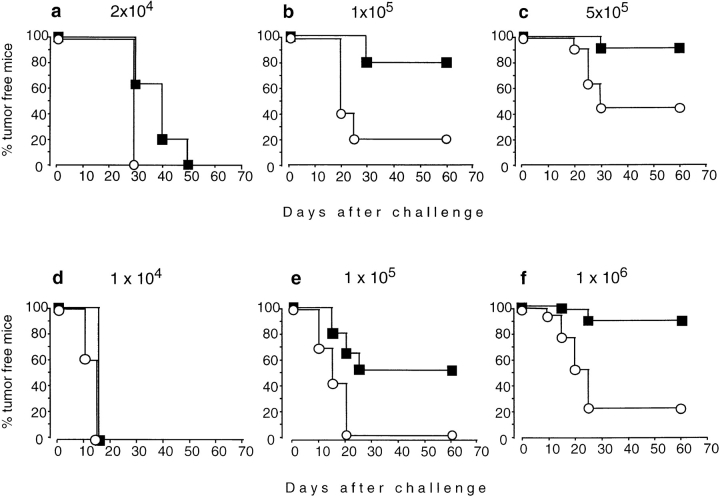
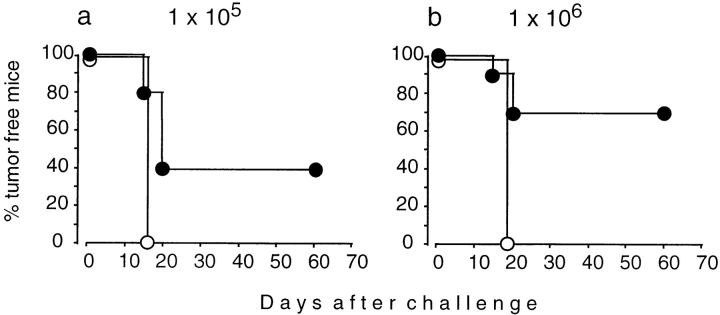
The ability to induce tumor immunity with nonproliferating tumor cells can depend on several variables, one of which is the number of tumor cells. To titrate the number of immunizing cells capable of inducing tumor immunity against the mammary adenocarcinoma TS/A, IL-4+/+ and IL-4−/− mice were immunized with increasing numbers of irradiated cells. 2 wk later, the mice were challenged with a constant number of TS/A cells sufficient to give rise to tumors in 100% of naive mice (Fig. 1, a–c). At the lowest immunization dose, 100% of IL-4+/+ and IL-4−/− mice developed tumors. With increasing vaccination doses, IL-4+/+ mice rejected the challenge tumor, whereas IL-4−/− mice were consistently less protected. These results were confirmed with a second tumor (CT-26; Fig. 1, d–f). 100% of challenge tumors grew in mice of both genotypes after immunization with the lowest cell number of CT-26. Increasing immunization doses induced protection in the majority of IL-4+/+ mice, whereas almost all IL-4−/− mice were unable to reject the challenge tumor. The defect of IL-4−/− mice to reject the challenge tumor was not due to generally enhanced tumor growth, because TS/A and CT-26 cells grew with similar kinetics in naive IL-4+/+ and IL-4−/− mice (Fig. 2). Therefore, IL-4 is required for the generation of protective tumor immunity.
Figure 1.
Generation of tumor immunity is impaired in IL-4−/− mice. IL-4+/+ (▪) and IL-4−/− mice (○) were immunized subcutaneously with irradiated TS/A (a–c) or CT-26 (d–f) with cell numbers as indicated. 2 wk later, mice were challenged contralaterally with 105 TS/A (a–c) or 106 CT-26 cells (d–f). Numbers of mice per group were 5 (a, b, d) or 15–18 (c, e, f; combined results from two to three experiments).
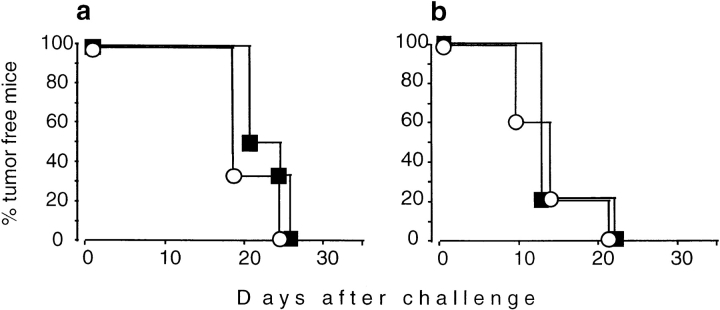
Figure 2.
Tumor growth does not differ in naive IL-4+/+ (▪) and IL-4−/− mice (○). Animals were injected subcutaneously with 105 viable TS/A cells (a; n = 6) or 106 viable CT-26 cells (b; n = 5). The tumor size was measured twice a week.
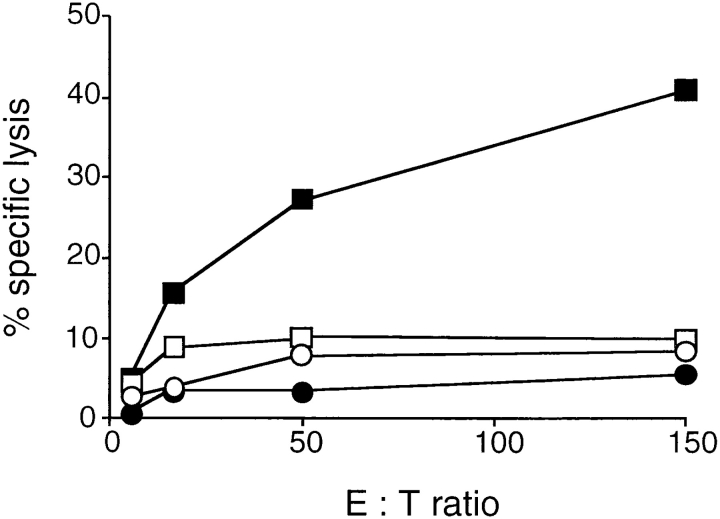
IL-4−/− Mice Have a Defective CTL Response.
Immunization with CT-26 induces tumor-reactive CTLs that are able to confer protection against challenge tumors (32). To find whether the defective tumor immunity in IL-4−/− mice was associated with reduced CTL activities, IL-4+/+ and IL-4−/− mice were immunized with CT-26 cells and tumor-specific lysis was measured. CTL activity of IL-4−/− splenocytes was undetectable, whereas splenocytes of IL-4+/+ mice contained substantial CTL activity (Fig. 3). Cytolytic activity against the NK target YAC-1 was negligible in spleen cells from both mouse strains, suggesting that lysis of CT-26 by IL-4+/+ CTLs was specific. Additionally, immunization with β-galactosidase–expressing TS/A cells resulted in clearly reduced β-galactosidase-specific CTL-activity in IL-4−/− mice (data not shown).
Figure 3.
The generation of cytotoxic T cells is impaired in IL-4−/− mice. IL-4+/+ (squares) and IL-4−/− mice (circles) were immunized twice subcutaneously at day 0 and day 21 with 106 irradiated CT-26 cells. 2 wk after the second injection (day 35), spleen cells were restimulated in vitro with CT-26 cells. After 5 d of culture, CTL activities of IL-4+/+ and IL-4−/− splenocytes were measured in a 4.5-h Cr- release assay. Lysis of CT-26 (filled symbols) and YAC-1 cells (open symbols) is shown.
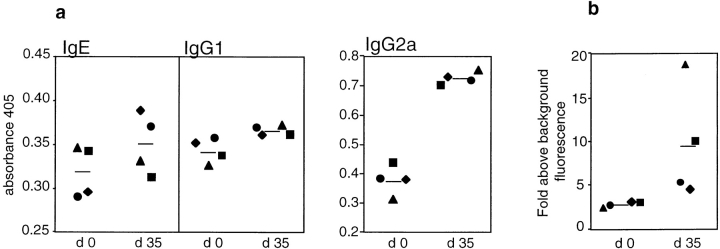
Tumor Immunity in IL-4+/+ Mice Is Associated with a Th1 Response.
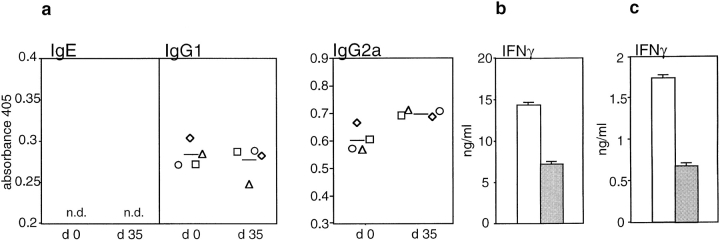
Changes in serum Ig isotype levels are an indication for ongoing Th1 or Th2 responses in vivo. We have shown that immunity to TS/A cells requires CD4+ T cells to be present during the priming phase (8). Similarly, immunization with recombinant vaccinia virus encoding β-galactosidase elicited maximal therapeutic effects to CT-26–β-galactosidase cells through the involvement of CD4+ T cells (33). Therefore, we analyzed total serum levels of different Ig isotypes before and after immunization of IL-4+/+ mice with CT-26 to evaluate if tumor immunity was associated with a dominant cytokine response (Fig. 4 a). Amounts of IgE and IgG1 remained largely unaltered, whereas IgG2a was significantly increased. To detect IgG2a antibodies reacting with tumor cells, CT-26 cells were stained with the same sera and the binding efficiency was measured by FACS® analysis. As shown in Fig. 4 b, sera of immunized mice showed, to varying extents, elevated amounts of tumor-reactive IgG2a compared with sera of naive mice indicating IFN-γ production in response to CT-26 cells. These data show that the immunization of IL-4+/+ mice with a sufficient amount of CT-26 cells initiated a typical Th1-associated response.
Figure 4.
Tumor immunity in IL-4+/+ mice is associated with a Th1 response. IL-4+/+ mice were immunized subcutaneously at day 0 and day 21 with 106 irradiated CT-26 cells. (a) Relative amounts of the indicated Ig subtypes before (day 0) and 14 d after the second immunization (day 35) in sera of individual IL-4+/+ mice were determined by ELISA. (b) Binding of serum IgG2a to CT-26 cells is shown for the same sera as in panel a. Fold above background fluorescence was calculated by dividing the median fluorescence of a stained sample by the median fluorescence of a sample incubated only with the primary and secondary antibody. Bold lines represent mean values for each experimental group.
IL-4−/− Mice Fail to Generate a Th1-associated Antitumor Response.
Next, Ig levels in IL-4−/− mice before and after injection of irradiated CT-26 cells were measured to evaluate if impaired T helper cell responses might account for reduced tumor immunity in IL-4−/− mice (Fig. 5 a). As reported previously, IgE is undetectable in naive IL-4−/− BALB/c mice (27) and was not induced by the injection of CT-26. The amount of IgG1 was not significantly altered in response to CT-26. Surprisingly, serum concentrations of IgG2a were only slightly enhanced after vaccination, although basal levels of IgG2a were elevated in comparison to IL-4+/+ mice. These results indicate a reduced IFN-γ production upon immunization in the absence of endogenous IL-4 (Fig. 4). To confirm this assumption, we cultured splenocytes of immunized IL-4+/+ and IL-4−/− mice in the presence of Con A and measured IFN-γ in culture supernatants (Fig. 5 b). Spleen cells of IL-4−/− mice produced twofold lower amounts of IFN-γ than those of IL-4+/+ mice. Similar results were obtained with CD8+-depleted spleen cells. These data are in accordance with the measurements of serum Ig isotypes, again suggesting that endogenous IL-4 is required for the induction of a Th1 antitumor immune response.
Figure 5.
Decreased tumor immunity in IL-4−/− mice is associated with an impaired Th1 response. (a) Relative amounts of the indicated Ig subtypes were determined by ELISA before (day 0) and 14 d after the second immunization (day 35) with 106 CT-26 cells. Each symbol represents serum Ig levels of individual animals and bold lines represent mean values for each experimental group. (b) IFN-γ production by total and (c) CD8+-depleted splenocytes of IL-4+/+ (white bars) and IL-4−/− (gray bars) mice after immunization with CT-26 was compared. Spleen cells obtained 14 d after the second immunization with 106 CT-26 cells (day 35) were cultured at 2 × 106/ml in the presence of 2.5 μg/ml Con A for 3 d. Amounts of IFN-γ in culture supernatants were determined by ELISA. n.d., not detectable.
The Establishment of Tumor Immunity Requires IL-4 Production during the Priming Phase.
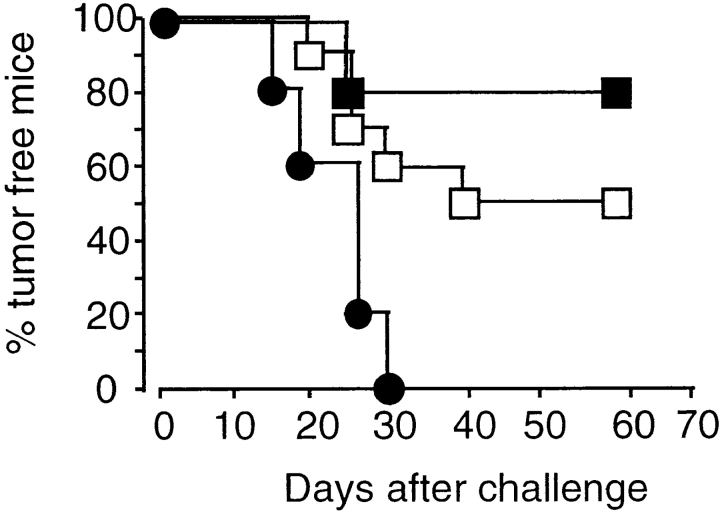
IL-4 is produced by naive CD4+ T cells under Th1 polarizing conditions in vitro within the first 24 h of antigen contact (34). Therefore, we questioned at which time IL-4 was required for the generation of tumor immunity. IL-4 was neutralized in IL-4+/+ mice by anti–IL-4 mAb during either the immunization or the challenge period. After immunization and challenge with TS/A cells, 50% of control mice but none of the animals treated with the anti–IL-4 mAb during the immunization phase rejected the challenge tumor (Fig. 6). IL-4 neutralization during the challenge phase did not impair tumor rejection. In fact, fewer mice developed a challenge tumor, the significance of which is not clear. These data indicate that memory effector cells do not require IL-4 to exert their tumoricidal activity, although their development is dependent on early IL-4 production.
Figure 6.
Generation of tumor immunity requires IL-4 during priming phase. IL-4+/+ mice were immunized with 5 × 105 irradiated TS/A cells and challenged 2 wk later with 105 TS/A cells. IL-4 was neutralized either during immunization (•, n = 5) or challenge phase (▪, n = 5) by intraperitoneal injection of mAb 11B11 at days −2, 0, 2, 4, and 6 of immunization or challenge, respectively. Mice of the control group (□, n = 10) were treated with PBS.
Exogenous IL-4 Restores Tumor Immunity in IL-4−/− Mice.
The data presented thus far do not clearly exclude that unknown developmental defects in IL-4−/− mice, such as reduced precursor frequencies of potential tumor reactive lymphocytes, rather than the absence of IL-4 during the priming phase, diminished vaccination efficiencies. To address this, IL-4−/− mice were immunized with different doses of CT-26 cells mixed with 106 IL-4–secreting NIH-3T3-IL-4 cells. As controls, IL-4−/− mice were injected with identical doses of CT-26 and parental, non-IL-4-secreting NIH-3T3. 2 wk later, mice were challenged with CT-26 cells and tumor incidence was measured. Depending on the number of CT-26 cells used for immunization, 40–70% (Fig. 7, a and b, respectively) of mice developed protective tumor immunity after immunization in the presence of exogenous IL-4, whereas none of the control animals was protected. These results show that tumor immunity can be induced in IL-4−/− mice, as long as IL-4 is provided during the immunization phase. As demonstrated for IL-4+/+ mice (Fig. 1), protection efficiency in IL-4−/− mice could also be modulated by the amount of antigen used for immunization. Therefore, these experiments suggest that IL-4−/− lymphocytes are functionally intact and emphasize the necessity of IL-4 during the T cell priming stage for the induction of tumor immunity.
Figure 7.
Exogenous IL-4 during immunization phase restores tumor immunity in IL-4−/− mice. Groups of IL-4−/− mice were immunized subcutaneously with the indicated numbers of irradiated CT-26 cells, which were mixed with either 106 NIH-3T3 (○) or 106 NIH-3T3-IL-4 (•) cells. Groups of 5 (a) or 10 mice (b) received the respective mixtures of cells. 2 wk later, mice were challenged contralaterally with 106 CT-26 cells.
Discussion
We have shown that IL-4−/− mice have a severe defect in the development of tumor immunity. Furthermore, the absence of tumor immunity in IL-4−/− mice was associated with the absence of a Th1 response to the tumor as shown by reduced serum IgG2a levels and IFN-γ production. In addition, we showed that IL-4 was required for the induction but not for the maintenance of tumor immunity. To understand this unexpected role of IL-4, the sequence of events leading to tumor immunity needs to be considered. Antigens derived from the immunizing tumor cells which are MHC class I+/II− must be taken up and processed by host APCs and presented by MHC class I and class II molecules to CD8+ and CD4+ T cells, respectively. Because CD4+ T cells are critical for the development of tumor immunity, the initial event is the activation of CD4+ T cells, which provide help for activation of CD8+ CTLs (3, 8). Like IL-4, CD4+ T cells are necessary in the priming phase. In the TS/A model, we have shown previously that once CTLs have been generated, CD4+ T cell depletion (at the time of challenge) did not impair CTL mediated tumor rejection (8). Therefore, it is likely that the defective CTL response in IL-4−/− mice is the result of defective help by Th1 cells. This help has been explained previously by providing cytokines to CTLs, probably Th1 cytokines like IL-2 or IFN-γ (17). Recently, however, it has been shown that CD4+ T cell help for CTL activation does not consist of a direct interaction between CD4+ and CD8+ T cells but rather by activation of APCs, perhaps dendritic cells (13–15). The cross-talk between CD4+ T cells and APCs is mediated by CD40–CD40 ligand interaction and T cell help for CTL generation can be replaced by in vivo application of an agonistic antibody that acts on APCs other than B cells (15). This mechanism not only obviates the need of CD4+ and CD8+ T cells to meet each other at the same APC but also the need of Th1 cytokines for CTL generation. It is possible that a similar mechanism is operative in our tumor models, a possibility that is supported by the finding that IL-4−/− mice can mount an undiminished CTL response to some viral antigens (35), which at least partially occurs independently of CD4+ T cell help (36, 37). Th1-independent CTL responses to viral antigens correlate with the observation that viruses can directly infect dendritic cells. Therefore, at the present time we cannot distinguish whether IL-4 (which is produced for a short period under Th1-polarizing conditions, see below) acts on the APC or the CD4+ T cells, or directly contributes to CTL generation during priming.
Although our results apparently contradict the Th1/Th2 paradigm, they do not if the time of IL-4 activity that is required for development of a Th1 response is considered. The inhibitory effect of IL-4 on Th1 development has been most clearly observed in vitro under conditions of prolonged addition of exogenous IL-4 or in vivo under conditions of chronic antigen exposure (18). Our results do not exclude the possibility that IL-4 has opposing effects in later stages of an antitumor response, e.g., in tumor-bearing animals. However, the fact that the development of a Th1 response and concomitant tumor immunity requires IL-4 at the time of immunization only (when T cell priming should happen) is reminiscent to the finding that CD4+ T cells cultured under Th1-polarizing conditions express IL-4 for the first 24 h (34). Also, the artificial expression of IL-4, either by tumor cells transfected to secrete IL-4 or in a transgenic mouse model, leads to induction of IFN-γ in vivo, in both T cell–competent and –deficient mice (23, 38). The importance of IFN-γ for the development of T cell–dependent tumor immunity (39) and for tumor growth inhibition of IL-4–secreting tumors has been demonstrated (38). Recently, it has been shown that IL-4−/− mice are impaired to resolve Candida albicans in the late stage of infection, which has been explained by the missing IL-4 to induce neutrophils for IL-12 production and subsequent Th1 development (40). The fact that the induction of an antitumor response induced by tumor cell–produced IL-4 is critically dependent on neutrophils (41) raises the possibility that a similar mechanism is operating during the generation of tumor immunity, although CTLs to C. albicans had not been measured.
The immunogenicity of tumor cells depends on a number of factors related to the host, e.g., the inhibitory effect of B cells (8), or to the tumor, e.g., the expression of immunosuppressive cytokines (42) or the amount of tumor cells used for immunization (Fig. 1). The failure to induce tumor immunity with low numbers of tumor cells could mean that it is simply the limiting amount of tumor- derived antigens available for the appropriate APCs, yet IL-4 is produced in a manner unrelated to the immunizing tumor cells (43), or that a certain number of tumor cells is required to induce IL-4. We favor the latter assumption, because Bosco et al. showed in the TS/A tumor model that in mice challenged with a low number of viable tumor cells which did not lead to a measurable antitumor response that tumor immunity could be induced by IL-4 injection around the tumor-draining lymph node where T cell priming probably occurs (44). IL-4 was injected for a period of 10 d, which is comparable to the estimated time of IL-4 production by the NIH-3T3-IL-4 cells, which when added to the immunizing tumor cells restored tumor immunity in IL-4−/− mice (Fig. 7). It is important to note that very low amounts of recombinant IL-4 (0.1 pg/d) were most effective (44), showing that not only the time point but also the amount of IL-4 required for induction of tumor immunity is critical. We also show that the lack of IL-4 is not compensated for by other cytokines such as IL-13 (45, 46) for the generation of tumor immunity. Our results are surprising because a number of studies in other experimental models with IL-4−/− mice showed that IL-4 was not necessary for a normal immune response, e.g., against parasites (47), viruses (35), or even diminished immune reactions, e.g., in retrovirus-induced immunodeficiency syndrome (MAIDS; reference 48). Whether this difference is due to Th-dependent CTL generation often required for tumor immunity remains to be established. In conclusion, our study reveals a novel role of IL-4 for the generation of tumor immunity that contributes to a better mechanistic understanding as a rationale for immunotherapy.
Acknowledgments
We thank M. Rösch and C. Westen for excellent technical assistance. 11B11 ascites were kindly made available by X. Cao, Second Military Medical University, Shanghai.
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF) (01 KV 9506) and the Deutsche Forschungsgemeinschaft (DFG) (Bl 288/3).
References
- 1.Van den Eynde B, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber, H. 1993. Tumor immunology. In Fundamental Immunology, Third Edition. W.E. Paul, editor. Raven Press, New York. 1143–1178.
- 3.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenstein T, Cayeux S, Qin Z. Genetic approaches to cancer immunotherapy. Rev Physiol Biochem Pharmacol. 1996;129:1–49. doi: 10.1007/3-540-61435-4_3. [DOI] [PubMed] [Google Scholar]
- 5.Hock H, Dorsch M, Kunzendorf U, Überla K, Qin Z, Diamantstein T, Blankenstein T. Vaccinations with tumor cells genetically engineered to produce different cytokines: effectivity not superior to a classical adjuvant. Cancer Res. 1993;53:714–716. [PubMed] [Google Scholar]
- 6.Cavallo F, Di Pierro F, Giovarelli M, Gulino A, Vacca A, Stoppacciaro A, Forni M, Modesti A, Forni G. Protective and curative potential of vaccination with interleukin-2-gene-transfected cells from a spontaneous mouse mammary adenocarcinoma. Cancer Res. 1993;53:5067–5070. [PubMed] [Google Scholar]
- 7.Uckert W, Kammertöns T, Haack K, Qin Z, Gebert J, Schendel DJ, Blankenstein T. Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther. 1998;9:855–865. doi: 10.1089/hum.1998.9.6-855. [DOI] [PubMed] [Google Scholar]
- 8.Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 9.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow- derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 11.Cayeux S, Richter G, Noffz G, Dörken B, Blankenstein T. Influence of gene-modified (IL-7, IL-4, and B7) tumor cell vaccines on tumor antigen presentation. J Immunol. 1997;158:2834–2841. [PubMed] [Google Scholar]
- 12.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 14.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 15.Schoenberger SP, Toes RE, van der Voort E, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 17.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 18.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 19.Fitch FW, McKisic MD, Lancki DW, Gajewski TF. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol. 1993;11:29–48. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- 20.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 21.Allen JE, Maizels RM. Th1-Th2: reliable paradigm or dangerous dogma? . Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 22.Toellner KM, Luther SA, Sze D, Choy R, Taylor DR, MacLennan I, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platzer C, Richter G, Überla K, Müller W, Blocker H, Diamantstein T, Blankenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992;22:1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- 24.Noble A, Kemeny DM. Interleukin-4 enhances interferon-gamma synthesis but inhibits development of interferon-gamma-producing cells. Immunology. 1995;85:357–363. [PMC free article] [PubMed] [Google Scholar]
- 25.Kamogawa Y, Minasi LA, Carding SR, Bottomly K, Flavell RA. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993;75:985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- 26.Noben-Trauth N, Köhler G, Burki K, Ledermann B. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 1996;5:487–491. doi: 10.1007/BF01980214. [DOI] [PubMed] [Google Scholar]
- 27.Noben-Trauth N, Kropf P, Müller I. Susceptibility to Leishmaniamajor infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 28.Nanni P, de Giovanni C, Lollini PL, Nicoletti G, Prodi G. TS/A: a new metastasizing cell line from a BALB/c spontaneous mammary adenocarcinoma. Clin Exp Metastasis. 1983;1:373–380. doi: 10.1007/BF00121199. [DOI] [PubMed] [Google Scholar]
- 29.Corbett TH, Griswold DJ, Roberts BJ, Peckham JC, Schabel FJ. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 30.Hu LJ, Ohara J, Watson C, Tsang W, Paul WE. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 31.Kiessling R, Petranyi G, Klein G, Wigzel H. Genetic variation of in vitro cytolytic activity and in vivo rejection potential of non-immunized semi-syngeneic mice against a mouse lymphoma line. Int J Cancer. 1975;15:933–940. doi: 10.1002/ijc.2910150608. [DOI] [PubMed] [Google Scholar]
- 32.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao JB, Chamberlain RS, Bronte V, Carroll MW, Irvine KR, Moss B, Rosenberg SA, Restifo NP. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996;156:3357–3365. [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-gamma-producing CD4+ T cells following activation of naive CD4+T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 35.Bachmann MF, Schorle H, Kühn R, Müller W, Hengartner H, Zinkernagel RM, Horak I. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J Virol. 1995;69:4842–4846. doi: 10.1128/jvi.69.8.4842-4846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahemtulla A, Fung LW, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 37.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platzer C, Richter G, Überla K, Hock H, Diamantstein T, Blankenstein T. Interleukin-4-mediated tumor suppression in nude mice involves interferon-gamma. Eur J Immunol. 1992;22:1729–1733. doi: 10.1002/eji.1830220710. [DOI] [PubMed] [Google Scholar]
- 39.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 40.Mencacci A, Del Sero G, Cenci E, d'Ostiani CF, Bacci A, Montagnoli C, Kopf M, Romani L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. . J Exp Med. 1998;187:307–317. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noffz G, Qin Z, Kopf M, Blankenstein T. Neutrophils but not eosinophils are involved in growth suppression of IL-4-secreting tumors. J Immunol. 1998;160:345–350. [PubMed] [Google Scholar]
- 42.Qin Z, Noffz G, Mohaupt M, Blankenstein T. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J Immunol. 1997;159:770–776. [PubMed] [Google Scholar]
- 43.Lange C, Schüler T, Blankenstein T. Interleukin 4 gene-defective mice reconstituted with wild-type bone marrow fail to produce normal immunoglobulin E levels. J Exp Med. 1998;187:1487–1493. doi: 10.1084/jem.187.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosco M, Giovarelli M, Forni M, Modesti A, Scarpa S, Masuelli L, Forni G. Low doses of IL-4 injected perilymphatically in tumor-bearing mice inhibit the growth of poorly and apparently nonimmunogenic tumors and induce a tumor-specific immune memory. J Immunol. 1990;145:3136–3143. [PubMed] [Google Scholar]
- 45.Urban J, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. . Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 46.Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–672. doi: 10.1016/s0960-9822(98)70256-8. [DOI] [PubMed] [Google Scholar]
- 47.von der Weid T, Kopf M, Köhler G, Langhorne J. The immune response to Plasmodium chabaudimalaria in interleukin-4-deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 48.Kanagawa O, Vaupel BA, Gayama S, Koehler G, Kopf M. Resistance of mice deficient in IL-4 to retrovirus-induced immunodeficiency syndrome (MAIDS) Science. 1993;262:240–242. doi: 10.1126/science.8211142. [DOI] [PubMed] [Google Scholar]