Abstract
Unmutated tumor antigens are chosen as primary candidates for tumor vaccine because of their expression on multiple lineages of tumors. A critical issue is whether unmutated tumor antigens are expressed in normal cells, and if so, whether such expression imposes special restrictions on cytotoxic T lymphocyte (CTL) responses. In this study, we use a transgenic approach to study the development and effector function of T cells specific for P1A, a prototypical unmutated tumor antigen. We report here that although P1A is expressed at low levels in normal tissues, including lymphoid tissues, the P1A-specific transgenic T cells develop normally and remain highly responsive to the P1A antigen. The fact that transgenic expression of P1A antigen in the thymus induces T cell clonal deletion demonstrates that normal hematopoietic cells can process and present the P1A antigen and that P1A-specific T cells are susceptible to clonal deletion. By inference, P1A-specific T cells must have escaped clonal deletion due to low expression of P1A in the thymus. Interestingly, despite the fact that an overwhelming majority of T cells in the T cell receptor for antigen (TCR)–transgenic mice are specific for P1A, these mice are no more resistant to a P1A-expressing plasmocytoma than nontransgenic littermates. Moreover, when the same TCR-transgenic mice were challenged simultaneously with B7-1+ and B7-1− tumors, only B7-1+ tumors were rejected. Therefore, even though P1A can be a tumor rejection antigen, the effector function of P1A-specific CTL is restrained in vivo. These results have important implications for the strategy of tumor immunotherapy.
Keywords: cytotoxic T lymphocytes, unmutated tumor antigen, T cell development, antitumor immunity, T cell receptor–transgenic mice
Afundamental question in tumor immunology is how tumor antigens induce T cell responses. Even though the existence of tumor antigens recognized by syngeneic T cells has been well demonstrated (reviewed in reference 1), the fundamental issue of whether these antigens are recognized as self antigen or foreign antigen remains unresolved (2, 3). Tumor antigens can be divided into two major categories: those that are mutated products of normal genes (4– 10) and those that bear no mutations (11–19). With the notable exception of mutations in several oncogenes that are directly responsible for tumorigenesis (4–7, 10), it is anticipated that the mutated tumor antigens are usually unique for individual tumors. Expression of unmutated tumor antigens, on the other hand, has been demonstrated in multiple tumors of the same lineage and among tumors of multiple lineages (12–16, 20). It has been suggested that this category of tumor antigens may offer valuable targets for tumor vaccines (21).
The basic knowledge of vaccination is derived from our experience with infectious agents, i.e., foreign antigens (22). However, tumor antigens, particularly unmutated tumor antigens, are not necessarily foreign to the immune system. Of the unmutated tumor antigens identified so far, genes encoding some of them, such as tyrosinase (23) and Pmel 17 (18), are expressed in normal tissues. Other genes, such as those for P1A in mice (11) and the MAGE and GAGE families in humans (12–16), are reportedly expressed only on tumors or perhaps in immunologically privileged sites such as testis (24, 25).
Multiple mechanisms have evolved to ensure self–nonself discrimination of T cells. T cells specific for self antigen in the thymus are deleted or rendered anergic (26, 27). In the periphery, tissue-specific antigens can also inactivate T cells by either one of these mechanisms (28–31). It has been documented that genes encoding a substantial number of unmutated tumor antigens are expressed in normal cells, but the immunological consequences have not been systematically analyzed. Two issues are particularly relevant. First, If vaccines based on unmutated antigens induce strong immunity, would such immune responses cause autoimmune disease? Although immunotherapy with tumor-infiltrating lymphocytes specific for gp100 and vaccination with GM–CSF-transduced renal carcinoma induced immune reaction to normal tissues, neither procedure resulted in severe autoimmune disease (2, 32, and 33). Immunization with unmutated tumor antigen P1A, which is expressed in murine testis, does affect fertility in male mice (24). Second, as self-reactive T cells are controlled at multiple checkpoints during the development, induction, and effector phase of T cell responses, it is of interest to determine if immune responses to unmutated tumor antigens are restrained at any of these checkpoints.
P1A was the first unmutated tumor antigen to be identified. Although it was originally described in mastocytoma cell lines (11), it was later observed that P1A is expressed in testis (24), but expression in other tissues was not reported. Using reverse transcriptase PCR (RT-PCR),1 we observed that P1A is expressed at low but significant levels in normal tissue including hematopoietic tissues. To determine whether unmutated tumor antigens can influence the development, induction, and effector function of antigen-specific T cells, we produced two types of transgenic mice: one that expresses the T cell receptor from a CTL clone that recognizes P1A, and another that overexpresses the tumor antigen P1A in hematopoietic tissues. These mouse models allowed us to monitor the effect of endogenous expression of tumor antigen on the development, induction, and effector function of T cells specific for an unmutated tumor antigen. Analysis of P1A-reactive T cells indicates that endogenous P1A antigen does not interfere with the development of P1A-specific T cells. Interestingly, the TCR-transgenic mice are no more resistant than their littermates to plasmacytoma J558. Moreover, even though immunization with P1A peptide induces an anti-P1A CTL response, it does not induce protection against P1A- expressing tumors. Thus, although P1A-specific CTL develop normally, their effector function is restrained in vivo.
Materials and Methods
Experimental Animals and Cell Lines.
BALB/cByJ, CByD2F1, C3H/J, and C57BL/6j mice were purchased from The Jackson Laboratory. Nude mice (nu/nu) were obtained from The National Cancer Institute. 6–18-wk-old male mice were used for the experiments. Plasmacytoma J558 was cultured in RPMI 1640 containing 5% FCS. J558 cells transfected with either pSRα (J558-Neo) or pSRα–πlnB7-1 (J558-B7) have been described (34). Anti-P1A CTL line P1CTL (35) was provided by Dr. Lieping Chen (Mayo Clinic, Rochester, MN).
Analysis of P1A Expression by RT-PCR.
Total RNA was isolated from testis, liver, lung, spleen, and thymus of normal BALB/c mice as well as J558 tumor cells. The first strand DNA was prepared using GAATTCTCGAGTCTAGATTTTTTTTTTTTTTTTTTT as a primer. In brief, the reaction mixture consisted of: 30 μl of total RNA (5 μg), 10 μl 5× first strand buffer, 3 μl 10 mM dNTP, 2 μl primer (100 μM), 2.5 μl H2O, and 2.5 μl RT (GIBCO BRL). Reverse transcription reactions were carried out at 42°C for 1 h. The RT was then inactivated by heating. The first strand DNA was denatured at 96°C for 1 min, and the P1A cDNA was then amplified by 40 cycles of PCR. Each cycle consisted of: 94°C for 1.5 min, 55°C for 1.5 min, and 72°C for 2 min. The following primers were used. P1A: TGGCCTGGATAGCCAGGCAAAGCAAGCGCA as forward primer and CTTCCGCTGTCCATTTCTTTCTTCTGGGCT as reverse primer; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): ATGGTGAAGGTCGGTGTGAACGGATTTGGC as forward primer and CATCGAAGGAAGAGTGGGAGTTGCTGT as reverse primer. The P1A primers used should amplify a 500-bp product from properly spliced P1A RNA. PCR product from genomic DNA should be 3.1 kb. The size of amplifed GAPDH cDNA should be 900 bp. The PCR products were separated on agarose gels and then transferred onto nylon membrane as described (36). P1A or GAPDH products were detected by 32P- labeled specific probes.
Cytotoxic T Cell Assays.
Cytotoxic T cell line P1CTL (35) specific for P1A35–43:Ld were used as effector cells and BALB/c spleen cells that had been stimulated with LPS (10 μg/ml), Con A (2 μg/ml), or anti-CD3 (0.25 μg/ml) for 48 h were used as target cells. As control, P388D1 (H-2d) pulsed with 1 μg/ml of P1A or control peptides were also used as targets. The lysis of target cells was determined by release of 51Cr after 6 h of coincubation with P1CTL. Data presented were specific lysis of target cells calculated according to the following formula: Specific lysis % = (cpm sample − cpm medium)/(cpm max − cpm medium) × 100. In other experiments, transgenic spleen cells were stimulated in vitro for 3 d with 1 μg/ml of P1A peptide and used as effectors.
Production of P1A Transgenic Mice.
We designed synthetic oligonucleotide primers corresponding to the ends of the P1A–open reading frame; the forward primer contained a HindIII restriction site and the reverse primer a ClaI site. These primers were used to amplify the P1A–open reading frame from pBS–P1A. We used the restriction sites at the end of the amplified fragment to clone it into the Eμmb–Id2 vector, a gift from Dr. Xiao-Hong Sun (New York University Medical Center, New York) which contains the IgM heavy chain enhancer (Eμ), the mb-1-promoter, an intron from the small T antigen gene, and an SV40 polyadenylation signal (37). The construct was linearized by digestion with NotI, and the fragment containing the P1A sequence was purified and used for microinjection into fertilized eggs of (B6 × D2)F1 origin.
Transgenic Mice Expressing TCR from a P1A-specific CTL Line.
Identification and cloning of the anti-P1A TCR and production of transgenic mice follow the basic procedure established by Kouskoff et al. (38). In brief, we cloned the VJ fragment of the TCR α chain, and DNA sequencing confirmed that the CTL line utilized Vα8 and J10. We used linked PCR to generate a PCR fragment containing the full length Vα8 and J10 as well as a small leading intronic sequence to ensure proper splicing of the TCR mRNA. This fragment was then inserted into a PTR-α vector provided by Dr. Mathis (Institut de Génétique et de Biologie et Cellulaire, Strasburg, France; 38). Using published primer sequences, we found that P1CTL express only Vβ1, a 10-bp D segment, Jβ2, and Cβ1. Transgenic vectors were produced as described (38). After removing the prokaryote DNA sequences, the transgenic vectors were injected into fertilized eggs from Swiss Webster (SW) mice.
Proliferation of Transgenic T Cells.
To test whether antigen-presenting spleen cells express P1A antigenic epitope, spleen mononuclear cells from BALB/c mice were depleted of T cells by two rounds of treatment with anti-Thy1.2 mAb plus complement. The T-depleted APC were cultured with either LPS (10 μg/ml) or medium alone for 16 h; after five washes with PBS, the viable spleen cells were irradiated for 2,000 rads and used as stimulator cells (2 × 105/well). T cells (6 × 104/well) purified from transgenic mice as described (36) were used as responders. After 66 h, the cultures were pulsed with 3H-TdR (1.25 μCi/ well) for 6 h. To test antigen specificity of the transgenic T cells, we added varying concentrations of P1A peptide or control NP peptide to 2 × 105/well of transgenic or nontransgenic spleen cells for 42 h and pulsed with 3H-TdR.
Tumorigenesis Assay.
Because the tumor cell lines used in this study originated from BALB/c mice, we used mice that carried at least one copy of the BALB/cByJ gene in all in vivo protection experiments to avoid immune responses against minor histocompatibility antigens. In brief, transgenic founder SW mice were crossed with BALB/cByJ mice for three generations, and the N2 mice were typed by flow cytometry for expression of the transgenic TCR-α chain using PE-conjugated anti-Vα8 mAb. Transgenic mice and their littermate controls were injected with 5 × 106 J558-B7 and J558-Neo subcutaneously in the left inguen. In other experiments, both types of tumor cells were injected into the same mice but on different sides. The tumor incidence was recorded every other day. Palpable tumors with a diameter of >3 mm were scored as positive.
Results
Tumor Antigen P1A Is Expressed at Low Levels in Normal Tissue.
To determine if the P1A gene is expressed in normal tissues, we isolated RNA from a number of normal mouse organs, including spleen, thymus, liver, lung, and testis, and measured P1A mRNA by both Northern blot and RT-PCR. Northern blot analysis did not reproducibly detect significant levels of P1A RNA expression in most tissues except in the testis (data not shown), which is consistent with an earlier study by Uyttenhove et al. (24). We therefore used the more sensitive method of RT-PCR to detect the expression of P1A RNA. The PCR products were detected by Southern blot using 32P-labeled probes. This procedure not only ensured the identity of the products amplified but also enhanced the sensitivity of the assay. The forward and reverse primers are in separate exons, and the products amplified from genomic DNA should be 3.1 kb, whereas those amplified from mRNA should be 500 bp. As shown in Fig. 1 a, a significant amount of P1A RNA was detected in most tissues by RT-PCR. The mRNA level was highest in the testis as has been reported (24). Lung and spleen tissues expressed more P1A RNA than liver tissue. Although P1A RNA can be detected in the thymus in some experiments, its thymic level was always at least 10-fold lower than in the spleen (data not shown). To compare the levels of P1A mRNA in spleen and tumor cells, we titrated the amount of cDNA prepared from either spleen or J558 tumor cells. As shown in Fig. 1 b, the relative abundance of the P1A cDNA in the spleen was ∼100– 1,000-fold lower than levels found in the tumor cells.
Figure 1.
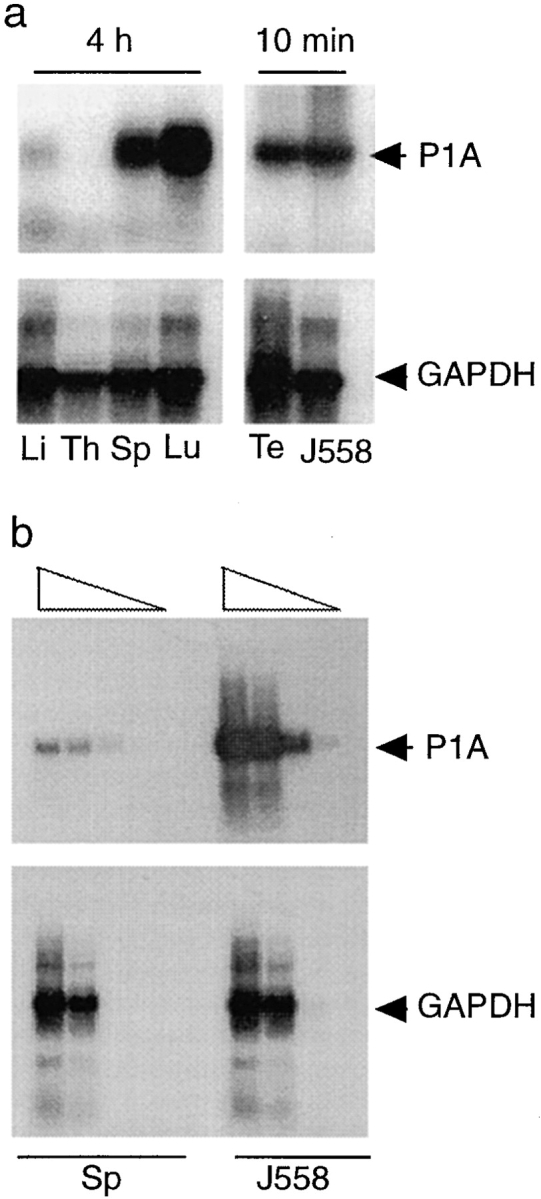
Expression of unmutated tumor antigen. (a) Expression of P1A RNA in normal tissues. Total RNA was isolated from liver (Li), thymus (Th), spleen (Sp), lung (Lu), and testis (Te) of normal BALB/c mice, and the expression of the P1A gene was determined by RT-PCR. The primers used detect a 0.5-kb fragment from mature P1A RNA but a 3.1-kb fragment from the genomic DNA. GAPDH primers were used as a control for the amount of cDNA used in each sample. The sources of RNA for different lanes are indicated at the bottom. Note that the autoradiograph of the left four lanes was exposed for 4 h, whereas that of the right two lanes was exposed for 10 min. (b) Comparison of the amounts of P1A RNA in normal spleens and J558 cells. First strand cDNA prepared from 5 μg of total RNA from either normal spleen or J558 cells was suspended in 50 μl of water. 1, 0.1, 0.01, or 0.001 μl of the cDNA was used as a template for PCR reaction using either P1A or GAPDH primers as described in Materials and Methods. 20 μl of PCR products was separated in agarose gels and the amount of specific products detected by Southern blot.
We used a P1A:Ld-specific CTL line, P1CTL, to determine if the P1A35–43:Ld epitope is presented on the normal lymphoid cells. The P1CTL was generated after immunization with P815 cells and has been restimulated in vitro for almost two years (35). All cells in the P1CTL line express the Vα8 chain on cell surface (data not shown). As shown in Fig. 2 a, P1A-specific CTL failed to kill syngeneic spleen cells that had been activated by either LPS, Con A, or anti-CD3 mAb. As a result we failed to detect P1A antigenic epitope on activated T and B cells by CTL assay. However, since direct cytolysis requires antigen expression on a substantial proportion of target cells, this assay cannot rule out the possibility that a small proportion of cells may express the antigen.
Figure 2.

Detection of P1A:Ld epitope in spleen cells by direct cytotoxicity assay (a) or proliferation assay (b). (a) Direct cytotoxicity of P1CTL to BALB/c spleen cells which were activated by LPS, Con A, or anti-CD3 mAb. Viable cells were isolated, labeled with 51Cr, and used as target cells. Macrophage P388D1 was pulsed with either P1A (P1A) or an unrelated Kd-binding peptide (KdP) and used as control. (b) Proliferation of transgenic T cells in response to T-depleted spleen antigen–presenting cells. T-depleted, BALB/c spleen cells were cultured with either medium or LPS for 16 h. Viable cells were isolated by centrifugation through a Ficoll-hypaque medium, washed five times with PBS, irradiated (2,000 rads), and used as stimulator cells. Transgenic spleen T cells from N2 of BALB/ cxSW TCR-TG founders were used as responder. Data shown were cpm incorporated by T cells when stimulated with activated APC (cpm[T+APC-A]) or resting APC (cpm[T+APC-R]). Sum of cpmT (T cells cultured alone) plus cpm– APC (APC cultured alone) was used as the negative control. Data shown were means of six replicates and the standard deviations.
We have recently produced transgenic mice that express the TCR from P1CTL. We therefore used proliferation assay to determine if antigen-presenting spleen cells from syngeneic mice can induce activation of P1A-specific T cells. As shown in Fig. 2 b, unstimulated spleen APC failed to induce proliferation of transgenic T cells. However, after stimulation with LPS, weak but significant proliferation of transgenic T cells could be detected. Thus, activated APC can present P1A epitope. The relatively weak proliferation could be due to either low frequency of APC that present P1A epitope or a low level of P1A epitope expression on all APC.
Normal Development and Induction of P1A-specific CTL.
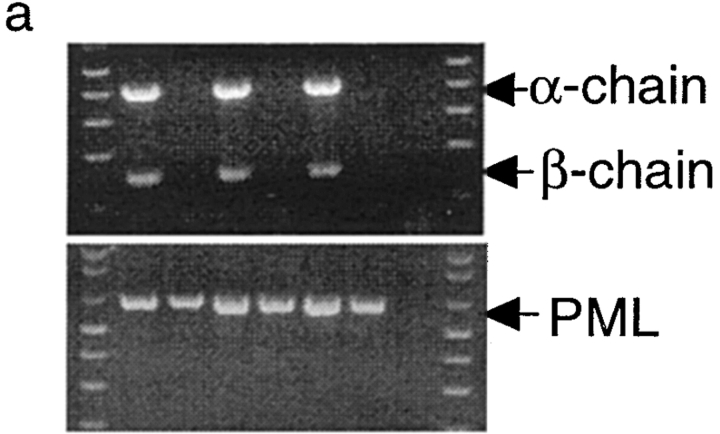
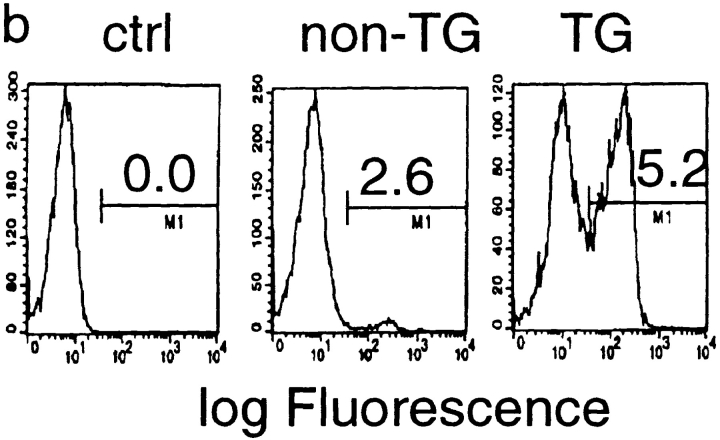
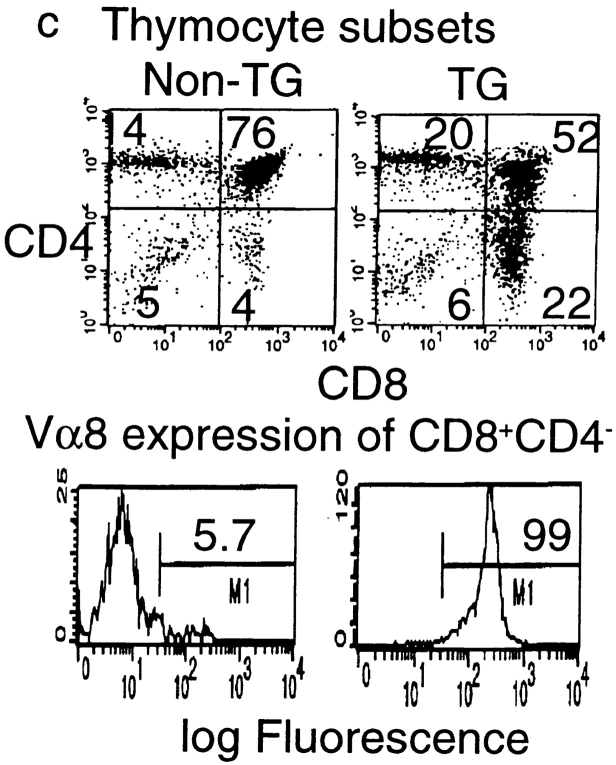
Given the fact that the P1A gene is expressed in hematopoietic cells, it is possible that endogenous expression of the P1A gene may cause either clonal deletion or clonal anergy of T cells. To analyze the development of P1A-specific T cells, we cloned the T cell receptor from a P1A-specific CTL line, P1A CTL. Using the cassette vector provided by Kouskoff et al. (38), we produced mice with integration of both α and β chains. Five independent founders were obtained that have cointegration of α and β chains, three of which are shown in Fig. 3 a. After breeding with BALB/c mice, all F1 mice that carried the transgenes expressed Vα chain on the surface of >50% of PBL (Fig. 3 b). Lacking a mAb specific for Vβ1, we were unable to directly measure expression of the β chain; however, expression of the β chains to which mAbs are available has been excluded, presumably due to transgenic expression of Vβ1 (data not shown). To determine whether endogenous expression of P1A impairs development of P1A-specific T cells, we analyzed the cellular composition of T cells in the thymus. As shown in Fig. 3 c, the transgenic mice contain a large proportion of CD4+CD8+ T cells and CD8+CD4− T cells. A skewing toward CD8+CD4− subset is consistent with the fact that transgenic TCR is restricted by MHC class I (39, 40). Moreover, essentially all CD8+CD4− T cells in the thymus express a high level of Vα8 on the surface. These results demonstrate that P1A-specific CD8+ T cells are not subject to clonal deletion in normal mice.
Figure 3.

Endogenous expression of unmutated tumor antigen does not interfere with development of transgenic T cells expressing P1A-specific TCR. (a) Cointegration of TCR α- and β-transgene in transgenic founder mice. Tail DNA from 22 independent founder mice were screened by PCR for integration of transgenes, six of which are shown. Top, integration of transgenes into mouse tail genomic DNA. PCR reactions for the two chains were carried out separately, but PCR products from the same mice were pooled and analyzed in the same lane. Bottom, the quality of the genomic DNA is confirmed by similar amplification of exon 3 of the PML gene. (b) Expression of transgenic α chain among the TCR-transgenic (TCR-TG), nontransgenic (non-TG), or control PBL. PBL were either left unstained (ctrl) or stained with FITC–labeled anti-Vα8 mAb. (c) Development of transgenic T cells in BALB/c × TCR–TG F1 mice. Thymi from TCR-TG+ and TCR-TG− mice were analyzed by three-color flow cytometry using FITC–anti-Vα8, PE–anti-CD4, and cychrome–anti-CD8 mAbs. Data presented are expression of CD4 and CD8 coreceptors among total thymocytes (top) and expression of TCR α chain among CD8+CD4− T cells (bottom). (d) CD8 T cells in the spleen expressing transgenic TCR maintain naive phenotype. Spleen cells from TCR-TG mice were analyzed by three-color flow cytometry using FITC–anti-Vα8, PE–anti-CD62L, and cychrome–anti-CD8 mAbs. Data presented are histograms of PE channel among the CD8+ Vα8− (top) and CD8+Vα8+ (bottom) cells. Numbers presented in the panels are percentages of cells that fall within the gate indicated.
To test whether endogenous P1A chronically stimulates P1A-specific T cells, we measured expression of L-selectin on T cells from the TCR-transgenic mice. L-selectin is expressed at a high level in naive T cells and is downregulated upon T cell activation (41, 42). Because antigen-experienced T cells remain L-selectinlow for a long period, this phenotype has been used as a marker for antigenic exposure of T cells. As shown in Fig. 3 d, spleen CD8 T cells that expressed the transgenic α chain are of L-selectinhigh phenotype. In contrast, in the same spleen, a substantial proportion of Vα8− CD8 T cells is of L-selectinlow phenotype. These results demonstrated that the transgenic T cells were not activated by the endogenous P1A antigen. This conclusion is consistent with the fact that in vitro syngeneic APC cannot stimulate transgenic T cells without LPS activation (Fig. 2 b).
To test whether the P1A-specific T cells are anergic, we stimulated the spleen cells from P1A-transgenic mice and their littermate controls with either P1A peptide or a control peptide from influenza virus. As shown in Fig. 4 a, spleen cells from P1A-transgenic mice mounted a vigorous, proliferative response to a low dose of P1A peptide. Maximal proliferation of T cells occurred with a dose of 10 ng/ml of peptide, indicating that the transgenic TCR must have high avidity for the tumor antigens. Moreover, after in vitro restimulation, transgenic T cells exhibited strong cytotoxic activity against P1A-expressing plasmacytoma (Fig. 4 b). Interestingly, B7-1+ tumor cells are more susceptible than their B7-1− counterparts, which is consistent with our previous observation of ex vivo, P1A-reactive CTL (34). Since J558-B7 and J558-Neo expressed comparable amounts of MHC class I (34) and P1A antigen (20), it is likely that after 4 d of in vitro stimulation, the effector function of transgenic T cells is dependent on B7 presence on the target cells.
Figure 4.
Normal induction of P1A-specific transgenic T cells. (a) Proliferation of spleen cells from transgenic and wild-type mice to tumor antigen P1A peptide or control peptide from influenza virus nucleoprotein (NP). (b) Cytotoxicity of transgenic T cells against J558-B7 or J558-Neo tumor cells. P388D1 cells (H–2d) pulsed with either P1A or NP (1 μg/ml) were used as controls. Transgenic spleen cells were stimulated for 72 h with P1A peptide and used as effectors.
Clonal Deletion of P1A-specific T Cells in Transgenic Mice Overexpressing P1A Gene in the Thymus.
The lack of clonal deletion and anergy of P1A-specific T cells is most likely due to low expression of the P1A gene in hematopoietic cells. We produced transgenic mice in which the expression of the P1A gene is under the control of mb-1 promoter and Eμ enhancer. We prepared cDNA from total thymic RNA and compared the amount of P1A mRNA in normal versus transgenic thymus by titration of cDNA. P1A mRNA was detectable in the thymi of transgenic mice after the cDNA reached 1:270 dilution, whereas 1:10 dilution of cDNA from nontransgenic littermates failed to yield any P1A product. Thus, P1A transgenic mice significantly overexpressed P1A. In fact, the levels of P1A mRNA in transgenic mice are comparable to those in J558 tumor cells (Fig. 5 a).
Figure 5.
Clonal deletion of P1A-specific transgenic T cells in mice overexpressing P1A in the thymus. (a) Overexpression of P1A mRNA in the thymus of P1A-transgenic mice (P1A-TG) as compared to that of nontransgenic mice (Non-TG). cDNA, prepared from total mRNA of J558 tumor cells, thymus P1A-TG, and Non-TG thymus, as described in the Fig. 1 legend, were diluted 1:10, 1:30, 1:90, or 1:270. 1 μl of the cDNA from each dilution was used for the PCR reaction. PCR products were transferred into nylon membrane, and the identity of the PCR products verified by 32P-labeled probes. The PCR products were not amplified from genomic DNA, as no P1A can be amplified in the absence of RT (data not shown). (b) Intrathymic clonal deletion of transgenic T cells in mice overexpressing tumor antigen in the thymus. Thymocytes (top) and spleen cells (bottom) from either TCR–TG or TCR/P1A–TG mice were analyzed by two-color flow cytometry for expression of CD4 vs. CD8 or CD8 vs. Vα8.
We bred the TCR-transgenic mouse (TG) with the P1A-TG to generate TCR/P1A–TG and analyzed the cell numbers and cell surface markers of TCR-TG and TCR/ P1A-TG thymi (Fig. 5 b). The total number of TCR/ P1A–TG thymocytes was ∼40% of that of TCR-TG (data not shown). In addition, a discrete population of CD8+ CD4− T cells in the TCR–TG is replaced by a population of cells that has downregulated both CD4 and CD8 coreceptors. This progressive downregulation of CD8 receptors is more obvious among the thymocytes that have expressed the highest level of transgenic TCR. Moreover, the relative number of CD4+CD8+ T cells is greatly reduced, and, as a result, an increase in the percentage of CD4−CD8− T cells is observed. These results demonstrate that P1A-specific T cells are being deleted in the TCR/P1A–TG. Consequently, the CD8+Vα8+ T cells are reduced by >80% in the TCR/P1A–TG compared to TCR-TG. Thus, transgenic T cells specific for unmutated tumor antigen are susceptible to clonal deletion in the thymus. Under normal circumstances, these T cells must have escaped clonal deletion because of low or no P1A expression in the thymus.
The Effector Function of P1A-specific CTL Is Restrained In Vivo.
To test whether the transgenic, P1A-specific cytotoxic T cells can efficiently reject the J558 tumor cells, we bred the transgenic founder mice with BALB/cByJ for three generations and tested the tumorigenicity of B7-transfected (J558-B7) or vector-transfected (J558-Neo) tumor cells in the N2 mice. Since all N2 mice carry at least one copy of the BALB/c genes, minor histoincompatibility should not cause tumor rejection. As shown in Table I, despite the fact that an overwhelming majority of the T cells in the transgenic mice express the P1A-specific T cell receptor, TCR-TG+ mice were no more resistant to the plasmacytoma than their TCR-TG− littermates. The rejection of J558-B7 is mediated by T cells, as J558-B7 and J558-Neo gave similar tumor onset (not shown) and incidences (Table I) in nu/nu mice.
Table I.
Transgenic Mice Rejected B7-1+ but Not B7-1− J558 Tumor Cells
| Mice | Tumors | Mice with tumors/ mice without tumors | ||
|---|---|---|---|---|
| TCR-TG+ | J558-B7 | 0/3 | ||
| J558-Neo | 5/9 | |||
| TCR-TG− | J558-B7 | 1/4 | ||
| J558-Neo | 4/8 | |||
| nu/nu | J558-B7 | 3/4 | ||
| J558-Neo | 3/4 |
N2 of BALB/cByJ × TCR−TG+ SW founder were screened for transgenic expression of the transgenic receptor by flow cytometry. Staining with PE–conjugated anti-Vα8 mAb revealed that ∼50% of PBL in the TG+ mice expressed the Vα8, whereas TG− mice have <2% of the Vα8+ T cells in the PBL. 6–12-wk-old mice were inoculated in the left inguen with 5 × 106 B7-1+ (J558-B7) or B7-1− (J558-Neo) tumor cells. Tumor incidences were determined by physical examination with a 2–3-d interval. Data presented are tumor incidences at 25–27 d after tumor cell inoculation.
As the J558-Neo tumor lacks costimulatory activity that is essential for T cell activation, it is possible that lack of tumor rejection is due to poor activation of the cytotoxic T cells in vivo. To rule out this possibility, we injected J558-B7 in the right inguens and J558-Neo in the left inguens of the same mice and monitored the tumor incidence. As shown in Table II, most of the mice that rejected J558-B7 tumors developed J558-Neo tumors. Both incidence (Table II) and growth kinetics (data not shown) of the J558-Neo tumor were unaffected by the J558-B7 injected into the same mice. Thus, the failure of the TCR-TG mice to reject J558-Neo tumors cannot be attributed to a lack of T cell activation in vivo, and the results presented in this section indicate that the effector function of the transgenic T cell is restrained in vivo.
Table II.
Mice that Rejected B7+ Tumors Did Not Reject B7− Tumors
| Mice | Mice with tumors/mice without tumors | |||
|---|---|---|---|---|
| Right inguen (J558-B7) | Left inguen (J558-Neo) | |||
| TCR-TG+ | 0/7 | 4/7 | ||
| TCR-TG− | 1/6 | 4/6 | ||
The experiment was carried out as described in the Table I legend except that the same mice were injected with J558-B7 in the right inguen and J558-Neo in the left inguen.
Discussion
Successful identification of tumor antigens marks the first important step toward the development of tumor vaccines. However, much of our current understanding of vaccination is based on experience with infectious agents that are immunologically distinct from self antigens (22). Even though such knowledge may be directly applicable to mutated tumor antigens (4–10) that are, by definition, nonself, a major issue is whether such approaches can be directly applicable to unmutated antigens, many of which may turn out to be self antigens (11–19). To address this important issue, we used P1A as a model tumor antigen and took a transgenic approach to study the development and effector function of P1A-specific T cells. We have made two major observations. First, endogenous expression of tumor antigen does not interfere with the development and induction of P1A-specific CTL. P1A was the first unmutated tumor antigen to be identified. Although it was originally described as expressed only in mastocytoma, later studies indicated that it is expressed in testis (24) and in other lineages of tumors (20). Here we used RT-PCR and were able to reproducibly detect P1A mRNA in normal tissues, including hematopoietic tissues. Two earlier studies based on Northern blot analysis and RT-PCR analysis of P1A RNA have concluded that in tissues other than testis, the level of P1A mRNA is <1% of that in P815 cells (24). Our finding that P1A RNA in the spleen is ∼100-fold lower than that of J558 tumor cells does not contradict findings in earlier reports. Moreover, though resting APC fail to activate P1A-specific T cells, significant proliferation of transgenic T cells is induced by activated syngeneic APC. Nevertheless, P1A is unlikely to be expressed on a large proportion of spleen cells. If so, it would have been detected by CTL assay using anti-P1A CTL, which generally requires less ligand but a wider distribution of antigen among the target population than is required for proliferation or IL-2 production (43).
Given the expression of the unmutated tumor antigen in normal tissues, it is possible that a T cell repertoire specific for such unmutated tumor antigens may have been selected so that the high affinity TCR are eliminated as in the case of T cells for some self antigens such as myelin basic protein (44). Although this is an attractive hypothesis, clonal deletion of T cells specific for unmutated tumor antigens has not been directly addressed due to lack of an experimental model. To our knowledge, this is the first report that has evaluated clonal deletion of a transgenic TCR specific for a natural tumor antigen. Our data clearly demonstrate that despite expression in normal tissues, P1A product does not induce clonal deletion of P1A-specific T cells. Since the transgenic T cells require <10 ng/ml of P1A peptide for maximal proliferation, it is likely that the TCR–Ld/ P1A35–43 interaction is of high avidity. It follows that endogenous P1A epitope does not cause clonal deletion of T cells with high avidity for P1A:Ld complex, in contrast to the epitope on myelin basic protein (44). Our conclusion is supported by the fact that P1A-specific CTL clones characterized by other researchers also have high avidity for the peptide–Ld complex (39). Our results suggest that TCR with high affinity for unmutated tumor antigen are not eliminated from the T cell repertoire.
The transgenic T cells maintain a naive phenotype for >6 mo (the age at which mice were killed for the study), although a substantial proportion of T cells utilizing endogenous α chain have experienced antigenic stimulation from the environment during the same period. It is therefore most likely that the P1A epitope is essentially ignored by T cells under physiological conditions. In this regard, it should be emphasized that although P1A mRNA is detected in normal tissue, we have not analyzed expression of P1A protein in these tissues due to lack of reagents. The P1A epitope is detected on the spleen cells only after LPS activation.
Clonal deletion requires less TCR ligand than T cell activation (45, 46). Lack of a discernible effect of endogenous P1A on the development of P1A-specific T cells strengthens the conclusion that endogenous P1A epitope is not present in significant amounts, particularly in the thymus.
Our second major observation was that the effector function of P1A-reactive CTL is restrained in vivo. Four lines of evidence strongly suggest that P1A can be a tumor rejection antigen. First, classic studies by Uyttenhove et al. reported that tumor cells that escaped immunity induced by the tum−P815 had lost P1A antigen (47). Second, immunization with P1A cDNA induces protection against subsequent challenge of P815 cells, although the authors noticed that the correlation between the strength of the recall CTL response in vitro and the anti-tumor immunity was poor (48). Third, adoptive transfer of P1A-specific CTL line (35) or polyclonal CTL (49) protects against subsequent challenge of P815 cells. Fourth, in mice challenged with P815 tumor, a strong correlation was observed between expansion of recurrent TCR repertoire and tumor rejection (50). Thus, although there is a strong case that P1A can induce immunity against tumors that express the antigen, it is less clear whether P1A-specific CTL generated in vivo are responsible for tumor rejection.
It is intriguing that active immunization with P1A, either by adenoviruses engineered to express P1A in vivo (49) or by P1A peptide (our unpublished results) which induces anti-P1A CTL, does not confer immunity against P1A-expressing tumors. In addition, we have previously demonstrated that multiple lineages of tumors that share the P1A antigen are not crossprotected (20). A critical issue is whether the poor CTL-mediated protection is due to insufficient clonal expansion of P1A-specific CD8 T cells or to diminished effector function of these T cells. To address this issue, we have produced transgenic mice in which the overwhelming majority of CD8 T cells are specific for P1A. We report here that unless B7-1 is present on the tumor cells, no immunity is conferred by the cytotoxic T cells. Moreover, when the same transgenic mouse is injected with both B7-1+ and B7-1− tumors, B7+ tumors are rejected but B7-1− tumors grow progressively. Our finding supports and extends findings by Wick et al. (51), who have recently reported that a tumor expressing an alloantigen was not rejected in mice that carry transgenic T cells specific for the antigen, although the same mice could reject skin grafts with the same alloantigen. Even though both studies highlight a restrained T cell effector function, our study emphasized that such restriction can be overcome by expressing B7-1 on the tumor cells.
Theoretically, at least four mechanisms can be proposed to account for the apparent restraint of the effector function of P1A-specific CTL. First, T cells specific for unmutated tumor antigen may be of intrinsically low affinity because high affinity P1A-reactive CTL may have been deleted due to endogenous expression of the tumor antigen. We show here that the high avidity, P1A-specific T cells were not deleted, unless P1A is overexpressed in the thymus as a transgene, which would rule out this possibility. Second, endogenous P1A may have rendered P1A- reactive T cells anergic. This is also unlikely, as we have shown that transgenic P1A-reactive T cells remain fully responsive to low concentrations of P1A and exhibit strong cytotoxicity against P1A-expressing tumors after in vitro stimulation. Third, it is possible that endogenous P1A has prevented full maturation of P1A-reactive CTL. The fact that fully active, P1A-specific CTL generated in vitro can be therapeutic in vivo (35, 49) and that the effector function of ex vivo tumor-infiltrating lymphocytes recovered from P1A-expressing tumors requires B7 on target cells for optimal cytolysis (34) support this contention. Fourth, we have recently demonstrated that anti-tumor CD8 T cells do not exhibit cytotoxicity until after they have reached the tumor site (52). The requirement of B7-1 for tumor rejection in the transgenic mice can also be explained in part by the need for local activation of anti-tumor CTL.
Since tissue destruction by some autoreactive transgenic T cells also requires expression of B7 in the target tissues (53–55), it is tempting to suggest that endogenous expression of unmutated tumor antigen may contribute to the restrained effector function of transgenic T cells. This concept can be formally tested when mice lacking endogenous P1A are generated.
In summary, we have used a transgenic model to study the development, induction, and effector function of P1A-specific CTL. Our results demonstrate that the endogenous P1A antigen does not cause clonal deletion of high avidity, P1A-specific T cells in vivo. Even though P1A may well be a tumor rejection antigen, the effector function of P1A-specific T cells is restrained. Since P1A is the prototype of unmutated tumor antigen, the findings presented here have important implications: although this type of unmutated antigen can be used as a target for tumor vaccines and tumor immunotherapy, more emphasis must be placed on the strategy to induce full activation of T cells to achieve optimal effector function.
Acknowledgments
We thank Dr. John Hirst (New York University Medical Center, New York) for assistance with flow cytometry.
Abbreviations used in this paper
- Eμ
IgM heavy chain enhancer
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- RT
reverse transcriptase
- SW
Swiss Webster
- TG
transgenic mouse
Footnotes
This study is supported by grants from the National Institutes of Health (NIH) (CA58033 and CA69091), Kaplan Comprehensive Center of New York University Medical Center, and Ohio State University Comprehensive Cancer Center. Part of the study was carried out when Supria Sarma was supported by NIH training grant CA09161.
Present addresses are as follows: S. Sarma, Howard Hughes Medical Institute, Rockefeller University, 1230 York Ave., New York, NY 10021; Y. Guo, Hoechst Marion Rousse, Inc., CNS-Molecular Biology, Bridgewater, NJ 08807; C. Lee, 3161 Broadway, Apt. 6C, New York, NY 10027; X.-F. Bai, Department of Pathology, Ohio State University Medical Center, 146 Hamilton Hall, 1645 Neil Ave., Columbus, OH 43210; and Y. Guilloux, Institut de Biologie, INSERM U463, 9 Quai, Moncousu, 44093, Nantes cedex 1, France.
S. Sarma, Y. Guo, Y. Guilloux, and X.-F. Bai contributed equally to this study.
References
- 1.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Nanda NK, Sercarz EE. Induction of anti-self– immunity to cure cancer. Cell. 1995;82:13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 3.Houghton AN. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung S, Schluesener HJ. Human T lymphocytes recognize a peptide of single point–mutated, oncogenic ras proteins. J Exp Med. 1991;173:273–276. doi: 10.1084/jem.173.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peace DJ, Chen W, Nelson H, Cheever MA. T cell recognition of transforming proteins encoded by mutated ras proto-oncogenes. J Immunol. 1991;146:2059–2065. [PubMed] [Google Scholar]
- 6.Skipper J, Stauss HJ. Identification of two cytotoxic T lymphocyte–recognized epitopes in the Ras protein. J Exp Med. 1993;177:1493–1498. doi: 10.1084/jem.177.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noguchi Y, Chen YT, Old LJ. A mouse mutant p53 product recognized by CD4+ and CD8+T cells. Proc Natl Acad Sci USA. 1994;91:3171–3175. doi: 10.1073/pnas.91.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Buschenfelde KH, Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 9.Dubey P, Hendrickson RC, Meredith SC, Siegel CT, Shabanowitz J, Skipper JC, Engelhard VH, Hunt DF, Schreiber H. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase P68. J Exp Med. 1997;185:695–705. doi: 10.1084/jem.185.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Peace DJ, Rovira DK, You SG, Cheever MA. T-cell immunity to the joining region of p210BCR–ABL protein. Proc Natl Acad Sci USA. 1992;89:1468–1472. doi: 10.1073/pnas.89.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J Exp Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traversari C, van der Bruggen P, Van den Eynde B, Hainaut P, Lemoine C, Ohta N, Old L, Boon T. Transfection and expression of a gene coding for a human melanoma antigen recognized by autologous cytolytic T lymphocytes. Immunogenetics. 1992;35:145–152. doi: 10.1007/BF00185107. [DOI] [PubMed] [Google Scholar]
- 13.van der Bruggen P, Szikora JP, Boel P, Wildmann C, Somville M, Sensi M, Boon T. Autologous cytolytic T lymphocytes recognize a MAGE-1 nonapeptide on melanomas expressing HLA-Cw*1601. Eur J Immunol. 1994;24:2134–2140. doi: 10.1002/eji.1830240930. [DOI] [PubMed] [Google Scholar]
- 14.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethe B, Brasseur F, Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P, Boon T, van der Bruggen P. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 16.Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH, Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 19.Guilloux Y, Lucas S, Brichard VG, Van Pel A, Viret C, De Plaen E, Brasseur F, Lethe B, Jotereau F, Boon T. A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanomas is encoded by an intron sequence of the N-acetylglucosaminyltransferase V gene. J Exp Med. 1996;183:1173–1183. doi: 10.1084/jem.183.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramarathinam L, Sarma S, Maric M, Zhao M, Yang G, Chen L, Liu Y. Multiple lineages of tumors express a common tumor antigen, P1A, but they are not cross-protected. J Immunol. 1995;155:5323–5329. [PubMed] [Google Scholar]
- 21.Pardoll DM. Tumour antigens. A new look for the 1990s. Nature. 1994;369:357. doi: 10.1038/369357a0. [DOI] [PubMed] [Google Scholar]
- 22.Ada G. Prospects for a vaccine against HIV. Nature. 1989;339:331–332. doi: 10.1038/339331a0. [DOI] [PubMed] [Google Scholar]
- 23.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA–A2 melanomas. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Godfraind C, Lethe B, Amar-Costesec A, Renauld JC, Gajewski TF, Duffour MT, Warnier G, Boon T, Van den Eynde BJ. The expression of mouse gene P1A in testis does not prevent safe induction of cytolytic T cells against a P1A-encoded tumor antigen. Int J Cancer. 1997;70:349–356. doi: 10.1002/(sici)1097-0215(19970127)70:3<349::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 26.Blackman M, Kappler J, Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990;248:1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- 27.Marrack P, Blackman M, Burgert HG, McCormack JM, Cambier J, Finkel TH, Kappler J. T-cell repertoire and thymus. Cold Spring Harb Symp Quant Biol. 1989;54:105–110. doi: 10.1101/sqb.1989.054.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Burkly LC, Lo D, Kanagawa O, Brinster RL, Flavell RA. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989;342:564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- 29.Miller JF, Morahan G. Peripheral T cell tolerance. Annu Rev Immunol. 1992;10:51–69. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 30.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 32.Visseren MJ, van Elsas A, van der Voort EI, Ressing ME, Kast WM, Schrier PI, Melief CJ. CTL specific for the tyrosinase autoantigen can be induced from healthy donor blood to lyse melanoma cells. J Immunol. 1995;154:3991–3998. [PubMed] [Google Scholar]
- 33.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphas Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 34.Ramarathinam L, Castle M, Wu Y, Liu Y. T cell costimulation by B7/BB1 induces CD8 T cell–dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J Exp Med. 1994;179:1205–1214. doi: 10.1084/jem.179.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang G, Hellstrom KE, Mizuno MT, Chen L. In vitro priming of tumor-reactive cytolytic T lymphocytes by combining IL-10 with B7-CD28 costimulation. J Immunol. 1995;155:3897–3903. [PubMed] [Google Scholar]
- 36.Guo Y, Wu Y, Zhao M, Kong XP, Liu Y. Mutational analysis and an alternatively spliced product of B7 defines its CD28/CTLA4-binding site on immunoglobulin C–like domain. J Exp Med. 1995;181:1345–1355. doi: 10.1084/jem.181.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun XH. Constitutive expression of the Id1 gene impairs mouse B cell development. Cell. 1994;79:893–900. doi: 10.1016/0092-8674(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 38.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 39.Lethe B, van den Eynde B, van Pel A, Corradin G, Boon T. Mouse tumor rejection antigens P815A and P815B: two epitopes carried by a single peptide. Eur J Immunol. 1992;22:2283–2288. doi: 10.1002/eji.1830220916. [DOI] [PubMed] [Google Scholar]
- 40.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 41.Bradley LM, Atkins GG, Swain SL. Long-term CD4+memory T cells from the spleen lack MEL-14, the lymph node homing receptor. J Immunol. 1992;148:324–331. [PubMed] [Google Scholar]
- 42.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel R. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in transgenic model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Targoni OS, Lehmann PV. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J Exp Med. 1998;187:2055–2063. doi: 10.1084/jem.187.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yagi J, Janeway CA., Jr Ligand thresholds at different stages of T cell development. Int Immunol. 1990;2:83–89. doi: 10.1093/intimm/2.1.83. [DOI] [PubMed] [Google Scholar]
- 46.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 47.Uyttenhove C, Maryanski J, Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med. 1983;157:1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosato A, Zambon A, Milan G, Ciminale V, D'Agostino DM, Macino B, Zanovello P, Collavo D. CTL response and protection against P815 tumor challenge in mice immunized with DNA expressing the tumor-specific antigen P815A. Hum Gene Ther. 1997;8:1451–1458. doi: 10.1089/hum.1997.8.12-1451. [DOI] [PubMed] [Google Scholar]
- 49.Chen PW, Wang M, Bronte V, Zhai Y, Rosenberg SA, Restifo NP. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J Immunol. 1996;156:224–231. [PMC free article] [PubMed] [Google Scholar]
- 50.Levraud JP, Pannetier C, Langlade-Demoyen P, Brichard V, Kourilsky P. Recurrent T cell receptor rearrangements in the cytotoxic T lymphocyte response in vivo against the P815 murine tumor. J Exp Med. 1996;183:439–449. doi: 10.1084/jem.183.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L, Bluestone JA, Schreiber H. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maric M, Zheng P, Sarma S, Guo Y, Liu Y. Maturation of cytotoxic T lymphocytes against a B7-transfected nonmetastatic tumor: a critical role for costimulation by B7 on both tumor and host antigen-presenting cells. Cancer Res. 1998;58:3376–3384. [PubMed] [Google Scholar]
- 53.Soldevila G, Geiger T, Flavell RA. Breaking immunologic ignorance to an antigenic peptide of simian virus 40 large T antigen. J Immunol. 1995;155:5590–5600. [PubMed] [Google Scholar]
- 54.Harlan DM, Hengartner H, Huang ML, Kang YH, Abe R, Moreadith RW, Pircher H, Gray GS, Ohashi PS, Freeman GJ, et al. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T-lymphocyte unresponsiveness. Proc Natl Acad Sci USA. 1994;91:3137–3141. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allison J, Stephens LA, Kay TW, Kurts C, Heath WR, Miller JF, Krummel MF. The threshold for autoimmune T cell killing is influenced by B7-1. Eur J Immunol. 1998;28:949–960. doi: 10.1002/(SICI)1521-4141(199803)28:03<949::AID-IMMU949>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]