Abstract
Contact hypersensitivity (CHS) is a T cell–mediated skin inflammation induced by epicutaneous exposure to haptens in sensitized individuals. We have previously reported that CHS to dinitrofluorobenzene in mice is mediated by major histocompatibility complex (MHC) class I–restricted CD8+ T cells. In this study, we show that CD8+ T cells mediate the skin inflammation through their cytotoxic activity. The contribution of specific cytotoxic T lymphocytes (CTLs) to the CHS reaction was examined both in vivo and in vitro, using mice deficient in perforin and/or Fas/Fas ligand (FasL) pathways involved in cytotoxicity. Mice double deficient in perforin and FasL were able to develop hapten-specific CD8+ T cells in the lymphoid organs but did not show CHS reaction. However, they did not generate hapten-specific CTLs, demonstrating that the CHS reaction is dependent on cytotoxic activity. In contrast, Fas-deficient lpr mice, FasL-deficient gld mice, and perforin-deficient mice developed a normal CHS reaction and were able to generate hapten-specific CTLs, suggesting that CHS requires either the Fas/FasL or the perforin pathway. This was confirmed by in vitro studies showing that the hapten-specific CTL activity was exclusively mediated by MHC class I–restricted CD8+ T cells which could use either the perforin or the Fas/FasL pathway for their lytic activity. Thus, cytotoxic CD8+ T cells, commonly implicated in the host defence against tumors and viral infections, could also mediate harmful delayed-type hypersensitivity reactions.
Keywords: cytotoxic T lymphocyte, contact hypersensitivity, contact dermatitis, hapten, dinitrofluorobenzene, CD8+ T cells
Contact hypersensitivity (CHS)1 is a T cell–mediated cutaneous inflammatory reaction occurring after epicutaneous exposure to haptens in sensitized individuals (1– 3). In humans, it frequently manifests as an inflammatory dermatosis referred to as contact dermatitis. Haptens are low molecular weight chemicals which covalently bind to discrete amino acid residues on self or exogenous proteins (4). The sensitization phase, also referred to as the afferent phase, occurs after the first contact of the skin with the hapten. Hapten-modified proteins are loaded onto dendritic epidermal Langerhans cells (LCs) which migrate from the epidermis to the regional draining LNs, where priming of hapten-specific CD4+ and CD8+ T cells occurs (5, 6). The elicitation phase, also known as the efferent phase, develops within a few hours after subsequent contact with the hapten, and is mediated by the activation of hapten-specific T cells in the skin.
For many years, CHS was considered, like classical delayed-type hypersensitivity (DTH), to be mediated by CD4+ T cells (7). Recent studies have demonstrated that CHS to dinitrofluorobenzene (DNFB) was mediated by IFN-γ–producing CD8+ T cells only, whereas CD4+ T cells downregulate this response (8–10). Thus, CHS can be considered as an antigen-specific inflammation mediated by hapten-specific CD8+ T cells, which differs from classical DTH to protein antigens (1, 2). Although haptens are potent inducers of CD8+ CTLs (11, 12), it is not known whether CD8+ cells mediate the skin inflammation through such cytotoxic activity or through the secretion of type 1 cytokines.
CD8+ CTLs are major effector cells of the immune defence system against viruses and tumors (13) and exert their lytic functions through two main independent mechanisms (14–16). The secretory pathway involves the release of perforin and granzymes from cytolytic granules. The nonsecretory pathway involves interaction of the FasL upregulated during T cell activation, with the apoptosis-inducing Fas molecule on the target cell.
In this study, we investigated the contribution of CD8+ T cell–mediated cytotoxicity to the pathophysiology of CHS, using mice deficient in the Fas/FasL pathway (lpr and gld mice), the perforin pathway (perforin-deficient [P0/0] mice), and in both cytolytic pathways (P0/0 gld mice). The results provide evidence that CD8+ T cells mediate CHS through their cytolytic activity.
Materials and Methods
Mice.
C57BL/6 mice were purchased from IFFA Credo. Mice homozygous for lpr mutation (lpr) and lacking the Fas (CD95) molecule were obtained from Harlan. Mice homozygous for perforin gene disruption (P0/0) completely lack perforin- dependent cytotoxicity, while the Fas/FasL pathway remains fully functional (17, 18). Mice homozygous for the gld mutation (gld), homozygous for the gld mutation and heterozygous for the perforin deletion (P+/0 gld), and mice double deficient for perforin and carrying the gld mutation (P0/0 gld, unable to generate antigen-specific CTLs [19]) were provided by Michael Hahne (Institute of Biochemistry, Lausanne, Switzerland). P0/0 gld mice were obtained by mating P+/0 gld mice, and the offspring were tested for perforin deletion as described by Lowin et al. (18). Mice with a mutation in the β2 microglobulin gene (MHC class I–deficient [I0/0]) or in the I-Aβ gene (MHC class II–deficient [II0/0]) were provided by Christophe Benoist and Diane Mathis (20, 21).
All mutant mice were on a C57BL/6 (H-2b) background (backcrossed more than eight times with C57BL/6 mice) and were used between 8 and 12 wk of age. Lpr, gld, and P0/0 gld mice, which develop a diffuse lymphoproliferation by the age of 2 mo, which could interfere with the development of the CHS reaction, were used at the age of 6 wk, at a time when they show no clinical sign of disease and have normal sized lymphoid organs. P0/0, I0/0, and II0/0 mice were bred at the IFFA Credo/Transgenic Alliance specific pathogen–free facility (L'Arbresle, France).
Chemicals.
DNFB and its water soluble form, dinitrobenzene sulfonic acid (DNBS), were obtained from Sigma and used for in vivo and in vitro experiments, respectively.
Antibody.
Ascites from the anti–MHC class I (heavy chain) hybridoma 20.8.4.S was obtained from Jean-Pierre Abastado (Institut Pasteur, Paris, France).
Assay for CHS to DNFB.
DNFB was diluted in acetone/olive oil (4:1) immediately before use. The procedure used for the CHS, i.e., the mouse ear swelling test (MEST), has been described elsewhere (22). In brief, 25 μl of 0.5% DNFB solution was applied to a 2-cm2 area of shaved dorsal skin. After 5 d, test and control animals received 10 μl of 0.15% (nonirritant concentration) DNFB applied on both sides of the left ear, and the solvent (acetone/olive oil) alone on the right ear. Ear thickness was monitored using a micrometer (J15; Blet SA, France), before challenge and every day after challenge. The ear swelling was calculated as [(T − T0) left ear] − [(T − T0) right ear], where T and T0 represent values of ear thickness after and before challenge, respectively.
In each experimental group, some mice were killed at different time intervals after DNFB challenge for histological and PCR analysis.
RNA Extraction and Reverse Transcription PCR Analysis of CD8 and IFN-γ mRNA.
At different intervals after challenge, ear samples were collected from sensitized or unsensitized mice and frozen in liquid nitrogen. The detection of RNA was conducted as described in detail elsewhere (23). In brief, total RNA was extracted using an RNAXEL kit (Eurobio). After DNase I treatment, 1 μg of total mRNA was reverse transcribed using poly dT15 primers and Superscript II RT (90 min, 37°C; GIBCO BRL). The amount of RNA to be used for detection was normalized using the housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) as reference. The cDNA obtained was amplified using different sets of primers: for HPRT (5′ primer, 5′-GTA ATG ATC AGT CAA CGG GGG AC-3′; 3′ primer, 5′-CCA GCA AGC TTG CAA CCT TAA CCA-3′), for CD8 (5′ primer, 5′-AGG ATG CTC TTG GCT CTT CC-3′; 3′ primer, 5′-TCA CAG GCG AAG TCC AAT CC-3′), and for IFN-γ (5′ primer, 5′-GCT CTG AGA CAA TGA ACG CT-3′; 3′ primer, 5′-AAA GAG ATA ATC TGG CTC TGC-3′). The amplifications were carried out with 29 cycles for HPRT and 33 cycles for IFN-γ and CD8 (1 min at 94°C, 1 min 30 s at 60°C, 2 min at 72°C). The PCR products were analyzed on 1.5% agarose gel.
In Vitro Secondary T Cell Proliferation.
Spleen cells from DNFB-sensitized C57BL/6 and P0/0 gld mice were collected 5 d after sensitization. T lymphocytes were purified through negative selection using anti-Ig columns (Biotex) as described elsewhere (6). The resulting cell suspensions contained >90% CD3+ viable cells. CD8+ T cells were isolated from the spleen T cells by elimination of CD4+ T cells using columns coated with goat anti– mouse and goat anti–rat IgG and a rat anti–mouse CD4+ mAb (YTS191.1; Biotex). FACS® analysis of cells eluted from the column showed <0.5% contaminating CD4+ T cells. In vivo DNFB-primed unfractionated or CD8+ T cells (2.5 × 105/well) obtained on day 5 after DNFB sensitization were cocultured for 3 d at 37°C in 96-well plates with 106 mitomycin C–treated syngenic spleen cells from naive mice, that were either DNBS-derivatized as described (6) or left untreated. In brief, 107 cells were incubated for 30 min with 25 μg/ml of mitomycin C (Sigma), washed, and haptenated by 30-min incubation at 37°C with 4 mM DNBS, pH 8.0, in serum-free RPMI medium. The proliferative responses were assessed on day 3 of culture by [3H]thymidine incorporation (1 μCi/well) for the last 6 h of culture. The results are expressed as proliferation indices: (cpm in cultured T cells + DNBS-treated spleen cells)/(cpm in cultured T cells + untreated spleen cells).
IFN-γ Enzyme-linked Immunospot Assay.
Inguinal and axillary LNs were harvested 5 d after DNFB sensitization. Cell suspensions were restimulated in vitro by overnight culture in complete RPMI medium supplemented with 10% FCS and containing a final concentration of 0.4 mM DNBS. Control cultures included cells cultured overnight in medium supplemented with 0.2 mM of the irrelevant hapten TNBS, or in medium alone. The number of IFN-γ–producing cells was determined using an enzyme-linked immunospot (ELISPOT) assay. In brief, 96-well nitrocellulose plates (MAHA 45; Millipore) were coated overnight at 4°C with anti–IFN-γ antibody (R46A2) and blocked with PBS/2% BSA for 2 h at 37°C. The plates were washed three times with PBS/Tween 0.1% before use. The cell suspensions were washed, and incubated at different concentrations in duplicate wells for 4 h at 37°C, 5% CO2. Plates were washed three times with PBS/0.1% Tween and incubated with a biotinylated anti–IFN-γ antibody (AN18). IFN-γ spot-forming cells (SFCs) were developed using streptavidin–alkaline phosphatase (Boehringer Mannheim), incubated for 2 h, and extensively washed before adding the substrate (5-bromo-4-chloro-3-indolyl-phosphate; Sigma). The number of IFN-γ SFCs present in each well was counted using a microscope, and the results were expressed as IFN-γ SFCs/106 cells.
Production of CTLs.
DNBS-specific CTLs were recovered from splenocytes of various H-2b mice. Spleens were recovered 5 d after cutaneous sensitization with DNFB, perfused with RPMI to eliminate red blood cells. 107 splenocytes were used either fresh or after restimulation for 5 d in culture with 107 syngenic mitomycin C–treated, DNBS-derivatized spleen cells from either normal C57BL/6, I0/0, or II0/0 mice.
Target Cells.
Target cells included MBL2 and MBL2-Fas cell lines, provided by Maries van den Broeck (Institute of Experimental Immunology, University of Zurich, Zurich, Switzerland [24]), and EL-4 cells, all maintained in RPMI plus 10% FBS. Target cells were simultaneously haptenated and labeled by incubating 2 × 106 cells in 10 μl of RPMI with or without 40 mM DNBS and 100 μCi of Na2 51CrO4 (sodium chromate solution, 1 Ci/mM) for 1 h at 37°C with periodic mixing. Labeled targets were washed thoroughly before use.
Cytotoxicity Assays.
Various numbers of effector cells were incubated at 37°C for 4 h with 104 labeled target cells. Supernatants were then collected, and 51Cr release was counted in a γ counter. The percentage of cytotoxicity was calculated using the formula: 100 × (experimental cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm), where the maximal cpm and spontaneous cpm are the radioactivity released from targets exposed to 0.5 M HCl or medium, respectively. The results presented are representative of at least three independent experiments.
Statistical Analysis.
Data were examined for normality and equal variance, and groups were compared by a two-tailed Student's t test.
Results
CHS Reaction to DNFB Is Impaired in Perforin and FasL Double-knockout (P0/0 Gld) Mice.
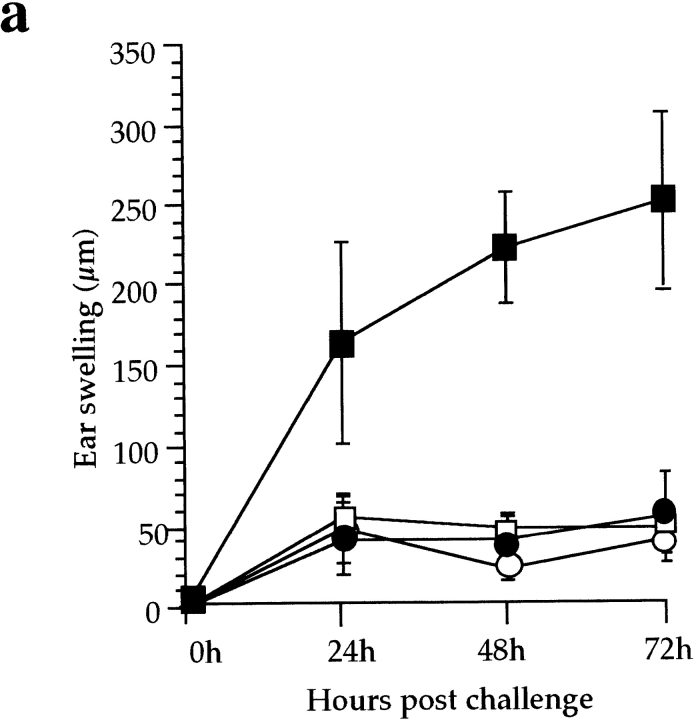
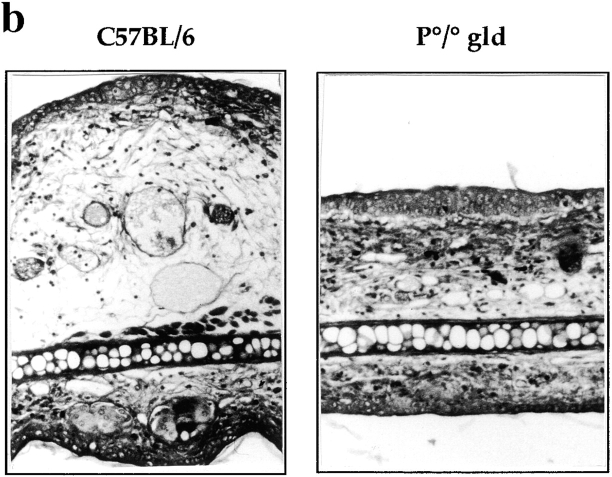
The contribution of CD8+ T cell–mediated cytotoxicity to the CHS reaction to DNFB was examined in mice double deficient for Fas/FasL and perforin (P0/0 gld), which are devoid of CTL activity (15). The CHS reaction was dramatically reduced in P0/0 gld mice compared with that observed in C57BL/6 mice (Fig. 1 a). No difference in ear swelling could be observed between the sensitized and unsensitized groups of P0/0 gld mice and the unsensitized C57BL/6 mice. Similarly, none of the characteristic pathological changes occurring during a normal CHS reaction, namely edema, vascular enlargement, and mononuclear cell infiltration, was observed in P0/0 gld mice (Fig. 1 b).
Figure 1.
CHS to DNFB is impaired in P0/0 gld mice. (a) CHS to DNFB was analyzed in groups of four wild-type C57BL/6 (squares) or P0/0 gld (circles) mice either sensitized with DNFB (filled symbols) or unsensitized (open symbols) and challenged 5 d later on the left ear. Results are expressed as the mean ear swelling (in μm) at different time points after challenge. Results are representative of three independent experiments. (b) Histological analysis of the CHS reaction 24 h after challenge in C57BL/6 and P0/0 gld mice. Major changes were observed in C57BL/6 mice, including edema of the dermis, mononuclear cell infiltration, and enlargement of blood vessels. No main histological modification was noted in the skin of P0/0 gld mice. Original magnification: ×100. (c) Cytotoxic activity of spleen cells from DNFB-sensitized C57BL/6 mice (▪), P0/0 gld mice (•), or naive C57BL/6 mice (□) was assessed after in vitro restimulation for 5 d with haptenated C57BL/6 mice spleen cells. Specific cytotoxic activity was determined by hapten-pulsed MBL2-Fas target lysis in a 4-h 51Cr-release assay. Results are representative of three independent experiments.
Spleen cells from sensitized animals were tested for their ability to lyse haptenated targets either directly or after in vitro restimulation. As shown in Fig. 1 c, T cells from DNFB-sensitized C57BL/6 mice exhibited a potent hapten-specific CTL activity after restimulation in vitro but which was already detectable directly ex vivo (data not shown). In contrast, DNFB-specific CTLs could never be demonstrated in double-deficient P0/0 gld mice ex vivo, nor after in vitro restimulation with DNBS-derivatized cells.
Thus, P0/0 gld mice cannot produce a CHS reaction to DNFB and are unable to develop any of the pathological changes associated with CHS, suggesting that hapten-specific CTL activity is mandatory for expression of the CHS reaction.
DNFB Can Prime for Specific CD8+ T Cells in the Lymphoid Organs of Perforin and FasL Double-deficient Mice.
To determine whether the lack of CHS reaction in P0/0 gld mice is secondary to an impairment in the priming of specific CD8+ T cells in the lymphoid organs, P0/0 gld and C57BL/6 mice were sensitized with DNFB, and lymphoid cells were recovered 5 d later and tested for hapten-specific proliferative responses and IFN-γ production.
Lymphocytes from P0/0 gld mice responded vigorously to hapten-treated syngenic cells in secondary proliferative responses, with stimulation indices identical to those observed with T cells recovered from sensitized C57BL/6 mice (Fig. 2 a). Similarly, CD8+ T cells from P0/0 gld mice exhibited hapten-specific responses indistinguishable from those of control C57BL/6 cells. Thus, CD8+ T cell priming has occurred in the lymphoid organs of P0/0 gld mice.
Figure 2.
Hapten-specific T cell responses in P0/0 gld mice. (a) Secondary proliferative responses. T cells and CD8+ T cells were purified from the spleen and LNs of DNFB-sensitized C57BL/6 and P0/0 gld mice and tested for proliferative responses to hapten-modified or unmodified syngenic spleen cells in a 6-h [3H]thymidine assay on day 3 of culture. Results are expressed as stimulation indices: ([3H] cpm incorporation of T or CD8+ cells cultured with DNBS-modified spleen cells/ [3H] cpm incorporation of T or CD8+ cells cultured with nonmodified spleen cells). Coculture with nonmodified spleen cells determines the level of nonspecific proliferation: TC57BL/6 = 894 (±78) cpm; CD8+ C57BL/6 = 672 (±266) cpm; TP0/0 gld = 936 (±300) cpm; CD8+ P0/0 gld = 775 (±256) cpm. Results are representative of three independent experiments. (b) Hapten-specific IFN-γ–producing cells were determined by ELISPOT assay in LN cells from DNFB-sensitized C57BL/6 mice, after overnight restimulation with DNBS (▪), TNBS (○), or medium alone. In some experiments, CD8 depletion of DNBS-restimulated LN cells was performed before the ELISPOT assay (□). No spots were detected in unsensitized mice after in vitro culture with or without haptens (not shown). (c) DNBS-specific IFN-γ SFCs in LNs from C57BL/6 mice and P0/0 gld mice after overnight restimulation with (black bars) or without (white bars) DNBS.
We next used an ELISPOT assay to determine the frequency of DNFB-specific, IFN-γ–producing LN cells in primed C57BL/6 and P0/0 gld mice. In C57BL/6 mice, the IFN-γ–producing cells were entirely contained in the CD8+ T cell subset, with a mean frequency of 3 IFN-γ SFCs/105 LN cells (Fig. 2 b). Interestingly, P0/0 gld mice exhibited comparable levels of hapten-specific, IFN-γ–producing cells (Fig. 2 c).
These data indicate that the lack of CHS in double-deficient P0/0 gld mice is not due to impaired priming of hapten-specific CD8+ T cells nor to an altered production of IFN-γ, but rather to the deficient CTL activity.
IFN-γ–producing CD8+ T Cells of P0/0 Gld Mice Migrate to the Challenged Skin but Are Unable to Induce the Recruitment of Inflammatory Cells.
Two possibilities could explain why P0/0 gld mice did not show a CHS response despite the presence of IFN-γ–producing DNFB-specific CD8+ T cells in the lymphoid organs: (a) primed CD8+ T cells lacking cytolytic activity are unable to migrate from the blood to the skin; (b) CD8+ T cells are able to infiltrate the skin but, due to lack of cytolytic functions, are unable to induce recruitment of inflammatory cells mandatory for the full development of CHS. Since the CHS reaction to DNFB is associated with the infiltration of IFN-γ–producing CD8+ T cells in situ a few hours after challenge (Akiba, H., manuscript in preparation), we examined the presence of CD8+ T cells and the expression of IFN-γ in the skin of P0/0 gld and C57BL/6 mice after sensitization and challenge (Fig. 3). PCR analysis demonstrated that CD8 and IFN-γ mRNAs were not found in the skin of naive mice or in hapten-painted ears of unsensitized mice. In contrast, upregulation of both CD8 and IFN-γ mRNA occurred as early as 6 h after challenge in both C57BL/6 and P0/0 gld mice, indicating that lack of both perforin and FasL did not affect the migration of CD8+ T cells to the skin. However, at 24 and 48 h after challenge, dramatic differences were noted. C57BL/6 mice showed a gradual increase in the intensity of the CD8 and IFN-γ mRNA expression, whereas no signal was observed in the skin of P0/0 gld mice. These results suggest that DNFB-specific CD8+ T cells from P0/0 gld mice are able to migrate from blood to skin, but cannot induce the specific signals necessary for the constitution of the inflammatory cellular infiltrate.
Figure 3.
Detection of CD8 and IFN-γ mRNA during the elicitation phase of the CHS. (a) CD8 and IFN-γ mRNA expression in situ was analyzed using semiquantitative RT-PCR: mRNA was obtained from the ears of mice at 6, 24, and 48 h after challenge. Controls include ears of untreated mice (naive) and ears of nonsensitized but challenged mice (unsensitized). (b) Histogram representation of the CD8 and IFN-γ mRNA relative quantities compared with HPRT mRNA as standard. Each band was analyzed by densitometry, and the results are expressed as ratios of optical densities to HPRT band. Results are representative of two independent experiments.
DNFB-specific CHS and CTL Responses Develop in Lpr Mice, Gld Mice, and P0/0 Mice.
To determine the relative contribution of the Fas/FasL and perforin pathways to the CHS response, we tested the ability of Fas-deficient (lpr), FasL-deficient (gld), and P0/0 mice to exhibit specific CHS and CTL responses after DNFB sensitization.
In marked contrast to double-deficient P0/0 gld mice, lpr, gld, or P0/0 mice developed a normal CHS reaction to DNFB (Fig. 4 a), with intensity, kinetics, and histological characteristics comparable to those observed in C57BL/6 mice. Mice homozygous for the gld mutation and heterozygous for the perforin deletion (P+/0 gld) also had a similar reaction to DNFB (data not shown). Interestingly, lpr, gld, and P0/0 mice exhibited a strong hapten-specific CTL response (Fig. 4 b) as well as normal quantities of DNFB-specific, IFN-γ–producing LN cells (data not shown).
Figure 4.
Normal CHS reaction of priming of specific CTLs in Fas-deficient (lpr), FasL-deficient (gld), and P0/0 mice. (a) Groups of five gld (squares), lpr (triangles), and P0/0 (circles) mice were sensitized with DNFB (filled symbols) or left unsensitized (open symbols), and challenged 5 d later on the left ear. Results are expressed as the mean ear swelling (in μm) at different time points after challenge. (b) Spleen cells from DNFB-sensitized gld (squares), lpr (triangles), and P0/0 (circles) mice were restimulated in vitro for 5 d with syngenic haptenated spleen cells. Specific cytotoxic activity was determined by hapten-pulsed MBL2-Fas target lysis in a 4-h 51Cr-release assay. Results are representative of three independent experiments.
Thus, exclusion of only one cytolytic pathway did not prevent the induction of hapten-specific CTLs nor the development of a CHS reaction to DNFB, indicating that CTLs can use either the Fas/FasL or the perforin pathway to mediate CHS.
Hapten-specific CTL Activity Is Found in the MHC Class I–restricted CD8+ T Cell Subset and Is Mediated by Both Perforin and Fas/FasL.
The above data demonstrating that CTLs mediate the CHS reaction to DNFB were obtained in mice genetically deficient for molecules involved in CTL activity, raising the question of the nature of the cells endowed with the specific CTL activity and of the existence of similar mechanisms in normal C57BL/6 mice, which have both functional perforin and Fas/FasL pathways.
Hapten-specific CTL activity was restricted to the MHC class I–restricted CD8+ T cell subset. Indeed, depletion of CD8+ cells, but not of CD4+ cells, totally abolished the CTL activity of primed C57BL/6 spleen cells (data not shown). MHC class I molecules were mandatory for the induction, expansion, and effector function of specific CD8+ CTLs, since: (a) primed spleen cells from C57BL/6 and II0/0, but not from I0/0, mice could lyse haptenated targets (Fig. 5 a); (b) hapten-specific lysis was not observed when effector cells were restimulated in vitro with haptenated APCs from I0/0 mice (Fig. 5 b); and (c) CTL activity of C57BL/6 spleen cells was suppressed by incubation with mAbs to the Kb MHC class I molecules (Fig. 5 c). These results indicate that the hapten-specific CTLs are “classical” MHC class I–restricted CD8+ T cells.
Figure 5.
MHC class I molecules are mandatory for the generation (a), expansion (b), and effector functions (c) of specific CTLs, which can lyse their targets through Fas/FasL- or perforin-mediated mechanisms (d). Specific cytotoxic activity was determined by lysis of hapten-pulsed EL-4 targets (a, b, and c) or MBL2 and MBL2-Fas targets (d) in a 4-h 51Cr- release assay. (a) Spleen cells from DNFB-sensitized C57BL/6 (squares), II0/0 (circles), and I0/0 mice (triangles) were restimulated in vitro for 5 d with haptenated C57BL/6 mice spleen cells. (b) Spleen cells from DNFB-sensitized C57BL/6 mice were restimulated in vitro for 5 d with haptenated spleen cells from naive C57BL/6 (squares), II0/0 (circles), or I0/0 mice (triangles). (c) Cytotoxic activity of C57BL/6 mice spleen cells against haptenated EL-4 cells (▪) was inhibited by addition of the 20.8.4.S mAb to the Kb MHC class I molecules (○). Antibody was used at 1:500 (solid line) and 1:100 (hatched line). (d) Spleen cells from sensitized C57BL/6 mice (squares) and P0/0 mice (circles) were restimulated in vitro for 5 d with syngenic haptenated spleen cells and tested for cytotoxic activity against hapten-pulsed Fas+ (MBL2-Fas; filled symbols) or Fas− (MBL2; open symbols) targets. Results are representative of three to four independent experiments.
The contribution of perforin or Fas/FasL to the CTL activity was tested using perforin+ (from C57BL/6 mice) and perforin-deficient (from P0/0 mice) effectors and targets comprising Fas+ (MBL2-Fas) and Fas-deficient (MBL2) cells. As shown in Fig. 5 d, perforin-deficient effectors could lyse hapten-treated Fas+ cells, but not Fas-deficient cells, whereas perforin+ CTLs were able to lyse Fas-deficient cells as efficiently as Fas-expressing cells. Thus, hapten-specific MHC class I–restricted CD8+ CTLs could use the Fas/FasL or the perforin pathway for their CTL activity, which could be abolished only by inactivation of both cytolytic pathways.
Discussion
This study demonstrates that CD8+ T cells require cytotoxic activity to mediate CHS. The invalidation of the two main cytolytic pathways, as observed in mice deficient in perforin and FasL (P0/0 gld), is responsible for the lack of generation of specific CTLs and for the abolition of the CHS reaction. Interestingly, the presence of a single cytolytic pathway, in lpr mice, gld mice, and P0/0 mice, is sufficient for the development of a CHS reaction and for the priming of specific CTLs, indicating that the Fas/FasL and the perforin pathways could be used independently and with similar efficiency for cytolytic activity. These results are in line with recent data on influenza virus infection showing that virus clearance by CTLs could be achieved only if one of the two main lytic pathways remained functional (25).
Until our study, cytokines, and especially IFN-γ and TNF-α, were thought to mediate skin inflammation through their ability to activate keratinocytes and endothelial cells (2, 26–29). Recent studies excluded a pivotal role for IFN-γ in the elicitation phase of CHS, since IFN-γ receptor–deficient mice developed a normal CHS reaction (30). In addition, studies in TNF-α–deficient mice indicated that the cytokine was not involved in the elicitation phase but played an important role in the sensitization phase by inducing the emigration of LCs to the draining LNs (29).
CHS develops in two phases, the sensitization (i.e., afferent) phase leading to the priming of hapten-specific CD8+ T cells and the elicitation (i.e., efferent) phase occurring after challenge and leading to the development of skin inflammation. MHC class I molecules expressed by LCs are mandatory for the priming of hapten-specific CD8+ T cells in the LNs during the afferent phase (6, 31). These MHC class I–restricted CD8+ T cells exert CHS through CTL activity using classical cytolytic pathways. That cytotoxicity is the effector mechanism responsible for the efferent phase of CHS is supported by the observation that hapten-primed, IFN-γ–producing CD8+ T cells are present in lymphoid organs of P0/0 gld mice and respond vigorously in secondary proliferative responses, demonstrating that the lack of CHS reaction is not due to an impairment of the sensitization phase of CHS, but rather reflects an alteration during the elicitation phase.
The mechanisms involved in the development of skin inflammation upon challenge in sensitized mice, i.e., during the elicitation phase, associates hapten-specific and nonspecific steps (2). First, lymphocytes have to emigrate from the blood to the skin. Infiltration of the skin by mononuclear cells has been reported to occur within a few hours after challenge (32). Haptens are able to directly induce expression of E- and P-selectins on endothelial cells 2 h after skin painting (33, 34). It is thought that circulating antigen-specific memory T cells which carry homing receptors (the cutaneous leukocyte-associated antigen [CLA] molecule) are able to enter the skin (35) through interaction with P- and E-selectins expressed on endothelial cells (36). Second, specific T cells are activated on hapten presentation by skin-resident cells, as revealed by detection of IFN-γ mRNA in situ between 4 and 8 h after challenge (37, 38). Third, activation of skin-resident cells by T cell cytokines results in the amplification of the inflammatory reaction leading to the cellular inflammatory infiltrate. Upon IFN-γ activation, keratinocytes upregulate intercellular adhesion molecule 1 (ICAM-1) and Ia molecules and produce a wide array of inflammatory cytokines and chemokines such as IL-8 (2, 26, 39, 40). Likewise, endothelial cell activation is followed by leukocyte migration from the blood vessels to the dermis, leading to the formation of inflammatory cellular infiltrate (2, 26). In this scheme, the hapten-specific limb of the CHS reaction corresponds to the activation of hapten-specific T cells and occurs ∼6 h after challenge, whereas the nonspecific phase corresponds to the infiltration of the skin by the polymorphic cellular infiltrate, which peaks 24 h after challenge.
It appears from our data that the failure of P0/0 gld mice to exhibit a CHS reaction is not due to the inability of CD8+ T cells to infiltrate the skin or to be activated, since IFN-γ–producing CD8+ T cells could be detected in the skin 6 h after challenge, at levels comparable to those found in C57BL/6 mice. However, these early parameters of the CHS reaction were not followed by an increase in cellular infiltration in situ, as observed in C57BL/6 mice at 24 and 48 h. These observations suggest that cytolytic function of hapten-specific CD8+ T cells is required for the recruitment of inflammatory cells and full development of the CHS response. Both epidermal dendritic LCs and keratinocytes express MHC class I molecules and may produce upon activation (or lethal hit) a wide array of inflammatory cytokines and chemokines. Destruction of haptenated LCs by CTLs may account for the recent observation that LCs undergo apoptotic cell death in CHS (41). Alternatively, keratinocytes, which represent >90% of epidermal cells, could be the targets of antihapten CTLs, the more so since IFN-γ, produced in situ during the course of CHS, upregulates Fas expression by keratinocytes (42).
In conclusion, our data demonstrate that CHS to DNFB is mediated by hapten-specific CTLs which may use either the Fas/FasL or the perforin pathway for the induction of cutaneous inflammation. The precise nature of the MHC class I–expressing skin cells able to present the hapten to CTLs in vivo during the elicitation phase is currently under investigation.
Acknowledgments
We thank Hans Hengartner, Jacques Banchereau, Anne-Marie Schmitt-Verhulst, and Pierre Goldstein for their critical reading of the manuscript and for suggestions. We are indebted to Christophe Benoist and Diane Mathis for donation of the I0/0 and II0/0 mice and for fruitful discussions, to Maria van den Broeck for donation of the MBL2 and MBL2-Fas cells, and to Jean-Pierre Abastado for donation of anti–MHC class I antibodies. We thank Dorothée Carvallo and Patrick Hardy from IFFA Credo/Transgenic Alliance for their help in obtaining and breeding the transgenic mice.
This work was supported by a grant from MGEN-INSERM (Mutuelle Générale de l'Education Nationale– Institut National de la Santé et de la Recherche Médicale) and by a grant from L'ORÉAL, Aulnay-Sous-Bois, France.
Abbreviations used in this paper
- CHS
contact hypersensitivity
- DNBS
dinitrobenzene sulfonic acid
- DNFB
dinitrofluorobenzene
- DTH
delayed-type hypersensitivity
- ELISPOT
enzyme-linked immunospot
- HPRT
hypoxanthine phosphoribosyltransferase
- I0/0
MHC class I–deficient
- II0/0
MHC class II–deficient
- P0/0
perforin-deficient
- LC
Langerhans cell
- SFC
spot-forming cell
References
- 1.Bour, H., M. Krasteva, and J.-F. Nicolas. 1997. Allergic contact dermatitis. In Skin Immune System. Cutaneous Immunology and Clinical Immunodermatology. J. Bos, editor. CRC Press, Boca Raton, FL. 509–522.
- 2.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in the elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 3.Krasteva M, Kehren J, Ducluzeau M-T, Sayag M, Cacciapuoti M, Akiba H, Descotes J, Nicolas J-F. Pathophysiology of contact sensitivity. Eur J Dermatol. 1999;9:65–77. [PubMed] [Google Scholar]
- 4.Lepoittevin JP, Leblond I. Hapten-peptide-T cell receptor interactions: molecular basis for the recognition of haptens by T lymphocytes. Eur J Dermatol. 1997;7:151–154. [Google Scholar]
- 5.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 6.Krasteva M, Kehren J, Horand F, Akiba H, Choquet G, Ducluzeau M-T, Tedone R, Garrigue JL, Kaiserlian D, Nicolas J-F. Dual role of dendritic cells in the induction and down-regulation of antigen-specific cutaneous inflammation. J Immunol. 1998;160:1181–1190. [PubMed] [Google Scholar]
- 7.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by Th1 clones. J Immunol. 1987;138:3688–3694. [PubMed] [Google Scholar]
- 8.Gocinski BL, Tigelaar R. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–4125. [PubMed] [Google Scholar]
- 9.Bour H, Peyron E, Gaucherand M, Garrigue J-L, Desvignes C, Kaiserlian D, Revillard J-P, Nicolas J-F. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized pattern of cytokine production: interferon γ–producing (Tc1) effector CD8+ T cells and interleukin (IL)-4/IL-10–producing (Th2) negative regulatory CD4+T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt-Verhulst AM, Pettinelli CB, Henkart P, Lunney JK, Shearer GM. H-2 restricted cytotoxic effectors generated in vitro by the addition of trinitrophenyl-conjugated soluble proteins. J Exp Med. 1978;147:352–368. doi: 10.1084/jem.147.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin S, Weltzien HU. T cell recognition of haptens. A molecular view. Int Arch Allergy Immunol. 1994;104:10–16. doi: 10.1159/000236703. [DOI] [PubMed] [Google Scholar]
- 13.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 14.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca+-independent T cell–mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kägi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Goldstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 16.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 17.Kägi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K, Podack E, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 18.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun MY, Lowin B, French L, Achaorbea H, Tschopp J. Cytotoxic T cells deficient in both functional Fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med. 1996;183:657–661. doi: 10.1084/jem.183.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 21.Koller B, Marrack P, Kappler J, Smithies O. Normal development of mice deficient in β2M, MHC class I proteins. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 22.Garrigue JL, Nicolas JF, Fraginals R, Benezra C, Bour H, Schmitt D. Optimization of the mouse ear swelling test for in vivo and in vitro studies of weak contact sensitizers. Contact Dermatitis. 1994;30:1868–1873. doi: 10.1111/j.1600-0536.1994.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 23.Delassus S, Coutinho GC, Saucier C, Darche S, Kourilsky P. Differential cytokine expression in maternal blood and placenta during murine gestation. J Immunol. 1994;152:2411–2420. [PubMed] [Google Scholar]
- 24.van den Broeck M, Kägi D, Ossendorp F, Toes R, Vamvakas S, Lutz W, Melief C, Zinkernagel R, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tropham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 26.Smith CH, Barker JNWN. Mechanisms of neutrophil accumulation in skin and inflammatory dermatoses. Eur J Dermatol. 1993;3:527–530. [Google Scholar]
- 27.Proudfoot AEI. The chemokine family. Potential therapeutic targets from allergy to HIV infection. Eur J Dermatol. 1998;8:147–157. [PubMed] [Google Scholar]
- 28.Kondo S, Wang B, Fujisawa H, Shivji GM, Echtenacher B, Mak TW, Sauder DR. Effect on generated mutation in TNF receptor (p55) on contact hypersensitivity and ultraviolet B-induced immunosuppression. J Immunol. 1995;155:3801–3805. [PubMed] [Google Scholar]
- 29.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF-α–deficient mice: critical requirement for TNF-α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saulnier M, Huang S, Aguet M, Ryffel B. Role of interferon-gamma in contact hypersensitivity assessed in interferon-gamma receptor-deficient mice. Toxicology. 1995;102:301–312. doi: 10.1016/0300-483x(95)03101-k. [DOI] [PubMed] [Google Scholar]
- 31.Kolesaric A, Stingl G, Elbe-Burger A. MHC class I+/II−dendritic cells induce hapten-specific immune responses in vitro and in vivo. J Investig Dermatol. 1997;109:580–585. doi: 10.1111/1523-1747.ep12337508. [DOI] [PubMed] [Google Scholar]
- 32.Flax MH, Caulfield JB. Cellular and vascular components of allergic contact dermatitis. Light and electron microscopic observations. Am J Pathol. 1963;43:1031–1053. [PMC free article] [PubMed] [Google Scholar]
- 33.Friedmann PS, Strickland I, Memon AA, Johnson PM. Early time course of recruitment of immune surveillance in human skin after chemical provocation. Clin Exp Immunol. 1993;91:351–356. doi: 10.1111/j.1365-2249.1993.tb05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goebeler M, Meinardus-Hager G, Roth J, Goerdt S, Sorg C. Nickel chloride and cobalt chloride, two common contact sensitizers, directly induce expression of ICAM-1, VCAM-1 and ELAM-1 by endothelial cells. J Investig Dermatol. 1993;100:759–765. doi: 10.1111/1523-1747.ep12476328. [DOI] [PubMed] [Google Scholar]
- 35.Santamaria-Babi LF, Moser R, Perez-Soler MT, Picker LJ, Blaser K, Hauser C. Migration of skin-homing T cells across cytokine-activated human endothelial cell layers involves interaction of cutaneous lymphocyte-associated antigen (CLA), the very late antigen-4 (VLA-4), and the lymphocyte function-associated antigen-1 (LFA-1) J Immunol. 1995;154:1543–1550. [PubMed] [Google Scholar]
- 36.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 37.Asada H, Linton J, Katz SI. Cytokine gene expression during the elicitation phase of contact sensitivity: regulation by endogenous IL-4. J Investig Dermatol. 1997;108:406–411. doi: 10.1111/1523-1747.ep12289700. [DOI] [PubMed] [Google Scholar]
- 38.Webb EF, Tzimas MN, Newsholme SJ, Griswold DE. Intralesional cytokines in chronic oxazolone- induced contact sensitivity suggest roles for tumor necrosis factor alpha and interleukin-4. J Investig Dermatol. 1998;111:86–92. doi: 10.1046/j.1523-1747.1998.00239.x. [DOI] [PubMed] [Google Scholar]
- 39.Abe M, Kondo T, Xu H, Fairchild RL. Interferon-gamma inducible protein (IP-10) expression is mediated by CD8+ T cells and is regulated by CD4+ T cells during the elicitation of contact hypersensitivity. J Investig Dermatol. 1996;107:360–366. doi: 10.1111/1523-1747.ep12363337. [DOI] [PubMed] [Google Scholar]
- 40.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolde G. Langerhans cells undergo apoptotic cell death in contact sensitization. Arch Dermatol Res. 1995;287:356–358. [Google Scholar]
- 42.Takahashi H, Kobayashi H, Hashimoto Y, Matsuo S, Iizuka H. Interferon-gamma-dependent stimulation of Fas antigen in SV40-transformed human keratinocytes. Modulation of the apoptotic process by protein kinase C. J Investig Dermatol. 1995;105:810–815. doi: 10.1111/1523-1747.ep12326577. [DOI] [PubMed] [Google Scholar]