Abstract
Previous studies have shown that autoimmune thyroiditis can be induced in normal laboratory rats after thymectomy and split dose γ-irradiation. Development of disease can be prevented by reconstitution of PVG rats shortly after their final irradiation with either peripheral CD4+CD45RC− T cells or CD4+CD8− thymocytes from syngeneic donors. Although the activity of both populations is known to depend on the activities of endogenously produced interleukin 4 and transforming growth factor β, implying a common mechanism, the issue of antigen specificity of the cells involved has not yet been addressed. In this study, we show that the regulatory T cells that prevent autoimmune thyroiditis are generated in vivo only when the relevant autoantigen is also present. Peripheral CD4+ T cells, from rats whose thyroids were ablated in utero by treatment with 131I, were unable to prevent disease development upon adoptive transfer into thymectomized and irradiated recipients. This regulatory deficit is specific for thyroid autoimmunity, since CD4+ T cells from 131I-treated PVG.RT1u rats were as effective as those from normal donors at preventing diabetes in thymectomized and irradiated PVG.RT1u rats. Significantly, in contrast to the peripheral CD4+ T cells, CD4+CD8− thymocytes from 131I-treated PVG donors were still able to prevent thyroiditis upon adoptive transfer. Taken together, these data indicate that it is the peripheral autoantigen itself that stimulates the generation of the appropriate regulatory cells from thymic emigrant precursors.
Keywords: autoimmunity, regulatory T cells, specificity, autoantigen
There is compelling evidence that the immune system is controlled by populations of T cells whose function is to inhibit the activation of T cells that are potentially dangerous to the host. These regulatory T cells (Treg) have been described in the prevention of inflammatory responses to dietary antigens (1, 2) and in the control of autoimmunity (3–6). The mode of action of these protective cells is not well understood, but a knowledge of their antigen specificity is an essential prerequisite for such understanding.
Studies of Treg in our own laboratory have exploited two different inducible models of autoimmunity. Autoimmune thyroiditis and insulin-dependent diabetes mellitus (IDDM) are induced in PVG.RT1c (hereafter PVG) and PVG.RT1u strain rats, respectively, by similar protocols of thymectomy and split dose γ-irradiation (TxX) (7, 8). Although both diseases are similarly induced, they are characterized by contrasting autoimmune processes. IDDM in PVG.RT1u rats involves a CD8+ T cell–dependent cell-mediated pathogenesis, whereas thyroiditis development in PVG rats results in high titers of Th2-associated IgG1 isotype anti-thyroglobulin (Tg) antibodies and does not require CD8+ T cells. However, in both cases, development of disease can be prevented by reconstitution of rats with either peripheral CD4+ T cells or mature CD4+CD8− thymocytes (8–10), and, at least in the prevention of thyroiditis, the suppressive activity of both subsets is dependent on IL-4 and TGF-β.
The Treg in the periphery that prevent autoimmunity are found exclusively among cells with a resting memory phenotype (CD4+CD45RC−TCR-α/β+RT6+) (8). Although it has been shown that the thymus is a potent source of Treg (9, 10), the observation that the peripheral cells have a primed phenotype suggests that recognition of specific antigen in the periphery is an essential developmental step. However, the identity of the antigen(s) is not known. Data from studies of oophoritis and prostatitis that develop in certain mice strains after thymectomy between 2 and 4 d after birth implicate a role for tissue specific antigens in the generation of Treg, since CD4+ T cells from mice lacking the relevant organs, either due to gender or surgical removal, are less effective at preventing disease development (11, 12). However, these data remain contentious since other studies report contradictory results (13) and it is possible that gender-specific organs still share tissue-specific antigens that may be the target of regulatory activity.
The aim of our study was to test the hypothesis that the encounter of Treg with peripheral self-antigen is essential for their successful development. To address this question, rats were generated in which the thyroid gland had been completely ablated by treatment with 131I in utero before significant export of T cells from the thymus had occurred (14). The data from this study show that T cells from these athyroid rats were selectively unable to prevent thyroid-specific autoimmunity while retaining the capacity to prevent autoimmune diabetes. In contrast, CD4+CD8− thymocytes from the same athyroid donors were as effective as those from normal rats at preventing thyroiditis. Implications for the development of Treg and the possibility for therapeutic intervention in this process are discussed.
Materials and Methods
mAbs.
The mouse mAbs used in these studies were as follows: OX6 (anti–rat class II MHC; reference 15), OX7 (anti–rat Thy-1; reference 16), OX8 (anti–rat CD8; reference 17), and OX12 (anti–rat κ chain; reference 18).
Animals and Induction of Thyroiditis and Diabetes.
3–12-wk-old female PVG and PVG.RT1u rats, which differ only in their MHC haplotype, were obtained from the specific pathogen-free breeding facilities of the Medical Research Council Cellular Immunology Unit (Oxford, UK). Thyroiditis was induced in female PVG rats as has been previously described (7). In brief, female rats aged 3 wk were thymectomized and given four 275-rad doses of 137Cs γ-irradiation at 2-wk intervals starting 1 wk after thymectomy. Development of thyroiditis was determined histologically and by the development of anti-Tg antibodies. Diabetes was induced in male PVG.RT1u rats by their thymectomy at 6 wk of age followed by four 250-rad doses of 137Cs γ-irradiation at 2-wk intervals starting 2 wk after thymectomy. Development of diabetes was determined by monitoring body weight and blood glucose levels.
Ablation of Rat Thyroid Glands.
Fetal PVG and PVG.RT1u rats were exposed to 131I (Amersham International plc., UK) at day 18 of gestation by intraperitoneal injection of the mother with 2 mCi of the radioactive isotope. The success of the ablation was determined both histologically and by assaying serum thyroid-stimulating hormone (TSH) levels by radio immunoassay (Amersham International plc.) in 8-wk-old treated rats.
Isolation of T Cell Subpopulations.
Mature rat CD4+ T lymphocytes were negatively selected from lymph node and spleen cells using magnetic beads (Dynal, Norway) by depletion of B cells, CD8+ T cells, and recent thymic emigrants using the mAbs OX12, OX8, OX7, and OX6. CD4+CD8− thymocytes were similarly purified from thymus by depletion of CD8+ cells. The purity of all isolated cells, analyzed on a FACScan® by labeling pre- and postdepletion samples with rabbit anti–mouse Ig FITC, was >98%.
Detection and Quantitation of Anti-Tg Antibodies in Sera of Rats.
Sera from TxX rats were assayed by specific ELISA using 96-well microtiter plates coated overnight with purified rat Tg (20 μg/ml). After incubation of serial dilutions of sera from individual rats for 2 h at room temperature, bound rat IgG was detected using anti–rat IgG alkaline phosphatase conjugate (Sigma Chemical Co.) for 1 h at room temperature. The assay was developed using enzyme substrate 4-nitro-phenyl phosphate (5 mg/ml; Sigma Chemical Co.) for 15 min at room temperature before reading the OD at 405 nm. Anti-Tg antibody titers were quantified by comparison with a standard serum pooled from TxX rats with thyroiditis and high anti-Tg antibody titers and expressed as a percentage of this standard. The level of nonspecific binding found in normal PVG serum represented a titer of ∼0.1% in the assay, and therefore only sera with titers >0.3% were considered to contain specific anti-Tg antibodies.
Histological Analysis.
Whole thyroids attached to thyroid cartilage were dissected out and fresh-frozen in O.C.T. embedding medium (Sakura Finetek U.S.A. Inc.). 10-μm sections were cut from frozen blocks and stained with hematoxylin and eosin.
Results and Discussion
Athyroid rats were generated after a modification of a previously described protocol using 131I (19). Rats were treated in utero with 131I on day 18 of gestation by injecting the pregnant mother with 2 mCi of the isotope into the peritoneal cavity. Serial sections of thyroid cartilage from 8-wk-old 131I-treated rats revealed extensive destruction of glands with little or no follicular thyroid tissue apparent (Fig. 1 B). The success of the ablation was confirmed by the detection of high levels of TSH in serum of treated animals. Thyroxine normally exerts negative feedback inhibition of TSH production via the hypothalamic-pituitary axis, such that in the absence of thyroxine TSH levels are allowed to increase unchecked. Serum TSH levels in PVG or PVG.RT1u rats treated with 131I in utero were >10 times higher than those of normal rats (Table I).
Figure 1.
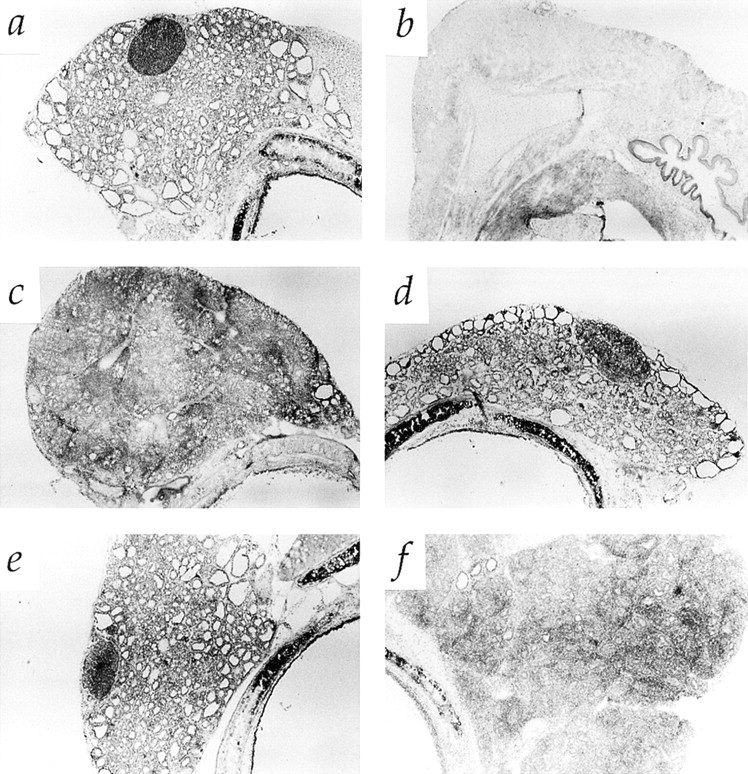
Morphology of thyroid glands in 131I-treated rats and TxX rats with thyroiditis. Pregnant PVG rats were injected intraperitoneally with 2 mCi of 131I on day 18 of gestation. Thyroid cartilage and attached tissue was removed from normal PVG rats (A) and 131I-treated rats (B) at 8 wk of age, sectioned and stained with hematoxylin and eosin. In the same series of experiments as those described in Fig. 2, whole thyroid glands attached to thyroid cartilage were taken at the time of peak disease from control TxX PVG rats (C), recipients of 107 CD4+ T cells from normal PVG rats (D), and recipients of either 5 × 106 CD4+CD8− thymocytes (E) or 107 CD4+ T cells (F) from the same athyroid donors. Thyroids were frozen, sectioned, and stained with hematoxylin and eosin and are shown at low magnification. (Original magnification: ×50.)
Table I.
Serum TSH Levels of 8-wk-old Normal PVG Rats and Rats Treated with 131I In Utero
| Treatment | Serum TSH ng/ml ± SD | Range | n | |||
|---|---|---|---|---|---|---|
| None | 7.7 ± 1.4 | 5.8–10.2 | 7 | |||
| 131I-treated PVGs | 116.7 ± 52.3 | 54.6–200.5 | 10 | |||
| 131I-treated PVG.RT1u rats | 171.8 ± 29.8 | 128.5–199.2 | 6 | |||
| All 131I-treated rats | 137.4 ± 52 | 54.6–200.5 | 16 |
Pregnant PVG and PVG.RT1u rats were injected intraperitoneally with 2 mCi of 131I on day 18 of gestation. Offspring of 131I-treated and normal untreated PVG rats were bled at 8 wk of age and serum TSH concentration was determined by RIA.
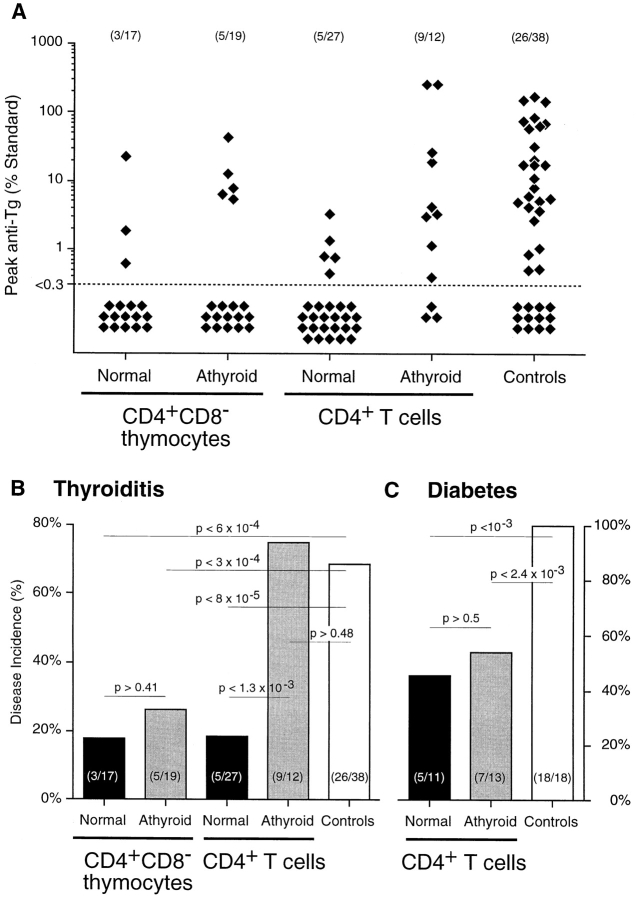
To determine whether thyroid antigen–specific Treg function had been affected by ablation of thyroid glands, CD4+ T cells from these rats were assayed for their ability to regulate thyroid-specific autoimmunity by their adoptive transfer into TxX PVG rats before development of thyroiditis. Although the protective capacity of CD4+ T cells has been shown previously to be mediated entirely by the CD45RC− subset (8, 10), unfractionated CD4+ T cells were assayed because, at least in principle, it was possible that in the absence of specific thyroid antigen in 131I-treated rats, Treg would still be functional but assume a naive CD4+CD45RC+ phenotype. Autoimmune thyroiditis was induced in PVG rats by their thymectomy at 3 wk of age followed by four doses of 275 rad 137Cs γ-irradiation at 2 wk intervals starting 1 wk after thymectomy. Groups of these rats were reconstituted shortly after the final irradiation with either 5 × 106 CD4+CD8− thymocytes or 107 CD4+ peripheral T cells purified from either normal or athyroid 8-wk-old PVG rats. Consistent with previous studies, a highly significant proportion of rats reconstituted with CD4+CD8− thymocytes or CD4+ T cells from normal rats were protected from development of both serological (Fig. 2 A) and histological (Fig. 1 D) signs of disease. In contrast, TxX rats reconstituted with CD4+ T cells from athyroid rats developed high titers of anti-Tg antibody (Fig. 2 A) and had extensive leukocytic infiltration of thyroid glands (Fig. 1 F). Significantly, CD4+CD8− thymocytes from the same athyroid rats were as effective as the same population from normal rats at preventing development of thyroiditis (Figs. 1 E and 2 A).
Figure 2.
Adoptive transfer of peripheral CD4+ T cells from athyroid donors into TxX rats prevents development of diabetes but not thyroiditis. Athyroid donor PVG and PVG.RT1u rats were generated after exposure to 131I in utero as described in Fig. 1. Normal PVG and PVG.RT1u rats were thymectomized at 3 and 6 wk of age, respectively, and given 1,100 and 1,000 rads of split dose γ-irradiation, respectively, in four equal doses at 2-wk intervals. Shortly after the last irradiation, groups of TxX PVG rats were reconstituted with 5 × 106 CD4+CD8− thymocytes purified from the thymus of either normal or athyroid 8-wk-old PVG donor rats. Further groups of TxX PVG rats were reconstituted with 107 CD4+ T cells purified from the same normal and athyroid PVG donors. Development of anti-Tg IgG responses was monitored for between 4 and 12 wk after the last irradiation by specific ELISA. Data represent peak anti-Tg IgG titers of individual TxX rats, expressed as percentage of standard (A). TxX PVG.RT1u rats were reconstituted shortly after their final irradiation with 107 CD4+ T cells purified from either normal or athyroid 8-wk-old PVG.RT1u rats. The disease incidence of thyroiditis in groups of TxX PVG rats (B) and diabetes in groups of TxX PVG.RT1u rats (C) reconstituted with different cell subsets from different donors are expressed as a percentage of the group. Numbers in parentheses show the actual incidence and group sizes. P values were calculated using Fisher's exact test.
One possible explanation for the failure of CD4+ T cells from athyroid rats to control thyroiditis was that the thyroxine deficiency that resulted from 131I treatment may have had an adverse effect on either thymopoiesis, and therefore normal repertoire selection, or on peripheral T cell function including that of Treg. Therefore, to test both the specificity of the regulatory deficit and the impact of thyroxine deficiency on Treg function, the ability of T cells from athyroid rats to prevent autoimmunity was assayed in a second autoimmune model not involving thyroid antigens. PVG.RT1u rats were induced to develop insulin- dependent diabetes by a similar protocol of thymectomy and split dose γ-irradiation. Shortly after the final irradiation, rats were reconstituted with 107 CD4+ peripheral T cells from either normal or athyroid syngeneic donors. Significantly, the level of protection from diabetes afforded to TxX PVG.RT1u rats reconstituted with 107 CD4+ T cells from athyroid syngeneic donors was almost identical to that observed in recipients of 107 CD4+ T cells from age-matched euthyroid controls. Approximately 50% of recipients were protected in both cases (Fig. 2 C), a level of protection essentially the same as that observed in previous studies (8). Furthermore, that intrathymic generation of Treg was not adversely affected by thyroxine deficiency in 131I-treated rats was evident from the observation that CD4+CD8− thymocytes from these rats were able to prevent thyroiditis in TxX PVG rats.
The most economical interpretation of these data is that peripheral autoantigen is itself responsible for the induction of Treg, and although other interpretations are possible, Treg appear to express TCRs specific for the relevant autoantigen. Athyroid rats were deficient only in Treg that control thyroid targeted autoimmunity, since cells from these rats could still suppress diabetes (Fig. 2, B and C). Such observations are compatible with others studies of thyroid autoimmunity that result after removal of thyroids during gestation, either surgically or by 131I ablation. Bilateral thyroidectomy but not hemithyroidectomy of lambs at 52 d of gestation results in a loss of self-tolerance to the same thyroid glands, retransplanted after their storage in nude mice (20). Similarly, rats exposed to 131I in utero reject syngeneic thyroid grafts as adults (19). However, when thyroid- ablated rats are surgically parabiosed with normal, syngeneic partners, self-tolerance to thyroid transplantation is restored (21). These results provide further evidence implicating a role for peripheral self-antigen in the generation of specific Treg. Experiments with Treg in mice, which prevent skin allograft rejection, are also compatible with this interpretation (22).
The observation of this study that CD4+CD8− thymocytes from athyroid rats retain the capacity to prevent thyroiditis, in stark contrast to their peripheral counterparts, has implications regarding the lineage development of these regulatory cells. Earlier studies demonstrated that CD4+CD8− thymocytes are a more potent source of Treg than are peripheral CD4+ T cells (9) and that this is unlikely to be attributed to differences in their mechanism of action, since protection from disease is dependent on IL-4 and TGF-β in both cases (10). One interpretation of these data is that maturation of Treg from thymic emigrants into the mature peripheral Treg population is subject to strict homeostatic regulation, such that only as many Treg undergo peripheral maturation as are required to suppress the activity of autoreactive T cells in the repertoire. Under conditions where the Treg pool has been perturbed, as occurs during the induction of autoimmunity in TxX rats, such a mechanism would stimulate the differentiation of all potential precursors among CD4+CD8− thymocytes. Since mature peripheral Treg have already undergone differentiation, no further recruitment is possible, and it is this difference in plasticity of the regulatory populations that results in the apparent potency of thymocytes compared with peripheral cells. That CD4+CD8− thymocytes from athyroid rats are able to prevent disease development shows that intrathymic generation of Treg during repertoire selection does not require peripheral thyroid tissue. Thymocytes from these animals have the potential to mature into specific Treg, but in the absence of peripheral antigen fail to do so. However, adoptive transfer of these cells into euthyroid TxX rats rescues Treg development and in doing so protects the recipients from disease development. These data suggest that the requirement for self-antigen in Treg maturation is also the point of homeostatic control, raising the possibility that it is the Treg themselves that regulate their own differentiation. With regard to the fate of the Treg precursor that emerges from the thymus of athyroid rats in the absence of autoantigen, it is not possible to conclude whether the regulatory deficit in these rats results from a failure of these cells to expand to a functionally significant frequency or a failure of the cells to survive in the absence of autoantigen.
The finding that the thymus is a potent source of Treg (9), together with the observation that mRNA for many “tissue-specific” autoantigens, including insulin and thyroglobulin, can be detected intrathymically (23–25) is suggestive of a role for positive selection of the T cell repertoire on self-antigens in the generation of Treg. There is some evidence that the self-antigens that mediate positive selection are antagonist peptides (26, 27). Significantly, recognition of synthetic antagonistic peptide has been shown in several studies to affect cytokine production, notably that of TGF-β (28). This raises the possibility that peripheral T cell recognition of the autoantigen on which they were selected intrathymically induces generation of Treg and evokes a regulatory response from them that acts to specifically suppress autoimmune responses (6).
Finally, previous studies suggest that there exists in the T cell repertoire a fine balance between autoreactive T cells and the Treg that control them (9). Thus, any adverse perturbation of this balance, either congenital or environmental, could result in autoimmunity. The conclusion of this study, that peripheral autoantigen is responsible for the induction of specific Treg from precursors found in abundance amongst mature thymocytes, raises the possibility of augmenting the physiological process with a therapeutic outcome. In principle, administration of autoantigens in an appropriate form may have the effect of increasing the induction of Treg and as such represent a basis for vaccination against autoimmune disease.
Footnotes
Thanks are due to Liz Darley for preparation and staining of histological sections and to Steve Simmonds and Michael Puklavec for technical assistance.
Benedict Seddon is supported by a Wellcome Trust Prize Fellowship.
References
- 1.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor β but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CD45RBlowCD4+T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi O, Nishizuka Y, Sakakura T, Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980;40:540–553. [PMC free article] [PubMed] [Google Scholar]
- 4.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomo A, Kehn P, Shevach E. Post-thymectomy autoimmunity: abnormal T-cell homeostasis. Immunol Today. 1995;16:61–67. doi: 10.1016/0167-5699(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 6.Saoudi A, Seddon B, Heath V, Fowell V, Mason D. The physiological role of regulatory T cells in the prevention of autoimmunity: the function of the thymus in the generation of the regulatory T cell subset. Immunol Rev. 1996;149:195–216. doi: 10.1111/j.1600-065x.1996.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 7.Penhale WJ, Farmer A, Irvine WJ. Thyroiditis in T cell-depleted rats. Influence of strain, radiation dose, adjuvants and antilymphocyte serum. Clin Exp Immunol. 1975;21:362–375. [PMC free article] [PubMed] [Google Scholar]
- 8.Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into pre-diabetic recipients. J Exp Med. 1996;184:2393–2398. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor β and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+CD45RC− cells and CD4+CD8−thymocytes. J Exp Med. 1999;189:279–288. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirements of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taguchi O, Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self-tolerance. J Exp Med. 1987;165:146–156. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith H, Sakamoto Y, Kasai K, Tung KS. Effector and regulatory cells in autoimmune oophoritis elicited by neonatal thymectomy. J Immunol. 1991;147:2928–2933. [PubMed] [Google Scholar]
- 14.Ema H, Cumano A, Kourilsky P. TCR-beta repertoire development in the mouse embryo. J Immunol. 1997;159:4227–4232. [PubMed] [Google Scholar]
- 15.McMaster WR, Williams AF. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979;9:426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- 16.Mason DW, Williams AF. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980;187:1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brideau RJ, Carter PB, McMaster WR, Mason DW, Williams AF. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SV, Fowler MH. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinet. 1981;14:445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 19.Eishi Y, McCullagh P. Acquisition of immunological self-recognition by the fetal rat. Immunology. 1988;64:319–323. [PMC free article] [PubMed] [Google Scholar]
- 20.McCullagh P. Interception of the development of self tolerance in fetal lambs. Eur J Immunol. 1989;19:1387–1392. doi: 10.1002/eji.1830190806. [DOI] [PubMed] [Google Scholar]
- 21.McCullagh P. Curtailment of autoimmunity following parabiosis with a normal partner. Immunology. 1990;71:595–597. [PMC free article] [PubMed] [Google Scholar]
- 22.Modigliani Y, Coutinho A, Pereira P, Le Douarin N, Thomas-Vaslin V, Burlen-Defranoux O, Salaun J, Bandeira A. Establishment of tissue-specific tolerance is driven by regulatory T cells selected by thymic epithelium. Eur J Immunol. 1996;26:1807–1815. doi: 10.1002/eji.1830260822. [DOI] [PubMed] [Google Scholar]
- 23.Morahan G, Allison J, Miller JF. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989;339:622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- 24.Antonia SJ, Geiger T, Miller J, Flavell RA. Mechanisms of immune tolerance induction through the thymic expression of a peripheral tissue-specific protein. Int Immunol. 1995;7:715–725. doi: 10.1093/intimm/7.5.715. [DOI] [PubMed] [Google Scholar]
- 25.Heath VL, Moore NC, Parnell SM, Mason DW. Intrathymic expression of genes involved in organ specific autoimmune disease. J Autoimmunity. 1998;11:309–318. doi: 10.1006/jaut.1998.0210. [DOI] [PubMed] [Google Scholar]
- 26.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 27.Hsu BL, Evavold BD, Allen PM. Modulation of T cell development by an endogenous altered peptide ligand. J Exp Med. 1995;181:805–810. doi: 10.1084/jem.181.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windhagen A, Scholz C, Hollsberg P, Fukaura H, Sette A, Hafler DA. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity. 1995;2:373–380. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]