Abstract
This study investigated the role of natural killer (NK) cells as effectors of an immune response against autologous cells modified by gene therapy. T lymphocytes were transduced with LXSN, a retroviral vector adopted for human gene therapy that carries the selectable marker gene neo, and the autologous NK response was evaluated. We found that (i) infection with LXSN makes cells susceptible to autologous NK cell–mediated lysis; (ii) expression of the neo gene is responsible for conferring susceptibility to lysis; (iii) lysis of neo-expressing cells is clonally distributed and mediated only by NK clones that exhibit human histocompatibility leukocyte antigen (HLA)-Bw4 specificity and bear KIR3DL1, a Bw4-specific NK inhibitory receptor; and (iv) the targets are cells from HLA-Bw4+ individuals. Finally, neo peptides anchoring to the Bw4 allele HLA-B27 interfered with KIR3DL1-mediated recognition of HLA-B27, i.e., they triggered NK lysis. Moreover, neo gene mutations preventing translation of two of the four potentially nonprotective peptides reduced KIR3DL1+ NK clone–mediated autologous lysis. Thus, individuals expressing Bw4 alleles possess an NK repertoire with the potential to eliminate autologous cells modified by gene therapy. By demonstrating that NK cells can selectively detect the expression of heterologous genes, these observations provide a general model of the NK cell–mediated control of viral infections.
Keywords: natural killer cells, killer cell inhibitory receptor, gene therapy, retroviral vectors, peptides
NK cells express receptors that, upon interaction with their MHC class I ligands, produce a signal inhibiting killing of the autologous cell (reviewed in references 1 and 2). One type of receptor is a lectin-like heterodimer, CD94-NKG2A, that recognizes the nonclassical MHC class Ib molecule HLA-E (3). The other is a family of Ig-like receptors designated killer cell inhibitory receptors (KIRs)1 (4–6). KIRs with two Ig domains (KIR2D) identify HLA-C allotypes: KIR2DL1 (formerly designated p58.2) recognizes group 2 HLA-C allotypes, whereas KIR2DL2 (p58.1) recognizes group 1 HLA-C allotypes. A KIR with three Ig domains, KIR3DL1 (p70/NKB1), is specific for HLA-B alleles sharing the Bw4 supertypic specificity. Therefore, the diversity of human MHC class I allotypes collapses to only three well-defined KIR supertypic ligands: group 1 HLA-C, group 2 HLA-C, and HLA-Bw4. In addition, a homodimer of molecules with three Ig domains, KIR3DL2 (p70/140), has been shown to be specific for HLA-A3 and -A11 (7). Thus, NK cells are activated in response to the missing expression of self-class I molecules on target cells, such as some allogeneic cells or tumor or virus-infected cells. KIRs also distinguish peptides bound to MHC class I molecules. NK cell recognition of class I alleles on target cells is prevented, and, consequently, NK lysis is triggered by amino acid substitutions along protective peptides loaded onto empty class I molecules at the surfaces of transporter for antigen presentation (TAP)-deficient cells (8–12). Exogenously loaded viral peptides also abrogate NK cell recognition of MHC ligands (13). Consequently, the NK cell killing of virus-infected cells (14) may be triggered by replacement of self-peptides with endogenously synthesized viral peptides.
Human gene therapy uses replication-deficient, recombinant viral vectors (reviewed in references 15 and 16). Lack of sustained exogenous gene expression may be due to several mechanisms, including specific immune reactions to the genetically modified cells. Infection of immunocompetent mice with replication-defective adenoviruses elicits a CD8+ CTL response that eliminates virus-infected cells (17–22). In humans reinfused with retrovirally infected cells, specific CTL immunity against the transgene product has been implicated in sharp reductions in survival of autologous transduced cells (23, 24). Therefore, immunogenicity of genetically modified cells may elicit specific immunity in the host and limit the stability of gene expression in vivo.
In this study, we explored the role of NK cells in the immune response to genetically modified autologous cells and used the LXSN retroviral vector because it, or other similar vectors, is currently adopted for human gene therapy protocols (24–27). These vectors are potential targets for immune recognition, as they express neo, a heterologous antibiotic (G418)-resistance gene used for selection of transfected packaging cell lines and gene marking studies in vivo (28).
We found that (i) infection with the LXSN retroviral vector makes human cells susceptible to autologous NK cell– mediated lysis, (ii) expression of the neo gene is responsible for conferring susceptibility to lysis, (iii) the effectors are NK cells that bear KIR3DL1 and exhibit HLA-Bw4 specificity, and (iv) the targets are cells from HLA-Bw4+ individuals. Finally, we found that neo protein peptides anchor to Bw4 alleles (such as B27) and interfere with KIR3DL1-mediated recognition of Bw4 alleles, i.e., they trigger NK lysis. Moreover, neo gene mutations preventing translation of two of the four potentially nonprotective peptides reduced KIR3DL1+ NK clone–mediated autologous lysis.
Materials and Methods
Immunofluorescence and Flow Cytometry.
Indirect immunofluorescence with primary mAb plus secondary fluorochrome-conjugated goat anti–mouse Ig antibodies (Southern Biotechnology Associates) and flow cytometry (FACSCalibur™; Becton Dickinson) determined NK cell phenotypes. NK cell clones were identified using an anti-CD16 mAb (Immunotech). Expression of KIRs recognizing group 1 HLA-C alleles (KIR2DL2), group 2 HLA-C alleles (KIR2DL1), and HLA-Bw4 alleles (KIR3DL1) was determined with mAb EB6, GL183, and Z27, respectively (29) (provided by L. Moretta, University of Genova, Italy). Anti-HLA class I mAb and ME1 and B27M2 anti–HLA-B27 mAb were obtained from American Type Culture Collection (ATCC).
Preparation of NK Cells and NK Cell Clones.
NK cell–enriched preparations were obtained from PBMC by negative immunomagnetic selection (Dynal) of T cells, B cells, and monocytes with anti-CD3, -CD20, and -CD14 mAb (Immunotech) and were cultured with 100 U/ml rIL-2 for 4–6 d before cell-killing assays. For NK cell cloning, PBMC depleted of T cells by negative immunomagnetic selection with anti-CD3 mAb (OKT3; obtained from ATCC) were plated at the concentration of 10 cells/well in 96-well microtiter plates, activated with PHA, and cultured with IL-2 and irradiated feeder cells as described elsewhere (30). Bulk-cultured and -cloned NK cells were used as effectors in standard (30) 51Cr-release cytotoxicity assays using autologous gene–transferred T cells as targets. E/T ratio was 10:1.
Analysis of NK Clone Allospecificity.
Standard 51Cr-release cytotoxicity assays against cell lines transfected with class I genes (provided by L. Moretta) determined NK clone allospecificity. Specificity for group 1 (Cw4-related) and group 2 (Cw3-related) HLA-C allotypes was analyzed using the HLA class I–negative cell line 721.221 and 721.221 cells transfected with Cw*0401 or Cw*0302 genes, respectively (31). Specificity for HLA-Bw4 allotypes (such as HLA-B27) was analyzed using untransfected C1R cells and C1R cells transfected with B*2705 gene (29). Specificity for the nonclassical MHC class Ib molecule HLA-E was analyzed using C1R cells expressing HLA-B7 (29) (cell surface expression of HLA-E is regulated by the binding of peptides derived from the signal sequence of some other MHC class I molecules, such as HLA-B7) (3). E/T ratio was 10:1. Results are presented as percent inhibition compared with lysis of the untransfected 721.221 or C1R cells. Clones lysed the untransfected cells at levels exceeding 60%, whereas lysis of autologous cells (PHA lymphoblasts) never exceeded 5%. NK clones were considered specific for a given allotype when cytotoxicity was <50% of control lysis obtained against untransfected cells.
Vector Preparation; Transfer and Selection of Transduced Cells.
The PA317 amphotropic packaging cell line was transfected with the pLXSN retroviral plasmid (32), G418-selected, and subcloned. A high retroviral titer clone was used to infect cells. To construct a retroviral vector unable to produce the neo protein, the SV40neo transcriptional unit was deleted from the LXSN plasmid by BamH1–Nae1 restriction digestion, Klenow blunting, and religation. The modified plasmid was transiently transfected in Phoenix amphotropic packaging cells (33) to produce retroviral particles. To construct a retroviral vector carrying a neo gene encoding a mutated protein that cannot produce potentially nonprotective peptides, the 209 cystine TGT codon of the neo gene was mutated to TGA to truncate the reading frame. The LXSN plasmid was consequently used as a template in a PCR reaction with the following mutagenizing oligonucleotides: 5′-TGCAAAAAGCTTGGGCTGCAGGTC, 3′-CCCAGCCGGCCTCAGTCGATGAATC. The PCR product was cloned and replaced in the original LXSN plasmid at the Hind III–NgoIV fragment. The final plasmid was sequenced to ensure that the open reading frame of the neo gene was maintained throughout the cDNA to the new stop codon. The plasmid was used to transiently transfect Phoenix cells and produce retroviral particles. PHA-activated, 48-h IL-2–cultured T cells (PBMC forming rosettes with SRBC) were infected by repeated cycles of centrifugation in the presence of viral supernatants. For cloning, T cells were plated at the concentration of 0.5 cells/well in 96-well microtiter plates and cultured with 100 U/ml IL-2 and irradiated feeder cells. The gene-transferred T cell clones were identified by PCR on DNA.
PCR.
For PCR on DNA, 105 lymphocytes were lysed in 100 ml of a buffer containing 10 mM Tris/HCl, 50 mM KCl, 2.5 mM MgCl2, 0.1% gelatin, 0.45% NP40, 0.45% Tween 20, and 100 mg/ml proteinase K. 10 ml of extract was used for a PCR reaction with the following ψ region primers: 5′-TGGTTCTGGTAGGAGACGAG, 3′-GCTTCCCAGGTCACGATGTA. Reverse transcriptase (RT)-PCR was performed with published neo primers (34).
Peptide Pulsing of RMA–S-B27 Cells.
Peptides were synthesized by solid-phase method on an automated multiple peptide synthesizer (AMS 422; Aimed) using F-moc chemistry (35). Purity was confirmed by reverse-phase HPLC. TAP2-deficient RMA-S cells transfected with the human β2m alone (referred to in this report as RMA-S) or in combination with the HLA-B*2705 class I gene (RMA–S–B27) were cultured for 24 h at 26°C (8). Peptides were added in two separate doses: 100 μM at the onset of the experiment and an additional 100 μM 12 h later. Peptide loading was documented by surface stabilization of HLA-B27 as measured by immunofluorescence and flow cytometry. RMA-S and RMA-S–B27 cells were 51Cr-labeled overnight during the peptide pulsing. After labeling, cells were used as targets in standard cell-killing assays with KIR3DL1+ NK clones as effectors (30). Some cytolytic assays were performed in the presence of Z27 anti-KIR3DL1 mAb (500 ng/ml) (29).
HLA Typing of Donors Used in This Study.
Donor 1: A2/A33, B52/B35, Cw4; donor 2: A2/A11, B51/B27, Cw2; donor 3: A2/A28, B44/B18, Cw5/Cw7; donor 4: A11/A28, B8/B35, Cw4/Cw7.
Results and Discussion
LXSN Infection Confers Susceptibility to Lysis by Autologous NK Cells.
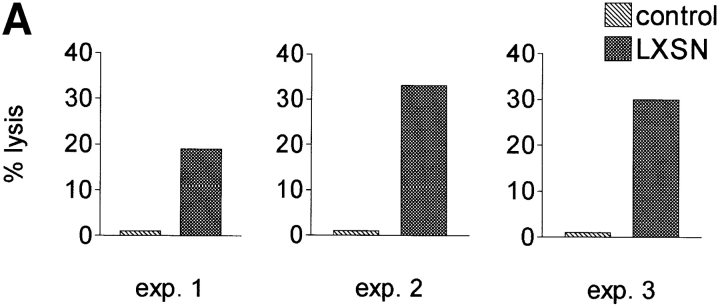
Peripheral blood T lymphocytes were infected with LXSN, and the transduced cells were selected in G418. The selected cells as well as nontransduced control cells were used as targets in cytotoxicity assays with autologous IL-2–cultured NK cells as effectors. Transfer of LXSN conferred susceptibility to lysis by autologous NK cells in three consecutive, independent experiments (Fig. 1 A).
Figure 1.
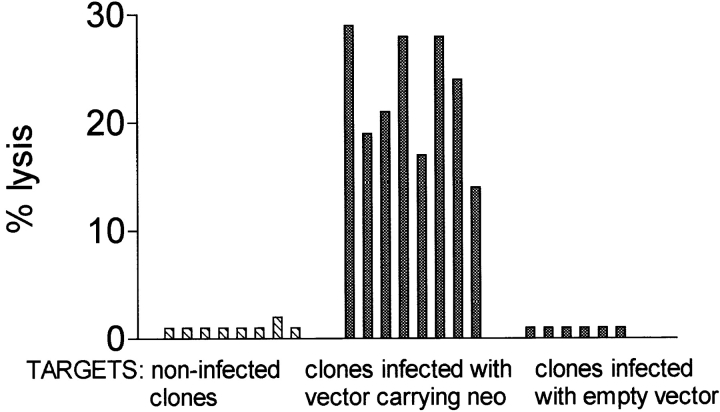
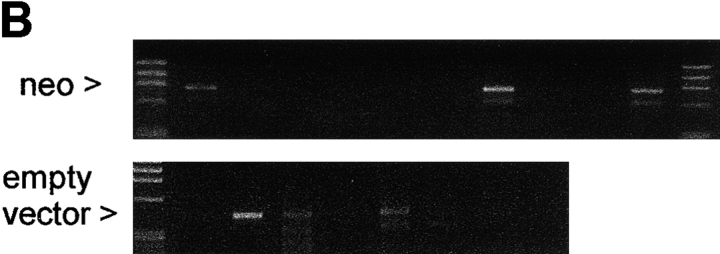
The antibiotic resistance gene neo within the retroviral vector LXSN makes LXSN-infected cells susceptible to autologous NK cell lysis. (A) NK cell cytotoxicity against autologous T cells infected with LXSN (E/T ratio, 10:1). LXSN infection conferred susceptibility to lysis by autologous NK cells. (B) NK cell lysis is not activated by autologous T cells infected with the same retroviral vector that does not contain the neo cDNA. Left panels, PCR on DNA identified neo-positive, empty vector–positive, and nontransduced T cell clones used as targets for autologous NK cell killing (neo expression was confirmed by RT-PCR). Right panel, NK cell cytotoxicity assay on PCR-selected clones (E/T ratio, 10:1). All clones infected with the LXSN vector carrying neo were lysed by autologous NK cells. All clones transduced with the empty vector and all nontransduced clones were resistant to lysis.
Immunofluorescence analysis of MHC class I expression showed that killing was not the consequence of a downregulation of MHC class I molecules at the surface of the transduced cells (not shown).
The Selectable Marker Gene, neo, Is Responsible for NK Cell Lysis of Autologous LXSN-infected Cells.
The next step was to identify whether expression of the heterologous selectable marker gene, neo, or the defective retroviral mRNA itself (through translation of short open reading frames) was responsible for susceptibility of LXSN-infected cells to lysis. To this end, we measured NK killing of autologous T lymphocytes transduced with the same retroviral vector that did not contain the neo cDNA. T cells underwent the gene transfer procedure and were cloned by limiting dilution. Cells infected with LXSN were also cloned by limiting dilution without antibiotic selection. PCR on DNA identified neo-positive, “empty” vector–positive, and nontransduced control targets for autologous NK cell–mediated killing (Fig. 2 B). neo expression was confirmed by RT-PCR. Remarkably, all of the neo-transduced clones and none of the empty vector–transduced clones (and none of the noninfected control clones) were killed by autologous NK cells. Therefore, within the LXSN retroviral vector, the neo gene was responsible for conferring susceptibility to NK lysis.
Figure 2.
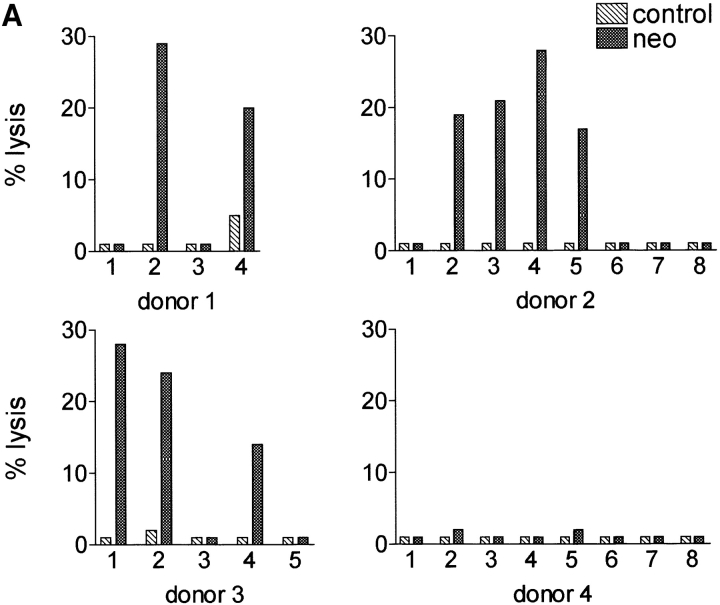
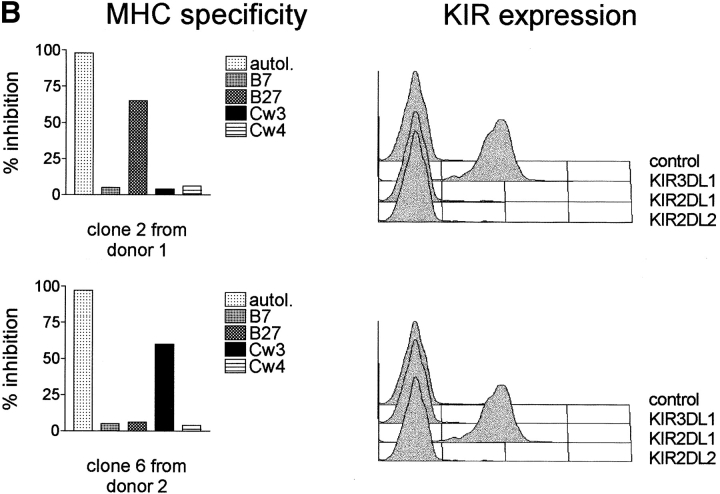
NK cell lysis of autologous neo gene–expressing cells is clonally distributed. It is a property only of NK clones bearing KIR3DL1 and exhibiting specificity for MHC class I alleles of the Bw4 group. (A) NK clones from four random donors (see Materials and Methods for HLA typing) were evaluated for their lytic ability against autologous cells expressing the neo gene (E/T ratio, 10:1). Some clones from donors 1, 2, and 3 lysed the autologous neo gene–expressing targets. No clone from donor 4 lysed the target. (B) KIR expression and MHC specificity of lytic and nonlytic NK clones. For analysis of MHC specificity, clones were used as effectors in cell-killing assays against cell lines transfected with the indicated class I allotypes. The results are presented as percent inhibition compared with lysis of the untransfected cells; “autol.” indicates inhibition of lysis mediated by autologous PHA lymphoblasts. All clones from donors 1, 2, and 3 that lysed the autologous neo gene–expressing targets, but none of the nonlytic clones, exhibited Bw4 specificity and expressed the Bw4 receptor KIR3DL1. An example of data from experiments with one lytic and one nonlytic clone from these three donors is shown (see Table I for a summary of data from all clones of donors 1, 2, and 3).
Lysis of neo-Expressing Cells Is Mediated by NK Clones Bearing KIR3DL1 and Exhibiting Specificity for HLA-Bw4.
The neo cDNA might include sequences that could be translated into heterologous peptides, replacing the autologous peptides at given MHC class I alleles, so that NK cells with the appropriate MHC receptors recognize the neo peptide– loaded alleles. To test this hypothesis, several NK clones were obtained from four random donors and used for cytotoxicity experiments with autologous LXSN-infected cells as targets. Interestingly, as illustrated in Fig. 2 A, some NK clones from donors 1, 2, and 3, but not from donor 4, killed the neo gene–expressing autologous targets. The three well-defined KIR specificities (1, 2) were presumably present in these donors, because donor 1 and donor 2 expressed group 1 HLA-C and Bw4 HLA-B alleles; donor 3 expressed group 1 HLA-C, group 2 HLA-C, and Bw4 HLA-B alleles; donor 4 expressed group 1 and group 2 HLA-C but not Bw4 alleles (this donor, in contrast to the other donors, was homozygous for HLA-B alleles of the Bw6 group, which is not recognized by KIR) (see Materials and Methods for HLA typing). Clones were, therefore, analyzed for these KIR specificities by the use of target cells expressing the HLA-Bw4 allele B27, the group 2 HLA-C allele Cw3, and the group 1 HLA-C allele Cw4 and for expression of the corresponding KIR (Fig. 2 B and Table I). Control targets, not recognized by KIRs, were cells expressing B7 (the binding of signal sequence peptides from certain class I alleles, such as B7, regulates the expression of HLA-E, a nonclassical MHC class Ib molecule recognized by CD94-NKG2A) (3). All clones from donors 1, 2, and 3 that lysed the neo-expressing autologous targets, but none of their nonlytic clones, exhibited Bw4 specificity and expressed KIR3DL1, a Bw4 receptor. Fig. 2 B illustrates data obtained from one lytic and one nonlytic clone, and Table I summarizes data from all clones of donors 1, 2, and 3. These three donors expressed alleles of the Bw4 group. In contrast, donor 4, whose LXSN-infected cells were resistant to autologous NK lysis, did not express Bw4 alleles. Thus, without exception, Bw4 specificity and the Bw4 receptor KIR3DL1 were the distinctive features of NK clones triggered to lyse genetically modified autologous targets. Moreover, and again without exception, lack of Bw4 specificity and failure to express KIR3DL1 defined clones that did not recognize the genetically modified autologous targets. As expected, several clones coexpressed multiple specificities and the corresponding KIRs. It was therefore remarkable that, in spite of the fact that a number of specificities might be involved, expression of the Bw4 receptor KIR3DL1 appeared to be both necessary and sufficient for recognition of the autologous gene-modified cells. Immunofluorescence analysis of HLA-B27 on the LXSN- infected cells from donor 2 revealed that susceptibility to lysis was not a consequence of selective downregulation of Bw4 molecules at the surface of the transduced cells (data not shown).
Table I.
MHC Specificities and KIR Expression of Clones from Donors 1, 2, and 3
| Clone No. | MHC specificity* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| non-KIR ligand§ | KIR ligands | KIR‡ | ||||||||||||
| HLA-B27 (Bw4 HLA-B) | HLA-Cw3 (group 2 HLA-C) | HLA-Cw4 (group 1 HLA-C) | KIR3DL1 (BW4 HLA-B) | KIR2DL1 (group 2 HLA-C) | KIR2DL2 (group 1 HLA-C) | |||||||||
| HLA-B7 | ||||||||||||||
| NK clones with lytic activity against autologous cells expressing the neo gene | ||||||||||||||
| 1.2 | − | + | − | − | + | − | − | |||||||
| 1.4 | − | + | + | − | + | + | − | |||||||
| 2.2 | − | + | + | + | + | + | + | |||||||
| 2.3 | + | + | + | − | + | + | − | |||||||
| 2.4 | − | + | − | + | + | − | + | |||||||
| 2.5 | + | + | − | − | + | − | − | |||||||
| 3.1 | − | + | + | − | + | + | − | |||||||
| 3.2 | − | + | − | − | + | − | − | |||||||
| 3.4 | − | + | + | − | + | − | − | |||||||
| NK clones without lytic activity against autologous cells expressing the neo gene | ||||||||||||||
| 1.1 | + | − | + | − | − | + | − | |||||||
| 1.3 | + | − | − | − | − | − | − | |||||||
| 2.1 | − | − | + | + | − | + | + | |||||||
| 2.6 | − | − | − | + | − | − | + | |||||||
| 2.7 | − | − | + | − | − | + | − | |||||||
| 2.8 | + | − | + | + | − | + | + | |||||||
| 3.3 | − | − | + | − | − | + | − | |||||||
| 3.5 | − | − | + | + | − | + | + | |||||||
+ indicates allotype specificity, i.e., that NK lysis was inhibited to >50% of control lysis by a target expressing the indicated allele (the corresponding allele group is indicated in parentheses). − denotes lack of allele specificity, i.e., that lysis was unaffected by that target.
+ and − denote positivity or negativity for expression of KIR (the allele group recognized by each KIR is indicated in brackets).
This HLA-B allele allows expression of the nonclassical MHC class Ib molecule HLA-E, which is not recognized by KIR (reference 3).
The coexpression of other KIRs besides KIR3DL1 by lytic clones suggests that neo gene expression might also lead to the production of peptides interfering with MHC recognition of other receptors. However, the pivotal role of KIR3DL1 in detecting expression of the neo gene is emphasized by the presence of one neo peptide with anchor residues for Cw4 and a residue at position 8 that prevents recognition of HLA-Cw4 by KIR2DL2 (i.e., QYDDAVYFL) (11). As shown in Table I, clones expressing KIR2DL2 did not kill the gene-transferred cells unless they coexpressed KIR3DL1.
Neo Peptides Prevent KIR3DL1 Recognition of HLA-Bw4 Alleles.
Recognition of the Bw4 allele HLA-B27 by KIR3DL1 is the most extensively studied model of peptide-specific recognition of MHC class I by NK cells (8– 10). A comparison of the amino acid sequence of the neo protein with sequences of peptides reported to block recognition of HLA-B27 by KIR3DL1 (Table II) showed that several nonamers within the neo protein possess (i) anchor residues at positions 2 and 9 that allow binding to HLA-B27 (36) and (ii) residues at positions 7 and/or 8 that are known to prevent KIR3DL1-mediated recognition of HLA-B27 (8–10). In addition, other P7 and/or P8 residues that prevent recognition of HLA-B27 were present within neo peptides anchoring to Bw4 alleles of the other donors (Table II).
Table II.
Neo Protein Peptides with Potential to Interfere with NK Cell Recognition of the Bw4 Alleles of Donors 1, 2, and 3
| Published HLA-B27 | RRISGVDRY | ARFGIRAK | TRYPILAGH | RRYQKSTEL | RRLPIFSRL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neo protein | ||||||||||
| HLA-B27 | GRLGVADRY | QRIAFYRLL | ARTRMEAGL | |||||||
| (donor 2) | HRIERARTR | |||||||||
| Neo protein | ||||||||||
| HLA-B52 | ||||||||||
| (donor 1) | LQDEAARLS | EQDGLHAGS | DQDDLDEEL | GQDLLSSHL | ||||||
| Neo protein | ||||||||||
| HLA-B44 | ||||||||||
| (donor 3) | VENGRFSGF | VENGRFSGF |
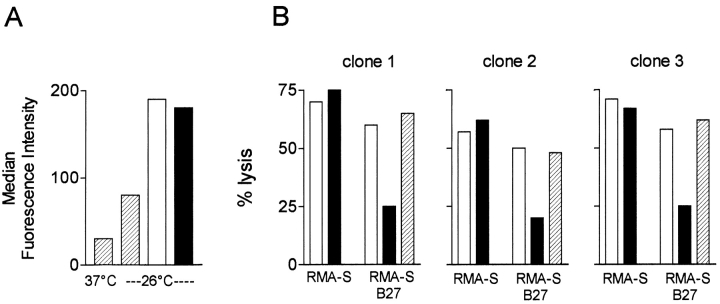
We therefore asked whether neo peptides actually bind Bw4 alleles and if this complex specifically affects interaction with KIR3DL1. To this end, we synthesized one of the HLA-B27–binding neo peptides shown in Table II, GRLGVADRY, and the analogue peptide GRLGVAIHY, in which aspartic acid and arginine at positions 7 and 8 had been replaced by isoleucine and histidine, respectively. As isoleucine and histidine at positions 7 and 8 are critical for the interaction between HLA-B27 and KIR3DL1 (8–10), the latter peptide was expected to be a protective version of the original neo peptide. Peptides were loaded onto HLA-B27 molecules of RMA-S cells, as indicated by the surface stabilization of HLA-B27 molecules shown in Fig. 3 A. Peptide-pulsed RMA-S–B27 and control RMA-S cells were used as targets for lysis by three randomly chosen KIR3DL1+ (Bw4-specific) NK clones (Fig. 3 B). Binding of the neo peptide was unable to protect RMA-S–B27 cells from NK lysis. In contrast, the analogue peptide conferred protection from lysis. Protection was mediated by HLA-B27 on target cells because no protection was conferred to control RMA-S cells and by KIR3DL1 on the NK clones because it was abrogated by the addition of anti-KIR3DL1 mAb. Similar results were obtained with the other HLA-B27–binding neo peptides shown in Table II. Therefore, exogenously loaded neo peptides prevent recognition of HLA-B27 by KIR3DL1.
Figure 3.
Neo peptides prevent recognition of HLA-B27 by KIR3DL1. (A) The neo peptide GRLGVADRY (shown in Table II) and the analogue-protective peptide GRLGVAIHY (in which aspartic acid and arginine at positions 7 and 8 had been replaced by isoleucine and histidine) were loaded onto HLA-B27 molecules of TAP-deficient RMA-S–B27 cells. Surface stabilization of HLA-B27 in the absence (hatched bars) or presence (open bar) of neo or analogue peptide (filled bar). (B) Binding of the neo peptide was unable to protect RMA-S–B27 cells from lysis by three randomly selected KIR3DL1+ NK clones (open bars). The analogue peptide conferred protection from lysis (filled bars). Protection required expression of HLA-B27 on target cells because it was not conferred to control RMA-S cells, and it was mediated by KIR3DL1 on NK clones because it was abrogated by the addition of anti-KIR3DL1 mAb (hatched bars).
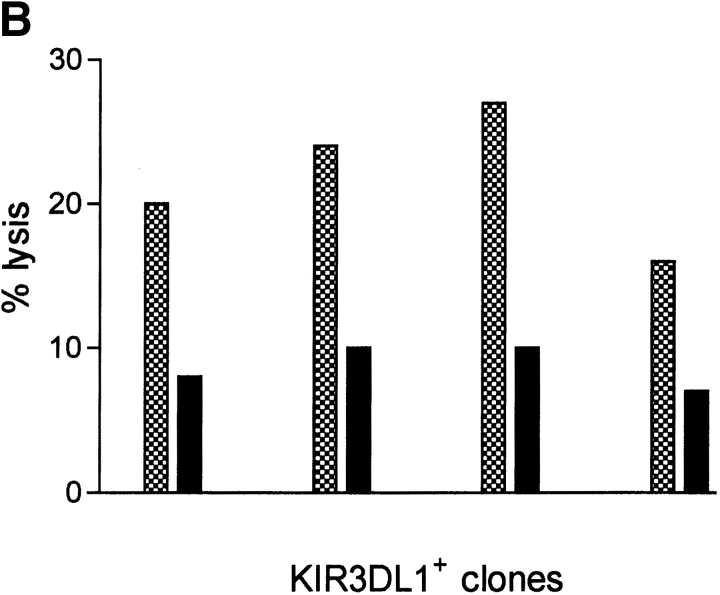
To document whether the endogenous production of these neo peptides is indeed capable of triggering autologous NK lysis, a stop codon preventing translation of the last 56/265 amino acids of the protein was created in the neo cDNA (Fig. 4 A). The resulting truncated neo protein does not contain two of the four HLA-B27–binding, nonprotective peptides shown in Table II and used for the experiments in Fig. 3 (GRLGVADRY and QRIAFYRLL). T lymphocytes from an HLA-B27+ donor were transduced with the truncated neo protein–expressing gene and used as targets for autologous NK lysis by KIR3DL1+ clones (Fig. 4 B). This deletion of ∼1/5 of the protein reduced lysis by over 60%. Importantly, the deleted fragment did not contain other HLA-B27–binding peptides with P7 and/or P8 residues that could have triggered autologous NK lysis. Therefore, the reduction of NK-mediated lysis of autologous T lymphocytes infected with the mutated vector must be attributed to the deletion of the two nonprotective peptides.
Figure 4.

Neo gene mutations that stop nonprotective peptide expression reduce autologous NK killing. (A) Neo protein amino acid sequence. The large box shows the truncated neo protein obtained by inserting a stop codon into the neo cDNA. The small boxes indicate the potentially nonprotective HLA-B27–binding peptides (shown in Table II and used for the experiments in Fig. 3). (B) KIR3DL1+ NK clone killing of autologous HLA-B27+ cells transduced with neo (checked bars) and with the mutated neo gene (filled bars).
Taken together, the experiments in Figs. 3 and 4 indicate that neo gene expression by LXSN-infected cells from HLA-B27+ individuals can generate peptides that prevent KIR3DL1-mediated recognition of HLA-B27 and trigger autologous NK lysis.
The fact that 721.221 or C1R cells commonly transfected with class I genes for NK recognition employ neo selection may seem perplexing and opens the question as to why these cells, when they express Bw4 alleles, are not rendered susceptible to lysis by neo peptides. Our data show that the susceptibility to lysis conferred by the neo gene is limited. On the other hand, the degree of protection conferred to 721.221 or C1R cells by class I transfection seldom reaches the 100% level of unmanipulated autologous cells, even when NK clones with single class I allele specificity are used. It is therefore plausible that the neo gene also partially antagonizes the protective effect of Bw4 allele expression in 721.221 or C1R cells.
The fact that clones with specificity for Bw4 allotypes were responsible for killing autologous LXSN-infected cells implies that it should be possible to predict that HLA-Bw4+ individuals may possess an NK cell repertoire with potential to clear gene therapy–modified cells. Individuals (like donor 4) homozygous for the reciprocal group of HLA-B allotypes, Bw6, should tolerate the engineered cells. Assessment of the impact of the present findings on survival of gene-transferred cells will require monitoring of gene expression at serial times after the reintroduction of engineered cells in vivo. We had the opportunity to evaluate two patients with adenosine deaminase deficiency undergoing adenosine deaminase gene therapy with the LXSN vector (carrying neo) (26). One patient expressed alleles of the Bw4 and Bw6 groups, and the other was homozygous for Bw6 alleles. Both patients had undergone gene therapy simultaneously 4 mo before our assessment. The HLA-Bw4+ patient, compared with the HLA-Bw6+ patient, exhibited 100-fold lower levels of the transduced gene.
Finally, our observations, by demonstrating that NK cells can selectively detect the expression of heterologous genes, provide a general model of the NK cell–mediated control of viral infections.
Acknowledgments
We thank Lorenzo Moretta (University of Genova, Italy) for anti-KIR antibodies, cell lines expressing class I alleles, and critical reading of the manuscript; Vincenzo Barnaba (University of Rome, “La Sapienza,” Italy) for peptides; Mauro Malnati (Department of Biological and Technological Research, Istituto Scientifico H.S. Raffaele, Milan, Italy) for RMA-S and RMA-S–B27 cells and for useful suggestions and discussion; Pier-Giuseppe Pelicci (European Institute of Oncology, Milan, Italy) for critical reading of the manuscript; and Geraldine Anne Boyd for assistance in the writing of the manuscript.
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) and from the Telethon Foundation. I. Volpi and L. Ruggeri are supported by fellowships from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
Abbreviations used in this paper
- KIRs
killer cell inhibitory receptors
- RT
reverse transcriptase
- TAP
transporter for antigen presentation
References
- 1.Moretta A. Molecular mechanisms in cell-mediated cytotoxicity. Cell. 1997;90:13–18. doi: 10.1016/s0092-8674(00)80309-8. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Natural killer cells: from no receptors to too many. Immunity. 1997;6:371–378. doi: 10.1016/s1074-7613(00)80280-0. [DOI] [PubMed] [Google Scholar]
- 3.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 4.Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati M, Vitale M, Bottino C, Moretta L, Moretta A, Long EO. Molecular clones of the p58 natural killer cell receptor reveal Ig-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2:439–449. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 5.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 6.D'Andrea A, Chang C, Franz-Bacon K, McClanahan T, Phillips JH, Lanier LL. Molecular cloning of NKB1: a natural killer cell receptor for HLA-B allotypes. J Immunol. 1995;155:2306–2310. [PubMed] [Google Scholar]
- 7.Pende D, Biassoni R, Cantoni C, Verdiani S, Falco M, Di Donato C, Accame L, Bottino C, Moretta A, Moretta L. The natural killer cell receptor specific for HLA-A allotypes: a novel member of the p58/p70 family of inhibitory receptors that is characterized by three immunoglobulin-like domains and is expressed as a 140-kD disulphide-linked dimer. J Exp Med. 1996;184:505–518. doi: 10.1084/jem.184.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, Moretta A, Long EO. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–1018. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 9.Peruzzi M, Wagtmann N, Long EO. A p70 killer inhibitory receptor specific for several HLA-B allotypes discriminates among peptides bound to HLA-B*2705. J Exp Med. 1996;184:1585–1590. doi: 10.1084/jem.184.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peruzzi M, Parker KC, Long EO, Malnati M. Peptide sequence requirements for the recognition of HLA-B*2705 by specific natural killer cells. J Immunol. 1996;157:3350–3356. [PubMed] [Google Scholar]
- 11.Rajagopalan S, Long EO. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J Exp Med. 1997;185:1523–1528. doi: 10.1084/jem.185.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zappacosta F, Borrego F, Brooks AG, Parker KC, Coligan JE. Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc Natl Acad Sci USA. 1997;94:6313–6318. doi: 10.1073/pnas.94.12.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandelboim O, Wilson SB, Valés-Gòmez M, Reyburn HT, Strominger JL. Self and viral peptides can initiate lysis by autologous natural killer cells. Proc Natl Acad Sci USA. 1997;94:4604–4609. doi: 10.1073/pnas.94.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman DS, Schoon RA, Liebson PL. Role for major histocompatibility complex class I in regulating natural killer cell-mediated killing of virus-infected cells. Proc Natl Acad Sci USA. 1992;89:8337–8341. doi: 10.1073/pnas.89.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AD. Human gene therapy comes of age. Nature. 1992;357:455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- 16.Verma IM, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 17.Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Xiang Z, Ertl HC, Wilson JM. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Y, Schwarz EM, Gu D, Zhang W, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Ertl J, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds TC, Corey L, et al. T-cell mediated rejection of gene modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 24.Bonini C, Ferrari G, Verzelletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1996;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. Gene transfer into humans: immunotherapy of patients with advanced melanoma, using tumor infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 26.Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 27.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, et al. T-lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 28.Emmons RV, Doren S, Zujewski J, Cottler-Fox M, Carter CS, Hines K, O'Shaughnessy JA, Leitman SF, Greenblatt JJ, Cowan K, et al. Retroviral gene transduction of adult peripheral blood or marrow-derived CD34+cells for six hours without growth factors or on autologous stroma does not improve marking efficiency assessed in vivo. Blood. 1997;89:4040–4046. [PubMed] [Google Scholar]
- 29.Vitale M, Sivori S, Pende D, Agugliaro R, Di Donato C, Amoroso A, Malnati M, Bottino C, Moretta L, Moretta A. Physical and functional independency of p70 and p58 natural killer (NK) cell receptors for HLA class I: their role in the definition of different groups of alloreactive NK cell clones. Proc Natl Acad Sci USA. 1996;93:1453–1457. doi: 10.1073/pnas.93.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albi N, Ruggeri L, Aversa F, Merigiola C, Tosti A, Tognellini R, Grossi CE, Martelli MF, Velardi A. Natural killer (NK)-cell function and antileukemic activity of a large population of CD3+/CD8+T cells expressing NK receptors for major histocompatibility complex class I after “three-loci” HLA-incompatible bone marrow transplantation. Blood. 1996;87:3993–4000. [PubMed] [Google Scholar]
- 31.Biassoni R, Falco M, Cambiaggi A, Costa P, Verdiani S, Pende D, Conte R, Di Donato C, Parham P, Moretta L. Amino acid substitutions can influence the natural killer (NK)-mediated recognition of HLA-C molecules. Role of serine-77 and lysine-80 in the target cell protection from lysis mediated by “group 2” or “group 1” NK clones. J Exp Med. 1995;182:605–609. doi: 10.1084/jem.182.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–982. [PMC free article] [PubMed] [Google Scholar]
- 33.Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 34.Bregni M, Magni M, Siena S, Di Nicola M, Bonadonna G, Gianni AM. Human peripheral blood hematopoietic progenitors are optimal targets of retroviral- mediated gene transfer. Blood. 1992;80:1418–1422. [PubMed] [Google Scholar]
- 35.Salemi S, Caporossi AP, Boffa L, Longobardi MG, Barnaba V. HIVgp120 activates autoreactive CD4-specific T cell responses by unveiling of hidden CD4 peptides during processing. J Exp Med. 1995;181:2253–2257. doi: 10.1084/jem.181.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rammensee HG, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]