Abstract
Invasion of the malarial parasite into a vector mosquito begins when the motile ookinete transverses the gut epithelium. Adhesive proteins that may mediate this invasive process have not been identified to date. We found that a molecule with an adhesive protein–like structure was expressed in the ookinete of Plasmodium berghei. This protein is structurally homologous to circumsporozoite protein and thrombospondin-related adhesive protein (TRAP)-related protein, CTRP, of Plasmodium falciparum. We named it P. berghei CTRP (PbCTRP) and report here its structure and manner of expression. PbCTRP has six integrin I region–like domains and seven thrombospondin-like domains in its putative extracellular region. This structure is similar to that of CTRP and TRAPs of malaria sporozoite. The putative transmembrane and cytoplasmic regions of PbCTRP, CTRP, and TRAP also have conserved amino acid sequences. PbCTRP is produced at least 10 h after fertilization when zygotes begin transformation to ookinetes. In the mature ookinete, PbCTRP is located mainly in the anterior cytoplasm. The staining pattern was also similar to TRAP in the sporozoite. We suggest that PbCTRP may play a role in ookinete invasive motility and belongs to a protein family together with TRAP and other structurally related proteins of apicomplexan parasites.
Keywords: malaria, Plasmodium berghei, ookinete, TRAP, CTRP
Anopheline mosquitoes are vectors of human and rodent malarial parasites. After gamete fertilization in the midgut lumen of this insect, malarial parasites transform into ookinetes, the invasive form. Ookinetes attach to the surface of the midgut epithelium, penetrate it, and arrive at the basement membrane, where they develop into oocysts. Significant efforts have been made to elucidate the molecular mechanisms of this invasive process, in particular receptor-mediated interactions between ookinetes and the midgut epithelium. However, no molecule that may mediate this interaction has been identified.
Invasive-stage parasites of phylum Apicomplexa, including malarial ookinetes, are characterized by a common subcellular structure called the apical complex. Adhesive proteins that play a role in host cell recognition and invasion are expressed in this stage of the parasite. Most of them reported to date have common adhesive protein–related domains (1). These proteins bind to the host cell and are thought to be involved in invasive motility by creating a moving junction between the parasite apex and the surface of the host cell (1–3). They are located towards the anterior cytoplasm, especially in the secretory organelles called micronemes, and are thought to be secreted from the apical surface of parasites shortly after initial contact with a host cell (1, 4).
Here, we report the structure and expression of a protein of rodent malarial parasite, Plasmodium berghei, that is likely involved in ookinete invasive motility. This protein is termed PbCTRP1 because it has a structural homologue in the human malarial parasite, Plasmodium falciparum, called CTRP (circumsporozoite [CS] protein and thrombospondin [TSP]-related adhesive protein [TRAP]-related protein) (5). PbCTRP is also structurally related to a sporozoite protein, TRAP, and other adhesive proteins of apicomplexan parasites (6–8).
Materials and Methods
Ookinete Culture.
Balb/c mice infected with P. berghei ANKA strain were prepared by a peritoneal injection of the infected blood samples maintained at −70°C. Infected mice were used within one blood passage for ookinete culture. The culture conditions were as described (9). Parasites were collected from the culture at different time intervals, purified by erythrocyte lysis in 0.83% NH4Cl, and used for further analysis.
Genomic Library Construction.
Infected blood was collected from anesthetized rats by a cardiac puncture and applied to a CF 11 column (Whatman Biosystems) to remove white blood cells. This blood was cultured by a candle-jar method for 24 h, and erythrocytes containing merozoites were purified by density gradient (10). Parasites were purified by erythrocyte lysis, and genomic DNA was extracted. The genomic DNA was partially digested by restriction enzyme Sau3AI, ligated to BamHI-digested arms of lambda phage vector, λFix II (Stratagene, Inc.), by a partial fill-in method, and packed in vitro.
Genomic DNA Cloning.
Degenerated primers were designed based on amino acid sequences of CTRP (GDDCFC and APKVTILF), and related sequences were amplified from the genomic DNA of P. berghei by PCR under low-stringency conditions (annealing conditions, 47°C for 1 min). The amplified fragment (380 bp) was subcloned to a plasmid vector, pBluescript II (Stratagene, Inc.), and the DNA sequence was determined. The deduced amino acid sequence of this fragment has 68% identity with the second integrin-like domain of CTRP and ∼30% identity with plasmodial TRAPs (5, 8). It was labeled with [32P] dCTP and used for screening of a genomic library of P. berghei. Screening of the library yielded four positive phage clones. The insert DNA of a positive phage was digested into two fragments with restriction enzymes, SalI and EcoRI, separately subcloned into the plasmid vector, pBluescript II, and sequenced.
Southern Blot Analysis.
Genomic DNA of P. berghei was digested with restriction enzymes, separated on an agarose gel, fragmented in 0.25 M HCl, and transferred to nylon membrane. The blot was hybridized with a [32P]dCTP-labeled HindIII/MunI- digested DNA fragment (0.8 kb) of PbCTRP, and visualized by the BAS 2000 system (Fuji Film and Photo, Inc.).
Northern Blot Analysis.
Poly A(+) RNA was extracted using a microprep mRNA purification kit (Amersham Pharmacia Biotech) from blood-stage parasites and ookinetes cultured for 16 h after fertilization from the same infected blood. Poly A(+) RNA (0.1 μg per lane) was separated on an agarose gel and transferred to nylon membrane. The blot was hybridized with a [32P]dCTP-labeled HindIII/EcoRI-digested DNA fragment of PbCTRP (3.2 kb) and rehybridized with a control probe for Pbs 21, the transcript of which is known to be present in both gametocytes and ookinetes (11).
Production of Recombinant Protein and Antibody Preparation.
The DNA fragment encoding the NH2-terminal region of PbCTRP (amino acids 1–270) was subcloned into the baculovirus transfer vector, pAcYM1. The construction of recombinant virus and production of recombinant protein were performed as described (12). Recombinant protein was purified from the culture medium by gel-filtration HPLC and anion exchange HPLC as described (12).
The DNA fragment encoding the putative cytoplasmic domain (amino acids 1864–1904) was subcloned into Escherichia coli expression vector, pGEX 2T (Amersham Pharmacia Biotech), and produced as a glutathione S-transferase (GST) fusion protein. This recombinant protein was affinity purified on glutathione-Sepharose (Amersham Pharmacia Biotech), emulsified in TiterMax Gold (CytRx Corp.), and used for immunizing rabbits. Antibodies against GST were removed from the rabbit antisera by incubation with GST-conjugated Sepharose. Specific antibodies were affinity purified on GST fusion protein–conjugated Sepharose. The specificity of purified antibodies was checked by Western blot analysis.
Metabolic Labeling of Ookinete Proteins and Immunoprecipitation.
Parasites were cultured in ookinete culture medium without methionine and cysteine. Pro-mix [35S] (Amersham Pharmacia Biotech) was added to medium (50 μCi/ml in the final concentration) 5 h after exflagellation and cultured for an additional 17 h. Parasites were collected by centrifugation (400 g, 6 min), washed in PBS, and homogenized in extraction buffer (20 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM PMSF, 10 mg/ml aprotinin, and 10 mg/ml leupeptin). After centrifugation (15,000 g, 30 min), supernatant was recovered. Labeled PbCTRP was bound to antibody-conjugated protein G–Sepharose (Amersham Pharmacia Biotech) and eluted by sample buffer for SDS-PAGE. Samples were separated by SDS-PAGE in a linear gradient (5–20%) and visualized by the BAS 2000 system.
Western Blot Analysis.
Parasites were purified by erythrocyte lysis and homogenized in sample buffer. Parasite proteins were separated by SDS-PAGE in a linear gradient (5–20%) and transferred to nitrocellulose membrane. Blotted membrane was blocked in PBS containing 5% skim milk, and incubated with primary antibody against PbCTRP (10 μg/ml in PBS containing 0.05% Tween 20) and then with alkaline phosphatase–conjugated anti– rabbit IgG (GIBCO BRL).
Immunocytochemistry.
After culture for ookinete development, parasites were purified by erythrocyte lysis and fixed in acetone for 2 min on a glass slide. The samples were washed three times in PBS, preincubated in PBS containing 1% BSA for 10 min, and incubated with primary antibody against PbCTRP (6 μg/ml in PBS containing 1% BSA) for 1 h at room temperature. After washing three times in PBS, samples were incubated with FITC-conjugated goat anti–rabbit IgG (GIBCO BRL) for 1 h at room temperature, washed three times in PBS again, and examined under a fluorescence microscope. For control, nonimmune rabbit serum (diluted 1:100) was used as the primary antibody instead of anti-PbCTRP antibody.
Results
Structure of PbCTRP.
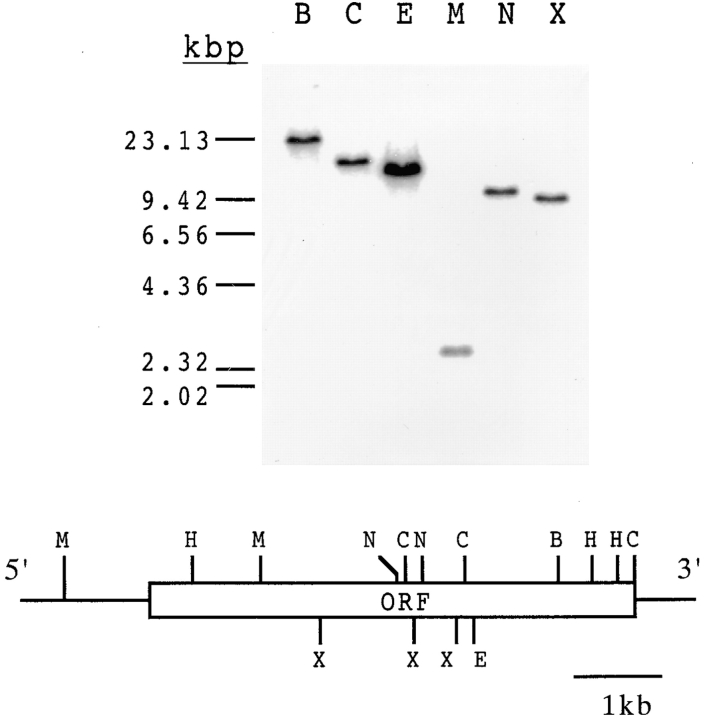
Cloned genomic DNA contains a single open reading frame of 5715 bp, which encodes a 1905–amino acid protein. Southern blot analysis showed it was a single-copy gene (Fig. 1). The hydropathy plot and the TMpred prediction (13, 14) showed that this protein has a transmembrane-like structure composed of a large putative extracellular region, a transmembrane-like domain, and a small putative cytoplasmic domain (Fig. 2). The putative extracellular region of PbCTRP is composed of six integrin I region–like domains and seven TSP-like domains. The NH2-terminal 21 amino acid residues of this protein probably encode a signal peptide. This is supported by the observation that the recombinant protein (amino acids 1–270) produced using the baculovirus–insect cell system is cleaved at this site (data not shown). The molecular mass of the mature protein is estimated to be 212 kD. We named this protein PbCTRP, because, in the overall structure and amino acid sequence, it showed striking similarity to CTRP of P. falciparum (PfCTRP). For example, amino acid sequence identities between PbCTRP and PfCTRP in the first integrin I region–like domain, the first TSP-like domain, and the putative cytoplasmic domain are 62.7, 64.9, and 80.5%, respectively. Conservation of amino acid sequences was also found among the putative cytoplasmic and transmembrane domains of PbCTRP, TRAPs, and PfCTRP (Fig. 3).
Figure 1.
Southern blot analysis of P. berghei genomic DNA (1 μg) digested with six different restriction enzymes. The blot was probed with the HindIII/MunI-digested fragment (0.8 kb) of the PbCTRP gene that is not cut by these enzymes. The bottom panel shows a restriction map of P. berghei genomic DNA around the open reading frame (open box, ORF) encoding PbCTRP. B, BglII; C, ClaI; E, EcoRI; M, MunI; N, NdeI; X, XbaI; H, HindIII.
Figure 2.
Hydrophilicity/hydrophobicity plot (top) and domain structure (bottom) of PbCTRP. The plot was generated according to the coefficients proposed by Kyte and Doolittle (reference 13).
Figure 3.
Comparison of amino acid sequences of PbCTRP, PfCTRP, and P. berghei TRAP in the putative transmembrane and cytoplasmic domains. Identical residues are highlighted in black. These sequence data are available from EMBL/Genbank/DDBJ under accession nos. AJ238798, AF149771, and AB027129.
Expression of PbCTRP during Ookinete Development.
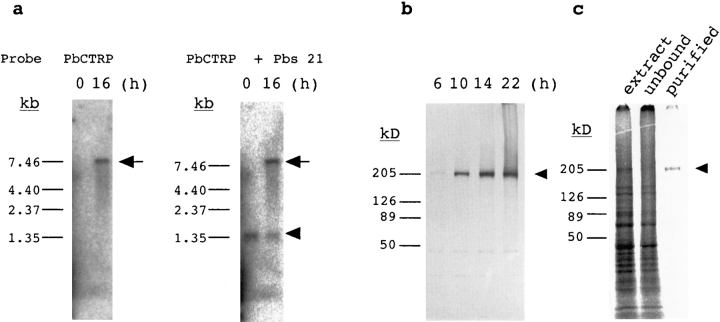
To investigate expression of PbCTRP, we performed Northern blot analysis of RNA preparations obtained from intraerythrocytic-stage parasites and ookinetes. Transcripts of PbCTRP were absent from blood-stage parasites but were detected in ookinetes (Fig. 4 a). The size of the transcript was 7.5 kb. We prepared polyclonal antibodies against recombinant protein (amino acids 1864–1904). Western blot analysis using this antibody showed that PbCTRP was abundantly present 10 h after exflagellation when zygotes had just begun transformation to ookinetes (Fig. 4 b and Fig. 5 a). After this, PbCTRP gradually increased during ookinete maturation. The apparent molecular mass of a detected protein was 210 kD, which is in good agreement with that estimated from the amino acid sequence. In addition, we metabolically labeled ookinete proteins by adding [35S]methionine/cysteine to the culture medium, immunoprecipitated labeled PbCTRP, and analyzed it on SDS-PAGE (Fig. 4 c). PbCTRP was identified as a distinct band of 210 kD among labeled proteins, which showed that it was actively produced during ookinete development. By SDS-PAGE analysis under reducing and nonreducing conditions, no apparent mobility change of the purified protein was observed (data not shown).
Figure 4.
Expression of PbCTRP during ookinete maturation. (a) Induction of PbCTRP transcript (arrow) by in vitro culture of the ookinete. Poly A(+) RNA was extracted from parasites that were purified from the same infected blood (parasitemia 10.5%) before (0 h) and after ookinete culture (16 h). Northern blot (0.1 μg per lane) was hybridized with a probe for PbCTRP (left) and rehybridized with a control probe for Pbs 21 (arrowhead, right). (b) PbCTRP production during ookinete maturation. The infected blood was cultured for ookinete maturation and collected at different time intervals as indicated above each lane. Parasites were purified by erythrocyte lysis in 0.83% NH4Cl and lysed in SDS-PAGE sample buffer. The samples (containing ∼106 ookinetes per lane) were separated by SDS-PAGE (in a 0–20% gradient) under nonreducing conditions and blotted onto the nitrocellulose membrane for immunodetection using anti-PbCTRP rabbit polyclonal antibody and then alkaline phosphatase–conjugated goat anti–rabbit IgG. (c) Metabolic labeling of ookinete proteins and immunoprecipitation of PbCTRP. Parasites were cultured with [35S]methionine/cysteine (from 5–22 h after exflagellation) and homogenized in the extraction buffer. Labeled PbCTRP (arrowhead) was immunoprecipitated from the extracted proteins using anti-PbCTRP antibody. Samples were separated by SDS-PAGE (in a 0–20% gradient) and visualized in the BAS 2000 system. Lane 1, labeled ookinete proteins (from ∼5 × 104 ookinetes). Lane 2, the same protein extract after absorbing PbCTRP with anti-PbCTRP antibody. Lane 3, purified labeled PbCTRP by immunoprecipitation (from ∼3 × 105 ookinetes).
Figure 5.
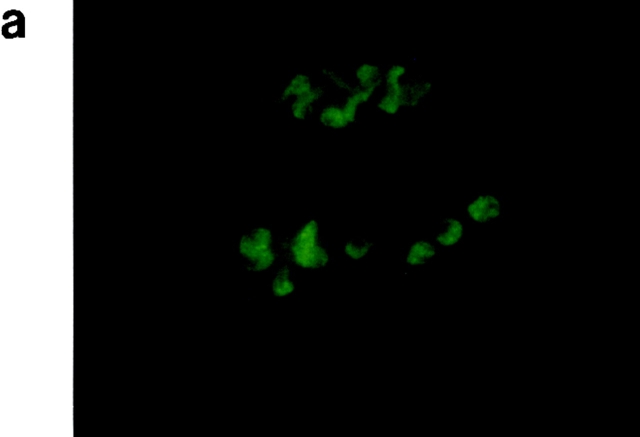
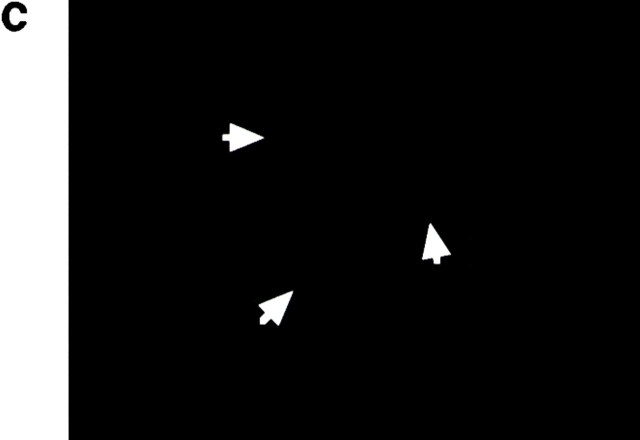
Localization of PbCTRP. Parasites were collected 10 and 22 h after exflagellation, purified by erythrocyte lysis, and fixed in acetone for 2 min. Specific antigen was revealed using an antiserum against recombinant PbCTRP as described in Materials and Methods. (a) 10 h after exflagellation; (b) 22 h after exflagellation; (c) 22 h after exflagellation, stained using nonimmune rabbit serum (as control). Arrows indicate mature ookinetes in control. Original magnifications: ×200.
Immunolocalization of PbCTRP.
When cultured for 10 h after exflagellation, most zygotes showed an oval or budding shape, indicating they had just started transformation into the invasive form. In this stage, a strong signal was already detected over the whole zygote (Fig. 5 a). When cultured for an additional 12 h, most parasites had transformed to ookinetes and a strong signal was detected in the cytoplasm mainly anterior to the nucleus (Fig. 5 b). This staining pattern was similar to that reported for TRAP in the sporozoite (5, 15). In the retort form, the incompletely mature ookinete with a remnant of the spherical zygote, the signal was observed only in the posterior (zygote) side, but not in the anterior (ookinete) side (data not shown).
Discussion
In this study, we report the structure and expression profile of PbCTRP. PbCTRP is structurally homologous to CTRP of the human malarial parasite, P. falciparum, and is expressed in the mosquito invasive-stage parasite, the ookinete. The expression profile of PfCTRP has not yet been clearly established (5). However, striking amino acid identities between PbCTRP and PfCTRP indicate they may play identical roles in the ookinete stage.
The putative extracellular region of PbCTRP is composed of adhesive protein–related domains, that is, six integrin I region–like domains and seven TSP-like domains. The first four integrin I region–like domains contain a metal ion–dependent adhesion site (MIDAS) motif. In integrins, this motif is known to mediate the divalent cation binding of the I domain and play a role in ligand binding of I domain–containing integrins (16). It is also found in a malaria sporozoite protein, TRAP (5, 8). TSP-like domains contain a similar amino acid sequence to the TSP 1 motif in different identities, especially around the sequence, WSXWXXCSXTCGXXXRXR. TSP-like domains are also found in mammalian adhesive proteins like properdin (17) and F-spondin (18) and malarial proteins, CS proteins, and TRAPs (5, 8, 19). These structural relationships to adhesive proteins indicate its possible role in attachment to the mosquito midgut epithelium.
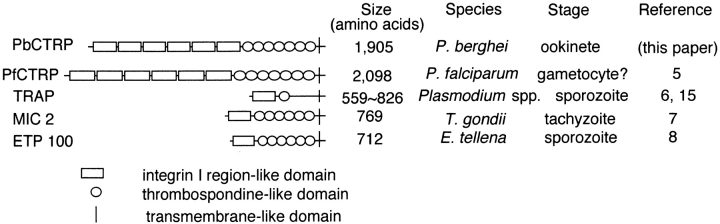
In addition, the structural similarity of PbCTRP to other apicomplexan proteins indicates its involvement in ookinete motility and in the invasion of the mosquito midgut. As shown in Fig. 6, PbCTRP bears a resemblance to TRAPs, the micronemal protein 2 (MIC 2) of Toxoplasma gondii, and the micronemal protein ETP 100 of Eimeria tenella (5–8). These proteins have single putative transmembrane structures and cytoplasmic domains. The extracellular regions are composed of tandemly aligned integrin I region– like domains and TSP-like domains. These proteins are specifically expressed in the invasive-stage parasites and are thought to play an essential role in invasive motility called gliding motility (1). It has been reported that the TRAP gene–disrupted malaria parasite lost its gliding motility and cell invasion abilities. PbCTRP is also expressed in the motile-stage parasite, the ookinete, indicating its possible role in motility through the mosquito midgut epithelium. The amino acid conservation observed in the transmembrane and cytoplasmic domains of PbCTRP and P. berghei TRAP might indicate that these domains carry important functions related to the movement mechanism common to these proteins (Fig. 3).
Figure 6.
Overall structures and expression stages of PbCTRP and other structurally related proteins of apicomplexan parasites. MIC, microneme protein of T. gondii; ETP 100, E. tenella micronemal protein.
Despite intensive studies, the invasive mechanisms of the mosquito midgut by malarial parasites remain unknown. PbCTRP is the first identified malaria protein that may be involved in ookinete invasion into the mosquito midgut. We think PbCTRP may be a clue for elucidating the invasive mechanisms of the mosquito midgut by malarial parasites, and we here establish the molecular basis of malarial parasite–mosquito cell interactions.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research on Priority Areas 08281103 and for Exploratory Research 10877043 from the Ministry of Education, Science, Culture, and Sports of Japan to Y. Chinzei, and by a Grant-in-Aid from the Mie Medical Research Foundation to M. Yuda.
Abbreviations used in this paper
- CS
circumsporozoite
- CTRP
CS protein and TRAP-related protein
- GST
glutathione S-transferase
- PbCTRP
Plasmodium berghei CTRP
- TSP
thrombospondin
- TRAP
TSP-related adhesive protein
References
- 1.Dubremetz JF, Garcia-Reguet N, Conseil V, Fourmaux MN. Apical organelles and host-cell invasion by Apicomplexa. . Int J Parasitol. 1998;28:1007–1013. doi: 10.1016/s0020-7519(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 2.Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, Nussenzweig RS. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 3.Naitza S, Spano F, Robson KJ, Crisanti A. The thrombospondin-related protein family of apicomplexan parasites: the gears of the cell invasion machinery. Parasitol Today. 1998;14:479–484. doi: 10.1016/s0169-4758(98)01346-5. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondiiaccompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 5.Trottein F, Triglia T, Cowman AF. Molecular cloning of a gene from Plasmodium falciparumthat codes for a protein sharing motifs found in adhesive molecules from mammals and plasmodia. Mol Biochem Parasitol. 1995;74:129–141. doi: 10.1016/0166-6851(95)02489-1. [DOI] [PubMed] [Google Scholar]
- 6.Robson KJ, Naiza S, Barker G, Sinden RE, Crisanti A. Cloning and expression of the thrombospondin related adhesive protein gene of Plasmodium berghei. . Mol Biochem Parasitol. 1997;84:1–12. doi: 10.1016/s0166-6851(96)02774-0. [DOI] [PubMed] [Google Scholar]
- 7.Wan KL, Carruthers VB, Sibley LD, Ajioka JW. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondiiencoding the micronemal protein MIC2. Mol Biochem Parasitol. 1997;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- 8.Tomley FM, Clarke LE, Kawazoe U, Dijkema R, Kok JJ. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. . Mol Biochem Parasitol. 1991;49:277–288. doi: 10.1016/0166-6851(91)90071-d. [DOI] [PubMed] [Google Scholar]
- 9.Ranawaka GR, Fleck SL, Blanko AR, Sinden RE. Characterization of the modes of action of anti-Pbs21 malaria transmission-blocking immunity: ookinete to oocyst differentiation in vivo. . Parasitology. 1994;109:403–411. doi: 10.1017/s0031182000080653. [DOI] [PubMed] [Google Scholar]
- 10.Waters AP, Thomas AW, van Dijk MR, Janse CJ. Transfection of malaria parasites. Methods. 1997;13:134–147. doi: 10.1006/meth.1997.0506. [DOI] [PubMed] [Google Scholar]
- 11.Vervenne RA, Dirks R, Ramesar J, Waters A, Janse CJ. Differential expression in blood stages of the gene coding for the 21 kilodalton surface protein of ookinetes of Plasmodium berghei as detected by RNA in situhybridisation. Mol Biochem Parasitol. 1994;68:259–266. doi: 10.1016/0166-6851(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 12.Yuda M, Hirai M, Miura K, Matsumura H, Ando K, Chinzei Y. cDNA cloning, expression and characterization of nitric-oxide synthase from the salivary glands of the blood-sucking insect Rhodnius prolixus. . Eur J Biochem. 1996;242:807–812. doi: 10.1111/j.1432-1033.1996.0807r.x. [DOI] [PubMed] [Google Scholar]
- 13.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann K, Stoffel W. TMbase: a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 15.Robson KJ, Frevert U, Reckmann I, Cowan G, Beier J, Scragg IG, Takehara K, Bishop DH, Pradel G, Sinden RE. Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:3883–3894. doi: 10.1002/j.1460-2075.1995.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickeson SK, Santoro SA. Ligand recognition by the I domain-containing integrins. Cell Mol Life Sci. 1998;54:556–566. doi: 10.1007/s000180050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt GD, Pangburn MK, Ginsburg V. Properdin binds to sulfatide [Gal(3-SO4)beta 1-1 Cer] and has a sequence homology with other proteins that bind sulfated glycoconjugates. J Biol Chem. 1990;265:2852–2855. [PubMed] [Google Scholar]
- 18.Klar A, Baldassare M, Jessell TM. F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell. 1992;69:95–110. doi: 10.1016/0092-8674(92)90121-r. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki LS, Svec P, Nussenzweig RS, Nussenzweig V, Godson GN. Structure of the Plasmodium knowlesigene coding for the circumsporozoite protein. Cell. 1983;34:815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]