Abstract
The mannose receptor (MR) has established roles in macrophage (Mφ) phagocytosis of microorganisms and endocytic clearance of host-derived glycoproteins, and has recently been implicated in antigen capture by dendritic cells (DCs) in vitro. MR is the founder member of a family of homologous proteins, and its recognition properties differ according to its tissue of origin. Given this heterogeneity and our recent discovery of a soluble form of MR in mouse serum, we studied the sites of synthesis of MR mRNA and expression of MR protein in normal mouse tissues. We demonstrate that synthesis and expression occur at identical sites, and that mature Mφ and endothelium are heterogeneous with respect to MR expression, additionally describing MR on perivascular microglia and glomerular mesangial cells. However, MR was not detected on DCs in situ, or on marginal zone or subcapsular sinus Mφ, both of which have MR-like binding activities. We also compared expression of MR to the binding of a recombinant probe containing the cysteine-rich domain of MR. We show that MR and its putative ligand(s) are expressed at nonoverlapping sites within lymphoid organs, consistent with a transfer function for soluble MR. Therefore, in addition to endocytic and phagocytic roles, MR may play an important role in antigen recognition and transport within lymphoid organs.
Keywords: mannose receptor, macrophage, dendritic cell, endothelium, mesangial cell
The mannose receptor (MR)1 was first identified as a specific uptake system in rat liver Kupffer cells for mannosylated/N-acetylglucosamine–terminal and fucosylated neoglycoproteins in vivo (1). Later studies demonstrated similar carbohydrate-specific binding by hepatic endothelium (2), alveolar macrophages (Mφ) (3), resident and elicited peritoneal Mφ (4), human monocyte–derived Mφ (5), and cultured bone marrow–derived Mφ (6). These were attributed to the MR by Ab reactivity in Western blots of purified receptor. MR has also been purified from human retinal pigment epithelium (7). Mφ expression of MR appears restricted to mature populations, is downregulated during classical activation, such as in response to IFN-γ (8), and is upregulated during an alternative form of activation by IL-4 characterized by enhanced MHC class II (MHCII) expression and reduced proinflammatory cytokine production (9). More recently, MR has been detected on cultured human dendritic cells (DCs) matured from CD14+ peripheral blood monocytes (10) and cord blood CD34+ hemopoietic progenitors with GM-CSF and IL-4 (11), although it is not known how closely these cells reflect the properties of DCs in situ. Freshly isolated murine Langerhans cells do not appear to express MR protein, although uptake of mannose-BSA and a mannan-inhibitable component of zymosan phagocytosis have been documented (12). In contrast, functional MR has been detected on freshly isolated human Langerhans cells (13). Uncharacterized receptors with similar binding activity to MR have been detected in lymph node subcapsular sinus Mφ of mouse (14) and rat (15) and splenic marginal zone Mφ of mouse (16) and rat (15).
The early studies on liver and mature Mφ suggested two major functions for MR, in endocytic clearance of host- derived glycoproteins and phagocytosis/endocytosis of microorganisms and soluble ligands, and evidence has accrued in support of both roles. MR mediates uptake of ligands for the purposes of both homeostasis and immunity. Homeostatic functions include uptake of tissue plasminogen activator (17, 18) and lysosomal hydrolases (3). MR also plays a major role in host defence. It is now widely accepted that the recognition and phagocytosis of many nonopsonized microorganisms, including bacteria, fungi, and protozoa by Mφ, is mediated by MR, through interactions with polysaccharide components of fungal cell walls such as yeast mannan, bacterial capsules, and some strains of LPS and lipoarabinomannan (19). Transfection of nonphagocytic COS cells with MR cDNA is sufficient to confer an ability to recognize and phagocytose Candida albicans (20) and Pneumocystis carinii (21). Ligation of MR in Mφ causes intracellular signaling resulting in functional changes, including increased superoxide anion release (22) and induction of cytokine synthesis (23). The immunological roles of MR may extend to specific immunity if the observed MR- mediated uptake of glycoconjugates by cultured human DCs for efficient presentation to T cells by MHCII (24–26) and CD1b (27) prove to have in vivo correlates.
At a biochemical level, polysaccharide recognition has been attributed to cooperative, calcium-dependent binding of the sugar moieties mannose, fucose, and N-acetylglucosamine by several of the eight C-type lectin domains within the ectodomain of MR. Carbohydrate recognition domains 4–8 show affinity for natural ligands comparable to that of MR itself (28). The phagocytic and endocytic activity is mediated by a 45–amino acid cytoplasmic tail and transmembrane domain (20). MR also contains a cysteine-rich domain (CR) with sequence similarity to the plant lectin Ricin B at the NH2 terminus and an adjacent fibronectin type II–like domain (29).
Our recent discovery of ligands of CR in mouse secondary lymphoid organs gave the first indication of a function for CR, the domain of MR most highly conserved between mice and humans (30). Tissues were probed with a chimeric probe consisting of CR fused to the Fc region of human IgG1, CR-Fc. Binding of CR-Fc to spleen marginal metallophilic Mφ and undefined cells in B cell areas, and to lymph node subcapsular sinus Mφ, was observed in naive animals, and a time–course study of a secondary immune response indicated apparent migration of CR-binding cells from the subcapsular sinus to sites of developing germinal centers. This suggested that MR could be directed to areas where affinity maturation of B cells occurs. We have recently purified ligands of CR-Fc from spleen and identified among these novel glycoforms of sialoadhesin (Sn) and CD45 (Martínez-Pomares, L., our unpublished results).
We have also documented the existence of a soluble form of MR (sMR) which may act as a mobile antigen capture protein for delivery to the marginal zone of spleen and lymph node subcapsular sinus, as well as to primary and secondary B cell follicles (31). sMR is generated by proteolysis of MR from cultured Mφ and is shed into the media where it retains calcium-dependent mannosyl binding activity. Immunoreactive sMR also occurs naturally in serum.
The roles of MR outlined above have all been assigned to a functionally homogeneous MR, but Fiete and Baenziger have recently revealed tissue heterogeneity in MR and a new lectin activity of CR. They identified a receptor within rat liver that recognizes and internalizes lutropin hormone bearing Asn-linked oligosaccharides terminating in SO4-4-GalNAcβ1,4GlcNAcβ1,2Manα (S4GGnM), with structural and antigenic properties similar to MR, although MR purified from lung did not recognize S4GGnM (32). A protein with the same properties as this receptor could be generated from the same cDNA as MR, and the ability to bind galNAc-4-SO4 appeared to be determined posttranslationally (33). The galNAc-4-SO4 binding site was then localized to the CR by binding studies of deletion mutants of MR (34).
In addition to heterogeneity within MR, a wider family of molecules with the same basic structure as MR exists. These are the phospholipase A2 receptors (35, 36), DEC-205 (37, 38), and a novel lectin (39). Each has CR and fibronectin type II–like domains, and either 8 or, in the case of DEC-205, 10, C-type lectin-like domains.
More specific methods to detect in situ expression of MR are required, given the heterogeneity of MR and the limitations of ligand-binding assays. Several mAbs recognizing human MR have recently been developed. Uccini et al. (40) demonstrated MR expression in various reticulo-endothelial tissues and in neoplasms of possibly endothelial origin. MR was detected in resident tissue Mφ, including those in spleen red pulp, lymph node paracortex, and thymus cortex. Sinus lining cells of spleen and lymph node also expressed MR and coexpressed Mφ and endothelial markers. Noorman et al. (41) surveyed MR antigen in human tissue, with broadly similar results. In mouse, Takahashi and co-workers surveyed MR protein in a range of tissues from fetal development to the adult, revealing expression in Mφ and some endothelial cells, although a precise definition of most of these cell types was not attempted (42).
Given the heterogeneity of MR and the existence of other MR family members, we studied expression of MR in the normal adult mouse by both in situ hybridization (ISH) and immunocytochemistry (ICC). In lymphoid organs, we used double ICC to define the phenotype and location of cells expressing MR. We compared MR with markers of Mφ, DCs, and endothelium, and with the binding of CR-Fc. We found MR by ISH and ICC at identical locations, in subsets of Mφ and endothelium; no expression was seen in Langerhans cells, other DCs, cells that express putative CR ligands, or in sites in spleen and lymph node that express mannosyl ligand binding activity. Cells at these sites therefore may express novel MR-like receptor(s).
Materials and Methods
Animals.
BALB/c and sv/ev129 mice were bred at the Sir William Dunn School of Pathology, and males and females were used at 8–10 wk of age.
Abs and Fc Chimeric Protein.
Primary Abs used in this study are described in Table I. MR polyclonal Abs raised against MR purified from the J774e cell line and mAbs F4/80, FA.11, and 3D6 were prepared in-house. CR-Fc, a recombinant protein consisting of the CR of mouse MR fused to the Fc region of human IgG1, was also prepared in our laboratory (30). The ERTR-9 mAb was a gift of Dr. C.D. Dijkstra (Free University, Amsterdam, The Netherlands). Other Abs were purchased as shown. N418 was biotinylated in-house for direct detection. The secondary Abs, biotinylated goat anti–rabbit IgG and biotinylated rabbit anti–rat IgG, were purchased from Sigma Chemical Co. Biotinylated mouse anti–human IgG (Fab′)2 was purchased from Jackson ImmunoResearch Labs.
Table I.
Antibodies Used in This Study
| Antibody | Isotype | Murine antigen | Reference/supplier | |||
|---|---|---|---|---|---|---|
| ERTR-9 | Rat IgG | Undefined antigen of marginal zone Mφ | 57 | |||
| FA.11 | Rat IgG2a | Macrosialin | 58 | |||
| F4/80 | Rat IgG2b | F4/80 | 59 | |||
| M5/114.15.2 | Rat IgG2b | I-Ab,d,k, I-Ed,k | 60 | |||
| MR | Rabbit polyclonal | MR | 61 | |||
| NLDC-145 | Rat IgG2a | DEC-205 | 37 | |||
| N418 | Armenian hamster IgG | CD11c | 62 | |||
| R6-60.2 | Rat IgG2a | IgM | PharMingen | |||
| 3D6 | Rat IgG2a | Sn | 63 | |||
| 390 | Rat IgG2a | PECAM-1 (CD31) | Serotec |
ICC.
Organs were collected and immersed in OCT compound (BDH Chemicals-Merck) and frozen in dry ice–cooled isopentane. Frozen sections were cut at 5 μm, air-dried for 1 h, and stored at −20°C. Before staining, slides were thawed at room temperature for 30 min, then hydrated in PBS for 5 min at room temperature followed by fixation for 10 min in 2% paraformaldehyde in Hepes-buffered isotonic saline at 4°C. The hydration step was found to be essential for binding of anti-MR to tissues, and avoids the requirement for protease treatment of sections described by Takahashi and co-workers (42).
Fixed sections were washed in 0.1% (vol/vol) Triton X-100 in PBS, and endogenous peroxidase activity was blocked with 10 mM glucose, 1 mM NaN3, 0.4 U/ml glucose oxidase (Sigma Chemical Co.) in phosphate buffer for 15 min at 37°C. Blocking steps used an avidin-biotin kit (Vector Laboratories) and a 30-min incubation with 5% normal serum of the species in which the secondary Ab was raised. 5% serum was used as the diluent for primary and secondary Abs, with which the sections were incubated for 60 and 30 min, respectively. Sections were then incubated with avidin-biotin-peroxidase complex (ABC Elite; Vector Laboratories). Peroxidase activity was finally detected with 0.5 mg/ml 3,3′-diaminobenzidine tetrahydrochloride (Polysciences, Inc.) and 0.024% H2O2 in 10 mM imidazole in PBS, pH 7.4. Sections were counterstained with 0.1% cresyl violet or methyl green. When double ICC was used to detect MR and other markers, the first staining step with the above protocol was used, with the substitution of 3-amino-9-ethylcarbazole (AEC) substrate kit for diaminobenzidine (Vector Laboratories). In the second step, avidin-biotin-alkaline phosphatase complex (ABC-AP) and Vector blue detection system (Vector Laboratories) were used.
ISH.
The probe templates were generated by subcloning regions of MR and Sn cDNA into pBS SK+/−, allowing sense and antisense transcription from T3 and T7 promoters. The 301-bp SSt1-BamH1 fragment of MR corresponding to 922– 1223 bp of the cDNA and the 311- and 357-bp BamH1-BamH1 fragments of Sn corresponding to 765–1076 and 1076–1433 bp of the cDNA, respectively, were chosen. 35S-labeled RNA probes were transcribed from linearized plasmids using a Stratagene RNA transcription kit. Probe was used at 3 × 106 to 1 × 107 cpm/ml in 50% formamide, 10 mM Tris, pH 8.0, 300 mM NaCl, 10 mM dithiothreitol, 100 mg/ml dextran sulphate, 200 g/ml Ficoll, 200 μg/ml polyvinylpyrrolidone, 200 μg/ml BSA, and 500 μg/ml tRNA. Mouse tissue was prepared by perfusion-fixation. After perfusion with heparinized physiological saline, fixation was commenced with chilled 4% paraformaldehyde, 100 mM NaOH, 100 mM sodium acetate, pH 6.5 with acetic acid, and followed by chilled 4% paraformaldehyde, 100 mM NaOH, 100 mM sodium tetraborate, pH 9.5 with HCl. Dissected organs were postfixed at 4°C in this latter fix for 3.5 h, then placed in 20% sucrose in PBS for 16 h before freezing in OCT compound over dry ice in isopentane. Tissues were sectioned at 10 μm onto slides pretreated with Vectabond (Vector Laboratories). Before hybridization, sections were rinsed in 100 mM triethanolamine, pH 8.0, then treated by acetylation for 10 min with 2.5 μl/ml acetic anhydride in 100 mM triethanolamine, pH 8.0, rinsed in 2× SSC, and dehydrated through ethanol. Probe was heated at 65°C for 10 min, spun at 4,000 rpm for 10 min, then hybridized to tissue sections for 16 h at 56°C. Several posthybridization washes were performed, including a 30-min 20 μg/ml RNAse A treatment, and high stringency washes for 30 min at 60°C in 0.1× SSC, 1 mM dithiothreitol. Slides were exposed to Ilford nuclear research emulsion for 10–17 d, and signal was detected by Ilford PQ Universal paper developer diluted 1:4 with distilled water and Unifix (Eastman Kodak Co.). Slides were counterstained with 0.1% cresyl violet and photographed under bright field microscopy.
Results
The specific recognition of MR may be hampered by the tissue heterogeneity of MR, the potential cross-reactivity of reagents with other members of the family of proteins with which it shares homology, and the existence of other receptors that share a similar ligand recognition profile. We have used two independent methods to detect MR specifically: ISH to define mRNA and therefore sites of synthesis, and ICC to define protein expression in a wide range of organs of normal adult mice. Specificity of mRNA detection was confirmed by performing control ISH with sense strand probes. The specificity of the polyclonal anti-MR Ab was examined by Western blotting of tissue lysates. There was some tissue-specific heterogeneity with respect to apparent molecular weight in the protein detected, but in the absence of anti-MR, no signal was detected (not shown). The treatment of tissues for ICC was mild, allowing double ICC detection of MR with markers of Mφ, DCs, and endothelium to define expression more closely. Of particular interest, we used double ICC in lymphoid organs to compare the expression of MR with that of the putative CR ligand, Sn, and other ligands of CR-Fc.
MR Expression in Lymphoid Organs: MR and Sn Expression by ISH
Peripheral Lymph Node.
MR mRNA expression was seen in the medullary cords (Fig. 1 A, arrow). The subcapsular sinus was clearly negative (Fig. 1 A, arrowhead), although this site and the medulla were strongly labeled by the Sn probe (Fig. 1 B). Control sections hybridized with sense probes of MR (Fig. 1 C) and Sn had low background (Fig. 1 D). Although Sn is expressed by medullary and subcapsular sinus Mφ, only the latter bear ligands of CR-Fc (30). Together these data clearly indicate that MR and its Sn ligand are not produced concurrently in the lymph node.
Figure 1.
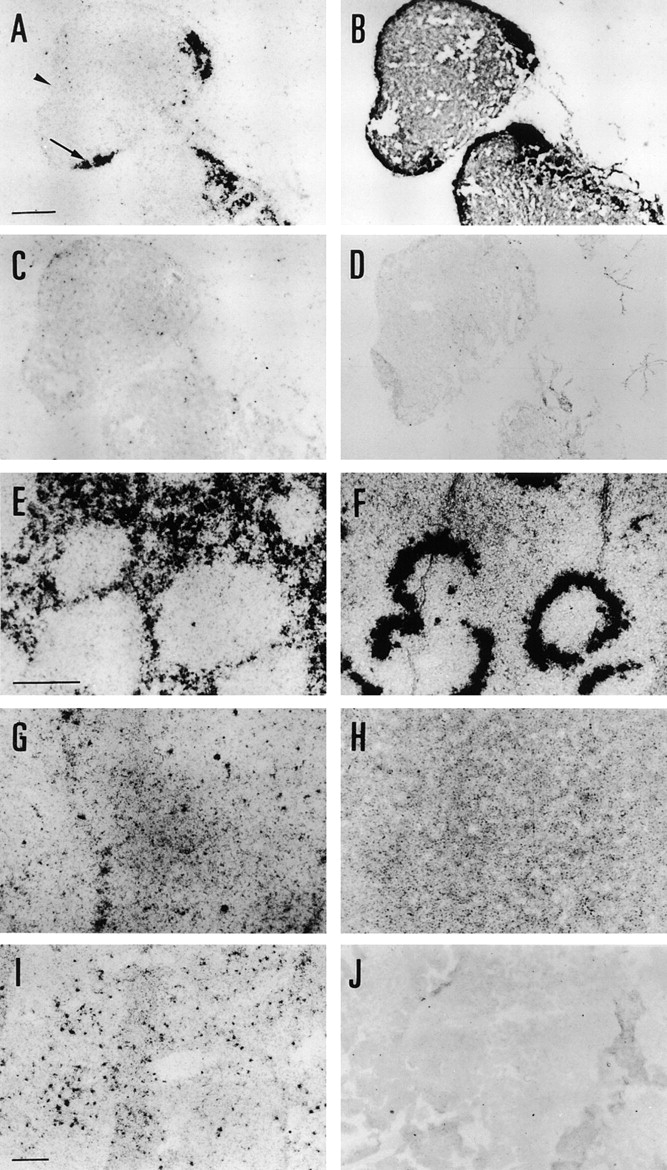
Localization of MR and Sn mRNA in lymphoid organs by ISH. (A) MR was expressed within the medulla of lymph node (arrow), but is absent from the subcapsular sinus (arrowhead). (B) Sn expression was also seen in the medulla of the lymph node, and additionally on the subcapsular sinus. (C and D) Adjacent control sections hybridized with sense orientation probes of MR and Sn, respectively, had low levels of background. (E) MR is expressed by the red pulp of spleen. (F) In spleen, Sn was abundantly expressed by the marginal zone, but was not detected in red pulp above background levels. (G and H) Adjacent control spleen sections hybridized with sense orientation MR and Sn probes, respectively, had homogeneous levels of background throughout. (I) MR was expressed by discrete cells of the thymus. (J) Background levels of hybridization of sense orientation probe were low in thymus. Bars = 100 μm, shown in A (for A–D), E (for E–H), and I (for I and J).
Spleen.
MR expression was observed throughout the red pulp by ISH, but appeared to be absent from the marginal zone and white pulp (Fig. 1 E). The marginal metallophilic zone was readily identified by its high expression of Sn (Fig. 1 F). The control sections for MR and Sn, respectively, probed with sense strand RNAs, had no significant background (Fig. 1, G and H). Although precise anatomical localization of the sites of synthesis was not possible by this method, these data are highly suggestive of MR and Sn synthesis occurring at distinct sites.
Thymus.
Discrete cells were labeled with MR probe within the thymus (Fig. 1 I), with very little background in the control (Fig. 1 J). Comparison with expression of Sn is not informative, since thymic Sn is not a ligand of CR-Fc.
MR Expression in Lymphoid Organs: MR Expression by ICC; Comparison with Markers of DC, Mφ, and Endothelial Cells
Peripheral Lymph Node.
MR antigen was found on medullary Mφ (m), sinus lining Mφ, and endothelium of the marginal sinus (ms), but not in T cell areas (t) (Fig. 2 A). No detectable background staining was found in these areas in the absence of MR Ab (Fig. 2 B). In contrast, DEC-205 was detected on interdigitating cells throughout the T cell areas of an adjacent section (Fig. 2 C). By double ICC, Sn and MR were shown to colocalize in medullary Mφ (m) (Fig. 3 A), but only Sn could be detected on the subcapsular sinus Mφ (Fig. 3 A, arrow). By contrast, no colocalization of CR-Fc and MR could be detected, CR-Fc being confined to subcapsular sinus Mφ and some germinal center cells (Fig. 3 B, arrowhead). Scattered cells expressing both MHCII and MR were detected in lymph nodes (Fig. 3 C) in the paracortex bordering the B cell follicle defined by reactivity to anti-IgM (Fig. 3 D). These cells did not express DC markers DEC-205 or CD11c, nor did they express the Mφ marker F4/80 (not shown). The elongated morphology of MR staining cells and location in the marginal sinus are characteristic of lymphatic endothelial cells, but expression of MR was restricted to a CD31− population, indicating that high endothelial venules do not express MR (Fig. 3 E). MR+ endothelial cells (arrow) did not coexpress the Mφ marker macrosialin, detected with mAb FA.11, although the intimately associated sinus lining Mφ (arrowhead) expressed both of these markers (Fig. 3 F).
Figure 2.
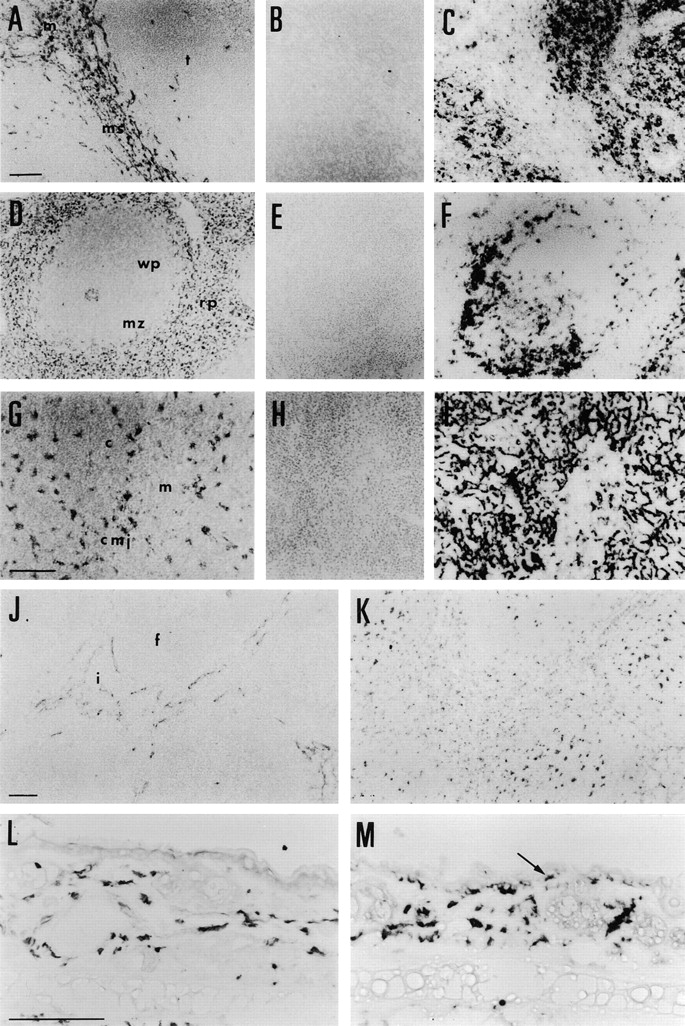
Localization of MR by ICC and comparison with markers of DCs. (A) In lymph node, MR was expressed by Mφ of the medulla (m) and sinus lining Mφ, and by endothelium of the marginal sinus (ms), but not by cells of the T cell area (t). (B) In the absence of MR Ab, background staining was not detected. (C) DEC-205–expressing DCs were abundant in the T cell area in lymph node. (D) In spleen, MR was detected in Mφ and venous sinus endothelial cells of the red pulp (rp), but appeared to be absent from the marginal zone (mz) and white pulp areas (wp). Central arteriole staining was variable. (E) A control spleen section had no background labeling. (F) DCs expressing CD11c were prominent at the border of the marginal zone with the white pulp. (G) In thymus, MR immunostaining was prominent in Mφ throughout the cortex (c) and corticomedullary junction (cmj). Mφ of the medulla appeared to be negative for MR or to express very low levels (m). (H) In the absence of MR Ab, background staining in thymus was absent. (I) DEC-205 was detected on cortical epithelial cells and medullary interdigitating cells. (J) In Peyer's patch, MR expression was confined to lymphatic endothelium in the interfollicular areas (i), but was absent from follicles (f). (K) In contrast, FA.11 staining was detected in Mφ and DCs of the follicles and interfollicular areas, but was absent from endothelial cells. (L) In skin, MR was expressed by dermal Mφ, but epidermal Langerhans cell staining was not detected. (M) F4/80 was expressed by both dermal Mφ and epidermal Langerhans cells (arrow) of the skin. Bars = 50 μm, shown in A (for A–F), G (for G–I), J (for J and K), and L (for L and M).
Figure 3.

Localization of MR with respect to markers of Mφ and endothelium by double ICC staining. MR was defined by a red product and the additional marker by blue. (A) There was some colocalization of MR with Sn, in the medullary Mφ of the lymph node (m), but Sn was also detected in MR− cells of the subcapsular sinus (arrow). (B) In lymph node, there was no colocalization between MR and CR-Fc, the latter staining only subcapsular sinus Mφ and a few cells in B cell follicles (arrowhead). (C) Scattered MR+ cells also express MHCII, and are located (D) in the paracortex adjacent to the B cell follicle defined by expression of IgM. (E) The endothelial cells that express MR in lymph node do not also express CD31, showing that only lymphatic endothelium and not high endothelial venules express MR. (F) These MR+ endothelial cells (arrow) do not coexpress macrosialin, although the intimately associated sinus lining Mφ express both markers (arrowhead). (G) In spleen, Sn was highly expressed by the marginal metallophilic Mφ and by marginal zone Mφ, and at low levels by red pulp Mφ which are strongly MR+. (H) As in lymph node, MR and CR-Fc defined two distinct populations in spleen. CR-Fc was localized in the marginal metallophilic Mφ and B cell areas of the white pulp, separated from the MR+ red pulp Mφ by the unlabeled outer marginal zone. (I) The boundary of the splenic marginal zone with the red pulp was defined by the marginal zone Mφ marker, ERTR-9. MR was absent from this population of Mφ, so its expression is restricted to the red pulp. (J) Spleen red pulp Mφ were stained with both anti-MR and FA.11, whereas the MR+ venous endothelium did not react with FA.11 (arrow). (K) In thymus, the majority of MR+ cells coexpressed the Mφ marker F4/80. (L) Staining of MR and CR-Fc in thymus revealed two distinct populations. CR-Fc was bound by large undefined cells of the medulla that may be part of the thymic epithelium, whereas MR appeared restricted to Mφ and possibly endothelium. Bars = 50 μm, shown in A (for A and B), C–F, G (for G and H), I (for I–K), and L.
Spleen.
Single immunostaining of MR in spleen revealed expression in red pulp (rp) Mφ but not in the white pulp (wp) or marginal zone (mz) (Fig. 2 D), while background staining in the absence of primary Ab was not detected (Fig. 2 E). This is in contrast to the expression of CD11c by DCs at the border of the white and red pulp (Fig. 2 F) and other DC subsets of the white pulp that are detectable with Abs to MHCII and DEC-205 (not shown). Expression of Sn, a CR-Fc ligand in spleen, was compared with that of MR by double ICC (Fig. 3 G). Sn alone was detected in marginal metallophilic Mφ, and there was additional low level expression in red pulp Mφ along with strong expression of MR. In contrast, CR-Fc bound to splenic marginal metallophilic Mφ, but not red pulp Mφ (Fig. 3 H). Therefore, MR and its putative ligand are expressed at nonoverlapping sites, separated by a clear region in the outer marginal zone. The absence of MR expression in this compartment was confirmed by double staining with ERTR-9, an mAb that specifically recognizes Mφ of the outer marginal zone (Fig. 3 I). Double ICC for MR and macrosialin with FA.11 defined two subsets of MR+ cells, double-positive Mφ (Fig. 3 J), and elongated venous sinus endothelial cells which did not express macrosialin (Fig. 3 J, arrow).
Thymus.
ICC revealed two distinct populations of cells that express MR. These were highly stained flattened Mφ lying beneath the capsule and along the connective tissue septa that penetrate the cortex (not shown), and less intensely stained Mφ with fine processes that were found throughout the cortex (c) and the corticomedullary junction (cmj) (Fig. 2 G). Staining of Mφ in the medulla (m) was very weak or negative (Fig. 2 G). A control section did not reveal any detectable background staining (Fig. 2 H). Cells expressing MR were quite distinct from the DEC-205–expressing cortical epithelial cells which have extensive dendrites, and the few rounded interdigitating cells of the medulla (Fig. 2 I). It seems likely that all MR+ cells in the thymus are Mφ. By double ICC it is apparent that most of them coexpress the Mφ marker, F4/80 (Fig. 3 K). As in spleen and lymph node, double staining with CR-Fc in thymus revealed that MR and CR-Fc ligand(s) are expressed by two distinct populations of cells (Fig. 3 L). CR-Fc bound to large undefined cells of the medulla which may be part of the thymic epithelium.
Peyer's Patch.
MR antigen appeared to be confined to the lymphatic endothelium of interfollicular areas (i) and was notably absent in follicles (f) (Fig. 2 J). Mφ and DCs in the interfollicular areas and follicles of an adjacent section that were identified with FA.11 did not express MR (Fig. 2 K). Control sections of Peyer's patch gave no background signal (not shown).
Skin.
MR was detected in dermal Mφ, but not in epidermal Langerhans cells (Fig. 2 L). In contrast, F4/80 stained both Mφ (Fig. 2 M) and Langerhans cells (Fig. 2 M, arrow). Again, no nonspecific staining was seen in control sections (not shown). We could not detect MR in isolated epidermal sheets of normal mice, using either the ICC method presented here, or the method of Takahashi and co-workers (42; data not shown).
MR Expression in Nonlymphoid Organs
We confirmed by ISH and ICC previous studies demonstrating expression of MR in hepatic endothelium and Mφ of liver (Kupffer cells), gut, lung, and resident tissue Mφ of other organs (not shown). We describe here the novel finding of MR in perivascular microglia of brain and glomerular mesangial cells of kidney.
Brain.
Mφ and related cells of the brain perform specialized functions in tissue homeostasis, inflammation, and maintenance of the blood–brain barrier. They are phenotypically, functionally, and morphologically distinct, and thus deserve special attention in their expression of MR. In addition, previous studies have suggested a role for an MR on vascular endothelium in regulating blood–brain barrier function. We observed that meningeal Mφ express MR by ISH (Fig. 4 A) and ICC (not shown). Perivascular microglia also express MR, but adjacent vessel endothelium does not, as shown by ISH (Fig. 4 B) and ICC (Fig. 4 C). Confirmation that these cells are perivascular microglia was deduced from their expression of F4/80 (Fig. 4 D). No signal was detected in the meninges or brain parenchyma in control sections examined by ISH or ICC (not shown). Like perivascular microglia, astrocytes are also associated with vessels, whereas more differentiated microglia are deeper in the parenchyma. Neither of these cell types appeared to express MR in normal brain (Fig. 4, B and C).
Figure 4.
Expression of MR in brain. (A) MR was detected in meningeal Mφ by ISH, here shown within an infolding of the meninges into the cerebellum. (B) By ISH, MR was detected in cells adjacent to blood vessels, the perivascular microglia. (C) ICC revealed expression of MR protein in perivascular microglia. (D) Expression of F4/ 80 by perivascular microglia confirmed that these cells were Mφ. Bars = 50 μm, shown in A (for A and B) and C (for C and D).
Kidney.
MR was observed in kidney glomeruli, both by ISH (Fig. 5 A) and ICC (Fig. 5 B) in repeated experiments. Control sections for ISH (Fig. 5 C) and ICC (Fig. 5 D) have low background, verifying the authenticity of these observations. An example of a glomerulus stained for MR and observed at high magnification indicated that expression is present on the mesangial cells (Fig. 5 E). No expression of Mφ markers F4/80, FA.11, or Sn was observed on glomeruli, nor did they bind CR-Fc (not shown).
Figure 5.

MR is expressed by renal glomerular mesangial cells. (A) Clusters of cells in the outer cortex of the kidney were detected by ISH using MR anti-sense probe. (B) By ICC, clusters of cells expressing MR were more easily determined to be within glomeruli. (C) A control section hybridized with MR sense probe showed no specific binding. (D) Similarly, a control section treated without MR Ab had low background staining, and none associated with glomeruli. (E) A single glomerulus stained for MR is shown at higher magnification, showing expression of MR on mesangial cells. Bars = 100 μm, shown in A (for A – D), and 50 μm, shown in E.
Discussion
We have used two independent methods to examine the expression of MR mRNA and protein in normal adult mouse. In all tissues studied, sites of synthesis of mRNA, examined by ISH, and expression of antigen, detected by ICC, were identical, suggesting that protein transfer between cells did not contribute to the staining observed. Both methods demonstrated MR expression by subsets of Mφ and endothelial cells. We confirmed previous studies of mature Mφ labeling in mice (42) and humans (40, 41), although a closer analysis of Mφ in spleen and lymph node revealed an unexpected anomaly. An MR-like binding activity has been described on marginal zone Mφ in mouse (16) and rat (15) spleen. Similarly, the subcapsular sinus Mφ of the lymph node have also been documented as having an MR-like binding activity in mice (14) and rats (15), although no specific carbohydrate receptor has been characterized structurally or antigenically in either case. Here we demonstrate clearly that MR is not responsible for these activities. Double ICC staining of MR with the marginal zone Mφ marker ERTR-9 confirmed that the cells of the marginal zone do not express MR. Similarly, we did not detect MR on subcapsular sinus Mφ by ISH or ICC. These binding activities, which are described as calcium dependent and of high affinity for ligands such as a linear β-1,2–linked tetramannose from C. albicans (16), Trypanosoma cruzi amastigotes (14), mannose/fucose/N-acetylglucosamine-BSA (15), and mannan (43) must therefore be mediated by some additional unknown receptor(s).
We found endothelium to be heterogeneous with respect to expression of MR. MR was detected on endothelial cells of spleen red pulp and liver, whereas blood vessel and high endothelial cells were negative. However, lymphatic endothelium appeared to express MR widely, consistent with a constitutive function, possibly endocytosis. Our finding contrasts with that in humans, in which lymphatic endothelium appeared negative, although coexpression of MR with endothelial markers CD31, VE-cadherin, and von Willebrand factor was observed in sinus lining cells of the spleen and lymph node (40). There may be additional phenotypic differences between human and murine endothelial cells. In contrast to humans, we noted the absence of CD31 expression by lymphatic endothelium and sinus lining cells of murine lymph node. Further studies are needed to establish the functional significance of heterogeneity in MR expression by selected vascular and lymphatic endothelium in different species.
MR has been implicated in T cell immunity, after the discovery of its expression on cultured human blood monocyte–derived DCs (24) and on DCs expanded from cord blood hemopoietic progenitors (11). Isolated DCs use MR to endocytose mannosylated ligands for presentation to T cells by MHCII (24) and CD1b (27). MR-mediated antigen uptake confers a greatly enhanced efficiency of presentation to T cells, of the order of 100 (25) and 200–10,000-fold (26). MR may be a marker of immature DCs, since it is downregulated in vitro by inflammatory stimuli (10). However, we found no expression of MR on DCs in vivo in thymus, lymph node, spleen, and Peyer's patch of normal mice. In particular, the CD11c+ cells of the spleen, which are thought to represent an immature population of myeloid-derived DCs, did not express MR. Likewise, we did not observe expression of MR by resting Langerhans cells of skin epidermis. This observation is consistent with the study by Reis e Sousa et al. (12), in which MR could not be detected on lysates of purified murine Langerhans cells by Western blotting, although a mannose-specific uptake by these cells was identified. Similarly, ICC studies in human tissue did not detect expression of MR in Langerhans cells (40, 41), although freshly isolated Langerhans cells did express functional MR (13). We did detect a subpopulation of MR+ cells of lymph nodes in the T cell areas bordering the B cell follicles which express MHCII, but these are unlikely to represent a known population of DCs, as they did not express DEC-205 or CD11c (not shown). Further studies are required to determine whether MR is expressed by DCs after immunization, and to characterize the mannose-specific binding activity of Langerhans cells, which may be due to a distinct receptor. The apparent lack of expression of MR on resting murine DCs in situ should be cautionary for those working on cultured DC populations.
We compared expression of MR with that of the putative endogenous ligand(s) of the CR, those that bind CR-Fc. Previously we hypothesized that a soluble form of MR or MR+ cells may interact with CR-Fc binding cells of spleen marginal metallophilic Mφ, lymph node subcapsular sinus Mφ, and germinal center cells (31). This would allow transfer of MR-bound carbohydrate antigen to cells strategically positioned at sites of generation of B cell responses to carbohydrate antigens. Here, we show that cells that bind CR-Fc in spleen and lymph node do not coexpress MR; indeed, the receptor and the ligand(s) are at spatially distinct sites within these organs, consistent with a transfer function via sMR. Although we did not detect sMR bound to the subcapsular sinus Mφ or marginal metallophilic Mφ, it may be present at levels below detection or may depend on immune stimulation. Intriguingly, we also observed scattered CR-Fc binding cells in the thymic medulla, where a role in capture of antigen-laden sMR would be unexpected. Thymic epithelial cells synthesize a variety of glycoprotein hormones (44), and our recombinant protein may recognize one of these in the thymus. Our CR-Fc, like the CR-Fc prepared by Fiete and co-workers (34), binds to bovine lutropin hormone, a glycoprotein bearing terminal galNAc-4-SO4 (Linehan, S.A., and L. Martínez-Pomares, unpublished data).
We have made a wider survey of MR expression than had previously been undertaken, including brain and kidney. We identified MR expression in perivascular microglia of murine brain by ISH and ICC. Perivascular microglia lie on the parenchymal side of arterioles, and MR at this location may be appropriately placed to endocytose glycoproteins that have traversed the blood–brain barrier. These specialized Mφ also express class A scavenger receptors and take up modified low density lipoprotein injected into the blood or cerebral ventricles (45). Those authors also showed that horseradish peroxidase, a known ligand of MR, can be endocytosed by perivascular microglia (45). In another study, liposomes labeled with mannose passed through the murine blood–brain barrier more efficiently than those labeled with fucose or galactose (46). Similarly, the ependymal cell layer lining the cerebral ventricles regulates solute transport between the cerebrospinal fluid and brain tissue, and in rat this can be dissociated by mannose- but not glucose- or galactose-BSA (47). However, we found that neither the ependymal cells nor the endothelial cells of the blood–brain barrier expressed MR. Astrocytes and more differentiated microglia of the parenchyma do not express MR in normal brain. Both of these cell types have a tendency to upregulate various Mφ markers when cultured in vitro or stimulated in vivo, so a definitive study of their phenotype requires further in situ analysis.
We also demonstrated expression of both MR mRNA and protein in glomerular mesangial cells of the kidney in situ. The glomerulus is the site at which blood is first filtered in the kidney. MR mRNA and protein have been observed on in vitro–cultured mouse mesangial cells stimulated with the inflammatory cytokines TNF-α and IL-1α, but were absent from unstimulated cells (48). An endocytic role for MR on mesangial cells is consistent with clearance of the MR ligand COOH-terminal propeptide of type 1 procollagen labeled with nondegradable 125I-tyramine-cellobiose, in which 20% of the label was found in the kidneys while 70% was recovered from liver (49). Glomerular mesangial cells share some features of the reticulo-endothelial system, including the ability to phagocytose apoptotic cells (50, 51). Cultured human mesangial cells also express components of NADPH oxidase (52) and FcγRIII and FcεRIγ chain (53). However, murine mesangial cells lacked all of the Mφ markers used in this study apart from MR (not shown), and are not believed to share a common lineage with hemopoietic and endothelial cells, which can both be generated from embryonic mesodermal cells (54, 55). Another cell type that is not hemopoietic or endothelial, but has been reported to express MR, is retinal pigment epithelium (7). Although the expression of MR in myeloid cells appears to be regulated by the transcription factors PU.1 and Sp1 (56), the detection of MR in mesangial cells and retinal pigment epithelium suggests that other transcription factors must be involved in these distantly related cell types.
In conclusion, we have characterized murine MR expression in situ in subsets of Mφ and endothelial cells, but not DCs, describing novel expression in perivascular microglia and renal mesangial cells. We demonstrate that MR-like binding activities of spleen marginal zone Mφ and lymph node subcapsular sinus Mφ, and possibly Langerhans cells, in situ are not due to MR. The expression pattern of MR in lymphoid organs is consistent with a model of antigen capture by MR and transfer to sites of anticarbohydrate immunity by a soluble form of MR that may recognize cells at these sites by their expression of ligands of the cysteine-rich domain of MR. Overall, the MR is widely expressed by distinct cell types involved in potential clearance functions. Further studies are needed to investigate the regulation of MR expression by these cells and the posttranslational modification of MR protein in different tissue microenvironments, as well as to characterize other MR-like activities.
Acknowledgments
We thank Dr. Ann Harris and Dr. Colm Reid (Institute of Molecular Medicine, Oxford, UK) for assistance with setting up the ISH protocol, and Dr. Paul Crocker (Dundee University, Dundee, UK) for providing Sn cDNA. We thank Dr. Christine Dijkstra (Free University, Amsterdam, The Netherlands) for providing ERTR-9 mAb. We are also grateful to Mrs. Liz Darley for preparation of tissue, and Mr. Lance Tomlinson for photography.
This work was supported by grants from the Arthritis Research Campaign and the Medical Research Council, UK.
Abbreviations used in this paper
- CR
cysteine-rich domain of MR
- DC
dendritic cell
- ICC
immunocytochemistry
- ISH
in situ hybridization
- MHCII
major histocompatibility complex class II
- Mφ
macrophage(s)
- MR
mannose receptor
- Sn
sialoadhesin
- sMR
soluble MR
References
- 1.Schlesinger PH, Doebber TW, Mandell BF, White R, DeSchryver C, Rodman JS, Miller MJ, Stahl PD. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosamine, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978;176:103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubbard AL, Wilson G, Ashwell G, Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. J Cell Biol. 1979;83:47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl PD, Rodman JS, Miller MJ, Schlesinger PH. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci USA. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stahl PD, Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982;93:49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd VL, Campbell EJ, Senior RM, Stahl PD. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothelial Soc. 1982;32:423–431. [PubMed] [Google Scholar]
- 6.Shepherd VL, Konish MG, Stahl PD. Dexamethasone increases expression of mannose receptors and decreases extracellular lysosomal enzyme accumulation in macrophages. J Biol Chem. 1985;260:160–164. [PubMed] [Google Scholar]
- 7.Shepherd VL, Tarnowski BI, McLaughlin BJ. Isolation and characterization of a mannose receptor from human pigment epithelium. Invest Ophthalmol Vis Sci. 1991;32:1779–1784. [PubMed] [Google Scholar]
- 8.Mokoena T, Gordon S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokines, gamma and alpha interferons, and dexamethasone. J Clin Invest. 1985;75:624–631. doi: 10.1172/JCI111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein ML, Keshav S, Harris N, Gordon S. IL-4 potently enhances macrophage mannose receptor (MMR) activity in mouse peritoneal macrophages. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1114. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caux C, Massacrier C, Vandervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha. II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 12.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condaminet B, Péguet-Navarro J, Stahl PD, Dalbiez-Gauthier C, Schmitt D, Berthier-Vergnes O. Human epidermal Langerhans cells express the mannose-fucose binding receptor. Eur J Immunol. 1998;28:3541–3551. doi: 10.1002/(SICI)1521-4141(199811)28:11<3541::AID-IMMU3541>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Kahn S, Wleklinski M, Aruffo A, Farr A, Coder D, Kahn M. Trypanosoma cruziamastigote adhesion to macrophage is facilitated by the mannose receptor. J Exp Med. 1995;182:1243–1258. doi: 10.1084/jem.182.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms G, Dijkstra CD, Hardonk MJ. Glycosyl receptors in macrophage subpopulations of rat spleen and lymph node. Cell Tissue Res. 1990;262:35–40. doi: 10.1007/BF00327742. [DOI] [PubMed] [Google Scholar]
- 16.Li R-K, Cutler JE. Chemical definition of an epitope/adhesin molecule on Candida albicans. . J Biol Chem. 1993;268:18293–18299. [PubMed] [Google Scholar]
- 17.Smedsrød B, Einarsson M, Pertoft H. Tissue plasminogen activator is endocytosed by mannose and galactose receptors of rat liver cells. Thromb Haemost. 1988;59:480–484. [PubMed] [Google Scholar]
- 18.Otter M, Kuiper J, Bos R, Rijken DC, van Berkel TJC. Characterization of the interaction both in vitro and in vivo of tissue-type plasminogen activator (t-PA) with rat liver cells. Effects of monoclonal antibodies to t-PA. Biochem J. 1992;284:545–550. doi: 10.1042/bj2840545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 20.Ezekowitz RAB, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezekowitz RAB, Williams DJ, Koziel H, Armstrong MYK, Warner A, Richards FF, Rose RM. Uptake of Pneumocystis cariniimediated by the macrophage mannose receptor. Nature. 1991;351:155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 22.Berton G, Gordon S. Modulation of macrophage mannosyl-specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology. 1983;49:705–715. [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto Y, Klein TW, Friedman H. Involvement of mannose receptor in cytokine interleukin-1 (IL-1), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1 (MIP-1), MIP-2, and KC responses, caused by attachment of Candida albicansto macrophages. Infect Immun. 1997;65:1077–1082. doi: 10.1128/iai.65.3.1077-1082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Cella M, Danielli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 26.Tan MCAA, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJM, Verwoerd D, Mulder AAJ, van der Heiden AN, Scheidegger D, Oomen LCJM, et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 27.Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, Brenner MB, Modlin RL, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 28.Taylor ME, Drickamer K. Structural requirements for high affinity binding of complex ligands by the mannose receptor. J Biol Chem. 1993;268:399–404. [PubMed] [Google Scholar]
- 29.Harris N, Peters LL, Eicher EM, Rits M, Raspberry D, Eichbaum QG, Super M, Ezekowitz RAB. The exon-intron structure and chromosomal localization of the mouse macrophage mannose receptor gene Mrc1: identification of a Ricin-like domain at the N-terminus of the receptor. Biochem Biophys Res Commun. 1994;198:682–692. doi: 10.1006/bbrc.1994.1099. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S, Bonnefoy J-Y, Gordon S. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Pomares L, Mahoney JA, Káposzta R, Linehan SA, Stahl PD, Gordon S. A functional soluble form of the murine mannose receptor is produced by macrophages in vitroand is present in mouse serum. J Biol Chem. 1998;273:23376–23380. doi: 10.1074/jbc.273.36.23376. [DOI] [PubMed] [Google Scholar]
- 32.Fiete D, Baenziger JU. Isolation of the SO4-4-GalNAcβ1,4GlcNAcβ1,2Manα-specific receptor from rat liver. J Biol Chem. 1997;272:14629–14637. doi: 10.1074/jbc.272.23.14629. [DOI] [PubMed] [Google Scholar]
- 33.Fiete D, Beranek MC, Baenziger JU. The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAc or Man at independent sites. Proc Natl Acad Sci USA. 1997;94:11254–11261. doi: 10.1073/pnas.94.21.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiete DJ, Beranek MC, Baenziger JU. A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4binding. Proc Natl Acad Sci USA. 1998;95:2089–2093. doi: 10.1073/pnas.95.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishizaki J, Hanasaki K, Higashino K-i, Kishimo J, Kikuchi N, Ohara O, Arita H. Molecular cloning of pancreatic group I phospholipase A2 receptor. J Biol Chem. 1994;269:5897–5904. [PubMed] [Google Scholar]
- 36.Ancian P, Lambeau G, Mattéi M-G, Lazdunski M. The human 180 kDa receptor for secretory phospholipases A2. J Biol Chem. 1995;270:8963–8970. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- 37.Kraal G, Breel M, Janse M, Bruin G. Langerhans cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 39.Wu K, Yuan J, Lasky LA. Characterization of a novel member of the macrophage mannose receptor type C lectin family. J Biol Chem. 1996;271:21323–21330. doi: 10.1074/jbc.271.35.21323. [DOI] [PubMed] [Google Scholar]
- 40.Uccini S, Sirianni MC, Vincenzi L, Topino S, Stoppacciaro A, Lesnoni La Parola I, Capuano M, Masini C, Cerimele D, Cella M, et al. Kaposi's sarcoma cells express the macrophage-associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi's sarcoma patients. Am J Pathol. 1997;150:929–938. [PMC free article] [PubMed] [Google Scholar]
- 41.Noorman F, Braat EAM, Barrett-Bergshoeff M, Barbé E, van Leeuwen A, Lindeman J, Rijken DC. Monoclonal antibodies against the human mannose receptor as a specific marker in flow cytometry and immunohistochemistry for macrophages. J Leukocyte Biol. 1997;61:63–72. doi: 10.1002/jlb.61.1.63. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K, Donovan MJ, Rogers RA, Ezekowitz RAB. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 1998;292:311–323. doi: 10.1007/s004410051062. [DOI] [PubMed] [Google Scholar]
- 43.Weston SA, Parish CR. Evidence that mannose recognition by splenic sinusoidal cells plays a role in the splenic entry of lymphocytes. Eur J Immunol. 1992;22:1975–1981. doi: 10.1002/eji.1830220804. [DOI] [PubMed] [Google Scholar]
- 44.Dardenne M, Savino W. Control of thymus physiology by peptidic hormones and neuropeptides. Immunol Today. 1994;15:518–523. doi: 10.1016/0167-5699(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 45.Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci USA. 1996;93:3269–3274. doi: 10.1073/pnas.93.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umezawa F, Eto Y. Liposome targeting to mouse brain: mannose as a recognition marker. Biochem Biophys Res Commun. 1988;153:1038–1044. doi: 10.1016/s0006-291x(88)81333-0. [DOI] [PubMed] [Google Scholar]
- 47.Kuchler S, Graff M-N, Gobaille S, Vincendon G, Roche A-C, Delaunoy J-P, Monsigny M, Zanetta J-P. Mannose dependent tightening of the rat ependymal cell barrier. In vivo and in vitrostudy using neoglycoproteins. Neurochem Int. 1994;24:43–55. doi: 10.1016/0197-0186(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 48.Liu ZH, Striker GE, Stetler-Stevenson M, Fukushima P, Patel A, Striker LJ. TNF-α and IL-1α induce mannose receptors and apoptosis in glomerular mesangial but not endothelial cells. Am J Physiol. 1996;270:C1595–C1601. doi: 10.1152/ajpcell.1996.270.6.C1595. [DOI] [PubMed] [Google Scholar]
- 49.Smedsrød B, Melkko J, Risteli L, Risteli J. Circulating C-terminal propeptide of type I procollagen is cleared mainly via the mannose receptor in liver endothelial cells. Biochem J. 1990;271:345–350. doi: 10.1042/bj2710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savill J, Smith J, Sarraf C, Ren Y, Abbott F, Rees A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992;42:924–936. doi: 10.1038/ki.1992.369. [DOI] [PubMed] [Google Scholar]
- 51.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular “myofibroblast”? . J Am Soc Nephrol. 1992;2:S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 52.Radeke HH, Cross AR, Hancock JT, Jones OWT, Nakamura M, Kaever V, Resch K. Functional expression of NADPH oxidase components (α- and β-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991;266:21025–21029. [PubMed] [Google Scholar]
- 53.Radeke HH, Gessner JE, Uciechowski P, Mägert H-J, Schmidt RE, Resch K. Intrinsic human glomerular mesangial cells can express receptors for IgG complexes (FcγRIII-A) and the associated FcεRI γ-chain. J Immunol. 1994;153:1281–1292. [PubMed] [Google Scholar]
- 54.Shalaby F, Rossant J, Yamaguchi TP, Gertstein M, Wu X-F, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:423–431. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 55.Eichmann A, Corbel C, Nataf V, Vaigot P, Bréant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor 2. Proc Natl Acad Sci USA. 1997;94:5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eichbaum Q, Heney D, Raveh D, Chung M, Davidson M, Epstein J, Ezekowitz RAB. Murine macrophage mannose receptor is regulated by the transcription factors PU.1 and SP1. Blood. 1997;90:4135–4143. [PubMed] [Google Scholar]
- 57.Van Vliet E, Melis M, Van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985;33:40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]
- 58.Smith MJ, Koch GLE. Differential expression of murine macrophage surface glycoprotein antigens in intra-cellular membranes. J Cell Sci. 1987;87:113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- 59.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharya A, Dorf ME, Springer TA. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 61.Blum JS, Stahl PD, Diaz R, Fiani ML. Purification and characterization of the D-mannose receptor from J774 mouse macrophage cells. Carbohydr Res. 1991;213:145–153. doi: 10.1016/s0008-6215(00)90605-0. [DOI] [PubMed] [Google Scholar]
- 62.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crocker PR, Gordon S. Properties and distribution of a lectin-like haemagglutinin differentially expressed by stromal tissue macrophages. J Exp Med. 1986;164:1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]



