Abstract
Macrophage inflammatory protein (MIP)-1α, a CC chemokine, enhances proliferation of mature subsets of myeloid progenitor cells (MPCs), suppresses proliferation of immature MPCs, and mobilizes mature and immature MPCs to the blood. MIP-1α binds at least three chemokine receptors. To determine if CCR1 was dominantly mediating the above activities of MIP-1α, CCR1-deficient (−/−) mice, produced by targeted gene disruption, were used. MIP-1α enhanced colony formation of marrow granulocyte/macrophage colony-forming units (CFU-GM), responsive to stimulation by granulocyte/macrophage colony-stimulating factor (GM-CSF), and CFU-M, responsive to stimulation by M-CSF, from littermate control CCR1+/+ but not CCR1−/− mice. Moreover, MIP-1α did not mobilize MPCs to the blood or synergize with G-CSF in this effect in CCR1−/− mice. However, CCR1−/− mice were increased in sensitivity to MPC mobilizing effects of G-CSF. Multi-growth factor–stimulated MPCs in CCR1−/− and CCR1+/+ marrow were equally sensitive to inhibition by MIP-1α. These results implicate CCR1 as a dominant receptor for MIP-1α enhancement of proliferation of lineage-committed MPCs and for mobilization of MPCs to the blood. CCR1 is not a dominant receptor for MIP-1α suppression of MPC proliferation, but it does negatively impact G-CSF–induced MPC mobilization.
Keywords: CC chemokine receptor 1, macrophage inflammatory protein 1α, progenitor cells, proliferation, mobilization
Mature blood cells must be constantly replaced. These cells are replenished from hematopoietic stem and progenitor cells (1). Intrinsic to this process is an interacting network of cytokines that control production and movement of stem and progenitor cells. Identifying the cytokines and specific receptors involved in these processes is important for clinically modulating blood cell production and movement.
Macrophage inflammatory protein (MIP)-1α, a cysteine cysteine (CC) chemokine (2–5), enhances and inhibits proliferation of myeloid progenitor cells (MPCs) and mobilizes MPCs to the blood. MIP-1α was first shown to enhance colony formation of granulocyte/macrophage (CFU-GM) and macrophage (CFU-M) progenitor cells in vitro (6, 7), a direct effect on mature subsets of MPCs that responded to stimulation by GM-CSF or M-CSF. By itself, MIP-1α did not stimulate colony formation by any type of MPC. The enhancing effect of MIP-1α on lineage-committed cells was subsequently confirmed by others (8–10). MIP-1α was subsequently shown to suppress more immature subsets of cells, including CFU-spleen (CFU-S [11], considered a stem, but not long-term marrow repopulating, cell) and immature MPCs (CFU-GM, erythroid [BFU-E], and multipotential [CFU-GEMM] progenitors) that proliferated in response to erythropoietin (Epo), IL-3, GM-CSF, and steel factor (SLF) (7–10, 12, 13). The suppressive effects were direct on MPCs (7, 12, 13). MIP-1α suppression was substantiated in vivo in mice (8, 14–17) and was confirmed in patients with breast cancer undergoing a phase I clinical trial with BB10010 (15), an MIP-1α analogue. BB10010 was also a modest mobilizing agent for stem and progenitor cells into the blood of mice (18); BB10010 synergized in this effect with G-CSF (18), a stem and progenitor cell mobilizer used clinically (1). The MPC mobilizing capacity of BB10010 was confirmed in a human clinical trial (15).
Chemokines act through seven transmembrane G protein–linked receptors (2–4). 16 chemokine receptors have been identified that bind known chemokines, including 9 in the CC chemokine group (2–5). Since MIP-1α binds chemokine receptors CCR1, CCR5, and D6, it is not clear through which chemokine receptor(s) MIP-1α activities are mediated. Chemokines do not appear to signal through D6 (19), suggesting that CCR1, CCR5, and/or an unknown receptor for MIP-1α may be involved in the above-noted activities of MIP-1α on MPCs. CCR1−/− mice have been developed by targeted gene disruption (20), and have demonstrated certain nonredundant functions in hematopoiesis, host defense, and inflammation. These mice were used in this study to demonstrate that CCR1 is a dominant receptor for enhancement of proliferation of mature MPCs and mobilization of MPCs to blood, but not for suppression of proliferation of immature MPCs.
Materials and Methods
Mice.
Generation of CCR1−/− and littermate control CCR1+/+ mice has been described (20). The mice used in this study were from an F1 or F6 backcross of 129/Sv with C57BL/6 mice. Results with both were similar and averaged. Age- and weight-matched CCR1−/− and CCR1+/+ mice were used. C3H/HeJ mice were purchased from The Jackson Laboratory.
Cytokines.
Purified recombinant preparations of cytokines were used. Human (hu) and murine (mu) MIP-1α, muMIP-2, and hu preparations of monocyte chemotactic protein (MCP-1), IL-8, platelet factor 4 (PF4), growth-related oncogene (GRO)-γ, also known as MIP-2β, neutrophil-activating peptide (NAP)-2, RANTES (regulated upon activation, normal T cell expressed and secreted), MIP-1β, and muM-CSF were purchased from R&D Systems. huExodus-1 (21) was a gift from Dr. Robert Hromas (Indiana University School of Medicine). huENA-78 was a gift from Dr. M.-S. Chang (Amgen Corp., Thousand Oaks, CA). huIFN-γ inducible protein (IP)-10 was a gift from Dr. Andreas Sarris (M.D. Anderson Tumor Hospital, Houston, TX). huGRO-α, muGM-CSF, and muSLF were gifts from Immunex Corp. (Seattle, WA). huEpo was purchased from Amgen Corp. Hemin was purchased from Eastman Kodak Co. PWM mouse spleen cell–conditioned medium (PWMSCM, a source of numerous growth factors, including GM-CSF and IL-3) was prepared as described (22).
MPC Assays.
Colony assays were done as described elsewhere (22). Unseparated bone marrow (5 × 104 cells/ml) and low-density blood cells (1–2 × 105 cells/ml, obtained after density cut procedure) were isolated from mice (22). To assess whether MIP-1α stimulates or enhances colony formation, marrow cells were plated in 0.3% agar (Difco) culture medium in the presence of 10% FBS (Hyclone, Inc.) with or without muGM-CSF (100 U/ml) or muM-CSF (100 U/ml) and with or without mu or huMIP-1α (100 ng/ml) (6, 7). Marrow cells were plated in agar with or without muGM-CSF (100 U/ml) plus muSLF (50 ng/ml) and with or without chemokines (100 ng/ml each) to evaluate inhibitory effects on multi-growth factor–stimulated colony formation by CFU-GM (12). Inhibitory assays were also done on marrow cells growing in 1% methylcellulose culture medium with 30% FBS, huEpo (1 U/ml), muSLF (50 ng/ml), PWMSCM (5%), and 0.1 mM hemin for effects on colony formation by CFU-GM, BFU-E, and CFU-GEMM. Results for CFU-GM suppression were similar for assays done in agar and methylcellulose and were pooled. Absolute numbers of MPCs in the blood were calculated based on the number of viable low-density nucleated cells, and the number of colonies was scored per number of cells plated in methylcellulose culture medium with Epo, SLF, PWMSCM, and hemin at the above-noted concentrations. The concentrations of cytokines chosen were predetermined to be maximally effective. Three plates were scored per point, and colonies were scored after 7 d incubation in a humidified environment at 5% CO2 and lowered (5%) O2.
In Vivo MPC Mobilization Assay.
Mice were given either control diluent, huG-CSF, huMIP-1α, or G-CSF plus MIP-1α. Timing and dosages were based on reports by others (18) and our own preliminary studies. Mice were injected subcutaneously with either control diluent (pyrogen-free saline; used at the same volume and timing as for injections of G-CSF plus huMIP-1α), 2.5 μg G-CSF given two times per day for 2 d, or 5 μg MIP-1α administered 12 h after the last injection of either control diluent or G-CSF. Mice were bled 30 min after injection of MIP-1α (or the control diluent for MIP-1α) and then killed.
Statistical Analysis.
Results are given as mean ± SEM, and Student's t test was used to analyze the data. P values < 0.05 designated significant differences between test points.
Results
Effects of MIP-α on Colony Formation by MPCs.
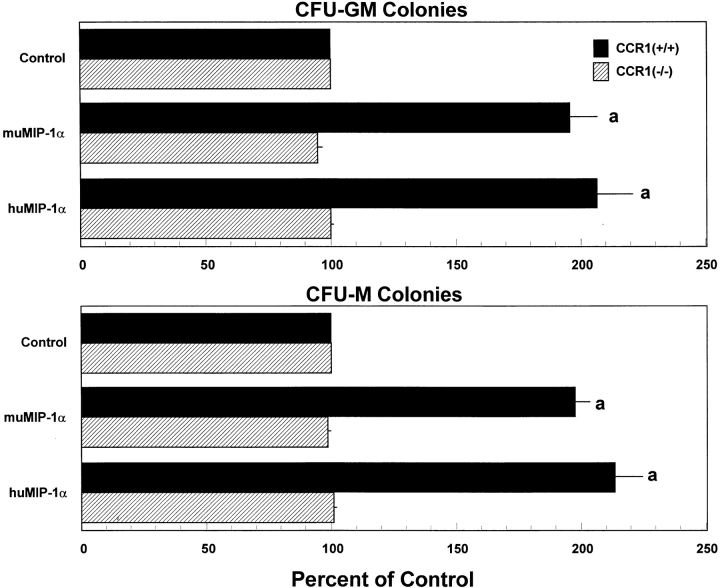
To determine if CCR1 was a dominant receptor for MIP-1α enhancement of colony formation (6, 7), we tested mu and hu forms of MIP-1α. As shown in Fig. 1, mu and huMIP-1α significantly enhanced colony formation by CCR1+/+, but not by CCR1−/−, marrow cells stimulated to proliferate by either GM-CSF or M-CSF. Colonies formed in the presence of GM-CSF, with or without MIP-1α, were composed mainly of granulocytes and macrophages with <20% of the colonies containing only granulocytes or macrophages. No shifts in colony types were noted in the absence or presence of MIP-1α. Colonies formed with M-CSF, with or without MIP-1α, were composed of macrophages. No colonies formed in the absence of GM-CSF or M-CSF whether or not MIP-1α was added to the plates. This suggests that CCR1 acts as a dominant receptor for the MIP-1α enhancing effects on MPCs stimulated by GM-CSF or M-CSF.
Figure 1.
Effects of mu and huMIP-1α on colony formation by CFU-GM stimulated with muGM-CSF and CFU-M stimulated with muM-CSF from marrow of CCR1+/+ compared with CCR1−/− mice. Results shown are mean percentage of control ± 1 SEM of five separate experiments in which each experiment used cells pooled from two to four mice. Control colony numbers for CCR1+/+ and CCR1−/− cells ranged from 25–41 and 26–37, respectively, for CFU-GM, and 39–66 and 42–61, respectively, for CFU-M for individual experiments. a P < 0.001, significant change from control diluent, of the indicated mouse strain.
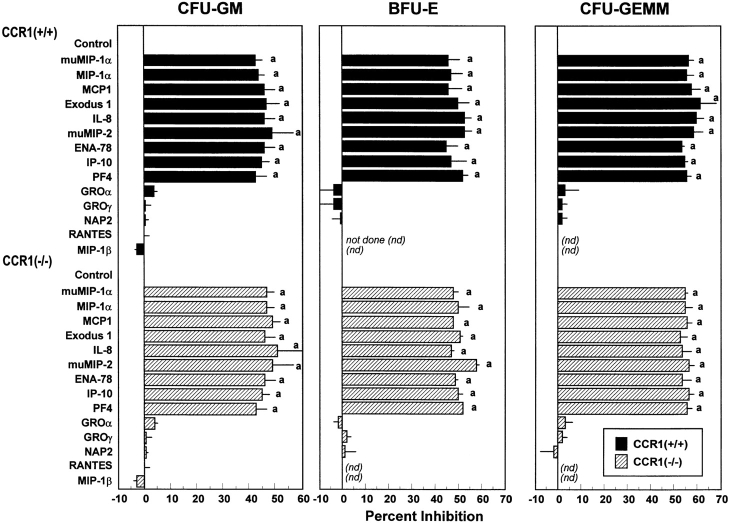
MIP-1α suppresses MPCs stimulated to proliferate by combinations of growth factors (7, 12, 13). One report using antibodies to CCR1 suggested that the suppressing effects of MIP-1α on colony formation by BFU-E were mediated by CCR1 (23). To determine if CCR1 was a dominant receptor for MIP-1α suppression, we analyzed the effects of MIP-1α on colony formation by marrow cells from CCR1+/+ and CCR1−/− mice stimulated to proliferate with Epo, PWMSCM, SLF, and hemin. As shown in Fig. 2, both mu and huMIP-1α were equally potent in suppressing colony formation by CFU-GM, BFU-E, and CFU-GEMM from CCR1+/+ and CCR1−/− marrow cells. As controls, other CC (MCP-1 and Exodus-1) and CXC (IL-8, muMIP-2, ENA-78, IP-10, and PF4) chemokines known to be inhibitory under these conditions (5) were tested and found to be equally suppressive on CCR1+/+ and CCR1−/− MPCs (Fig. 2). Chemokines known to be nonsuppressive (5; GRO-α, GRO-γ, NAP-2, RANTES, and MIP-1β) did not inhibit colony formation of CCR1+/+ or CCR1−/− MPCs (Fig. 2). These results suggest that CCR1 is not a dominant receptor for MIP-1α suppression of multi-growth factor–stimulated MPCs.
Figure 2.
Influence of chemokines on colony formation of CFU-GM, BFU-E, and CFU-GEMM from bone marrow of CCR1+/+ compared with CCR1−/− mice. All chemokines are human unless designated as murine (mu). Results shown are percent inhibition ± 1 SEM for two to seven separate experiments, each using cells pooled from two to four mice. Results for mu and huMIP-1α are from seven (CFU-GM), three (BFU-E), and three (CFU-GEMM) experiments. Other results for CFU-GM are from two to five experiments and for BFU-E and CFU-GM from two experiments. Control colony numbers for CCR1+/+ and CCR1−/− cells, respectively, ranged from 55 to 152 and 63 to 145 for CFU-GM, from 18 to 25 and 20 to 24 for BFU-E, and from 11 to 15 and 12 to 15 for CFU-GEMM. a P < 0.001, statistically significant change from control diluent of a particular subset of MPCs in either CCR1+/+ or CCR1−/− mice.
Effects of MIP-1α on Mobilization of MPCs to the Blood in the Absence or Presence of G-CSF.
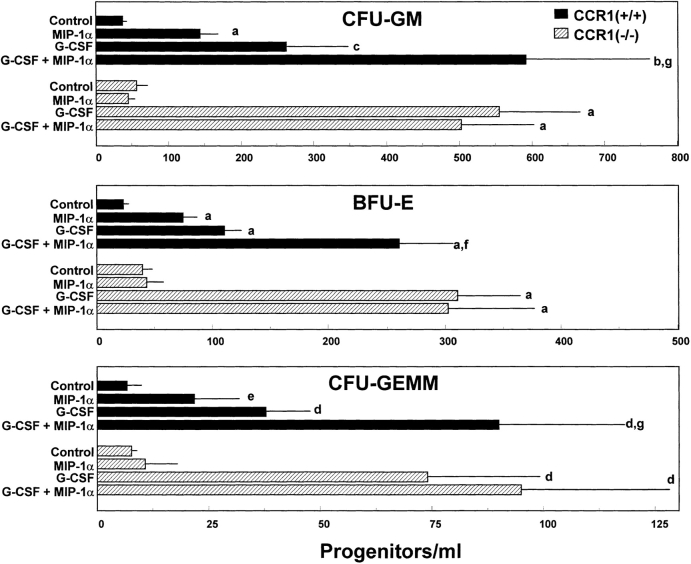
G-CSF and BB10010, an MIP-1α analogue, induce mobilization of stem and MPCs to blood (1, 18) although with different kinetics, and G-CSF and BB10010 in combination are additive or synergize in this mobilization (18). In preliminary experiments using C3H/Hej mice, we confirmed that huMIP-1α induction of mobilization of MPCs to blood was rapid (within 15 min to 1 h) and reversible. huRANTES did not have a mobilizing effect on MPCs in these same experiments. We thus assessed the in vivo MPC mobilizing effects of MIP-1α and G-CSF. As shown in Fig. 3, MIP-1α and G-CSF each significantly mobilized MPCs to the blood of CCR1+/+ mice, and the combination of G-CSF with MIP-1α showed greater mobilization than either cytokine alone. In contrast, MIP-1α did not significantly enhance mobilization of MPCs to the blood of CCR1−/− mice (Fig. 3). Moreover, MIP-1α did not act with G-CSF to enhance mobilization further in CCR1−/− mice. However, MPCs in CCR1 −/− mice were more sensitive to the mobilizing effects of G-CSF alone than in CCR1+/+ mice. In 3 separate experiments in which 4–5 mice per group per experiment were assessed, 1.3-, 1.7-, and 11.6-fold more CFU-GM, 1.5-, 2.3-, and 4.9-fold more BFU-E, and 2.6- and 3.4-fold more CFU-GEMM were mobilized in CCR1−/− compared with CCR1+/+ mice. In one experiment, we did not detect greater mobilization of CFU-GEMM in CCR1−/− mice. These results suggest that CCR1 is a dominant receptor for the MPC mobilizing effects of MIP-1α; moreover, CCR1 appears to play a negative role in G-CSF–induced mobilization of MPCs to the blood.
Figure 3.
Mobilization of CFU-GM, BFU-E, and CFU-GEMM in CCR1+/+ compared with CCR1−/− mice after administration of G-CSF, huMIP-1α, or G-CSF plus huMIP-1α. Results are the average of eight to nine mice, each individually evaluated, from a total of two complete experiments. Significant change from control: a P < 0.001; b P < 0.003; c P < 0.005; d P < 0.01; e P < 0.05. Significant change from G-CSF group: f P < 0.008; g P < 0.05.
Discussion
Since several chemokine receptors can bind more than one chemokine and some chemokines can bind more than one chemokine receptor (2–5), it is not always clear which chemokine receptor mediates the effects of specific chemokines. MIP-1α binds to three chemokine receptors: CCR1, CCR5, and D6. Although D6 does not elicit a Ca2+ influx signal in response to MIP-1α or other chemokines that bind this receptor (19), it is possible that other intracellular signals are activated through D6 in response to certain chemokines, and there is the possibility that additional receptors will be identified that bind MIP-1α. The availability of CCR1−/− mice (20) allowed us to assess if CCR1 served as a dominant receptor for three previously reported functions of MIP-1α. The results presented here clearly demonstrate that in cells without functional CCR1, MIP-1α did not enhance proliferation of CFU-GM stimulated by GM-CSF, or CFU-M stimulated by M-CSF, nor did MIP-1α induce in vivo mobilization of MPCs to the blood, implicating CCR1 as a dominant receptor for these activities. The MIP-1α– induced MPC mobilization effects complement our previous studies in which CCR1 was shown to be a dominant receptor for bacterial LPS–induced movement of MPCs between bone marrow, spleen, and blood and for MIP-1α mobilization of neutrophils to the blood (20).
Our current studies with MIP-1α confirm the mobilization effects on MPCs noted by others using an MIP-1α analogue, BB10010 (18), as well as the additive/greater than additive mobilization apparent when BB10010 is given as a single injection to mice previously injected with G-CSF. G-CSF mobilizes stem and progenitor cells for autologous and allogeneic transplantation (1), and enhancement of this may be clinically important. We also found that CCR1−/− mice were more sensitive to the MPC mobilizing effects of G-CSF than were CCR1+/+ mice. This unexpected finding implicates CCR1 as a negative component in G-CSF– induced MPC mobilization. How CCR1 would negatively mediate such an effect is not clear. It is known through the use of G-CSF receptor (G-CSFR)–deficient mice that the G-CSFR is crucial for G-CSF–induced MPC mobilization (24). Interestingly, no increase in circulating CFU-GM was detected in G-CSFR−/− mice in the absence of added G-CSF and after administration of IL-8 (24). Thus, CXC chemokine receptors have been linked to MPC mobilization by G-CSF through a G-CSFR.
Although CCR1 has been implicated in MIP-1α suppression of BFU-E proliferation in vitro (23), our studies suggest that CCR1 is not a dominant receptor for suppression of MPCs. We have previously shown no significant difference in cycling of MPCs in CCR1−/− versus CCR1+/+ marrow (20), consistent with CCR1 not being a dominant receptor for negative regulation of MPC proliferation. This contrasts with studies in other chemokine receptor–deficient mice. CCR2 binds several chemokines, including MCP-1 and its murine analogue, JE (25). The use of CCR2−/− mice demonstrated that CCR2 was a dominant receptor for suppression of MPCs by MCP-1 and JE (25). The relevance of CCR2 as a receptor involved in negative regulation of MPCs was confirmed by the fact that the cycling status of MPCs in CCR2−/− marrow is greater than that in CCR2+/+ marrow (26). IL-8 and muMIP-2 bind CXCR2, and the use of CXCR2−/− mice demonstrated that IL-8 and muMIP-2 did not inhibit proliferation of MPCs from CXCR2−/− marrow, and that enhanced proliferation of MPCs was apparent in CXCR2−/− compared with CXCR2+/+ mice (27).
In conclusion, our data identify CCR1 as the dominant receptor responsible for MIP-1α enhancement of growth factor–stimulated MPC proliferation and MIP-1α–induced MPC mobilization to peripheral blood, but rule out CCR1 as a dominant receptor for negative regulation of hematopoiesis by MIP-1α. In addition, the results identify an unexpected role for CCR1 as a negative regulator of G-CSF– dependent MPC mobilization to blood. Together with previous work, our results indicate that CCR1 is a highly versatile receptor able to mediate a broad range of MIP-1α actions, including specific steps in MPC proliferation, development, and distribution, as well as specific leukocyte trafficking. Delineation of specific downstream signaling events will be needed to understand the molecular basis for these diverse functional responses mediated by MIP-1α activation of CCR1.
Acknowledgments
We thank Cynthia Booth for typing this manuscript, and Stephen Braun and Jennifer Jayne for help with plating one set of experiments.
These studies were supported by U.S. Public Health Service grants R01 HL56416 and R01 DK53674 from the National Institutes of Health to H.E. Broxmeyer.
References
- 1.Broxmeyer, H.E. 1998. The hematopoietic system: principles of therapy with hematopoietically active cytokines. In Cytokines in the Treatment of Hematopoietic Failure. A. Ganser and D. Hoelzer, editors. Marcel Dekker, Inc., New York. 1–37.
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Murphy PM. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 4.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 5.Broxmeyer, H.E., and C.H. Kim. 1999. Chemokines and hematopoiesis. In Chemokines and Cancer. B.J. Rollins, editor. Humana Press, Inc., Totowa, NJ. 263–291.
- 6.Broxmeyer HE, Sherry B, Lu L, Cooper S, Carow C, Wolpe SD, Cerami A. Myelopoietic enhancing effects of murine macrophage inflammatory proteins 1 and 2 in vitro on colony formation by murine and human bone marrow granulocyte-macrophage progenitor cells. J Exp Med. 1989;170:1583–1594. doi: 10.1084/jem.170.5.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Sherry B, Lu L, Cooper S, Oh K-O, Tekamp-Olson P, Kwon BS, Cerami A. Enhancing and suppressing effects of recombinant murine macrophage inflammatory proteins on colony formation in vitroby bone marrow myeloid progenitor cells. Blood. 1990;76:1110–1116. [PubMed] [Google Scholar]
- 8.Clements JM, Craig S, Gearing AJH, Hunter MG, Heyworth CM, Dexter TM, Lord BI. Biological and structural properties of MIP-1α expressed in yeast. Cytokine. 1992;4:76–82. doi: 10.1016/1043-4666(92)90040-x. [DOI] [PubMed] [Google Scholar]
- 9.Keller JR, Bartelmez SH, Sitnicka E, Ruscetti FW, Ortiz M, Gooya JM, Jacobson SEW. Distinct and overlapping direct effects of macrophage inflammatory protein-1α and transforming growth factor β on hematopoietic progenitor/stem cell growth. Blood. 1994;84:2175–2181. [PubMed] [Google Scholar]
- 10.Van Ranst PCF, Snoeck H-W, Lardon F, Lenjou M, Nijs G, Weekx SFA, Rodrigus I, Berneman ZN, Van Bockstaele DR. TGF-β and MIP-1α exert their main inhibitory activity on very primitive CD34++CD38− cells but show opposite effects on more mature CD34+CD38+human hematopoietic progenitors. Exp Hematol. 1996;24:1509–1515. [PubMed] [Google Scholar]
- 11.Graham GJ, Wright EG, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, Lorimore S, Pragnell IB. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 12.Broxmeyer HE, Sherry B, Cooper S, Lu L, Maze R, Beckmann MP, Cerami A, Ralph P. Comparative analysis of the suppressive effects of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. J Immunol. 1993;150:3448–3458. [PubMed] [Google Scholar]
- 13.Lu L, Xiao M, Grigsby S, Wang WX, Wu B, Shen R-N, Broxmeyer HE. Comparative effects of suppressive cytokines on isolated single CD34+++stem/progenitor cells from human bone marrow and umbilical cord blood plated with and without serum. Exp Hematol. 1993;21:1442–1446. [PubMed] [Google Scholar]
- 14.Dunlop DJ, Wright EG, Lorimore S, Graham GJ, Holyoake T, Kerr DJ, Wolpe SD, Pragnell IB. Demonstration of stem cell inhibition and myeloprotective effects of SCI/rhMIP-1α in vivo. . Blood. 1992;79:2221–2225. [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Orazi A, Hague NL, Sledge GW, Jr, Rasmussen H, Gordon MS. Myeloid progenitor cell proliferation and mobilization effects of BB10010, a genetically engineered variant of human macrophage inflammatory protein-1α, in a phase I clinical trial in patients with relapsed/refractory breast cancer. Blood Cells Mol Dis. 1998;24:14–30. doi: 10.1006/bcmd.1998.0167. [DOI] [PubMed] [Google Scholar]
- 16.Maze R, Sherry B, Kwon BS, Cerami A, Broxmeyer HE. Myelosuppressive effects in vivoof purified recombinant murine macrophage inflammatory protein-1 alpha. J Immunol. 1992;149:1004–1009. [PubMed] [Google Scholar]
- 17.Lord BI, Dexter TM, Clements JM, Hunter MA, Gearing AJH. Macrophage-inflammatory protein protects multipotent hematopoietic cells from the cytotoxic effects of hydroxyurea in vivo. Blood. 1992;79:2605–2609. [PubMed] [Google Scholar]
- 18.Lord BI, Woolford LB, Wood LM, Czaplewski LG, McCourt M, Hunter MG, Edwards RM. Mobilization of early hematopoietic progenitor cells with BB10010: a genetically engineered variant of human macrophage inflammatory protein-1α. Blood. 1995;85:3412–3415. [PubMed] [Google Scholar]
- 19.Nibbs RJ, Wylie SM, Yang J, Landau NR, Graham GJ. Cloning and characterization of a novel promiscuous human beta chemokine receptor D6. J Biol Chem. 1997;272:32078–32083. doi: 10.1074/jbc.272.51.32078. [DOI] [PubMed] [Google Scholar]
- 20.Gao JL, Wynn TA, Chang Y, Lee E, Broxmeyer HE, Cooper E, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1–type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hromas R, Gray PW, Chantry D, Krathwohl M, Fife K, Bell GI, Takeda J, Aronica S, Gordon M, Cooper S, et al. Cloning and characterization of exodus, a novel β-chemokine. Blood. 1997;89:3315–3322. [PubMed] [Google Scholar]
- 22.Cooper, S., and H.E. Broxmeyer. 1996. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin and the potent co-stimulating cytokines steel factor and Flt-3 ligand. In Current Protocols in Immunology, Suppl. 18. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, W. Strober, and R. Coico, editors. John Wiley & Sons, Inc., New York. 6.4.1–6.4.12.
- 23.Su SB, Mukaida N, Wang JB, Zhang Y, Takami A, Nakao S, Matsushima K. Inhibition of immature erythroid progenitor cell proliferations by macrophage inflammatory protein-1α by interacting mainly with a C-C chemokine receptor, CCR1. Blood. 1997;90:605–611. [PubMed] [Google Scholar]
- 24.Liu F, Poursine-Laurent J, Link DC. The granulocyte colony-stimulating factor receptor is required for the mobilization of murine hematopoietic progenitors into peripheral blood by cyclophosphamide or interleukin-8 but not Flt-3 ligand. Blood. 1997;90:2522–2528. [PubMed] [Google Scholar]
- 25.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid S, Ritchie A, Boring L, Cooper S, Hangoc G, Charo IF, Broxmeyer HE. Enhanced myeloid progenitor cell cycling and apoptosis in mice lacking the chemokine receptor, CCR2. Blood. 1999;93:1524–1533. [PubMed] [Google Scholar]
- 27.Broxmeyer HE, Cooper S, Cacalano G, Hague NL, Bailish E, Moore MW. Interleukin-8 receptor is involved in negative regulation of myeloid progenitor cells in vivo: evidence from mice lacking the murine IL-8 receptor homolog. J Exp Med. 1996;184:1825–1832. doi: 10.1084/jem.184.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]