Abstract
We investigated the role of antigen-presenting cells in early interferon (IFN)-γ production in normal and recombinase activating gene 2–deficient (Rag-2−/−) mice in response to Listeria monocytogenes (LM) infection and interleukin (IL)-12 administration. Levels of serum IFN-γ in Rag-2−/− mice were comparable to those of normal mice upon either LM infection or IL-12 injection. Depletion of natural killer (NK) cells by administration of anti-asialoGM1 antibodies had little effect on IFN-γ levels in the sera of Rag-2−/− mice after LM infection or IL-12 injection. Incubation of splenocytes from NK cell–depleted Rag-2−/− mice with LM resulted in the production of IFN-γ that was completely blocked by addition of anti–IL-12 antibodies. Both dendritic cells (DCs) and monocytes purified from splenocytes were capable of producing IFN-γ when cultured in the presence of IL-12. Intracellular immunofluorescence analysis confirmed the IFN-γ production from DCs. It was further shown that IFN-γ was produced predominantly by CD8α+ lymphoid DCs rather than CD8α− myeloid DCs. Collectively, our data indicated that DCs are potent in producing IFN-γ in response to IL-12 produced by bacterial infection and play an important role in innate immunity and subsequent T helper cell type 1 development in vivo.
Keywords: recombinase-activating gene 2 knockout mouse, nonobese diabetic-severe combined immunodeficiency disease mouse, γc knockout mouse, natural killer cells, Listeria monocytogenes
Dendritic cells (DCs) are bone marrow (BM)-derived professional APCs. Peripheral DCs are characterized by high capability for antigen capture and processing, migration to lymphoid organs, and expression of various costimulatory molecules for antigen-specific lymphocyte activation. Cytokine secretion by DCs initiates and enhances both innate and acquired immunity (1).
Activation of macrophages and DCs by infectious agents leads to secretion of IL-12, which subsequently induces IFN-γ production by NK cells and directs Th1 development. IFN-γ, in turn, acts on monocytes to augment IL-12 secretion and to produce nitric oxide that eradicates infected microbes (2, 3). Thus, IL-12 and IFN-γ comprise a positive feedback system, which is probably required for optimal production of IL-12 in vivo (4, 5). Studies using neutralizing Abs against IFN-γ and mice deficient for IL-12 or IFN-γ have confirmed the importance of these cytokines for innate immunity and Th1 development for controlling intracellular pathogens (6–11).
It was generally assumed that the only cells producing IFN-γ in response to IL-12 are NK and T cells. However, recent studies have shown that IFN-γ is also produced by peritoneal macrophages in response to IL-12 and by BM-derived macrophages in response to a combination of IL-12 and IL-18, suggesting the presence of an autocrine activation pathway (12, 13).
In the study presented here, we examined IFN-γ production pathways in NK cell–depleted recombinase activating gene 2–deficient (Rag-2−/−) mice upon Listeria monocytogenes (LM) infection or IL-12 administration. We found that the levels of IFN-γ produced in the sera of these mice were unaltered as compared with those of Rag-2−/− mice with NK cells, suggesting an important role for a non-T, -B, and/or -NK cell type(s) in producing IFN-γ in vivo. We show here that purified DCs were capable of producing significant amounts of IFN-γ in response to IL-12. Among DCs, CD8α+ lymphoid DCs are the major producers of IFN-γ. Thus, DCs produce IFN-γ in an autocrine manner by responding to the IL-12 they produce upon bacterial infection, and such autocrine production of IFN-γ likely plays an important role in both innate and acquired immunity in vivo.
Materials and Methods
Mice.
B10.D2 and C57BL/6 mice were purchased from Sankyo Labo Service Co. Inc. (Japan). B10.D2–Rag-2−/− mice, generated by backcross of Rag-2−/− mice to B10.D2/nSnJ for 10 generations (reference 14 and unpublished data) were obtained from Taconic Farms. C57BL/6-Rag-2−/− mice which also lack the cytokine receptor common γ chain [hereafter C57BL/6-γc −/−(Y) Rag-2−/− mice], were made as follows. Rag-2−/− (14) and γc −/−(Y) mice (15) were backcrossed to C57BL/6 for eight generations and intercrossed to generate C57BL/6-Rag-2−/− and C57BL/6-γc −/−(Y) mice. These mice were further bred to generate C57BL/6-γc −/−(Y) Rag-2−/− mice. NOD/LtSz-scid/scid mice (16) were provided by N. Hozumi of Science University of Tokyo (Tokyo, Japan) and J. Hata of Keio University School of Medicine (Tokyo, Japan). All mice were maintained in our specific pathogen-free animal facility, and experiments were performed on mice between 6 and 12 wk of age, in accordance with our Institutional Guidelines.
Listeria monocytogenes.
Listeria monocytogenes EGD strain (LM) was provided by M. Mitsuyama of Kyoto University (Kyoto, Japan). The bacteria had been passed through C57BL/6 mice and colonies were obtained from the spleens of infected mice on agar plates with trypto-soy broth (Eiken Chemical Co., Japan). Bacteria were then grown in trypto-soy broth overnight at 37°C. Aliquots of bacteria suspension were stored at −80°C until use. After thawing, 2 × 106 LM were injected into mice intraperitoneally. In some in vitro experiments, 106 collagenase-treated splenocytes were cultured with 4 × 105 LM in a 96-well flat-bottomed plate. After 45 min, penicillin and streptomycin were added to the culture media at final concentrations of 100 U/ml and 100 μg/ml, respectively, to limit the growth of LM, and culture supernatants were collected after 72 h and subjected to ELISA.
Cell Preparation.
Splenocytes were prepared by homogenizing collagenase-treated spleens in all experiments. DCs were prepared from spleens as previously described (17). In brief, collagenase-treated spleens (Collagenase D; Boehringer Mannheim) were homogenized and suspended in a dense BSA solution (P = 1.080), overlaid with 1 ml of RPMI medium, and centrifuged in a swing bucket rotor at 9,500 g for 10 min at 4°C. DCs and monocytes at the interface were collected, washed, and allowed to adhere to plastic dishes for 2 h. Cells were incubated for an additional 18 h to allow DCs to detach from the plastic dishes. Nonadherent cells containing DCs were then collected and contaminated B cells were further excluded by anti–mouse Ig(H+L)-beads (Perseptive Biosystems) using a MACS magnet (Miltenyi Biotech). After removing DCs, adherent macrophages were detached from the plastic dishes by a cell scraper (Sumitomo Bakelite Co. Ltd., Japan). NK cells were enriched by a combination of PK136-biotin (anti-NK1.1) and Streptavidin-MicroBeads (Miltenyi Biotech). These fractions were stained with appropriate mAbs and were further purified by cell sorting on a FACS Vantage™ (Becton Dickinson).
Antibodies and Flow Cytometric Analysis.
The following mAbs were purchased from PharMingen: 145-2C11–FITC and 145-2C11–PE (anti-CD3ε); DX5-FITC (anti-Pan NK); PK136-PE and PK136-biotin (anti-NK1.1); HL3-FITC and HL3-PE (anti-CD11c); AF6-120.1-PE (anti-I-Ab); 53-6.7-PE and 53-6.7-biotin (anti-CD8α); M1/70-biotin (anti-CD11b); and PO3-biotin (anti-CD86). F4/80-FITC (anti-pan macrophage) was purchased from Caltag Labs. An mAb, Y3P (anti-pan-I-A), was purified from hybridoma culture supernatants and conjugated with FITC. Biotinylated mAbs were detected with streptavidin–Red 670 (GIBCO BRL). 1–2 × 106 cells were stained in PBS/2% FCS, washed, and analyzed on a FACScan® using the CELLQuest program (Becton Dickinson). Rabbit polyclonal anti-asialoGM1 (αASGM1) and anti–IL-12 Abs were purchased from Wako Pure Chemical Industries, Ltd. and PharMingen, respectively.
Cytokine Assays.
To induce IFN-γ in vivo, 0.5 μg IL-12 or 2 × 106 LM were intraperitoneally injected into mice. In in vivo experiments, cells were cultured in the presence of 1 ng/ml IL-12 for 72 h or 4 × 105 LM as described above. Titers of IFN-γ in the sera and culture supernatants were determined by Quantikine M ELISA Kit (R&D Systems).
Intracellular Immunofluorescence Analysis.
Immunofluorescence staining of intracellular IFN-γ was conducted as previously described (18). Sorted DCs were grown on coverslips coated with Cell-Tak7 (Becton Dickinson Labware) and fixed for 15 min with 3.7% paraformaldehyde in PBS. After surface staining with FITC-conjugated mAb against CD11c, cells were permeabilized with 0.5% saponin/1% BSA in PBS for 30 min. Cells were further incubated with polyclonal rabbit anti–mouse IFN-γ Ab (Pestka Biomedical Labs.), polyclonal rabbit anti–mouse IL-12Rβ Ab (Santa Cruz Biotechnology, Inc.), or normal rabbit serum as a negative control. Specimens were further developed with Rhodamine-conjugated goat anti–rabbit IgG (ICN Pharmaceuticals, Inc.). Samples on coverslips were mounted onto glass slides with Mowiol (Calbiochem Corp.) and examined under a Fluorescence Microscope Axiovert 100 (Carl Zeiss, Inc.) equipped with an image analysis system (Signal Analytics Co.).
Results
Production of IFN-γ in the Sera of NK Cell–depleted Rag-2−/− Mice.
Several studies have demonstrated that IFN-γ produced by NK as well as Th1 cells is a crucial cytokine for limiting and clearing infectious intracellular agents such as protozoan and bacterial pathogens (6–8, 19–22). To examine the role of NK cells in the early production of IFN-γ, we injected polyclonal αASGM1 Abs into B10.D2 or B10.D2– Rag-2−/− mice to deplete NK cells as previously demonstrated (23). Consistent with previous studies, NK cells were absent in the spleen of both B10.D2 and B10.D2–Rag-2−/− mice 3 d after administration of 300 μg αASGM1 Ab (Fig. 1 A). These mice were then injected with 2 × 106 LM or 0.5 μg IL-12, and serum IFN-γ levels were examined after 48 and 24 h, respectively. As shown in Fig. 1 B, IFN-γ was detected in the sera of NK cell–depleted B10.D2 mice at a level comparable to those in untreated B10.D2 mice upon LM infection (Fig. 1 B, top). As both T and B cells (24) are able to produce IFN-γ, we performed the same experiment with B10.D2–Rag-2−/− mice lacking both T and B cells. To our surprise, comparable levels of IFN-γ were induced in NK cell–depleted B10.D2–Rag-2−/− mice and in untreated B10.D2–Rag-2−/− mice (Fig. 1 B, top).
Figure 1.
IFN-γ production in the sera of NK cell–depleted Rag-2−/− mice. (A) 300 μg αASGM1 Ab were injected into B10.D2 and B10.D2–Rag-2−/− mice. Spleen cells were collected on day 3 and stained for 145-2C11–PE (anti-CD3ε) and DX5-FITC (anti-pan NK). (B) Saline, 0.5 μg IL-12, or 2 × 106 LM were intraperitoneally injected into B10.D2 or B10.D2– Rag-2−/− mice with or without treatment with αASGM1 3 d before injection (top), or into C57BL/6-γc −/−(Y)Rag-2−/− and NOD/LtSz-scid/scid mice (bottom). Sera were collected 24 h later from IL-12– injected mice and 48 h later from LM- infected and control mice, and the amounts of IFN-γ were measured by ELISA. (C) In vitro IFN-γ production from NK cell– depleted Rag-2−/− splenocytes cultured with LM. 106 collagenase-treated splenocytes were obtained from NK cell–depleted B10.D2, B10.D2–Rag-2−/−, and C57BL/ 6-γc −/−(Y)Rag-2−/− mice. The cells were infected with 4 × 105 LM and cultured for 3 d with or without either 10 μg/ml anti– IL-12 mAb (top) or 1 ng/ml IL-12 (bottom). The amounts of IFN-γ were measured by ELISA.
Because IL-12 is important in inducing IFN-γ, we also injected recombinant IL-12 into these mice and observed IFN-γ production in the sera independent of NK cell depletion (Fig. 1 B, top). IFN-γ was not detected in the sera of mice injected with PBS or αASGM1 alone. These data suggest that the contribution of NK cells to early IFN-γ production in response to LM infection or IL-12 administration is minimal. We further examined C57BL/6-γc −/−(Y)Rag-2−/− mice lacking T, B, and NK cells, as well as NOD/LtSz-scid/ scid mice lacking T and B cells and having functional defects in NK cells and monocytes/macrophages (16). After 24 h of IL-12 administration, only a small amount of serum IFN-γ was detected in C57BL/6-γc −/−(Y)Rag-2−/− mice, whereas substantial amounts of IFN-γ were produced by NOD/LtSz-scid/scid mice. IFN-γ production in NOD/ LtSz-scid/scid mice was also unaffected by pretreatment with αASGM1 Ab (Fig. 1 B, bottom, and data not shown).
To identify the IFN-γ–producing cells in αASGM1-treated Rag-2−/− mice, splenocytes were prepared from mice treated with αASGM1 and infected LM in vitro. As shown in Fig. 1 C, IFN-γ was produced by Rag-2−/− splenocytes in the absence of NK cells, and the production of IFN-γ was completely blocked by the addition of anti–IL-12 Ab, indicating that IL-12 plays a critical role in IFN-γ production upon LM infection. These results further indicate the presence of IFN-γ–producing cells other than T, B, and NK cells. In contrast, amounts of IFN-γ produced by C57BL/ 6-γc −/−(Y)Rag-2−/− splenocytes were 1–5% of those from NK-depleted Rag-2−/− splenocytes upon either Listeria infection or IL-12 administration, suggesting that IFN-γ production is impaired in C57BL/6-γc −/−(Y)Rag-2−/− mice.
IFN-γ Production by DCs.
To further identify IFN-γ– producing cells, DCs as well as macrophages and NK cells were freshly isolated from collagenase-treated spleen cells of unprimed mice by cell sorting. Highly purified CD11c+ I-A+, Mac1+F4/80+, and CD3-NK1.1+ cells were used as DCs, macrophages, and NK cells, respectively (Fig. 2 A and data not shown). These cells were cultured for 3 d in the presence of 1 ng/ml IL-12 in vitro. As shown in Fig. 2 B, significant amounts of IFN-γ were detected in the culture supernatants of DCs and macrophages. The amounts of IFN-γ from DCs and macrophages were significantly higher than those from NK cells. DCs cultured in the absence of IL-12 produced IFN-γ to a certain level, probably due to the cross-linking of surface MHC class II molecules by the use of anti–I-A mAb for DC preparation (25). Consistent with this interpretation, DCs purified using anti-CD86 mAb (26) instead of anti–I-A mAb did not produce IFN-γ without IL-12 (see Fig. 3 B).
Figure 2.
IFN-γ production from isolated DCs. (A) Isolation of splenic DCs. CD11c+I-Ab+ DCs were isolated from C57BL/6 splenocytes on a FACS Vantage™, and the purity of the cells was checked. (B) IFN-γ production in culture supernatants of DCs, macrophages, and NK cells. DCs (5 × 104), NK cells (5 × 104), and macrophages (2 × 104) were cultured for 3 d in the presence of 1 ng/ml IL-12. Culture supernatants were harvested and subjected to ELISA. Asterisk indicates the amount of IFN-γ produced by 5 × 104 equivalent macrophages.
Figure 3.
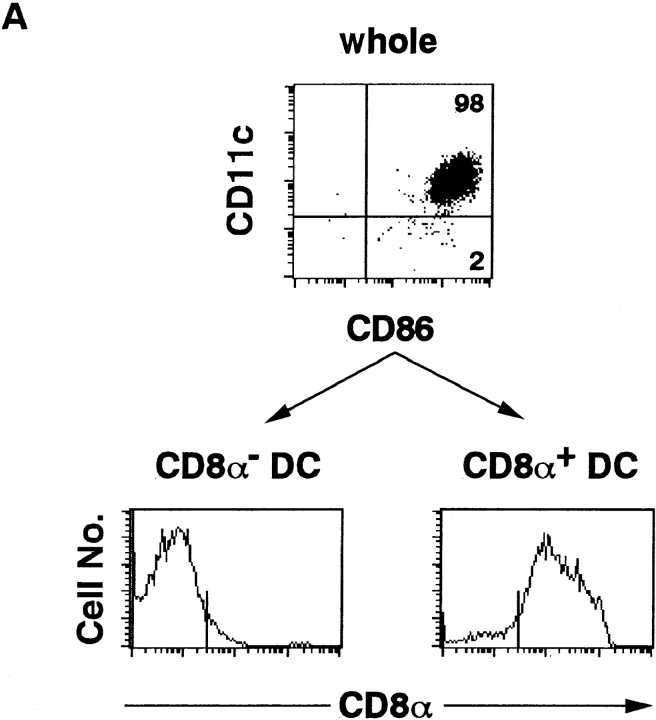
IFN-γ production by DC subpopulations. (A) Isolated splenic DCs were stained with anti-CD11c–FITC, anti-CD8α–PE, and anti-CD86– biotin, followed by streptavidin–Red 670, and subjected to cell sorting to purify CD8α−CD11c+CD86+ (myeloid) DCs and CD8α+CD11c+CD86+ (lymphoid) DCs. Purity of CD11c+CD86+ whole DCs, CD8α− DCs and CD8α+ DCs were 98, 95, and 90%, respectively. (B) Whole DCs, CD8α−CD11c+CD86+ DCs, and CD8α+ CD11c+CD86+ DCs (5 × 104) were cultured for 3 d in the presence of 1 ng/ml IL-12, and the amounts of IFN-γ in culture supernatants were measured by ELISA. No difference in viability was observed between CD8α+ and CD8α− DCs.
The CD8α+ Lymphoid DC Population Is a Major Source of IFN-γ Production.
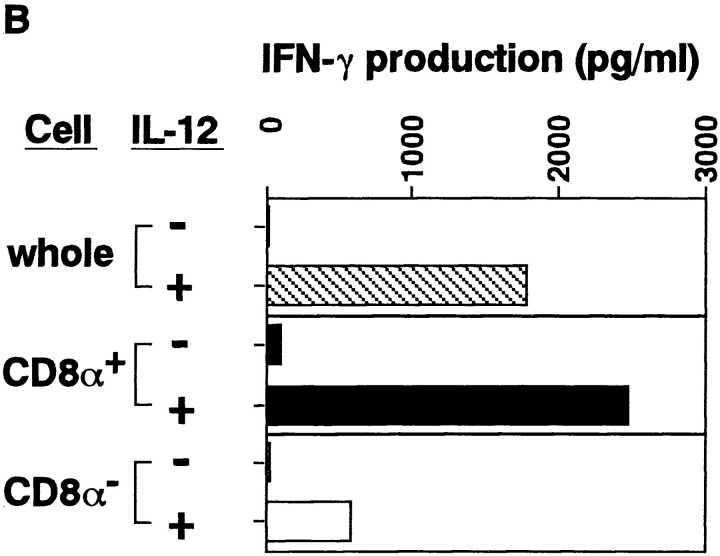
There are two different types of DCs in the spleen of an adult mouse (27–30). They differ in surface phenotypes (CD8α−DEC-205−CD11b+ versus CD8α+ DEC-205+CD11b−), origin (myeloid versus lymphoid), requirement of cytokines for their development (GM-CSF versus IL-3), and biological function. To this end, we examined IFN-γ production from DC subpopulations. CD8α− DCs (myeloid DCs) and CD8α+ DCs (lymphoid DCs) were isolated by cell sorting and cultured with 1 ng/ml IL-12 for 3 d. As shown in Fig. 3, CD8α+ DCs were found to produce an approximately fivefold higher level of IFN-γ than do CD8α− DCs, indicating that CD8α+ lymphoid DCs are the major IFN-γ producers in response to IL-12 stimulation.
Detection of Intracellular IFN-γ in DCs.
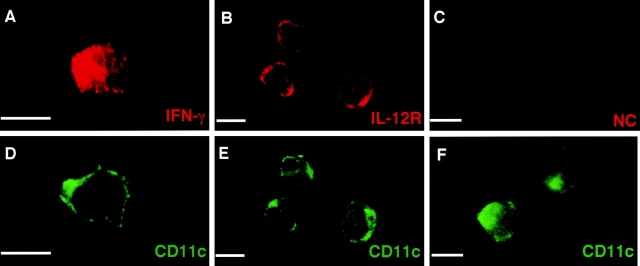
Immunofluorescence microscopy was conducted to directly detect the expression of IFN-γ protein in DCs. Purified CD11c+ CD86+ splenic DCs were cultured in the presence of IL-12 for 3 d, fixed on coverslips, and subjected to intracellular immunofluorescence microscopic analysis. As shown in Fig. 4, IFN-γ proteins were clearly detected in the cytoplasm of CD11c+ DCs (Fig. 4, A and D), whereas staining was undetectable with the control rabbit serum (Fig. 4, C and F). Consistent with a previous study (31), expression of IL-12Rs was readily observed on the cell surface of DCs (Fig. 4, B and E). IFN-γ was not detected in freshly isolated DCs but was detected in splenic macrophages upon IL-12 stimulation by immunofluorescence microscopy (data not shown).
Figure 4.
Detection of intracellular IFN-γ in IL-12–stimulated DCs. Purified DCs were cultured in the presence of 1 ng IL-12 for 3 d. After surface staining with FITC-conjugated mAb against CD11c, cells were fixed and permeabilized. Cells were then incubated with rabbit polyclonal Ab against IFN-γ (A and B) and normal rabbit serum (E and F). Freshly isolated DCs were also incubated with rabbit polyclonal Ab against IL-12R (C and D). Samples were further stained with Rhodamine-conjugated goat anti-rabbit IgG. Bars, 10 μm.
Discussion
We presented here evidence that NK cells play a small role in the production of IFN-γ at early stages of LM infection or IL-12 administration, and that DCs and macrophages produce IFN-γ. Among DC subpopulations, CD8α+ lymphoid DCs are major producers of IFN-γ in response to IL-12. Recent studies have also reported the ability of macrophages to produce IFN-γ (12, 13). Amounts of IFN-γ produced by DCs and macrophages were substantially larger than the amount produced by NK cells.
It has long been assumed that IL-12 is initially produced by macrophages in response to various intracellular pathogens and later by DCs (32, 33), based on the observations that DCs produce IL-12 through ligation of CD40 on DCs by CD40L on activated T cells, or through cross-linking of MHC class II molecules by the TCR (25, 34). However, it has been shown recently that phagocytosis of microparticle-adsorbed proteins stimulates DCs to synthesize IL-12 without interacting with T cells (35), and that DCs but not macrophages produce IL-12 in vivo in microbial infection such as Toxoplasma gondii (36). Furthermore, accumulating evidence has indicated that resting macrophages are unable to produce IL-12 in response to bacteria or microbial products such as LPS without prior activation by certain cytokines such as IFN-γ (4, 37, 38).
In this paper we showed that DCs are able to produce IFN-γ upon IL-12 stimulation. Because DCs produce IL-12 upon phagocytosis and microbial infection, and IL-12 in turn augments the production of IL-12 itself from DCs (31, 35, 36), it is likely that DCs produce IL-12 and IFN-γ by an autocrine manner once they have been triggered by microbial infection. The fact that the addition of anti–IL-12 Ab completely blocked the IFN-γ production by NK cell– depleted Rag-2−/− splenocytes upon LM infection supports this notion. In addition to IL-12, IL-18 and IL-1β are also likely to be involved in augmenting IFN-γ production from DCs in vivo, as observed in T and NK cells (39, 40).
Our results on C57BL/6-γc −/−(Y)Rag-2−/− mice are consistent with a recent paper by Andersson et al. that reports that γc −/−(Y)Rag-2−/− mice produce minimal amount of IFN-γ (41). Since these mice lack NK cells as well as T and B cells, it was concluded that NK cells are the major producers of IFN-γ. Flow cytometric analysis showed the presence of normal numbers of DCs and macrophages in the spleens of C57BL/6-γc −/−(Y)Rag-2−/− mice (Ohteki, T., and S. Koyasu, unpublished results). It is likely that APCs in the γc −/−(Y)Rag-2−/− mice have some functional rather than developmental defects that remain to be examined.
It is likely that the IFN-γ derived from DCs plays a key role in priming and activating macrophages to produce IL-12 in response to intracellular pathogens. DC-derived IFN-γ, together with IL-12, may also be important in upregulation of surface molecules on DCs such as MHC class II. Once IL-12 and IFN-γ are produced by DCs, a positive feedback pathway(s) would be activated between DCs and macrophages even in the absence of NK cell–derived IFN-γ. Macrophages then secrete IFN-γ in response to IL-12 or a combination of IL-12 and IL-18 (12, 13), which also activates macrophages in an autocrine manner to produce nitric oxide. In microbial infection such pathways would be quicker than the pathway through NK cell–derived IFN-γ, and thus important, although not sufficient, for an early stage of innate immune response.
DCs are divided into at least two subpopulations by origins, surface molecules, and the requirement of cytokines for their development (27–30). One subpopulation is myeloid DCs without CD8α expression, and the second is lymphoid DCs expressing CD8α. It has been shown that CD8α+ lymphoid DCs primarily produce IL-12 in vivo in intracellular protozoan infection (36). Given that the CD8α+ DCs produce IFN-γ in response to IL-12 (Fig. 3) and predominantly localized in the T cell area of the spleen (30), lymphoid CD8α+ DCs rather than myeloid CD8α− DCs are probably the most efficient initiators for innate immune response upon infection of intracellular microorganisms, as well as the directors of subsequent Th1 differentiation in vivo.
Acknowledgments
We thank Dr. Mitsuyama for providing LM, and Drs. N. Hozumi and J. Hata for providing NOD/ LtSz-scid/scid mice. We also thank A. Sakurai for excellent animal care.
This work was supported by a grant from the KANAE Foundation for Life & Socio-Medical Science to T. Ohteki, a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan (10153261), a National Grant-in-Aid for the Establishment of a High-Tech Research Center in a Private University, a Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects, and a grant from the Japan Society for the Promotion of Science (JSPS-RFTF 97L00701) to S. Koyasu. K. Suzue is supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 3.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, Chow JM, Gri G, Carra F, Gerosa SF, Wolf R, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubin M, Chow JM, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor-α, and IL-1β production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- 6.Buchmeier NA, Schreiber RD. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenesinfection. Proc Natl Acad Sci USA. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenesin the absence of IFNγ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 8.Dai WJ, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-γ receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 9.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFN γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 10.Magram J, Sfarra J, Connaughton S, Faherty DA, Warrier R, Carvajal D, Wu CY, Sarmiento U, Gately MK. IL-12 deficient mice are defective but not devoid of type 1 cytokine responses. Ann NY Acad Sci. 1996;795:60–70. doi: 10.1111/j.1749-6632.1996.tb52655.x. [DOI] [PubMed] [Google Scholar]
- 11.Wakil AE, Wang Z-E, Ryan JC, Fowell DJ, Locksley RM. Interferon γ derived from CD4+T cells is sufficient to mediate T helper cell type 1 development. J Exp Med. 1998;188:1651–1656. doi: 10.1084/jem.188.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puddu P, Fantuzzi L, Borghi P, Barbara V, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf SF, Belardelli F, Gessani S. IL-12 induces IFN-γ expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 13.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 15.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katuki M, Takahashi K, et al. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor γ chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 16.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, Leiter EH. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 17.Steinman, R.M., W.C. Van Voorhis, and D.M. Spalding. 1986. Dendritic cells. In Handbook of Experimental Immunology. D.W. Weir, L.A. Herzenberg, C. Blackwell, and L.A. Herzenberg, editors. Blackwell, London. 49.1–49.9.
- 18.Rovere P, Zimmermann VS, Forquet F, Demandolx D, Trucy J, Ricciardi-Castagnoli P, Davoust J. Dendritic cell maturation and antigen presentation in the absence of invariant chain. Proc Natl Acad Sci USA. 1998;95:1067–1072. doi: 10.1073/pnas.95.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bancroft GJ, Schreiber RD, Unanue ER. Natural immunity: a T cell independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 20.Laskay T, Rollinghoff R, Solbach W. Natural killer cells participate in the early defense against Leishmania majorinfection in mice. Eur J Immunol. 1993;23:2237–2241. doi: 10.1002/eji.1830230928. [DOI] [PubMed] [Google Scholar]
- 21.Scharton TM, Scott P. Natural killer cells are a source of interferon that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania majorin mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denkers EY, Gazzinelli RT, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of IFN-γ dependent immunity to Toxoplasma gondiiin MHC class I–deficient mice. J Exp Med. 1993;178:1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu YY, Kummar V, Bennett M. Murine natural killer and marrow graft rejection. Annu Rev Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–747. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Witmer-Pack M, Inaba M, Hathcock S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vremec D, Zorbas M, Scolly R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Li C-L, Shortman K. Thymic dendritic cell precursors: relationship to the T-lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 30.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 31.Grohmann U, Belladonna M, Bianchi R, Orabona C, Ayroldi E, Fioretti M, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 32.Locksley RM. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci USA. 1993;90:5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 34.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheisher C, Mehlig M, Dienes H-P, Reske K. Uptake of microparticle-adsorbed protein antigen by bone marrow-derived dendritic cells results in up-regulation of interleukin-1α and interleukin-12 p40/p35 and triggers prolonged, efficient antigen presentation. Eur J Immunol. 1995;25:1566–1572. doi: 10.1002/eji.1830250615. [DOI] [PubMed] [Google Scholar]
- 36.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flesch IE, Hess JH, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann SH. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon γ and tumor necrosis factor α. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skeen MJ, Miller MA, Shinnick TM, Ziegler HK. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 39.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 40.Hunter CA, Chizzonite R, Remington JS. IL-1β is required for IL-12 to induce production of IFN-γ by NK cells. A role for IL-1β in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 41.Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-γ production and innate immunity during Listeria monocytogenesinfection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]