Abstract
Intradermal administration of short overlapping peptides derived from chain 1 of the cat allergen Fel d 1 (FC1P) that did not cross-link IgE, elicited isolated late asthmatic reactions with no visible early or late cutaneous response in 9/40 cat-allergic asthmatics. Four of the nine were human histocompatibility leukocyte antigen DR13–positive, as compared with only 1/31 nonreactors. The other five reactors expressed either DR1 or DR4. To confirm major histocompatibility complex restriction, fibroblast cell lines transfected with HLA-DR molecules were used to present FC1Ps to cat allergen–specific T cell lines derived from subjects before peptide injection. FC1P3 (peptide 28–44 of Fel d 1 chain 1) was recognized in the context of DR13 alleles (DRB1*1301, 1302) and induced specific T cell proliferation and IL-5 production. T cells from a DR1+ responder proliferated and produced IL-5 in the presence of FC1P3 and DR1 (DRB1*0101) fibroblast cell lines, whereas T cells from a DR4+ subject recognized FC1P2 (peptide 22–37) when presented by DRB1*0405. We conclude that short, allergen-derived peptides can directly initiate a major histocompatibility complex–restricted, T cell–dependent late asthmatic reaction, without the requirement for an early IgE/mast cell–dependent response, in sensitized asthmatic subjects.
Keywords: T lymphocyte, Fel d 1, allergen, allergy, human histocompatibility leukocyte antigen
Allergen challenge of sensitized atopic individuals results in an early response, which in the skin is seen as the immediate wheal and flare reaction, and, after inhalation, as a decrease in airway caliber occurring within minutes (1). These acute reactions are generally considered to be due to the release of histamine and other pharmacological agents from mast cells subsequent to IgE cross-linking. 3–8 h after allergen challenge, there follows a late-phase reaction (LPR)1 characterized in the skin by swelling and induration (1) and in the lung by the late asthmatic reaction (LAR). LPRs are accompanied by inflammatory cell infiltration, in particular eosinophils and Th2 cells (2, 3), and have been used as models of chronic allergic inflammation. There is continued debate as to the relative contributions of IgE-dependent versus T cell–dependent mechanisms in the pathogenesis of the LPR. On the one hand, a cutaneous LPR could be transferred by IgE or injection of anti-IgE (4, 5), whereas we and others have provided extensive evidence that CD4+ T cells are associated with the LPR and chronic asthma (3, 6–8). Furthermore, in animal models, airway eosinophil responses and bronchial hyperresponsiveness to inhaled antigen challenge could be adoptively transferred by CD4+ T cells alone (9, 10).
To provide evidence that the LPR can be provoked by T cell activation independently of the early IgE response, we have administered peptide epitopes derived from the major cat allergen, Fel d 1, to cat-allergic asthmatics. The peptides were capable of inducing T cell proliferation but did not cross-link IgE, as shown by the basophil histamine release assay. We hypothesized that the peptides would elicit isolated late cutaneous reactions after intradermal injection, without a preceding wheal and flare reaction. Unexpectedly, we observed that intradermal injection of the T cell peptide epitopes produced isolated LARs with no visible cutaneous response. These reactions were only observed in a minority of the subjects challenged. In this report, we describe these isolated LARs provoked by T cell peptide epitopes and provide evidence that they are HLA restricted. We also present preliminary observations on hyporesponsiveness (“tolerance”) after subsequent rechallenge with the peptides.
Materials and Methods
Human Subjects.
40 cat-allergic asthmatic patients were recruited from the Allergy Clinic, Royal Brompton Hospital, London and by advertisement. The study was approved by the Royal Brompton Hospital Ethics Committee, and informed consent was obtained from all patients. Subjects were nonsmokers and had no other significant illness. Inhaled corticosteroids were discontinued at least 2 d before assessment, and oral corticosteroids were discontinued 3 mo before assessment. Patients were specifically instructed not to take antihistamines, corticosteroids, or any other medication for the duration of the study. Volunteers had a history of wheezing on exposure to cats and demonstrated airway hyperresponsiveness, with a provocative concentration (PC)20 of <4 mg/ml methacholine, and >20% reversibility with inhaled β2 agonists. Cat allergy was confirmed by a positive radio allergo sorbent test (>0.35 IU/ml; Pharmacia) and skin prick test to whole cat dander (ALK) and a late phase skin reaction of >3 cm diameter at 6 h, after the intradermal testing of 30 biological units of whole cat dander (containing 4.5 ng Fel d 1) in 0.03 ml. The vehicle for whole cat dander extract contained 0.3 mg/ml human serum albumin and 4 mg/ml phenol (ALK diluent; ALK).
Study Design.
On the first visit (control day), subjects were skin tested with intradermal cat dander. Peripheral blood (150 ml) was taken for HLA typing, histamine release assays, and proliferation assays. Local reactions were observed after 5, 15, and 30 min and hourly for 6 h thereafter. At these times, FEV1 and peak expiratory flow rate were also recorded. On the second visit, patients received 40 μg intradermally of the three peptides comprising FC1P, contained in 25 μl, into each forearm. Local reactions were observed at the same time points as the control day. A positive reaction was defined as a fall in forced volume in 1 s (FEV1) of 20% or more. The time interval between the two study days ranged from 4 to 56 d (average, 17 d). Six of the nine subjects who developed LARs after peptide injections had a further visit in which 0.03 ml of diluent (vehicle) was injected intradermally. Three subjects, having developed LAR after FC1P administration, were recalled for a repeat administration of the peptides 2–6 wk after the initial challenge. An additional three subjects received a repeat administration of FC1P a minimum of 12 mo after the first injection.
Statistical Analysis of LARs.
FEV1 data was summarized over time for each subject for the control and peptide days. Areas under each curve were calculated using the trapezoidal rule to generate area under curve (AUC). Differences in the AUC between the control day and the peptide day were analyzed by paired t test.
Peptide Synthesis.
The three FC1Ps for injection were synthesized by F-moc chemistry and supplied as acetate salts. They were purified to >95% by HPLC, subjected to sequence and mass analysis, and subjected to independent analysis for sterility and the absence of endotoxin. Peptides were reconstituted in ALK diluent at 1.6 mg/ml. Peptides for in vitro analysis were synthesized using F-moc chemistry at the Advanced Biotechnology Centre, Imperial College School of Medicine, Charing Cross Campus. Peptide sequences used in this study were: FC1P1, LFLTGTPDEYVEQVAQY; FC1P2, EQVAQYKALPVVLENA; and FC1P3, KALPVVLENARILKNCV. Cat allergen extract was a gift of ALK Abello, Copenhagen, Denmark.
Histamine Release Assays.
In 11 subjects, PBMCs isolated by density centrifugation from 50 ml of blood were washed three times with Ca2+- and Mg2+-free HBSS. 100-μl aliquots of cell suspension at 5 × 106 cells/ml were combined with an equal volume of peptide (0.02–100 μg/ml) or cat dander (0.02–200 μg/ml). Samples were incubated at 37°C for 60 min, followed by centrifugation. Supernatants were transferred to glass microfiber–coated microtiter plate wells (REFLAB), together with 50 μl of a histamine standard solution (50 ng/ml). Plates were incubated at 37°C for 60 min, washed twice (45 s) in distilled water, and air dried before measurement of histamine by spectrofluorimetry. Percentage of histamine release for each control and test substance was calculated from the mean histamine release (ng/ml) values using the equation: % release = [(sample − spontaneous)/(total − spontaneous)] × 100.
Transfected Fibroblast Cell Lines.
Murine and human fibroblast cell lines transfected with HLA-DR molecules were obtained as gifts from a number of sources: DRB1*0101, 0401, 0403, 0404, and 0406 from Prof. R.I. Lechler and Dr. G. Lombardi (Imperial College School of Medicine [ICSM], Hammersmith Campus, London), DRB1*0402 and 0405 from Prof. J.R. Lamb (University of Edinburgh, UK) and Dr. A. Verhoef (ICSM at National Heart and Lung Institute [NHLI], London), and DRB1*1301, 1302, 1303, 1304, and 1305 from Drs. J.R. Richert and C. Katovich Hurley (Georgetown University Medical Center, Washington, DC). Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 μg/ml G418 (all from GIBCO BRL).
Allergen-specific T Lymphocyte Lines.
PBMCs were isolated by density gradient centrifugation from all subjects at the initial screening visit. PBMCs were either frozen for subsequent use as APCs or cultured for 10 d in the presence of cat allergen extract (100 μg/ml; ALK) with addition of purified human IL-2 (Lymphocult LF; Biotest) at a final concentration of 10% on day 6 of culture. Viable cells were harvested and restimulated weekly with cat allergen extract and IL-2, with a further addition of IL-2 on day 3 of the restimulation cycle. Before assay, cells were rested overnight in culture medium in the absence of exogenous antigen or IL-2.
Primary PBMC Proliferation Assays.
PBMCs were separated from 100 ml whole blood in 25 patients by density centrifugation. PBMCs were washed and resuspended in RPMI supplemented with 5% vol/vol AB-serum (Sigma Chemical Co.), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and cultured at 105 cells/well in 96-well plates. 16 replicate wells for each of three concentrations were established for each peptide. Purified protein derivative (PPD; 10 μg/ml) was used as positive control. Cells were cultured for 6 d before addition of 1 μCi (37 kBq) tritiated methyl-thymidine per well for 8 h. Cells were harvested, and thymidine incorporation was determined by liquid scintillation spectroscopy. Individual data points for 16 peptide culture wells were compared with those containing medium alone. Statistical evaluation was performed using Mann-Whitney analysis. Results are expressed as delta counts per minute (observed cpm − medium-alone cpm).
T Cell Line Proliferation Assays in the Presence of Transfected Fibroblast Lines.
Transfected fibroblast lines were cultured overnight in the presence of the relevant peptide at a concentration of 100 μg/ml before harvesting and incubation with mitomycin C (50 μg/ml) for 1 h. Cells were washed extensively before seeding in 96-well, flat-bottomed plates at 3 × 104 cells/well. Allergen-specific T lymphocytes were added (104 cells/well) and cultured for 48 h. Culture supernatants were harvested for cytokine analysis before pulsing wells with 37 kBq/well of tritiated methyl-thymidine and cultured for a further 8 h. Proliferation as correlated with thymidine incorporation was measured by scintillation spectroscopy (TopCount; Canberra Packard).
ELISA for Cytokines.
IL-5 was measured in the laboratory of Dr. D. Huston (Baylor Medical Center, Houston, TX) according to a previously described technique (11). The sensitivity of the assay was 3 pg/ml.
Results
Incubation of FC1P with Basophil-enriched PBMCs Did Not Induce Histamine Release.
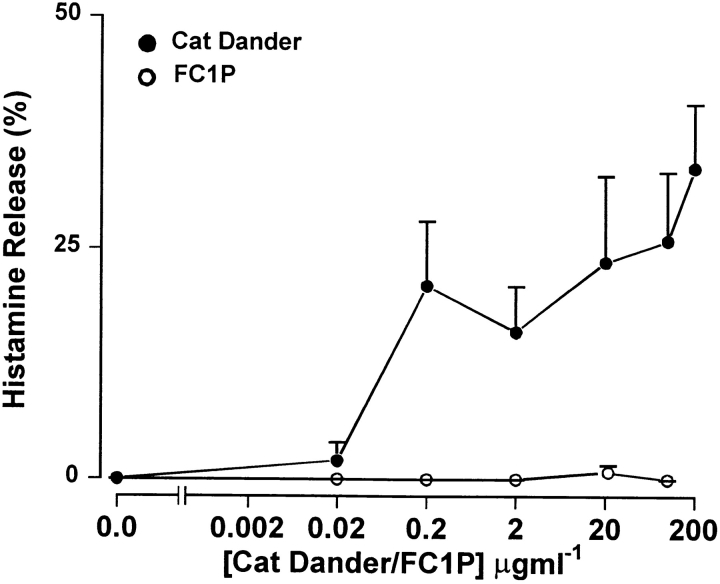
In contrast to whole cat extract (ALK cat), pooled FC1Ps did not release histamine from basophil-enriched mononuclear cells in vitro. Cells from 11 subjects (5 FC1P reactors and 6 nonreactors) were incubated with FC1Ps at five increasing concentrations up to 100 μg/ml (∼5 × 10−5 M of each of the pooled peptides). Whole cat dander extract served as a positive control, releasing histamine in all subjects assayed (Fig. 1). No immediate skin or asthmatic reactions were observed in vivo, further demonstrating that FC1P did not bind IgE.
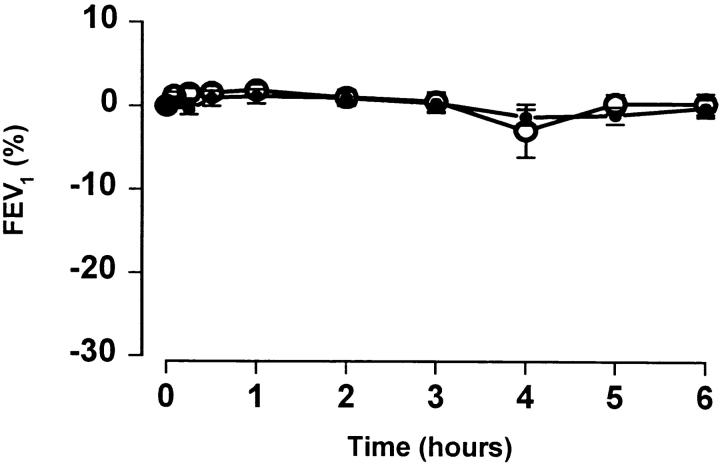
Figure 1.
FC1Ps do not induce histamine release from basophil- enriched PBMC in vitro. Mean percentage histamine release induced by whole cat dander extract (ALK; 0.2, 2, 20, 100, and 200 μg/ml) and FC1P (five increasing concentrations up to 100 μg/ml [5 × 10−5 M]) from basophil-enriched leukocytes (n = 11; five LAR responders and six nonresponders).
FC1Ps Induce Primary Proliferative Responses in PBMC Culture.
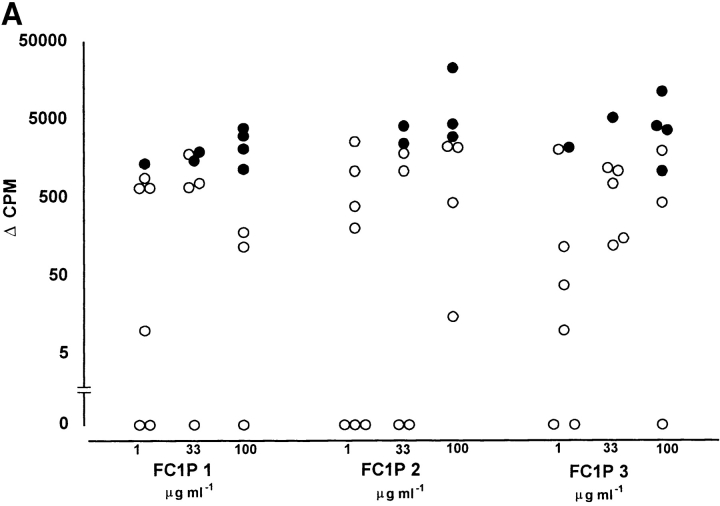
The ability of FC1Ps to stimulate T cell responses was assessed, initially by primary in vitro proliferation assays. All individuals assayed responded to one or more of the three peptides, although none of the peptides induced proliferation in all individuals, suggesting MHC-restricted recognition. Each of the peptides was recognized by PBMCs from both the group developing LARs (n = 7) and the nonresponder group (n = 19). As shown in Fig. 2, each peptide was able to induce proliferative responses, in a dose-dependent fashion, in PBMCs from representative (26/40) cat-asthmatic subjects from whom cells were available. Proliferative responses from individuals who developed LARs were generally of greater magnitude than those that did not; however, this difference was not statistically significant. All subjects responded to the positive control recall antigen, PPD.
Figure 2.
Proliferation occurs in primary PBMC cultures from reactors and nonreactors in response to peptides FC1P 1, FC1P 2, and FC1P 3. T cell reactivity to FC1P in (A) subjects developing LARs and (B) nonreactors. Delta counts per minute (Δcpm) from PBMC proliferating to peptides FC1P 1, 2, and 3 at up to three concentrations. All patients reacted to tuberculin (PPD; positive control) with a mean Δcpm of 65,112 (data not shown). Background counts ranged from 680 to 4,465 cpm. •, statistically significant proliferative responses (see Materials and Methods) compared with background; ○, nonstatistically significant proliferative responses. Each peptide was capable of eliciting a proliferative response, in a dose-dependent fashion, in individuals from both groups (responders, n = 7; nonresponders, n = 19); however, none of the peptides induced proliferation in all individuals.
Intradermal Administration of Peptides Derived from Fel d 1 Induced Isolated LARs.
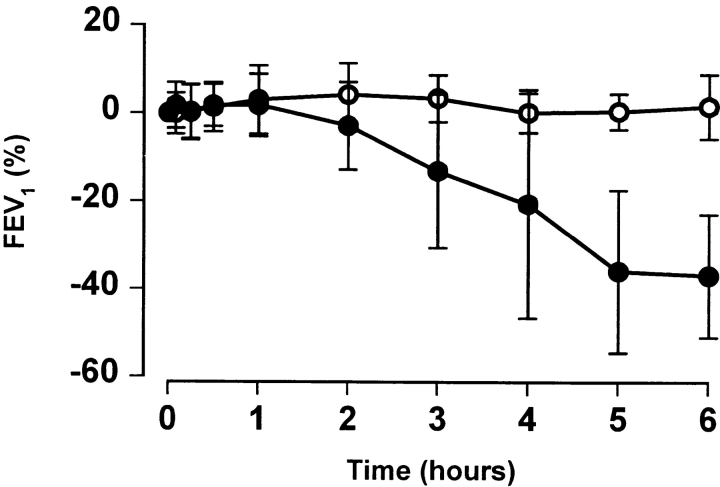
Inhaled whole allergen induces both an immediate early asthmatic reaction and an LAR. To determine whether T cell activation could trigger an isolated LAR, sensitized volunteers were challenged with peptides derived from Fel d 1 chain 1. In 9/40 subjects, 80 μg of FC1P did not produce a visible immediate or late skin reaction but did elicit a progressive decline in FEV1, associated, in all responders, with symptoms of chest tightness and wheezing. The remaining subjects did not develop any immediate or late bronchial or cutaneous reactions to the peptides. The reaction commenced at hours 2–3 and reached a plateau by hour 6 (Fig. 3). The decrease in FEV1 observed ranged from 21 to 64% of baseline. AUC analysis to compare percentage change in FEV1 on control and FC1P days in nine patients was statistically significant, at P = 0.0004. Subjects were monitored for a minimum of 6 h and treated with β2 agonists and inhaled corticosteroids before leaving the hospital. The mean maximum change in FEV1 observed on the whole cat dander control day was −3.83% (range −14 to +4%). Additionally, six of the nine reactors had a further control day in which 0.03 ml of the diluent (vehicle) was injected intradermally. The mean maximum change in FEV1 was −6.3% (range from 0 to –13%). 31 individuals showed no evidence of LAR (Fig. 3).
Figure 3.
Induction of isolated LARs by intradermal injection of peptides derived from FC1P. The mean percent changes in FEV1 after intradermal administration of FC1P (•) and during a control day (○) of 9 responders (left) and 31 nonresponders (right). AUC analysis to compare percent change in FEV1 on control and FC1P days in nine patients was statistically significant, at P = 0.0004. Error bars, SD.
Subjects Who Developed an LAR Demonstrated a Higher Frequency of HLA-DR13 than Nonreactors.
To determine whether the reactions were HLA restricted, subjects were typed for HLA-DRB1. HLA analysis revealed an increased frequency of HLA-DR13 in those who developed LAR (4/9 subjects), compared with only 1/31 subjects who did not react, suggesting that DR13 may restrict T cell responses to one or more of the injected peptides (P = 0.006, Fisher's exact test). Patient characteristics and HLA-DRB1 types are shown in Table I.
Table I.
Clinical Characteristics of Patients Injected with FC1P
| Sex | Age | PC20 | SPT Cat | RAST Cat | DRB1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/ml | mm | IU/ml | ||||||||||
| Responders | ||||||||||||
| 1 | F | 26 | 0.8 | 9 | 5.77 | 1, 7 | ||||||
| 2 | F | 22 | 1.5 | 10 | 6.25 | 1, 1302 | ||||||
| 3 | F | 41 | 2 | 11 | 42.8 | 7, 1301 | ||||||
| 4 | F | 28 | 2.5 | 8 | 0.54 | 17, 13 | ||||||
| 5 | M | 48 | 1.6 | 7 | 100 | 15, 1303 | ||||||
| 6 | M | 27 | 2.7 | 9 | 100 | 0408, 0801-11 | ||||||
| 7 | F | 46 | 2 | 5 | 34 | 0301, 0405 | ||||||
| 8 | F | 30 | <2 | 6 | 12.1 | 0401, 0701 | ||||||
| 9 | F | 36 | 0.95 | 6 | 9.66 | 0101-2, 0104, 1101-16, 18-22 | ||||||
| Nonresponders | ||||||||||||
| 1 | F | 29 | 3 | 8 | 1.7 | 01501-5, 0401 | ||||||
| 2 | M | 22 | <4 | 5 | 1 | 0101-2, 0104, 0701 | ||||||
| 3 | M | 23 | 2 | 10 | 3.09 | 1301-1322 | ||||||
| 4 | M | 33 | 0.3 | 6 | 0.62 | 0301, 1201-3 | ||||||
| 5 | M | 22 | 2 | 10 | 1.13 | ND | ||||||
| 6 | F | 24 | 3.85 | 8 | 7.37 | 17, 4 | ||||||
| 7 | M | 19 | <2 | 7 | 3.88 | 1501-5, 0405 | ||||||
| 8 | M | 35 | 3.22 | 9 | 3.21 | 1501-5, 0701 | ||||||
| 9 | M | 25 | 4 | 12 | 3.02 | 1701, 0301 | ||||||
| 10 | F | 24 | 2 | 7 | 1.39 | 0301/0304, 0408 | ||||||
| 11 | F | 37 | 2 | 6 | 4.65 | 0401-22, 0801-11 | ||||||
| 12 | F | 22 | <2 | 6 | 2.54 | 1501-5, 0301 | ||||||
| 13 | F | 25 | 3 | 5 | 2.03 | 0301, 0701 | ||||||
| 14 | F | 31 | 1.6 | 5 | 1.35 | 7, 11 | ||||||
| 15 | F | 31 | 3.5 | 8 | 4.99 | 16, 11 | ||||||
| 16 | M | 38 | 3 | 9 | 7.54 | 4, 7 | ||||||
| 17 | M | 25 | 2 | 7 | 3.68 | 0403, 0701 | ||||||
| 18 | M | 27 | 3.8 | 9 | 2.38 | ND | ||||||
| 19 | F | 23 | 3 | 9 | 6.94 | 1, 11 | ||||||
| 20 | F | 52 | 1.6 | 8 | 32.3 | 3(17), 4 | ||||||
| 21 | F | 38 | 2 | 12 | 3.91 | 4, 17 | ||||||
| 22 | M | 41 | 1.4 | 11 | 13.8 | 4, 11 | ||||||
| 23 | F | 34 | 1.7 | 9 | 3.04 | 1401, 08 | ||||||
| 24 | F | 25 | 3.6 | 6 | 1.29 | 0103, 7 | ||||||
| 25 | M | 23 | 3 | 8 | 60.4 | 0101, 0102, 0104, 1101-21 | ||||||
| 26 | F | 29 | 2 | 7 | >100 | ND | ||||||
| 27 | F | 24 | 1.3 | 13 | 1.38 | 11, 0311, 0306, 03011-12 | ||||||
| 28 | F | 30 | 2.88 | 8 | 5.76 | 11, 04 | ||||||
| 29 | M | 25 | 1 | 14 | 9.44 | 0403, 1101-1121 | ||||||
| 30 | F | 29 | 1 | 13 | >100 | ND | ||||||
| 31 | F | 23 | 3.6 | 8 | 2.54 | 04, 0701, 0703 |
RAST, radio allergo sorbent test; SPT, skin prick test.
Recognition of Peptides FC1P2 and 3 in the Context of DR13, DR1, and DR4.
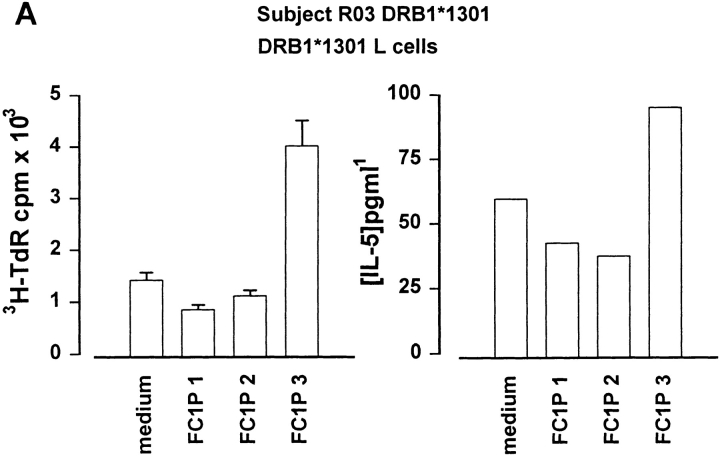
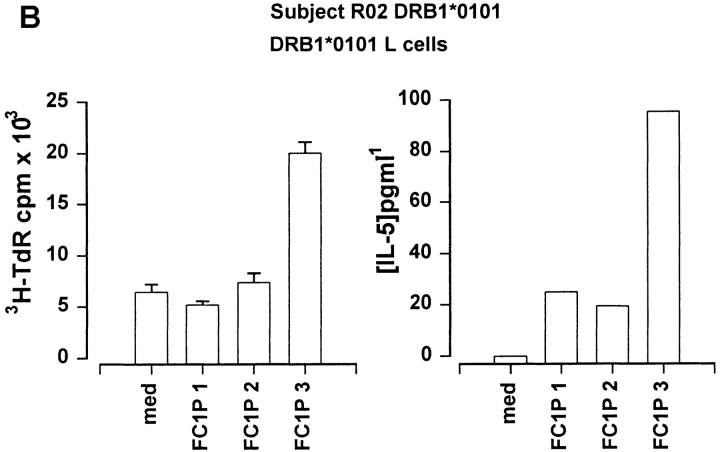
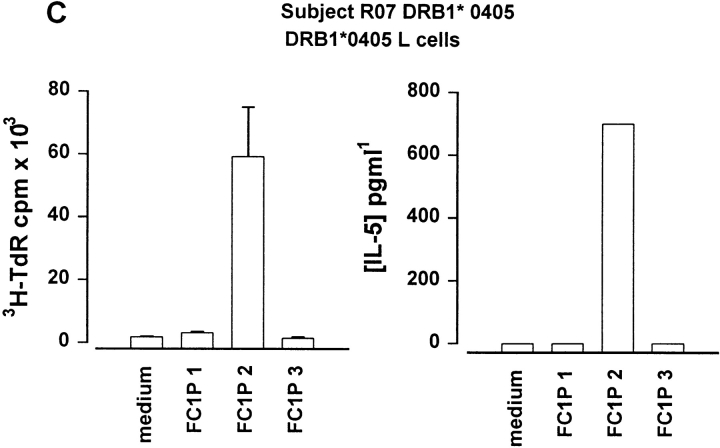
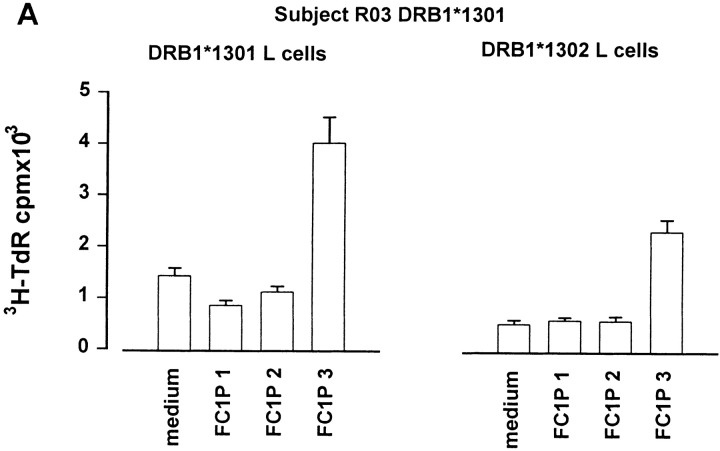
As peptides can be presented in vitro without intracellular processing, we determined whether Fel d 1 chain 1 peptides could be recognized in the context of MHC molecules expressed by the subjects developing asthmatic reactions. For this purpose, fibroblast cell lines transfected with relevant HLA molecules were cultured together with each peptide and T cell lines from the responding individuals. Cat dander extract–specific T cell lines were derived from a DR13+ subject (RO3; DRB1*1301) before induction of peptide-induced LAR. Culture of these cells with FC1Ps and a fibroblast cell line transfected with DRA and DRB1*1301 resulted in T cell proliferation and IL-5 secretion in response to FC1P3 but not to FC1P1 or FC1P2 (Fig. 4 A). Fibroblasts transfected with DRA and DRB1*0101 were able to present FC1P3 to a cat dander extract–specific T cell line derived from subject RO2 (DRB1*0101), resulting in proliferation and IL-5 production (Fig. 4 B). T cells from a cat dander extract–specific cell line from subject RO7 (DRB1*0405) were induced to proliferate and release IL-5 when cultured with FC1P2 and fibroblasts expressing DRB1*0405 (Fig. 4 C).
Figure 4.
Restriction of T cell responses to FC1P 2 and 3 in the context of DRB1*0405, DRB1*1301, and DRB1*0101. (A) Proliferation and IL-5 production by a cat allergen extract–specific T cell line from subject R03 (DRB1*1301) cultured with a fibroblast cell line transfected with DRB1*1301 in the presence of medium alone and peptides FC1P 1, 2, and 3. Presentation of FC1P 3 by DRB1*1301 results in proliferation and IL-5 production. (B) IL-5 production by a cat allergen extract– specific T cell line from subject R02 (DRB1*0101, 1302) cultured with a fibroblast cell line transfected with DRB1*0101 in the presence of medium alone and peptides FC1P 1, 2, and 3. Presentation of FC1P 3 by DRB1*0101 results in proliferation and IL-5 production. Responses in the context of DRB1*1302 were not detected in this subject (data not shown). (C) Proliferation and IL-5 production by a cat allergen extract– specific T cell line from subject R07 (DRB1*0405) cultured with a fibroblast cell line transfected with DRB1*0405 in the presence of medium alone and peptides FC1P 1, 2, and 3. FC1P 2 is presented by DRB1*0405, inducing proliferation and IL-5 production.
Degenerate T Cell Recognition of FC1P3 on Closely Related DR13 and DR4 Microvariants.
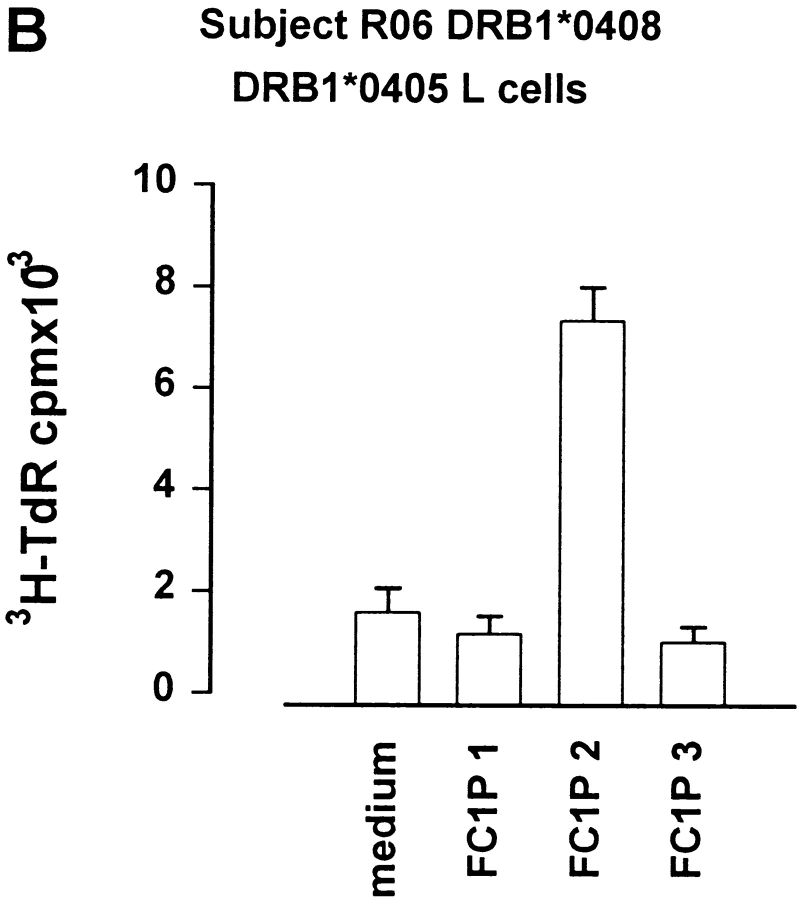
Fibroblast cell lines transfected with either DRB1*1301 or DRB1*1302 were assayed for their ability to present FC1P3 to allergen extract–specific T cells derived from a DRB1*1301 donor. As previously described, FC1P3 presented by DRB1*1301 induced a proliferative response in autologous T cells (Fig. 4 A). In addition, fibroblasts expressing DRB1*1302 were able to activate allergen-specific T cells from a DRB1*1301 donor (Fig. 5 A). In the absence of fibroblasts expressing DRB1*0408, cells expressing the closely related microvariant DRB1*0405 were assayed for their ability to present FC1P2 to cat allergen extract–specific T cells from a DRB1*0408 donor. As shown in Fig. 5 B, DRB1*0405, which differs from DRB1*0408 by only one residue in the peptide binding groove (position 57, serine→ aspartic acid), was able to present FC1P2, inducing proliferation in T cells from a DRB1*0408 donor.
Figure 5.
Presentation of FC1P 2 and 3 on more than one HLA-DR molecule. (A) Proliferation of a cat allergen extract–specific T cell line from subject R03 (DRB1*1301) cultured with fibroblast cell lines transfected with either DRB1*1301 or DRB1*1302 in the presence of medium alone and peptides FC1P 1, 2, and 3. FC1P 3 is presented by DRB1*1301 and DRB1*1302. The amino acid sequences of DRB1*1301 and DRB1*1302 (a) show the single amino acid difference at position 86 between the two molecules. (B) Proliferation of T cells from subject R06 (DRB1*0408) cultured with a fibroblast cell line transfected with DRB1*0405 in the presence of medium alone and peptides FC1P 1, 2, and 3. FC1P 2 is presented to DRB1*0408 T cells by DRB1*0405. The amino acid sequences of DRB1*0405 and DRB1*0408 (b) show the single amino acid difference in the peptide binding regions at position 57 of the two alleles. *Sequence not available. Dashes indicate homology with DRB1*0101.
Repeat Administration of Peptides Leads to Transient Attenuation of the Initial Reaction.
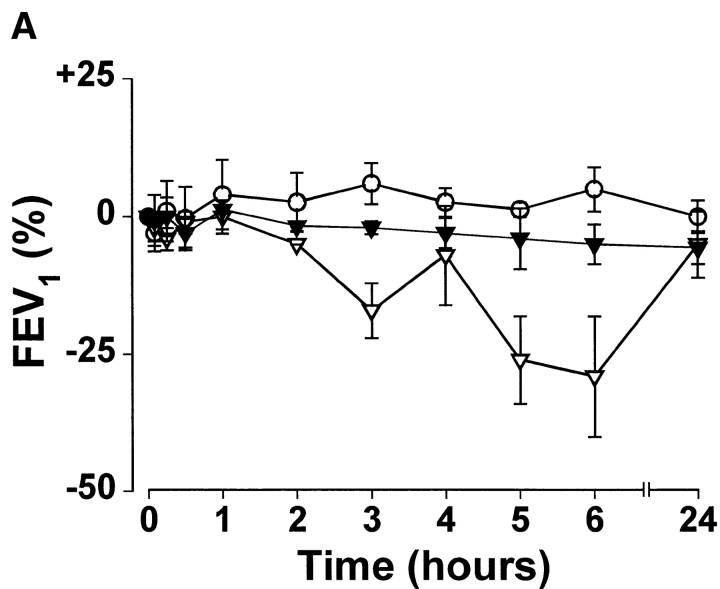
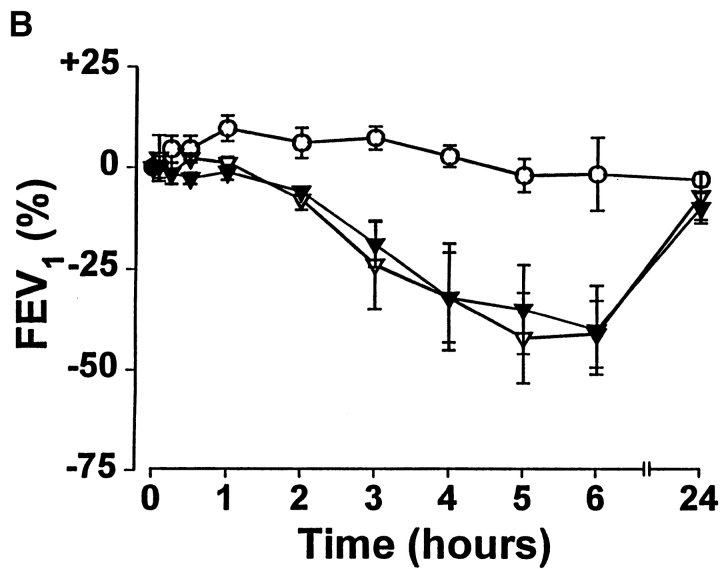
To study the reproducibility of peptide-induced LAR, three subjects (RO1, RO4, and RO5) received a second intradermal dose of 80 μg of FC1P between 2 and 6 wk after the first injection. A marked attenuation of the initial LAR was observed. The maximum percentage changes in FEV1 on the first peptide versus the second peptide challenge were −28 vs. −9 (R01), −21 vs. −10 (R04), and −51 vs. −9 (R05), respectively (Fig. 6). After an interval of at least 12 mo after the initial FC1P challenge, three subjects received a further challenge of the same dose. All experienced LARs of similar magnitude to those after the initial injection (Fig. 6 B).
Figure 6.
Repeat challenge with a second injection of FC1P demonstrates transient attenuation of the LAR. (A) Rechallenge with peptides after 2–6 wk. Three subjects were rechallenged with the same dose of FC1P as the first challenge (▿); no LAR was observed after intradermal injection of FC1P (▾). (B) Rechallenge with peptides after 12 mo. Three subjects were rechallenged with FC1P (80 μg) more than 12 mo after the initial challenge. LARs of similar magnitude were observed after the first (▿) and the second (▾) challenge. Control day, ○.
Discussion
In this study, we have demonstrated that peptides derived from a major allergen elicited an LAR in a proportion of sensitized individuals. That the peptides were most likely to initiate these reactions by direct activation of T cells, rather than mast cells or basophils, is supported by (a) the lack of IgE-mediated histamine release in vitro, (b) the absence of a wheal and flare reaction at the site of injection, and (c) the absence of an early asthmatic reaction. In addition, we have demonstrated HLA restriction of T cell responses to peptides FC1P2 and FC1P3 on the basis of fibroblast presentation assays. These observations provide direct evidence for the central role of T lymphocytes in the induction of the late asthmatic response and indicate that these reactions are MHC-restricted events. Although we had a control day for six of the nine responders that included the injection of human serum albumin, we did not specifically employ a control peptide. Some small peptides cause histamine release from mast cells, but because the action of histamine is immediate, this would not be relevant to the LAR, which commences at hour 2–3 and continues to hour 6. A peptide of “irrelevant” sequence would not be informative, as the biological actions are sequence dependent. On the other hand, as we demonstrate MHC restriction of T cell recognition, control peptide(s) would need to be immunologically inactive but capable of binding to the same range of MHC molecules as the FC1Ps and with similar affinities.
There is strong circumstantial evidence to support a role for T lymphocytes in the pathogenesis of asthma (12). This includes elevated numbers of activated T cells in the blood of patients with acute severe asthma (8), as well as studies using bronchoalveolar lavage (BAL) and bronchial biopsies from mild asthmatics in which increases in CD3/CD4+ cells expressing mRNA positive for IL-4 and IL-5 cells were demonstrated (3, 7, 13). In a provoked model of asthma, increased numbers of CD4+ cells in BAL after allergen challenge were also observed (14). Furthermore, the allergen-induced late-phase, but not the early-phase, reaction was inhibited by prior administration of cyclosporin A, a compound with major inhibitory effects on CD4+ cells (15). More recently, a single infusion of humanized anti-CD4 mAbs into patients with chronic asthma was associated with significant improvements in lung function (16). In Brown Norway rats, Watanabe et al. showed increases in airway resistance up to 8 h after adoptive transfer of CD4 but not CD8 T cells and subsequent allergen challenge (9). Adoptive transfer experiments in mice have also demonstrated that baseline airway hyperresponsiveness was T cell dependent (10).
In animal models, initial administration of peptides in sensitized mice can induce activation of primed T cells (17). However, subsequent administration leads to the induction of tolerance, not only to the peptide but to the whole allergen (18). Using cat-sensitive asthmatics, Norman et al. (19) attempted to induce the counterpart of murine experimental T cell tolerance in human atopic allergic individuals by subcutaneous injection of two peptides (termed IPC1 and IPC2) spanning a large proportion of chain 1 of Fel d 1. Although there was some subjective symptomatic improvement, many patients reported “allergic” symptoms that occurred between 10 min and 6 h after subcutaneous injections. Other groups, using the same peptides, reported similar results (20, 21). As IPC1 and IPC2 are 27 residues long (22), it is possible that the immediate reactions were the result of IgE binding, and the late reactions were due to T cell activation. For this reason, in this study, we specifically designed peptides of relatively small size (16/17 residues) and linear configuration to enable them to be presented to T cells in the absence of antigen processing and without binding to IgE.
This approach, using T cell–reactive peptide epitopes that do not cross-link IgE, provides evidence that asthma can be provoked without initial mast cell activation. The mechanism of T cell peptide–induced LPRs, however, remains to be elucidated. One explanation is that T cell activation in the airways leads to mobilization of eosinophils through the elaboration of type 2 and eosinophil active cytokines (i.e., IL-3, IL-4, IL-5, and GM-CSF). Together, these are known to promote selective eosinophil accumulation, migration, activation, and subsequent tissue injury, possibly through the generation of membrane-derived sulphidopeptide leukotrienes as well as the release of basic proteins from the eosinophil crystalloid granule (23). Alternatively, or in addition, T cell activation may be associated with the generation of histamine releasing factors, which in turn activate basophils and/or mast cells to release histamine with subsequent narrowing of already hyperresponsive airways (24). Direct triggering of bronchoconstriction by release of an unidentified T cell mediator may be another possible mechanism. Studies currently underway involving fiberoptic bronchoscopy with BAL and bronchial biopsy, performed at time intervals after peptide-induced LAR, will enable us to test these hypotheses. Our findings are thus of clinical significance, because they may help to identify the critical mediator(s) involved in T cell– as opposed to mast cell–dependent airway narrowing. This may have particular relevance to ongoing chronic asthma, which is believed to have an important T cell component (15, 16) but where the sequence of events between T cell activation and bronchial obstruction are incompletely understood.
It is intriguing that intradermal injection of peptides produced no visible local reaction in the nine subjects but caused marked changes in the airways. One explanation for a reaction at a distal site is that peptides are presented by skin Langerhans cells to circulating T lymphocytes that bear putative T cell homing receptors (analogous to the cutaneous lymphocyte-associated antigen described in patients with atopic dermatitis; reference 25) and that these then traffic to the airways. Alternatively, the peptide may be absorbed via the circulation or lymphatics and reach the airways, where they are taken up by bronchial APCs that in turn interact with locally sensitized T cells.
Related to the mechanism of T cell peptide epitope– induced asthma is the variation in responsiveness amongst the subjects studied, with only 9/40 responding with an isolated LAR. There was no association between the ability of a particular peptide to elicit a T cell–dependent proliferative response in vitro and its capacity to evoke LAR after intradermal injection in vivo, i.e., in vitro mitogenic responses were observed in the absence of an elicited in vivo effect (Fig. 2). These findings cannot be explained solely by the lack of the appropriate HLA haplotype in the nonresponders, as many of these also expressed DR1, DR4, or DR13 (Table I). We believe that responsiveness or nonresponsiveness was likely to have been a reflection of the dose of peptide administered, with the threshold for developing an LAR varying amongst individuals. It is clear that T cell reactivity is likely to have been dependent on HLA molecules restricting peptide presentation and T cell activation (26) to FC1P, with subsequent induction of isolated LARs in subjects in which a threshold dose was achieved. Of interest in this regard was the apparent clustering of HLA-DR13 amongst the subjects in whom these LPRs were observed. Haplotyping of reactors indicated that 4/9 reactors (44%) possessed a DR13 allele, compared with only one 1/30 (3%) nonreactors. Using transfected fibroblast cell lines, the ability of a variety of HLA-DR molecules to present FC1Ps to allergen-specific T cell lines raised from subjects who developed LARs has been assessed. Initially, based upon the high frequency of DR13 among those who developed LARs, we examined the ability of DRB1*1301 and 1302 to present one or more of the FC1Ps to cat allergen extract– specific T cell lines. We demonstrated that FC1P3 could be presented by both DRB1*1301 and 1302. These microvariants of DR13 differ by only one residue at position 86 (valine→ glycine; Fig. 6 A), and our results suggest, therefore, that this residue is not critical for the binding of this peptide. Furthermore, we have demonstrated the ability of DRB1*1302 to present FC1P3 to T cells from a DRB1*1301 donor, indicating a degree of degeneracy in T cell recognition of this peptide in the context of closely related microvariants of DR13. The ability of DRB1*1303, which differs from 1301 and 1302 more substantially, to present peptide FC1P3 is currently under investigation.
Investigation of the ability of DRB1*0101 to present FC1Ps revealed promiscuous binding of FC1P3 to both DR1 and DR13 molecules. As shown in Fig. 5 a, the sequences of DRB1*0101 and DRB1*1301/1302 differ substantially within the peptide binding region of the molecules. However, a precedent for promiscuous binding of a single peptide (influenza HA 307–319) to both DR1 and DR13 alleles has been described by Hickling et al. (27). Three of the nine individuals who developed peptide- induced LAR were DR4+, although each expressed a different microvariant of DR4 (Table I). The ability of DR4 microvariants to present FC1Ps was investigated, and it was established that DRB1*0405 was able to present FC1P2 to cat allergen extract–specific T cells from both DRB1*0405 (autologous) and DRB1*0408 individuals. Thus, in common with FC1P3, the second FC1P appears to be capable of binding to more than one HLA molecule. The ability of this peptide to bind to other DR4 microvariants is currently under investigation.
Our findings suggest that induction of the LAR results from MHC-restricted T cell activation. The limited clinical benefit that has so far been observed with peptide immunotherapy may therefore be explained by the fact that only a proportion of patients treated will have been capable of reacting to the peptides, as MHC restriction has not been accounted for (19–21). Furthermore, the observation that individual allergen peptides are capable of promiscuous HLA binding may hold promise for the development of MHC-restricted peptide-based therapies. All of the subjects developing asthmatic reactions expressed DR1, 4, or 13. However, a significant proportion of individuals who did not react also expressed these HLA types. The fact that these subjects did not progress to develop LARs may be due to a deficit in the subjects' T cell repertoire. Alternatively, the lowest possible dose that was found to induce an LAR was used in all individuals, and some of the volunteers who did not respond may have required a higher dose of FC1P to trigger an asthmatic reaction.
It should be noted that the amount of peptide required for systemic administration was ∼10,000 times that of the intact protein. However, there is clearly a dilutional effect in a situation where an intradermal injection elicits a reaction at a distal site. The minimum dose required to produce an LAR will be calculated more accurately in studies involving direct application of peptides into the airways. It should also be appreciated that any bronchial reaction elicited by intradermal injection of whole cat extract (which can very occasionally occur but did not in this study) would be predominantly anaphylactic in type, with an IgE/ mast cell–dependent bronchospasm occurring within 30 min and usually sooner (28).
Recent studies have demonstrated that an initial transient activation of T cells precedes the development of hyporesponsiveness (17). In support of this concept was the observation that in three of the patients studied, there was abrogation of the LAR on repeat administration of the same dose of peptide that previously caused a 20% or greater fall in FEV1 (Fig. 6 A). This is similar to the experience of Norman et al. (19), who also observed that there was a progressive decline in the incidence and severity of untoward symptoms after continued treatment of cat allergy with IPC1/IPC2, suggesting but not proving that some form of tolerance may have developed after repeated administration. In addition, three subjects who developed LARs in response to FC1P were readministered the same dose at an interval of greater than one year. All three developed LARs of approximately the same magnitude as those that accompanied the initial challenge (Fig. 6 B). These observations suggest that the induction of hyporesponsiveness after a single dose of FC1P is transient, lasting somewhere between a few weeks and one year. Further studies are currently underway to define the precise duration of peptide-induced hyporesponsiveness in humans. This may have further clinical significance if repeated administration of MHC class II–restricted T cell peptide epitopes produce safe and effective long-term “tolerance.”
In conclusion, we have demonstrated that intradermal injection of a linear peptide sequence within an allergen at a high dose can elicit a late-phase response by a presumed T cell–dependent mechanism in the absence of an IgE-mediated reaction. This appears to be the first demonstration of asthma provoked by MHC-restricted T cell activation.
Acknowledgments
We are grateful to Dr. Douglas Robinson for reviewing the manuscript, Prof. Robert Lechler (ICSM, Hammersmith Campus, London) and Dr. Meinir Jones (ICSM at NHLI, London) for performing MHC typing, Dr. Amanda Bennett and Prof. Martin Church (University of Southampton, UK) for measurement of histamine, and Dr. David Huston (Baylor College of Medicine, Houston, TX) and ALK (Horsholm, Denmark) for supplying cat dander extract.
This work was supported by the Medical Research Council (UK) and the National Asthma Campaign.
Abbreviations used in this paper
- AUC
area under curve
- BAL
bronchoalveolar lavage
- FEV1
forced volume in 1 s
- LAR
late asthmatic reaction
- LPR
late-phase reaction
- PPD
purified protein derivative
References
- 1.Kay, A.B. 1997. Concepts of allergy and hypersensitivity. In Allergy and Allergic Diseases, Vol. 1. A.B. Kay, editor. Blackwell Science, Oxford, UK. 23–35.
- 2.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham SR, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 4.Dolovich J, Hargreave FE, Chalmers R, Shier KJ, Gauldie J, Bienenstock J. Late cutaneous allergic responses in isolated IgE-dependent reactions. J Allergy Clin Immunol. 1973;52:38–46. doi: 10.1016/0091-6749(73)90119-x. [DOI] [PubMed] [Google Scholar]
- 5.Solley GO, Gleich GJ, Jordon RE, Schroeter AL. The late phase of the immediate wheal and flare skin reaction. Its dependence upon IgE antibodies. J Clin Invest. 1976;58:408–420. doi: 10.1172/JCI108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaga M, Frew AJ, Varney VA, Kay AB. Eosinophil activation and T lymphocyte infiltration in allergen-induced late phase skin reactions and classical delayed-type hypersensitivity. J Immunol. 1991;147:816–822. [PubMed] [Google Scholar]
- 7.Bentley AM, Meng Q, Robinson DS, Hamid Q, Kay AB, Durham SR. Increases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmatics. Am J Respir Cell Mol Biol. 1993;8:35–42. doi: 10.1165/ajrcmb/8.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Corrigan CJ, Kay AB. T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 1990;141:970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe A, Mishima H, Renzi PM, Xu LJ, Hamid Q, Martin JG. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+T cells in brown Norway rats. J Clin Invest. 1995;96:1303–1310. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deSanctis GT, Itoh A, Green FH, Qin S, Kimura T, Grobholz JK, Martin TR, Maki T, Drazen JM. Lymphocytes regulate genetically determined airway hyperresponsiveness in mice. Nat Med. 1997;3:460–462. doi: 10.1038/nm0497-460. [DOI] [PubMed] [Google Scholar]
- 11.Dickason RR, Huston MM, Huston DP. Enhanced detection of human IL-5 in biological fluids utilizing murine monoclonal antibodies which delineate distinct neutralizing epitopes. Cytokines. 1995;6:647–656. doi: 10.1016/1043-4666(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 13.Ying S, Humbert M, Barkans J, Corrigan CJ, Pfister R, Menz G, Larche M, Robinson DS, Durham SR, Kay AB. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and non atopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–3544. [PubMed] [Google Scholar]
- 14.Metzger WJ, Zavala D, Richerson HB, Moseley P, Iwamota P, Monick M, Sjoerdsma K, Hunninghake GW. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Description of the model and local airway inflammation. Am Rev Respir Dis. 1987;135:433–440. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- 15.Sihra BS, Durham SR, Walker S, Kon OM, Barnes NC, Kay AB. Effect of cyclosporin A on the allergen-induced late asthmatic reaction. Thorax. 1997;52:447–452. doi: 10.1136/thx.52.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon OM, Sihra BS, Compton CH, Leonard TB, Kay AB, Barnes NC. A double blind placebo controlled trial of a chimeric anti CD-4 monoclonal antibody, kleximab (IDEC CE9.1) in chronic severe asthma. Lancet. 1998;352:1109–1113. doi: 10.1016/S0140-6736(97)12261-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoyne GF, Askonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+T cells precedes the development of tolerance in vivo. Int Immunol. 1996;8:335–342. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 18.Briner TJ, Kuo MC, Keating KM, Rogers BL, Greenstein JL. Peripheral T-cell tolerance induced in naive and primed mice by subcutaneous injection of peptides from the major cat allergen Fel d 1. Proc Natl Acad Sci USA. 1993;90:7608–7612. doi: 10.1073/pnas.90.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norman PS, Ohman JL, Jr, Long AA, Creticos PS, Gefter MA, Shaked Z, Wood RA, Eggleston PA, Hafner KB, Rao P, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–1628. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 20.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–1945. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 21.Pene J, Desroches A, Paradis L, Lebel B, Farce M, Nicodemus CF, Yssel H, Bousquet J. Immunotherapy with Fel d 1 peptides decreases IL-4 release by peripheral blood T cells of patients allergic to cats. J Allergy Clin Immunol. 1998;102:571–578. doi: 10.1016/s0091-6749(98)70294-5. [DOI] [PubMed] [Google Scholar]
- 22.Rogers BL, Bond JF, Craig SJ, Nault AK, Segal DB, Morgenstern JP, Chen MS, Bizinkauskas CB, Counsell CM, Lussier AM. Potential therapeutic recombinant proteins comprised of peptides containing recombined T cell epitopes. Mol Immunol. 1994;31:955–966. doi: 10.1016/0161-5890(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266. doi: 10.1016/s0065-2776(08)60586-6. [DOI] [PubMed] [Google Scholar]
- 24.Kuna P, Reddigari SR, Schall TJ, Rucinski D, Sadick M, Kaplan AP. Characterization of the human basophil response to cytokines, growth factors, and histamine releasing factors of the intercrine/chemokine family. J Immunol. 1993;150:1932–1943. [PubMed] [Google Scholar]
- 25.Santamaria-Babi LF, Picker LJ, Perez-Soler MT, Drzimalla K, Flohr P, Blaser K, Hauser C. Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med. 1995;181:1935–1940. doi: 10.1084/jem.181.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinkernagel RM, Doherty PC. H-2 compatibility requirement for T cell–mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T cell specificities are associated with structures coded for in H-2K or H-2D. J Exp Med. 1975;141:1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickling JK, Fenton CM, Howland K, Marsh SG, Rothbard JB. Peptides recognized by class I restricted T cells also bind to MHC class II molecules. Int Immunol. 1990;2:435–441. doi: 10.1093/intimm/2.5.435. [DOI] [PubMed] [Google Scholar]
- 28.Valyasevi MA, Maddox DE, Li JT. Systemic reactions to skin tests at Mayo Clinic. J Allergy Clin Immunol. 1998;101:S30. . (Abstr.) [Google Scholar]