Abstract
We report that chlamydiae, which are obligate intracellular bacterial pathogens, can inhibit interferon (IFN)-γ–inducible major histocompatibility complex (MHC) class II expression. However, the IFN-γ–induced IFN regulatory factor-1 (IRF-1) and intercellular adhesion molecule 1 (ICAM-1) expression is not affected, suggesting that chlamydia may selectively target the IFN-γ signaling pathways required for MHC class II expression. Chlamydial inhibition of MHC class II expression is correlated with degradation of upstream stimulatory factor (USF)-1, a constitutively and ubiquitously expressed transcription factor required for IFN-γ induction of class II transactivator (CIITA) but not of IRF-1 and ICAM-1. CIITA is an obligate mediator of IFN-γ–inducible MHC class II expression. Thus, diminished CIITA expression as a result of USF-1 degradation may account for the suppression of the IFN-γ–inducible MHC class II in chlamydia-infected cells. These results reveal a novel immune evasion strategy used by the intracellular bacterial pathogen chlamydia that improves our understanding of the molecular basis of pathogenesis.
Keywords: interferon γ induction, major histocompatibility complex class II, chlamydia, upstream stimulatory factor 1, protein degradation
Tlymphocyte recognition of MHC–peptide complexes on target cells is essential for mounting an antigen-specific immune attack (1), which may in turn select pathogens able to evade immune recognition by suppressing MHC expression on the infected cells (2). Many intracellular pathogens have evolved various strategies for inhibiting MHC molecule expression on infected cells to avoid T lymphocyte recognition. For example, to escape CD8+ T cell recognition, a variety of viruses are found to suppress surface expression of MHC class I on the infected cells (3–12). To escape CD4+ T cell recognition, pathogens may need to inhibit the IFN-γ–inducible MHC class II expression. This is because IFN-γ induction is often required to upregulate MHC class II molecules on nonprofessional APCs, such as epithelial cells, that are usually the natural targets of intracellular pathogens. It has been demonstrated that IFN-γ– inducible MHC class II expression is inhibited in cells infected with various intracellular pathogens (13–18), which suggests that suppression of IFN-γ–inducible MHC class II may represent an immune evasion strategy used by intracellular pathogens.
Chlamydia is an obligate intracellular bacterial pathogen (19) and the causative agent of many important human diseases (20, 21). Although specific immune responses are provoked after a chlamydial infection, persistent infection often occurs (22, 23). We have recently demonstrated that chlamydia possesses a strong antiapoptotic activity (24), which may allow chlamydiae to escape CD8+ T cell attack. However, CD4+ T cell–mediated immunity also plays very important roles in controlling many intracellular pathogen infections (25, 26). As viruses that suppress IFN-γ–inducible MHC class II expression on the infected cells can evade CD4+ T cell recognition (16), we hypothesize that chlamydia may have also evolved strategies for inhibiting IFN-γ induction of MHC class II, which may partially contribute to the persistent infection. To test this hypothesis, we evaluated the effect of chlamydial infection on IFN-γ– inducible MHC class II antigen expression. We have found that chlamydial infection can indeed suppress IFN-γ– inducible MHC class II expression by selective disruption of IFN-γ signaling pathways. We further demonstrated that a chlamydia-dependent proteasome-like activity is responsible for the chlamydial inhibitory effect. These observations reveal a novel immune evasion strategy used by the intracellular bacterial pathogen chlamydia.
Materials and Methods
Chlamydial Infection and IFN-γ Stimulation.
The following human cell lines were used: MCF-7 (a mammary epithelium line, provided by Dr. Arnold Greenberg of the Manitoba Cancer Foundation); MRC-5 (fibroblast; American Type Culture Collection [ATCC]); 2C4 (fibroblast; provided by Dr. George Stark of the Cleveland Clinic Foundation); and HeLa (cervical epithelium; ATCC). Cells were infected with Chlamydia trachomatis LGV2 strain at a multiplicity of infection (MOI)1 of 5 or as indicated and for 24 h or as indicated in individual experiments (24). Cells with or without infection were stimulated with human IFN-γ (PharMingen) at 200 U/ml or as indicated for another 10 h (for reverse transcriptase [RT]-PCR analysis) or 20–24 h (for flow cytometry and Western blot analysis).
Flow Cytometry.
Cell samples were stained with mouse anti– HLA-DRα (L243; ATCC), mouse anti–human intercellular adhesion molecule (ICAM)-1 (HA58; PharMingen), or normal mouse IgG (Zymed Labs., Inc.). Primary antibody binding was detected using goat anti–mouse IgG conjugated with FITC (Caltag Labs.) and analyzed with a FACSCalibur™ equipped with CellQuest software (Becton Dickinson). Dead cells were excluded by propidium iodine staining.
Western Blot Assay.
Western blot assay was carried out as we previously described (24). Rabbit antibodies were used to detect IFN-γR (SC-700; Santa Cruz Biotechnology), tyrosine-phosphorylated signal transducers and activators of transcription (STAT)1α (9171S; New England Biolabs, Inc.), IFN regulatory factor (IRF)-1 (SC-497), upstream stimulatory factor (USF)-1 (SC-229) and USF-2 (SC-862; all from Santa Cruz Biotechnology). Mouse antibodies were used to detect Janus tyrosine kinase (JAK)-1 (J24320; Transduction Labs.) and STAT1α (SC-464; Santa Cruz Biotechnology), HLA-DRα (DA6.147; provided by Dr. Peter Cresswell, Yale University; reference 27), and a chlamydial major outer membrane protein (MOMP; clone MC22, our unpublished data). Primary antibody binding was detected with horseradish peroxidase–conjugated goat anti–mouse IgG or –rabbit IgG, depending on the source of the primary antibodies, and visualized using an ECL kit (Amersham Corp.).
RT-PCR Assay.
Cell samples were collected for RNA extraction using the Rneasy Mini Kit from QIAGEN, Inc. 2 μg of total RNA was used for each cDNA synthesis with random primers and the 1st Strand cDNA synthesis kit from Boehringer Mannheim. Aliquots of the cDNA samples were used as a template for amplifying specific gene fragments by PCR reactions (28, 29). The primers used for amplification of DRα (18), DMα (29), invariant chain p41 (IP41) (29), and IRF-1 (18) were previously described. The other primers used in this study were: for class II transactivator (CIITA) amplification, 5′-GACACGGTGGCGCTGTGGGAGTC-3′ (forward) and 5′-GGCAGCCGTGAACTTGTTGTACTGG-3′ (reverse); for USF-1 amplification, 5′-TGGCACTGGTCAATTCTTTGTG (forward) and 5′-GTTGCTGTCATTCTTGATGAC (reverse); for STAT1 amplification, 5′-TAGAGTTGCTGAATGTCACTG-3′ (forward) and 5′-GGAGTGAAGCTCTTCAGTAAC-3′ (reverse); for indoleamine 2,3-dioxygenase (IDO) gene amplification, 5′-ATGCATCACCATGGCATA-3′ (forward) and 5′-GCTTCCCGCAGGCCAGCATCA-3′ (reverse); and for β-actin amplification, 5′-GTGGGGCGCCCCAGGCACCA-3′ (forward) and 5′-CTCCTTAATGTCACGCACGATTTC-3′ (reverse). β-actin mRNA detection was used as an internal control for the amount of cDNA synthesized. To ensure the specificity of the mRNA detection, all primers were designed to cover at least two exons, and parallel samples without RT were run as negative controls. The amplified DNA products were run on an agarose gel and visualized with ethidium bromide staining.
Results
Chlamydial Infection Inhibits IFN-γ–inducible MHC Class II Gene Expression.
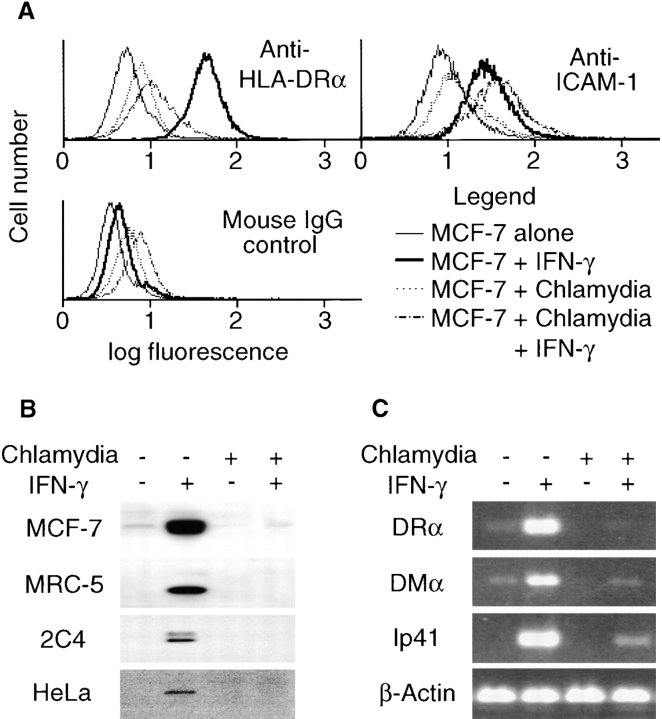
To investigate whether chlamydia possesses the ability to evade the IFN-γ–induced immune recognition mechanism, we evaluated IFN-γ–inducible MHC class II antigen expression in cells with or without chlamydial infection. IFN-γ significantly upregulated HLA-DR surface expression on uninfected cells, whereas the chlamydia- infected cells displayed a minimal level of DR, regardless of IFN-γ exposure (Fig. 1 A). However, chlamydial infection did not affect the IFN-γ–induced ICAM-1 surface expression (Fig. 1 A). These observations suggest that chlamydia selectively inhibits IFN-γ–inducible DR expression rather than preventing all IFN-γ dependent signaling or generally suppressing surface protein expression. Furthermore, the total cellular protein level of IFN-γ–induced HLA-DRα was also significantly diminished in chlamydia-infected cells as compared with uninfected cells (Fig. 1 B), suggesting that the suppression of surface expression of HLA-DR was not due to an alteration in intracellular trafficking. The chlamydial inhibition of IFN-γ–inducible HLA-DRα was reproduced in many other human cell lines, including HeLa, MRC-5, and 2C4 (Fig. 1 B), demonstrating that the inhibitory effect is not a cell line–specific phenomenon. To determine whether the chlamydial inhibition of HLA-DR expression occurs at the transcription or translation level, MHC class II mRNA levels were evaluated by semiquantitative RT-PCR. IFN-γ dramatically induced the expression of DRα, DMα, and Ip41 mRNA in the uninfected but not the infected cells (Fig. 1 C), suggesting that chlamydial inhibition of MHC class II occurred at the transcription level. Because the genes encoding the MHC class II presentation–related molecules DRα, DMα, and Ip41 share similar promoter structures and CIITA is a master regulator for the expression of these genes (30), we hypothesize that chlamydia may inhibit CIITA function or CIITA gene expression.
Figure 1.
Chlamydial infection selectively inhibits IFN-γ–inducible MHC class II expression. MCF-7 cells with or without chlamydial infection were stimulated with IFN-γ and collected for flow cytometry (A), Western blot (B), and RT-PCR (C) analysis. (A) Chlamydial infection prevents IFN-γ–inducible HLA-DR but not ICAM-1 surface expression. (B) Chlamydial infection suppresses the total cellular protein level of IFN-γ–inducible HLA-DRα in various human cell lines. MRC-5, 2C4, and HeLa cells were stimulated with IFN-γ at 400 U/ml. HLA-DRα was detected in a Western blot assay. (C) Chlamydial infection inhibits HLA-DRα, DMα, and Ip41 mRNA expression. Gene-specific primers were used to amplify random primer–directed cDNA templates in an RT-PCR reaction as described in Materials and Methods.
IFN-γ–inducible CIITA Expression Is Inhibited in Chlamydia-infected Cells.
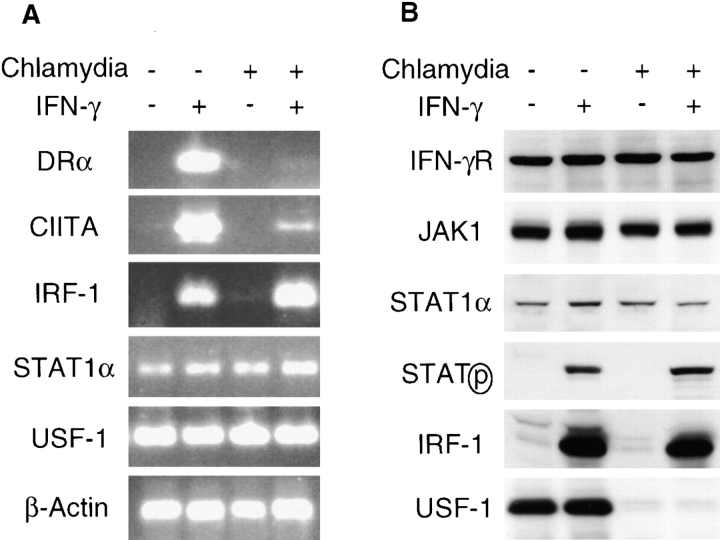
Although CIITA is constitutively expressed in professional APCs, such as dendritic cells and B cells, IFN-γ stimulation is often required for the expression of CIITA in nonprofessional APCs, such as epithelial cells (31). CIITA mRNA expression was induced by IFN-γ in uninfected MCF-7 cells (Fig. 2 A). However, CIITA mRNA expression was significantly lower in chlamydia- infected and IFN-γ–treated cells (Fig. 2 A), in accord with chlamydial inhibition of MHC class II gene expression. To investigate how CIITA gene expression was inhibited, we measured mRNA levels for three transcription factors, USF-1, STAT1, and IRF-1, all of which are required for IFN-γ–inducible transcription of the CIITA gene (32). Both USF-1 and STAT1 mRNAs were constitutively expressed, and IRF-1 mRNA was induced by IFN-γ in MCF-7 cells regardless of chlamydial infection (Fig. 2 A), suggesting that chlamydial infection did not affect transcription of the genes for these nuclear factors. Because the three transcription factors are considered to be sufficient and necessary for the IFN-γ induction of CIITA (32), we evaluated the protein levels of these transcription factors as well as upstream molecules in the IFN-γ signaling pathway. We found that IFN-γR, JAK-1, and STAT1 protein levels were not altered by chlamydial infection (Fig. 2 B). Chlamydia did not affect IFN-γ–induced STAT1 tyrosine phosphorylation (Fig. 2 B). Furthermore, IFN-γ induced both IRF-1 and ICAM-1 expression in chlamydia-infected cells (Figs. 1 A and 2 B). As STAT1 is required for the expression of both IRF-1 and ICAM-1 genes (32, 33), we conclude that STAT1 is transcriptionally functional in chlamydia-infected cells. The IFN-γ–induced IRF-1 in chlamydia-infected cells may also be transcriptionally functional, as we found that IFN-γ induced expression of IDO gene in chlamydia-infected cells (data not shown), and it is known that IRF-1 is required for IFN-γ induction of IDO (34). Therefore, the failure of the IFN-γ–inducible CIITA expression in chlamydia-infected cells is likely due to the deficiency in USF-1. We found that the USF-1 protein was not detectable in chlamydia-infected cells (Fig. 2 B), despite normal USF-1 mRNA expression (Fig. 2 A). We next determined the cause of the USF-1 protein loss.
Figure 2.
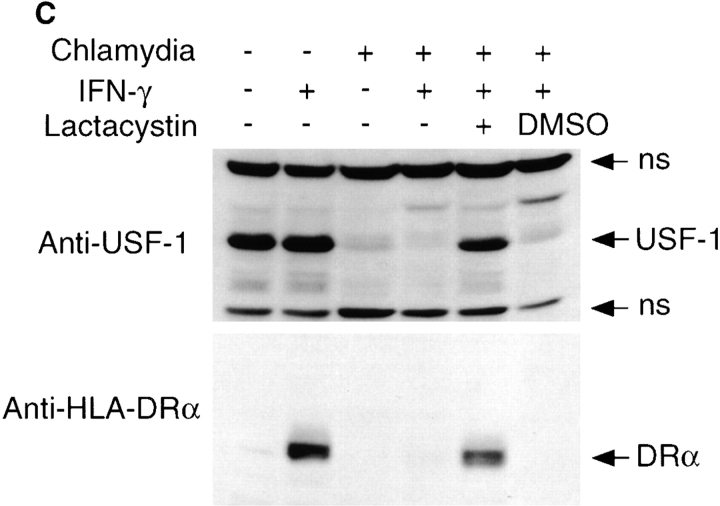
Chlamydial infection disrupts IFN-γ intracellular signaling pathways. (A) Chlamydial infection suppresses both HLA-DRα and CIITA but not IRF-1, STAT1α, or USF-1 mRNA expression in MCF-7 cells. The mRNA levels were analyzed by RT-PCR as described in Materials and Methods. (B) USF-1 protein is not detected in chlamydia-infected MCF-7 cells. The protein levels of IFN-γR, JAK-1, STAT1α, tyrosine-phosphorylated STAT1α, IRF-1, and USF-1 were detected using a Western blot assay. (C) Lactacystin prevents USF-1 degradation and preserves HLA-DRα expression in chlamydia-infected MCF-7 cells. 10 h before IFN-γ stimulation, a chlamydia-infected cell sample was treated with lactacystin (Calbiochem) at a final concentration of 75 μM or with an equivalent amount of solvent DMSO and kept in culture during IFN-γ stimulation. HLA-DRα and USF-1 were detected in a Western blot assay. ns, nonspecific binding.
USF-1 Is Degraded by a Proteasome-like Activity in Chlamydia-infected Cells.
Because USF-1 mRNA is expressed in chlamydia-infected cells with or without IFN-γ stimulation, the lack of USF-1 protein may be due to either the inhibition of translation of USF-1 mRNA or the accelerated degradation of USF-1 protein. We tested the protein degradation hypothesis by using protease inhibitors. We found that the proteasome inhibitor lactacystin prevented USF-1 protein degradation in chlamydia-infected cells (Fig. 2 C). Furthermore, the lactacystin treatment also preserved the IFN-γ–inducible HLA-DR expression in chlamydia-infected cells (Fig. 2 C). These observations not only demonstrate that a proteasome-like activity is responsible for the loss of USF-1 protein in chlamydia-infected cells but also suggest that USF-1 degradation may be responsible for the chlamydial suppression of MHC class II expression.
Both USF-1 Degradation and MHC Class II Suppression in Chlamydia-infected Cells Are Dependent on Chlamydial but not Host Protein Synthesis.
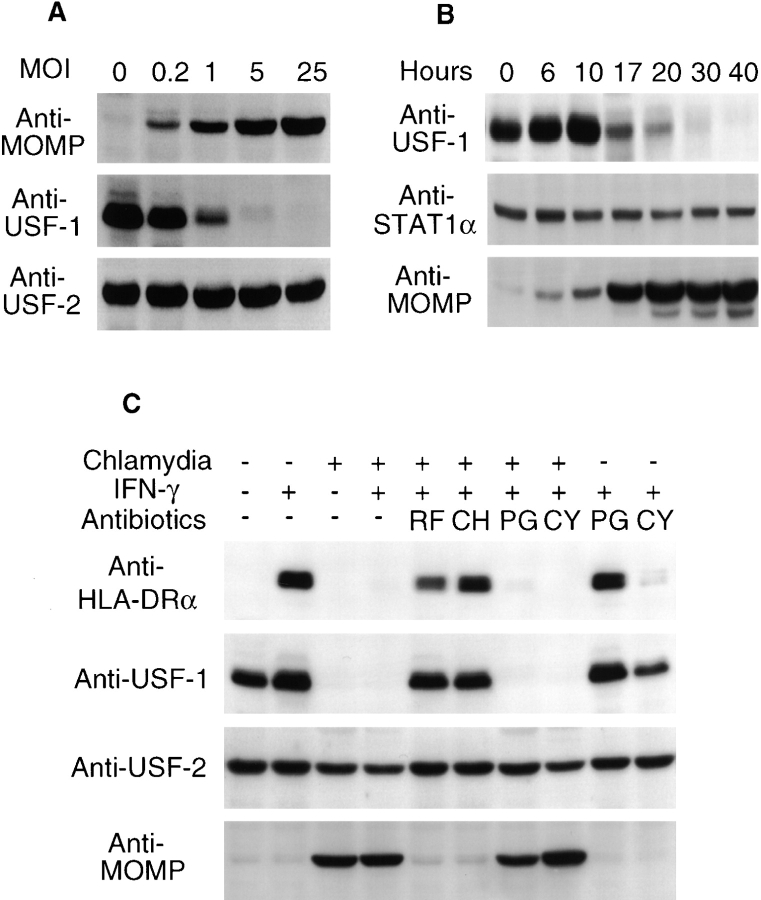
To examine whether the USF-1 protein degradation in chlamydia-infected cells is dependent on chlamydial growth and protein synthesis, we first evaluated the relationship between the chlamydial infection dose and USF-1 degradation. As MOI (ratio of number of organisms versus number of host cells) increased, more chlamydial protein was produced and less USF-1 protein was detected (Fig. 3 A). This effect was selective, as USF-2 was not degraded, regardless of the infection dose (Fig. 3 A). The time course relationship between chlamydial growth and USF-1 degradation was also analyzed (Fig. 3 B). Although the STAT1 protein level was not affected by chlamydial infection at any time points examined, significant USF-1 degradation was detected 17 h after chlamydial infection, when chlamydial protein synthesis approached its maximum (Fig. 3 B). The role of chlamydial and host protein synthesis in USF-1 degradation was examined using antibiotics specifically inhibiting either prokaryotic or eukaryotic protein synthesis. We found that both rifampin (inhibiting prokaryotic transcription) and chloramphenicol (inhibiting prokaryotic translation) blocked chlamydial protein synthesis (Fig. 3 C). More importantly, these antibiotics also prevented USF-1 degradation and preserved HLA-DRα expression in chlamydia-infected cells (Fig. 3 C). However, treatment with penicillin failed to prevent USF-1 degradation and preserve HLA-DRα expression (Fig. 3 C). Penicillin only blocks chlamydial particle assembly without inhibiting chlamydial protein synthesis (35). Penicillin did not alter the constitutively expressed USF-1 protein level and IFN-γ–inducible HLA-DRα expression in uninfected cells (Fig. 3 C). Together, these observations demonstrate that chlamydial protein synthesis, but not particle assembly, is necessary for chlamydia-induced degradation of USF-1 protein and suppression of HLA-DRα expression. Finally, cycloheximide treatment did not affect the chlamydia- induced degradation of USF-1 (Fig. 3 C). Because cycloheximide did not affect chlamydial protein synthesis but completely inhibited new protein synthesis by the host cell, for example, production of IFN-γ–induced HLA-DRα (Fig. 3 C), we conclude that newly synthesized host proteins are not required for chlamydia-induced degradation of USF-1.
Figure 3.
Chlamydial protein synthesis is required for both USF-1 degradation and inhibition of HLA-DRα expression. (A) Correlation between infection dose and USF-1 degradation. 24 h after chlamydial infection at various MOI, MCF-7 cells were analyzed for chlamydial MOMP and host USF-1 and USF-2 protein levels in a Western blot assay. As the USF-2 protein level was not altered by chlamydial infection, it served as an internal control. (B) Time course relationship between chlamydial growth and USF-1 degradation. At various time points after infection, MCF-7 cells were lysed for the detection of USF-1, STAT1α, and MOMP in a Western blot assay. (C) Inhibition of chlamydial but not host protein synthesis prevents USF-1 degradation and preserves HLA-DRα expression. Rifampin (RF; final concentration, 0.1 μg/ml), chloramphenicol (CH; 60 μg/ml), and penicillin (PG; 100 μg/ml) were added at the beginning of chlamydial infection and maintained throughout the culture. Cycloheximide (CY; 10 μg/ml) was added to the culture 10 h before IFN-γ treatment and maintained during the IFN-γ stimulation. The MCF-7 cells were analyzed for protein levels of HLA-DRα, USF-1, USF-2, and chlamydial MOMP in a Western blot assay.
Discussion
We have demonstrated that the obligate intracellular bacterial pathogen chlamydia can inhibit IFN-γ–inducible MHC class II expression. This inhibitory effect has also been found with other intracellular pathogens, including leishmania (13), listeria (14), cowdria (15), and cytomegalovirus (16–18). CD4+ T cell–mediated immunity plays an important role in host defense against various intracellular infections (36–38). Recognition of the infected cells by CD4+ T cells often requires IFN-γ induction of MHC class II expression, because many pathogen-targeted cells, such as epithelial cells, are generally MHC class II–negative. Suppression of IFN-γ–inducible MHC class II expression may represent an efficient immune evasion strategy used by intracellular pathogens to escape host defenses. Thus, chlamydial inhibition of IFN-γ–inducible MHC class II may contribute to the persistent infection caused by chlamydia in humans (22).
It has been demonstrated that cytomegalovirus can prevent IFN-γ–inducible class II expression in infected cells by both IFN-β–mediated inhibition (17, 39) and disruption of IFN-γ intracellular signaling pathways (16, 18). However, mechanisms of IFN-γ–inducible MHC class II inhibition by many other intracellular pathogens are still not clear (13–15). It was recently proposed that chlamydia may suppress IFN-γ–inducible MHC class II expression by stimulating host cells to release IFN-β (40). Here we show that the intracellular bacterial pathogen chlamydia has evolved a more specific mechanism for disrupting IFN-γ signaling pathways and inhibiting MHC class II expression. Chlamydia degrades USF-1, a downstream transcription factor required for IFN-γ–inducible MHC class II but not IRF-1 and ICAM-1 expression (Fig. 4). USF-1 degradation may represent an efficient means of interrupting IFN-γ– inducible MHC class II expression by chlamydia. Although the constitutively and ubiquitously expressed USF-1 is a member of the basic helix-loop-helix family consisting of multiple transcription factors, including Myc and USF-2, only USF-1 is both necessary and sufficient for binding to the E box within the CIITA promoter IV and cooperating with STAT1 and IRF-1 for promoting transcription of CIITA (32). Therefore, the constitutively and ubiquitously expressed USF-1 may serve as a convenient and efficient target for chlamydia-induced degradation. The correlation between the degradation of USF-1 and the suppression of IFN-γ–inducible MHC class II further confirms that USF-1 plays a critical role in IFN-γ induction of MHC class II (32). Besides its involvement in MHC class II expression, USF-1 also participates in many other cellular activities, including promoting the transcription of fatty acid synthase in response to insulin regulation (41), interfering with Ras transformation (42), and transactivating the promoter of the p53 tumor suppressor gene (43). Depletion of USF-1 may cause inhibition of host cell lipid biosynthesis and promotion of host cell survival, both of which are likely beneficial to the intracellular chlamydia organisms.
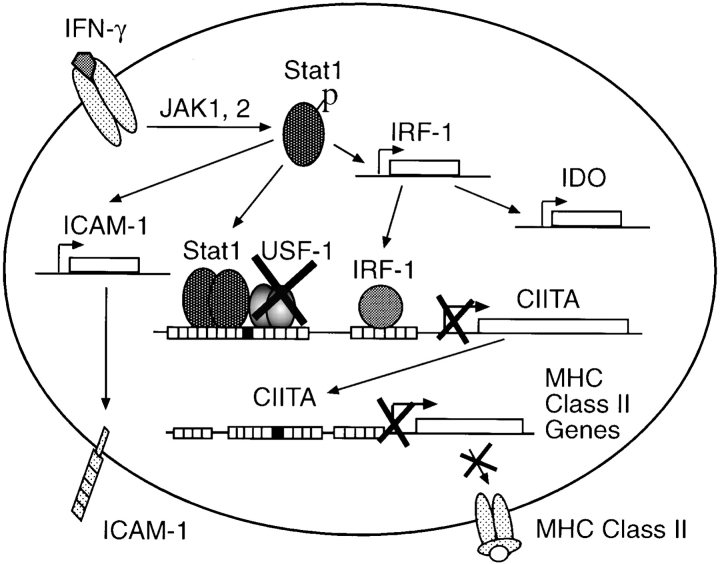
Figure 4.
Model for chlamydial inhibition of IFN-γ–inducible MHC class II expression. IFN-γ binding activates intracellular JAK/STAT pathways. USF-1 is a constitutively and ubiquitously expressed downstream transcription factor required for IFN-γ–inducible CIITA expression. Chlamydial infection degrades USF-1, which results in diminished expression of CIITA in chlamydia-infected cells. As CIITA is an obligate mediator for transcription of MHC class II genes, chlamydial degradation of USF-1 finally leads to suppression of IFN-γ–inducible MHC class II. However, because the upstream pathways of IFN-γ signaling are still intact in chlamydia-infected cells, expression of IRF-1, ICAM-1, and IDO is not affected by chlamydial infection.
Proteolysis is an important aspect of normal cellular physiology (44–46). Many viruses can take advantage of host proteolysis for the purposes of evading host defenses (2, 47). For example, human cytomegalovirus infection can induce degradation of JAK-1, a critical upstream kinase required for IFN-γ JAK/STAT signaling pathways, to suppress IFN-γ–inducible MHC class II on the infected cells (18). Furthermore, the cytomegalovirus-induced degradation can be inhibited by the proteasome inhibitor Z-L3VS (18, 48), suggesting that cytomegalovirus may be able to manipulate host proteasome activity. Because USF-1 degradation by chlamydia is inhibitable by lactacystin and lactacystin is a potent proteasome inhibitor (48, 49), we propose that chlamydia may also produce a factor(s) for manipulating host proteasomes. Efforts to identify the chlamydial factor(s) are underway.
Acknowledgments
We thank Dr. Ronald N. Germain for helpful discussions and critically reading the manuscript and Drs. Peter Cresswell, George Stark, and Arnold Greenberg for providing useful reagents for this work.
This work was supported by the Medical Research Council (MRC) of Canada. G. Zhong is the recipient of an MRC scholarship.
Abbreviations used in this paper
- CIITA
class II transactivator
- IDO
indoleamine 2,3-dioxygenase
- IRF
interferon regulatory factor
- JAK
Janus tyrosine kinase
- MOI
multiplicity of infection
- MOMP
major outer membrane protein
- RT
reverse transcriptase
- STAT
signal transducers and activators of transcription
- USF
upstream stimulatory factor
References
- 1.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 3.Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, Peterson PA, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 4.Hengel H, Flohr T, Hammerling GJ, Koszinowski UH, Momburg F. Human cytomegalovirus inhibits peptide translocation into the endoplasmic reticulum for MHC class I assembly. J Gen Virol. 1996;77:2287–2296. doi: 10.1099/0022-1317-77-9-2287. [DOI] [PubMed] [Google Scholar]
- 5.Lehner PJ, Karttunen JT, Wilkinson GW, Cresswell P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc Natl Acad Sci USA. 1997;94:6904–6909. doi: 10.1073/pnas.94.13.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones TR, Sun L. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J Virol. 1997;71:2970–2979. doi: 10.1128/jvi.71.4.2970-2979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machold RP, Wiertz EJ, Jones TR, Ploegh HL. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schust DJ, Tortorella D, Seebach J, Phan C, Ploegh HL. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J Exp Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 11.Hughes EA, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci USA. 1997;94:1896–1901. doi: 10.1073/pnas.94.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 13.Reiner NE, Ng W, Ma T, McMaster WR. Kinetics of gamma interferon binding and induction of major histocompatibility complex class II mRNA in Leishmania- infected macrophages. Proc Natl Acad Sci USA. 1988;85:4330–4334. doi: 10.1073/pnas.85.12.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuller S, Kugler S, Goebel W. Suppression of major histocompatibility complex class I and class II gene expression in Listeria monocytogenes-infected murine macrophages. FEMS Immunol Med Microbiol. 1998;20:289–299. doi: 10.1111/j.1574-695X.1998.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 15.Vachiery N, Trap I, Totte P, Martinez D, Bensaid A. Inhibition of MHC class I and class II cell surface expression on bovine endothelial cells upon infection with Cowdria ruminantium. . Vet Immunol Immunopathol. 1998;61:37–48. doi: 10.1016/s0165-2427(97)00129-3. [DOI] [PubMed] [Google Scholar]
- 16.Heise MT, Connick M, Virgin HWT. Murine cytomegalovirus inhibits interferon γ–induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II–associated genes. J Exp Med. 1998;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise MT, Pollock JL, O'Guin A, Barkon ML, Bormley S, Virgin HWT. Murine cytomegalovirus infection inhibits IFN-γ-induced MHC class II expression on macrophages: the role of type I interferon. Virology. 1998;241:331–344. doi: 10.1006/viro.1997.8969. [DOI] [PubMed] [Google Scholar]
- 18.Miller DM, Rahill BM, Boss JM, Lairmore MD, Durbin JE, Waldman JW, Sedmak DD. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grayston JT, Kuo CC, Campbell LA, Wang SP, Jackson LA. Chlamydia pneumoniae and cardiovascular disease. Cardiologia. 1997;42:1145–1151. [PubMed] [Google Scholar]
- 21.Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Beatty WL, Morrison RP, Byrne GI. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland MJ, Bailey RL, Conway DJ, Culley F, Miranpuri G, Byrne GI, Whittle HC, Mabey DC. T helper type-1 (Th1)/Th2 profiles of peripheral blood mononuclear cells (PBMC); responses to antigens of Chlamydia trachomatisin subjects with severe trachomatous scarring. Clin Exp Immunol. 1996;105:429–435. doi: 10.1046/j.1365-2249.1996.d01-792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geginat G, Lalic M, Kretschmar M, Goebel W, Hof H, Palm D, Bubert A. Th1 cells specific for a secreted protein of Listeria monocytogenesare protective in vivo. J Immunol. 1998;160:6046–6055. [PubMed] [Google Scholar]
- 26.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatisis mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 27.Arunachalam B, Pan M, Cresswell P. Intracellular formation and cell surface expression of a complex of an intact lysosomal protein and MHC class II molecules. J Immunol. 1998;160:5797–5806. [PubMed] [Google Scholar]
- 28.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albanesi C, Cavani A, Girolomoni G. Interferon-γ-stimulated human keratinocytes express the genes necessary for the production of peptide-loaded MHC class II molecules. J Invest Dermatol. 1998;110:138–142. doi: 10.1046/j.1523-1747.1998.00098.x. [DOI] [PubMed] [Google Scholar]
- 30.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 31.Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO (Eur Mol Biol Organ) J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B. Activation of the MHC class II transactivator CIITA by interferon-γ requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 33.Walter MJ, Look DC, Tidwell RM, Roswit WT, Holtzman MJ. Targeted inhibition of interferon-γ-dependent intercellular adhesion molecule-1 (ICAM-1) expression using dominant-negative Stat1. J Biol Chem. 1997;272:28582–28589. doi: 10.1074/jbc.272.45.28582. [DOI] [PubMed] [Google Scholar]
- 34.Chon SY, Hassanain HH, Pine R, Gupta SL. Involvement of two regulatory elements in interferon-gamma-regulated expression of human indoleamine 2,3- dioxygenase gene. J Interferon Cytokine Res. 1995;15:517–526. doi: 10.1089/jir.1995.15.517. [DOI] [PubMed] [Google Scholar]
- 35.Barbour AG, Amano K, Hackstadt T, Perry L, Caldwell HD. Chlamydia trachomatishas penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982;151:420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakhmilevich AL. Evidence for a significant role of CD4+ T cells in adoptive immunity to Listeria monocytogenesin the liver. Immunology. 1994;82:249–254. [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 38.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedmak DD, Chaiwiriyakul S, Knight DA, Waldmann WJ. The role of interferon β in human cytomegalovirus-mediated inhibition of HLA DR induction on endothelial cells. Arch Virol. 1995;140:111–126. doi: 10.1007/BF01309727. [DOI] [PubMed] [Google Scholar]
- 40.Rodel J, Groh A, Vogelsang H, Lehmann M, Hartmann M, Straube E. Beta interferon is produced by Chlamydia trachomatis-infected fibroblast-like synoviocytes and inhibits gamma interferon-induced HLA-DR expression. Infect Immun. 1998;66:4491–4495. doi: 10.1128/iai.66.9.4491-4495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Sul SH. Upstream stimulatory factor binding to the E-box at −65 is required for insulin regulation of the fatty acid synthase promoter. J Biol Chem. 1997;272:26367–26374. doi: 10.1074/jbc.272.42.26367. [DOI] [PubMed] [Google Scholar]
- 42.Aperlo C, Boulukos EK, Pognonec P. The basic region/helix-loop-helix/leucine repeat transcription factor USF interferes with Ras transformation. Eur J Biochem. 1996;241:249–253. doi: 10.1111/j.1432-1033.1996.0249t.x. [DOI] [PubMed] [Google Scholar]
- 43.Reisman D, Rotter V. The helix-loop-helix containing transcription factor USF binds to and transactivates the promoter of the p53 tumor suppressor gene. Nucleic Acids Res. 1993;21:345–350. doi: 10.1093/nar/21.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lupas A, Flanagan JM, Tamura T, Baumeister W. Self-compartmentalizing proteases. Trends Biochem Sci. 1997;22:399–404. doi: 10.1016/s0968-0004(97)01117-1. [DOI] [PubMed] [Google Scholar]
- 45.Glas R, Bogyo M, McMaster JS, Gaczynska M, Ploegh HL. A proteolytic system that compensates for loss of proteasome function. Nature. 1998;392:618–622. doi: 10.1038/33443. [DOI] [PubMed] [Google Scholar]
- 46.Gottesman S, Maurizi MR, Wickner S. Regulatory subunits of energy-dependent proteases. Cell. 1997;91:435–438. doi: 10.1016/s0092-8674(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 47.Ploegh HL. Destruction of proteins as a creative force. Immunol Today. 1997;18:269–271. doi: 10.1016/s0167-5699(97)80021-7. [DOI] [PubMed] [Google Scholar]
- 48.Bogyo M, Shin S, McMaster JS, Ploegh HL. Substrate binding and sequence preference of the proteasome revealed by active-site-directed affinity probes. Chem Biol. 1998;5:307–320. doi: 10.1016/s1074-5521(98)90169-7. [DOI] [PubMed] [Google Scholar]
- 49.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]