Abstract
The Kaposi's sarcoma–related herpesvirus (KSHV), also designated human herpesvirus 8, is the presumed etiologic agent of Kaposi's sarcoma and certain lymphomas. Although KSHV encodes several chemokine homologues (viral macrophage inflammatory protein [vMIP]-I, -II, and -III), only vMIP-II has been functionally characterized. We report here that vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8 that is preferentially expressed on Th2 T cells. Y3 cells transfected with CCR8 produced a calcium flux in response to vMIP-I and responded vigorously in in vitro chemotaxis assays. In competition binding experiments, the interaction of vMIP-I with CCR8 was shown to be specific and of high affinity. In contrast to its agonist activity at CCR8, vMIP-I did not interact with CCR5 or any of 11 other receptors examined. Furthermore, vMIP-I was unable to inhibit CCR5-mediated HIV infection. These findings suggest that expression of vMIP-I by KSHV may influence the Th1/Th2 balance of the host immune response.
Keywords: chemokines, cell trafficking, T helper cell type 1/T helper cell type 2, AIDS/HIV, inflammation
Kaposi's sarcoma–associated herpesvirus (KSHV), also designated human herpesvirus (HHV)-8, is a gammaherpesvirus linked to the etiology of Kaposi's sarcoma (1). KSHV has also been suggested to play a role in the pathology of primary effusion lymphoma (2, 3). The genome of KSHV has been shown to encode several chemokine-related proteins, including a constitutively active chemokine receptor and three viral chemokines, viral macrophage inflammatory protein (vMIP)-I, vMIP-II, and vMIP-III, all belonging to the CC or β family (4–7). Although two of these chemokines (vMIP-I and vMIP-II) bear a high degree of identity to one another (50% at the amino acid level; reference 4), only vMIP-II has been characterized to any significant extent. Thus, our understanding of the role that these chemokine-related genes play in viral biology is incomplete.
There is no direct demonstration of vMIP-I interacting with any receptor; however, Moore et al. reported that transient expression of vMIP-I and CCR5 in CD4+ cat kidney cells can block HIV infection (4), suggesting that vMIP-I might interact with this receptor. In contrast to these findings, a more recent report found no significant effect of exogenously added vMIP-I on HIV infection mediated by CCR3, CCR5, or CXCR4 (8). However, vMIP-I did inhibit macrophage-tropic HIV infection of PBMCs, suggesting the possibility that another chemokine receptor might mediate HIV entry into these cells (8).
Materials and Methods
Cell Lines.
CCR2, CCR6, CCR7, and CCR9 were stably expressed in murine BaF/3 cells (9) using pME18S-neo (CCR2, 6, 7) or a murine retroviral system (CCR9 [reference 10]), whereas CCR3 (11), CCR8, and HCR/L-CCR (12, 13) were stably expressed in rat Y3 cells using either pME18S-neo (CCR3, CCR8) or the murine retroviral system (HCR/L-CCR). CCR5, XCR1, GPR9-6, and STRL33 were stably expressed in human embryonic kidney (HEK) 293 cells using either pcDNA3.1 or pcDNA3.1/zeo(+) (Invitrogen). CXCR4 was analyzed as an endogenously expressed receptor present in the BaF/3 pre-B cell line. All lines were maintained in appropriate culture medium (RPMI or DMEM/10% FCS/10 ng/ml IL-3 for BaF/3 cells). Media for transfected cell lines also contained G418 (1 mg/ml) or zeocin (0.25 mg/ml; GIBCO BRL) and were periodically tested for their ability to flux calcium in response to known ligands.
Calcium Flux Assays.
BaF/3 cells were loaded with Fluo-3-AM (Sigma Chemical Co.) in appropriate culture medium (RPMI or DMEM/10% serum) for 1 h at 37°C, after which cells were washed three times in flux buffer (HBSS/20 mM Hepes/0.1% BSA) and aliquoted into a 96-well black-wall plates at a density of 105 cells/well. HEK 293 and Y3 cells were plated at a density of 5 × 104 cells/well 1 d in advance of assaying, loaded for 1 h in culture medium as above and washed four times. All plates were pre-coated with poly-l-lysine. Calcium flux was measured in all 96 wells simultaneously and in real time using a Fluorescent Imaging Plate Reader (FLIPR; Molecular Devices) and data was expressed as fluorescence units versus time. Chemokines were obtained commercially (R&D Systems or Peprotech) or produced by DNAX/Schering-Plough.
Assays for Chemotaxis and HIV Infection.
Chemotaxis was assayed in a 48-well microchamber (Neuroprobe) as previously described (14) using polycarbonate porous membranes (5-μm pore size). Assays were conducted over a 1-h period and cells were counted in an automated fashion on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/). Five high power (400×) fields were counted for each of the duplicate wells at a stated concentration.
For HIV infection assays, pseudotyped virus was used in a single cycle infection assay and infectivity was monitored as a measure of luciferase activity according to previously published methods (15). Pseudotyped HIV-1 stock was prepared by cotransfecting HEK 293T cells with the envelope-deficient pNL4-3-luc-E−R− construct and a plasmid encoding the ADA envelope glycoprotein (pADAenv). 48–72 h after transfection, media was harvested, filtered (0.22 μm), aliquoted, and stored at −80°C. Pseudotyped virus was added to HEK 293T cells that were transiently transfected with CD4 alone or CD4 and CCR5. After a 4-d incubation at 37°C, cells were washed with PBS and lysed in reporter lysis buffer (Promega). Lysates were assayed for luciferase activity according to the instructions of the manufacturer. For inhibition, chemokines were added at 100 nM at the same time as virus.
I-309/CCR8 Binding Assay.
CCR8-Y3 cells (1D-21, described above) were resuspended in binding buffer (125 mM NaCl, 25 mM Hepes, 1 mM CaCl2, 5 mM MgCl2, and 0.5% BSA, pH 7.0; 200,000 cells in 200 μl) with 0.1 nM 125I-labeled I-309 (100,000 cpm). Unlabeled vMip-I and I-309 were included as competitors where indicated. Reactions were incubated at room temperature for 3–5 h, harvested (Unifilter-96 Harvester; Packard Instrument Co.) onto 96-well GF/C filter plates (Packard Instrument Co.), and washed with 4°C binding buffer containing 500 mM NaCl. The filter plates were dried at room temperature overnight, scintillation cocktail (Microscint-0; Packard Instrument Co.) was added, and plates were counted (Topcount HTS; Packard Instrument Co.). Data was analyzed by nonlinear regression (GraphPad Prism; GraphPad Software, Inc.) and is expressed as the average of triplicates (± SD).
Results
Identification of CCR8 as a Specific Host-encoded Receptor for vMIP-I.
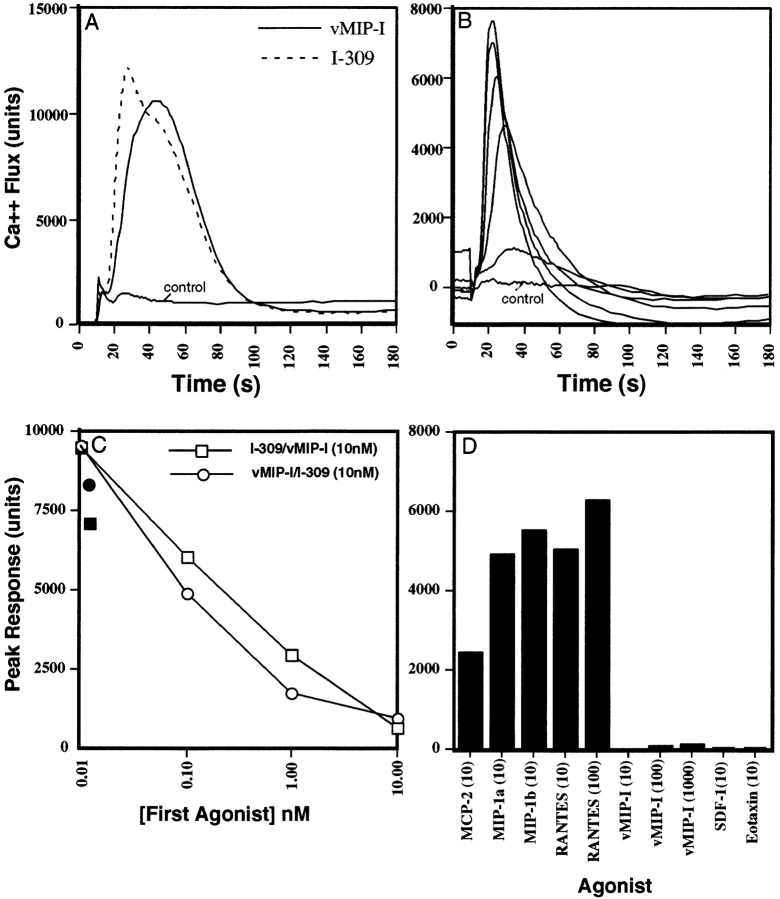
To identify a host-encoded receptor(s) for vMIP-I, we screened cell lines stably transfected with known or suspected chemokine receptors for calcium flux in response to vMIP-I and other chemokines. Among these cell lines we found only CCR8-Y3 cells to be highly responsive to vMIP-I (Fig. 1 A). These same cells also responded to I-309 (Fig. 1 A), a confirmed human ligand for CCR8 (16–19), but not to any of 39 other chemokines tested (see legend to Fig. 1). The calcium response to vMIP-I was dose dependent and observable at picomolar concentrations (Fig. 2 B). Prior incubation of CCR8-Y3 cells with vMIP-I also decreased subsequent signaling to I-309 in a dose-dependent manner (Fig. 2 C). Similarly, I-309 stimulation reduced subsequent signaling to vMIP-I (Fig. 2 C). At the lowest dose examined (0.01 nM first agonist), a slight enhancement of signaling was observed when the second agonist was added. 12 other receptors tested (CCR2, CCR3, CCR5, CCR6, CCR7, CCR9, CXCR3, CXCR4, XCR1, GPR9-6, STRL33, and HCR/L-CCR) failed to respond to either vMIP-I or I-309 with a calcium flux (data not shown).
Figure 1.
Calcium flux of CCR8-Y3 and CCR5-293 cells in response to various chemokines. (A) Various chemokines were used at concentrations ranging from 10 to 50 nM to induce a calcium flux in Y3-CCR8 cells. Chemokines tested included MCP-1, MCP-2, MCP-3, MCP-4, mMCP-5, eotaxin, MIP-1α, MIP-1β, DC-CK-1, RANTES, HCC-1, mMIP-1γ, m6Ckine, BCA-1, MIP-3α, MIP-3β, 6Ckine, fractalkine (soluble domain), TARC, MDC, MIP-4, mC10, I-309, TECK, Mig, IP-10, vMIP-I, SDF-1α, SDF-1β, IL-8, mJE, Gro-α, ENA-78, mTECK, mLptn, NAP-2, mGCP-2, mLIX, and mMIP-2. Only positive responses are shown as calcium flux (units) versus time and a vehicle control is indicated. (B) Dose–response of CCR8-Y3 cells to vMIP-I. CCR8-Y3 cells were stimulated with vMIP-I in a range of 1 μM–100 pM. A vehicle control is indicated. Each curve shown is the average of duplicate wells for each dose (SD did not exceed 10% of peak height). (C) Dose-dependent heterologous desensitization of I-309/vMIP-I signaling. CCR8-Y3 cells were exposed to increasing concentrations of an initial agonist, either I-309 (□) or vMIP-I (○), for 3 min before a second stimulation with the indicated chemokine at 10 nM. Preincubation in vehicle alone is indicated (vehicle/I-309, •; vehicle/vMIP-I, ▪). Results are graphed as peak response versus concentration of initial agonist. (D) The response of CCR5-293 cells to a panel of chemokines. The peak response of CCR5-293 cells to various chemokines is shown. The panel used was identical to that used in A except for the addition of 100 nM RANTES. In addition, vMIP-I was tested at 1 μM, 100 nM, and 10 nM. Only those chemokines producing a response and a few selected negative controls are shown.
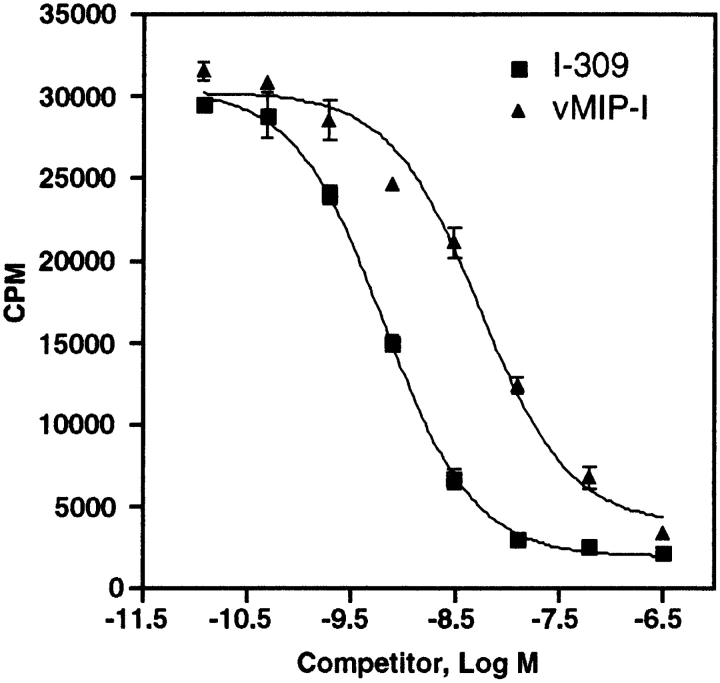
Figure 2.
vMIP-I inhibits 125I-labeled I-309 binding to CCR8. Competition binding of recombinant I-309 (squares) or vMIP-I (triangles) was assessed on CCR8-Y3 intact cells. K d for I-309 = 0.65 ± 0.17 nM (n = 2) and K i for vMIP-I = 4.68 ± 0.44 nM (n = 2). Results are expressed as total counts versus log concentration of competitor.
To characterize more fully the interaction between vMIP-I and CCR8, we examined the ability of vMIP-I to compete for 125I-labeled I-309 binding to CCR8-Y3 cells. As shown in Fig. 2, vMIP-I competed successfully for I-309 binding to CCR8-Y3 cells with a K i of 4.68 ± 0.44 nM, which was somewhat higher (sevenfold) than the K i observed for I-309 binding (0.65 ± 0.17 nM). In saturation binding experiments, I-309 bound to CCR8-Y3 cells with a K d of 0.40 ± 0.23 nM (n = 5, data not shown). Interestingly the EC50 for CCR8-Y3 cell calcium response was roughly equivalent for vMIP-I and I-309 stimulation (∼3.7 nM).
vMIP-I Does Not Antagonize CCR5 or CXCR4 Signaling.
Since previous reports have suggested that vMIP-I might interact with CCR5 (4), we examined CCR5–HEK 293 cells for a response to vMIP-I. These cells did not flux calcium in response to vMIP-I, SDF-1α, or eotaxin, but were responsive to monocyte chemoattractant protein (MCP)-2, MIP-1α, MIP-1β, and RANTES (Fig. 1 D). vMIP-II has been suggested to act as an agonist for CCR3 (8), yet antagonizes other receptors, including CCR5 (20). Therefore, we examined whether vMIP-I could antagonize CCR5 signaling in response to its natural ligands. Prior incubation of HEK 293–CCR5 cells with vMIP-I was unable to antagonize subsequent responses to any of the CCR5 ligands tested, even when present at 100-fold excess amounts (data not shown). In addition, preincubation of the other available receptor cell lines (as above) with vMIP-I failed to inhibit subsequent signaling of these receptors in response to their known ligands (data not shown). These data suggest that, unlike vMIP-II, vMIP-I is not a broad-spectrum chemokine antagonist.
Biological Activity of vMIP-I.
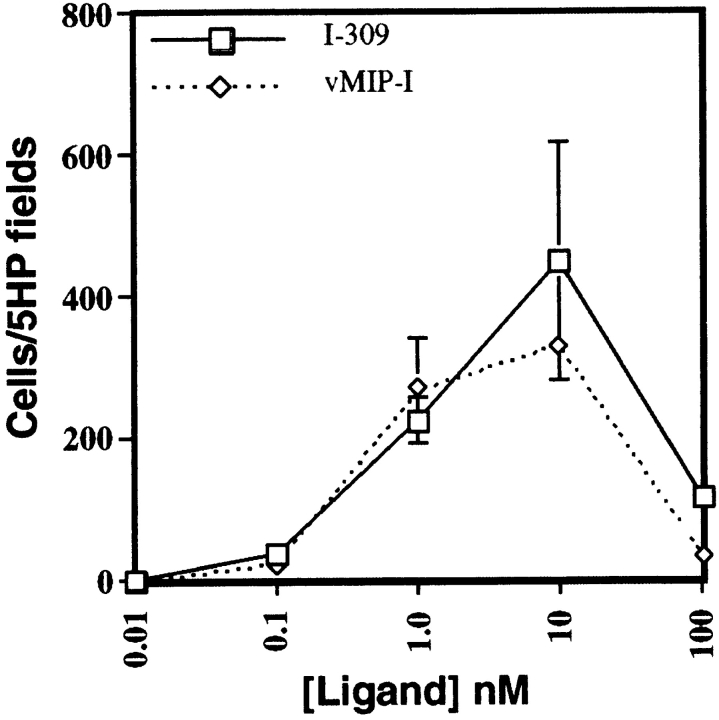
To determine whether vMIP-I binding to CCR8-Y3 cells could mediate directed cell migration, we performed in vitro chemotaxis assays. CCR8-Y3 cells responded vigorously to both vMIP-I and I-309 (Fig. 3). This response shows the typical bell-shaped curve previously observed in microchemotaxis assays with a maximal in the range of 1–10 nM for both vMIP-I and I-309. Background migration in this assay system was essentially zero, with fewer than five cells/five high power fields migrating in response to medium alone. These data demonstrate that vMIP-I acts as a CCR8 agonist for chemotaxis as well as calcium flux.
Figure 3.
Chemotactic response of CCR8-Y3 cells. The chemotactic response of CCR8-Y3 cells to either vMIP-I (⋄) or I-309 (□) was measured in the 48-well microchemotaxis assay. Chemokines were used at the indicated concentrations and results are shown as number of cells migrating/five high power (400×) fields versus concentration of ligand. The results are representative of three independent experiments and each data point is the average of duplicate wells. The range of counts for each concentration is indicated. Vehicle alone served as a negative control.
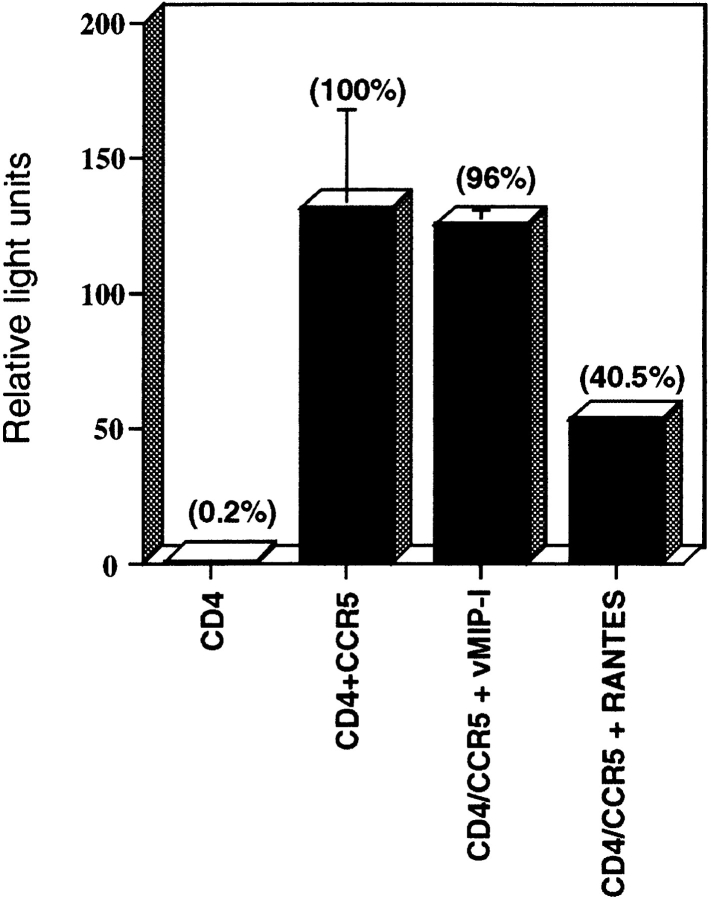
Because our data indicate that vMIP-I does not interact with CCR5, and because reports of the ability of vMIP-I to inhibit HIV infection are contradictory, we examined whether recombinant vMIP-I would block CCR5-mediated HIV entry. To address this question we used ADA envelope pseudotyped HIV-1 in a luciferase-based viral entry assay. HEK 293 cells transiently transfected with CD4 alone or CD4 and CCR5 were used as target cells. As expected, 293 cells transfected with CD4 alone did not permit viral entry (Fig. 4), whereas cells transfected with both CD4 and CCR5 were very efficient in allowing HIV entry. In contrast to the earlier results reported by Moore et al. (1, 4), but in agreement with Boshoff et al. (8), we found vMIP-I to have no detectable effect on CCR5-mediated HIV infection (Fig. 4). In contrast, RANTES was effective in blocking HIV infection (Fig. 4) of CD4/CCR5 double transfectants. This finding is consistent with our observation that vMIP-I is neither an agonist nor antagonist for CCR5 (Fig. 2).
Figure 4.
vMIP-I does not inhibit CCR5-mediated HIV infection. The ability of vMIP-I to inhibit entry of the ADA strain of HIV was compared with that of RANTES (positive control) and TECK (negative control). HEK 293 cells were transiently transfected with CD4 or CD4+ CCR5 as indicated. Chemokines were used at 100 nM. Results are representative of two experiments. Each bar is the mean of duplicate or triplicate data points and results are expressed as relative light units; percentage of control (CD4+CCR5) is also noted.
Discussion
Viruses have efficiently usurped various host genes in order to manipulate a variety of host-cell functions as well as to modify the host immune response. Through what amounts to in vivo recombinant genetics, virus-encoded molecules have been selected to retain and improve those functions that are advantageous to the virus, while abrogating those functions that are not (e.g., vIL-10). KSHV encodes several molecules that specifically target the chemokine subsystem of the immune response. We have provided the first functional characterization of one of these molecules, vMIP-I. The data presented here demonstrate that vMIP-I is a specific and potent agonist for the chemokine receptor CCR8, which is known to be preferentially expressed on Th2 cells. In addition we have addressed questions regarding the ability of vMIP-I to inhibit CCR5-mediated HIV infection.
Understanding the functional role of KSHV-encoded chemokines may help elucidate the mechanisms underlying the pathology of Kaposi's sarcoma. One function of vMIP-I and vMIP-II may be to influence the balance of the immune response toward a Th2 phenotype. Several lines of evidence support this hypothesis. Recently, CCR8 has been shown to be preferentially expressed on human and mouse Th2 cells and its natural ligand, I-309, attracts Th2-polarized T cells in vitro with considerable vigor (21, 22). Here, we have presented data that demonstrates KSHV- encoded vMIP-I is an agonist for CCR8 and that CCR8-transfected cells migrate vigorously in response to vMIP-I. In addition, KSHV-encoded vMIP-II has been reported recently to be chemotactic for CCR8-transfected Jurkat cells as well as Th2-polarized T cells (23). vMIP-II is also reported to interact with CCR3 (8), which is expressed on at least a subset of Th2 cells (24–26). Furthermore, vMIP-II is an antagonist for CCR5 and CXCR3 (20), which are preferentially expressed on Th1 cells (24, 26–28). Finally, Sozzani et al. have reported that CD4+ and CD8+ T cell clones generated from the neoplastic skin of patients with Kaposi's sarcoma are more heavily skewed toward a type II cytokine profile than are clones obtained from patients with alopecia areata or atopic dermatitis (23).
Another potential role for the vMIP-I–CCR8 interaction is in apoptosis. Van Snick et al. reported that I-309 and its murine homologue TCA-3 can block dexamethasone-mediated apoptosis of the BW5147 thymoma (29), suggesting a role for CCR8 in mediating this event. As a CCR8 agonist, vMIP-I might be used by KSHV to prevent apoptosis of a CCR8+ cell population. Alternatively, vMIP-I might be expressed in order to attract potential host cells for newly produced virus.
An important aspect of KSHV pathology is the interaction of this virus with HIV. vMIP-II is reported to inhibit viral entry through CCR5, CXCR4, and CCR3 (8, 20). The role of vMIP-I in this regard has been less clear. Using CD4+/cat kidney cells transiently expressing both CCR5 and vMIP-I, Moore et al. demonstrated a reduction in the ability of R5 HIV-1 strains M23 and SF162 to enter these cells and express p24 versus cells expressing only CCR5 (4). In a subsequent report, Boshoff et al. reported no effect of vMIP-I on HIV-1 entry/p24 expression in U87/CD4 cells stably expressing CCR5, CCR3, or CXCR4, although vMIP-I did inhibit infection of PBMCs (8). In support of these later findings, we were unable to observe a calcium flux in response to vMIP-I in 293 cells stably expressing CCR5 (Fig. 1 D). vMIP-I was also unable to antagonize RANTES, MIP-1α, or MIP-1β signaling in these cells. Furthermore, we did not observe any effect of vMIP-I on HIV infection of 293 cells transiently expressing both CCR5 and CD4, although RANTES was effective in abrogating viral entry (Fig. 4).
It is possible that differences in experimental systems can explain why neither our group nor Boshoff et al. was able to observe inhibition of CCR5-mediated HIV. Another possibility is that vMIP-I acts to inhibit HIV infection through some mechanism other than direct inhibition of binding. If this were true then a simple explanation would be that this mechanism simply does not operate in receptor-transfected 293 cells but is functional in cat kidney cells and PBMCs. Alternatively, it is possible that vMIP-I, when expressed endogenously by cat kidney cells, is posttranslationally processed in such a manner as to produce a chemokine that does interact with CCR5. However, this seems less likely, as Boshoff et al. used exogenously added recombinant vMIP-I to inhibit HIV entry into PBMCs (8). If this effect was mediated by CCR5, one would expect recombinant vMIP-I to be effective in transfected 293 cells as well.
Given the results reported here, one obvious hypothesis is that the HIV infection of PBMCs in these experiments was mediated by CCR8, which has been reported to allow entry of some strains of HIV, including the ADA strain used for the experiments presented in this report (30, 31). Indeed we investigated this possibility and have been consistently unable to observe infection of CCR8/CD4-transfected 293 cells by the ADA strain HIV, despite confirmed expression of both CCR8 and CD4. In any case, questions regarding the ability of vMIP-I to affect HIV pathology are clearly of interest and deserve further investigation.
A great deal has been learned about KSHV in the past few years. This virus, which appears to be the etiologic agent of Kaposi's sarcoma and primary effusion lymphoma (2, 3), recently has also been linked to the development of multiple myeloma (32). Expression of the KSHV G-protein–coupled receptor in rodent fibroblasts leads to a proliferative phenotype, suggesting a role for this constitutively active chemokine receptor in cellular transformation (5, 33). It has been reported that vMIP-I and vMIP-II are angiogenic (8), and that vMIP-II is a broad-spectrum chemokine antagonist (20). Understanding the role of vMIP-I in the context of these other molecules, particularly as a CCR8 agonist, should shed further light on our understanding of KSHV and HIV pathogenesis as well as on the role of chemokines in viral immunity.
Footnotes
The authors would like to acknowledge a number of individuals for providing various cell lines: Dan Lundell, Paul Zavodny, Chung-Her Jenh, and Chuan Chu Chou (Schering-Plough Research Institute, Kenilworth, NJ) for CCR2, CCR6, CCR7, and CXCR3; Albert Zlotnik and Wei Wang (DNAX Research Institute, Palo Alto, CA) for CCR9; Shelby Umland and Himanshu Shah (Schering-Plough Research Institute) for CCR3; and Kevin Moore, Kurt Gish, and Christi Parham (DNAX Research Institute) for HCR/ L-CCR cells. In addition, we would like to acknowledge Sergio Lira and Frederick Monsma for helpful discussions regarding this work. Finally, thanks to Bahige Baroudy, M. Motasim Billah, Robert Egan, Francis Cuss, Marvin Bayne, and Catherine Strader for their support.
M.J. Endres and C.G. Garlisi contributed equally to this manuscript.
References
- 1.Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, Garber R, Pellett PE, McGeoch DJ, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesarman E, Nador RG, Aozasa K, Delsol G, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am J Pathol. 1996;149:53–57. [PMC free article] [PubMed] [Google Scholar]
- 3.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 4.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein–coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 6.Nicholas J, Zong JC, Alcendor DJ, Ciufo DM, Poole LJ, Sarisky RT, Chiou CJ, Zhang X, Wan X, Guo HG, et al. Novel organizational features, captured cellular genes, and strain variability within the genome of KSHV/HHV8. J Natl Cancer Inst Monogr. 1998;1998:79–88. doi: 10.1093/oxfordjournals.jncimonographs.a024179. [DOI] [PubMed] [Google Scholar]
- 7.Nicholas J, Ruvolo VR, Burns WH, Sandford G, Wan X, Ciufo D, Hendrickson SB, Guo HG, Hayward GS, Reitz MS. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 8.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 9.Palacios R, Henson G, Steinmetz M, McKearn JP. Interleukin-3 supports growth of mouse pre-B-cell clones in vitro. Nature. 1984;309:126–131. doi: 10.1038/309126a0. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan GP. Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci USA. 1995;92:9146–9150. doi: 10.1073/pnas.92.20.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dairaghi DJ, Oldham ER, Bacon KB, Schall TJ. Chemokine receptor CCR3 function is highly dependent on local pH and ionic strength. J Biol Chem. 1997;272:28206–28209. doi: 10.1074/jbc.272.45.28206. [DOI] [PubMed] [Google Scholar]
- 12.Fan P, Kyaw H, Su K, Zeng Z, Augustus M, Carter KC, Li Y. Cloning and characterization of a novel human chemokine receptor. Biochem Biophys Res Commun. 1998;243:264–268. doi: 10.1006/bbrc.1997.7981. [DOI] [PubMed] [Google Scholar]
- 13.Shimada T, Matsumoto M, Tatsumi Y, Kanamaru A, Akira S. A novel lipopolysaccharide inducible C-C chemokine receptor related gene in murine macrophages. FEBS Lett. 1998;425:490–494. doi: 10.1016/s0014-5793(98)00299-3. [DOI] [PubMed] [Google Scholar]
- 14.Hedrick JA, Saylor V, Figueroa D, Mizoue L, Xu Y, Menon S, Abrams J, Handel T, Zlotnik A. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol. 1997;158:1533–1540. [PubMed] [Google Scholar]
- 15.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 16.Roos RS, Loetscher M, Legler DF, Clark-Lewis I, Baggiolini M, Moser B. Identification of CCR8, the receptor for the human CC chemokine I-309. J Biol Chem. 1997;272:17251–17254. doi: 10.1074/jbc.272.28.17251. [DOI] [PubMed] [Google Scholar]
- 17.Goya I, Gutiérrez J, Varona R, Kremer L, Zaballos A, Márquez G. Identification of CCR8 as the specific receptor for the human β-chemokine I-309: cloning and molecular characterization of murine CCR8 as the receptor for TCA-3. J Immunol. 1998;160:1975–1981. [PubMed] [Google Scholar]
- 18.Tiffany HL, Lautens LL, Gao JL, Pease J, Locati M, Combadiere C, Modi W, Bonner TI, Murphy PM. Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J Exp Med. 1997;186:165–170. doi: 10.1084/jem.186.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardini G, Hedrick J, Sozzani S, Luini W, Gaia S, Weiss M, Menon S, Zlotnik A, Mantovani A, Santoni A, Napolitano M. Identification of the CC chemokines TARC and macrophage inflammatory protein-1β as novel functional ligands for the CCR8 receptor. Eur J Immunol. 1998;28:582–588. doi: 10.1002/(SICI)1521-4141(199802)28:02<582::AID-IMMU582>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Luttichau HR, Gerstoft J, Clapham PR, et al. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma–associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 21.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 22.D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 23.Sozzani S, Luini W, Bianchi G, Allavena P, Wells TNC, Napolitani M, Bernardini G, Vecchi A, D'Ambrosio D, Mazzeo D, et al. The viral chemokine macrophage inflammatory proetin-II is a selective Th2 chemoattractant. Blood. 1998;92:4036–4039. [PubMed] [Google Scholar]
- 24.Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto H, Wang W, Strieter RM, Copeland NG, Gilbert DJ, Jenkins NA, Hedrick J, Zlotnik A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci USA. 1998;95:8205–8210. doi: 10.1073/pnas.95.14.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Snick J, Houssiau F, Proost P, Van Damme J, Renauld JC. I-309/T cell activation gene-3 chemokine protects murine T cell lymphomas against dexamethasone-induced apoptosis. J Immunol. 1996;157:2570–2576. [PubMed] [Google Scholar]
- 30.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, et al. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 31.Rucker J, Edinger AL, Sharron M, Samson M, Lee B, Berson JF, Yi Y, Margulies B, Collman RG, Doranz BJ, et al. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rettig MB, Ma HJ, Vescio RA, Pold M, Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, et al. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]
- 33.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gerhengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]