Abstract
The requirements for CD8 T cells to provide protection against a localized virus infection in models of adoptive immunotherapy are not well defined. Here we investigated the protective value of defined in vitro–generated hemagglutinin (HA) peptide-specific primary CD8 T cell effectors from the clone 4 T cell receptor transgenic mice, secreting type 1 or type 2 cytokines, against pulmonary infection with whole influenza virus. Cytotoxic T lymphocytes producing type 1 and type 2 cytokine (Tc1 and Tc2) populations were equally cytolytic, but Tc1 effectors and not Tc2 effectors reduced the pulmonary virus titer early during infection. Host recovery mediated by Tc1 effectors was found to be independent of interferon γ production. Tc2 effectors entered the lung with delayed kinetics as compared with Tc1 effectors, and after lung entry Tc2 effector cells did not localize near the infected airway epithelium as did Tc1 effectors but were found within clusters of inflammatory cells distant from the epithelium. We also show that the expression of several chemokine receptors was selectively regulated in the Tc1 and Tc2 subsets. Thus, the protective value of a CD8 cell population against pulmonary influenza virus infection is strongly correlated with its ability to exert its effector potential at the site of virus infection.
Keywords: CD8, subset, migration, influenza virus, protection
Avariety of factors are involved in the host defense against virus and in the recovery from pulmonary viral infections (1, 2). CD8 T cells have been shown to be important effector cells during pulmonary influenza virus infection, promoting host recovery and virus clearance. The adoptive transfer of influenza virus–immune cytotoxic T cells (3) or CD8 T cell clones (4) into lethally influenza- infected hosts resulted in a reduction of the pulmonary virus titer and prevention of death. The main effector mechanism of CD8 T cell populations against influenza was shown to be contact–dependent lysis (5). Additional requirements for a CD8 T cell population to be protective against influenza virus infection have not yet been defined.
CD8 T cells, which were initially described as cytolytic effector cells producing high levels of IFN-γ, have only recently been shown to be a source of a wider variety of cytokines, including both type 1 and type 2 cytokines (6, 7). Depending on the cytokines present during primary stimulation, CD8 T cells can be polarized into type 1 and type 2 CD8 T cells producing IFN-γ and IL-2 or IL-4, IL-5, IL-6, and IL-10, respectively (8–10). In most of the studies, including our own, both the Tc1 (CTLs producing type 1 cytokines)1 and Tc2 subsets were shown to be cytolytic (9– 11). Furthermore, we have demonstrated previously that Tc1 or Tc2 effectors can give rise to Tc1 or Tc2 memory cells, respectively, indicating that the type 1– and type 2–producing phenotypes are stable (10). There is substantial evidence that CD8 T cells secreting type 1 and type 2 cytokines also exist in vivo during infection with a number of pathogens, including influenza virus. IL-5–secreting CD8 T cells have been isolated from lesions from patients with lepromatous leprosy (12) and from HIV–infected individuals with a Job's-like syndrome (13). Lymphocytic choriomeningitis virus–specific CD8 T cells secreting IL-5 have been shown to be associated with airway eosinophilia (14) and IL-5– or IFN-γ–producing CD8 T cells were found in murine gut-associated tissues (15). During pulmonary influenza virus infection, both CD4 and CD8 populations isolated from the mediastinal lymph nodes concurrently produced IL-2, IFN-γ, and IL-10 as assessed by Elispot assay (16). In addition, both CD4 and CD8 T cells isolated from the respiratory tract contributed to the mRNA expression of the cytokines IFN-γ, IL-5, and IL-10 (17) and splenic CD4 and CD8 T cells from influenza-immune mice were found to produce IL-2, IL-4, and IFN-γ in vitro (18).
The observation that CD8 T cells can be a source of several cytokines suggests that they may carry out previously unrecognized in vivo functions in the regulation of immune responses. However, the relative protective value of CD8 T cell effectors producing different patterns of cytokines against pulmonary virus infection has not yet been addressed. The patterns of cytokines produced by CD8 Tc1 and Tc2 effectors might recruit cell populations with distinct effector functions to the site of infection. In addition, recent evidence suggests that the protective value of T cell populations might also strongly depend on their ability to migrate efficiently to infected tissue sites where they perform their effector function (19). For CD4 T cells, it was shown that not only the expression of adhesion molecules (20) but also the expression of chemokine receptors (21, 22) differed profoundly within human Th1 and Th2 clones, suggesting distinct migration patterns of CD4 subsets. Chemokine receptor expression on polarized CD8 T cell subsets and their migration patterns has not yet been investigated.
In this paper, we have studied the in vivo function, migration pattern, and chemokine receptor expression of polarized type 1 or type 2 CD8 T cells during pulmonary influenza virus infection. Primary hemagglutinin (HA) peptide-specific Tc1 and Tc2 effector cells from clone 4 TCR transgenic mice specific for a nonapeptide from the transmembrane region of the HA2 molecule from influenza virus were generated in vitro and adoptively transferred into hosts infected intranasally with 10 LD50 influenza virus (A/PR/8/34).
We show that Tc1 effectors were more effective in promoting virus clearance and host recovery than Tc2 effectors, despite the fact that both populations were equally cytolytic. Delayed entry or accumulation of Tc2 effectors into the lung and distinct localization of Tc2 effectors within clusters of inflammatory cells distant from the infected airway epithelium correlated with their ineffectiveness to clear virus early during infection. In contrast, Tc1 cells entered or accumulated in the lung early and migrated to the infected airway epithelium. Tc1 and Tc2 effectors exhibited striking differences in chemokine receptor expression. These data suggest that the localization of cytolytic effectors together with their cytokine production are likely to make a major contribution to the effectiveness of CD8 T cell populations against localized virus infections.
Materials and Methods
Mice.
Mice were purchased from the Animal Breeding Facility at the Trudeau Institute. Clone 4 Vβ8.2/Vα10 TCR transgenic mice (23) were provided by Dr. Linda Sherman (The Scripps Research Institute, La Jolla, CA). The clone 4 TCR transgenic mice bear the α and β chains of the clone 4 CTL specific for the transmembrane peptide, residues 518–528 (IYSTVASSL) of HA2 on H-2Kd. The clone 4 TCR transgenic mice were back-crossed for eight generations with BALB/c mice. They were also crossed to the IFN-γ−/− background on BALB/c. The IFN-γ−/− mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/c Thy 1.1 mice were a gift from Dr. Jon Sprent (The Scripps Research Institute, La Jolla, CA).
Preparation of Tc1 and Tc2 Effector Cells.
CD8 T cells from the spleens and lymph nodes of clone 4 TCR transgenic mice were enriched by passing through nylon wool and treating with anti-CD4 (RL172.4) and anti–heat stable antigen (J11D), and anti– class II MHC (D3.137, M5114, CA4) mAbs, and complement. Small resting CD8 T cells were harvested from the bottom interface of a four-layer Percoll gradient (Sigma Chemical Co.). The freshly isolated T cell populations were 90–95% CD8+Vβ8+ T cells. T cell–depleted splenocytes were prepared using anti-Thy1.2 (HO13.14 and F7D5), anti-CD4 (RL172.4), and anti-CD8 (3.155) mAbs, and complement, and stimulated with LPS (25 μg/ml) and Dextran sulfate (25 μg/ml) for 48 h. “APC blasts” were loaded with the HA peptide (11 μM) at 37°C for 30 min, treated with mitomycin C (50 μg/ml; Sigma Chemical Co.) at 37°C for 40 min, and washed three times before use. CD8 T cells were cultured in RPMI 1640 (Irvine Scientific Co.) supplemented with penicillin, streptomycin, glutamine (PSG), 2-ME, Hepes, and 10% FCS (Hyclone Labs.). For effector generation CD8 T cells from the clone 4 transgenic mice (2 × 105 cells/ml) were stimulated with HA peptide–loaded APCs (2 × 105 cells/ml) in the presence of IL-2 (20 U/ml, supernatant from the X63Ag.IL-2 murine cell line), IL-12 (9.2 U/ml, provided by Dr. Stanley Wolf, Genetics Institute, Inc., Cambridge, MA), and anti–IL-4 mAb (10 μg/ml, 11B11) for Tc1 cultures and in the presence of IL-2 (20 U/ml), IL-4 (200 U/ml, X63.Ag.IL-4 supernatant), and anti-IFN-γ mAb (XMG1.2, 20 μg/ml) for Tc2 cultures. On day 4 of culture, effectors were 99% CD8+Vβ8+.
Virus Infections and Cell Transfers.
The influenza virus preparation (A/PR/8/34) grown in the allantoic cavity of 10-d-old embryonated hen eggs was a gift of Drs. David Morgan and Linda Sherman (The Scripps Research Institute, La Jolla, CA). Each mouse was inoculated with a 10 LD50 dose of virus (corresponding to a 10−3 dilution of stock virus in 100 μl PBS) intranasally during light halothane (Halocarbon) anesthesia. Before adoptive transfer, Tc1 and Tc2 effector cells were washed twice in HBSS and, if not indicated otherwise, 107 effector cells were injected in 0.5 ml PBS via the tail vein within ∼1 h after intranasal virus infection.
Analysis of Cytokine Production.
Enriched CD8 T cells (2 × 105 cells/ml) were stimulated with mitomycin-treated P815 cells (1.2 × 106/ml) loaded or not with the HA peptide. IFN-γ, IL-4, and IL-5 were measured by specific ELISAs as previously described (10).
Cytotoxicity Assay.
P815 cells were used as targets and loaded or not with the indicated concentrations of the HA peptide for 30 min at 37°C. Subsequently, target cells (106 cells/400 μl RPMI medium containing 1% FCS) were incubated with 3.7 mBq 51Cr (specific activity 1.85 TBq/g; NEN™ Life Science Products) for 1 h at 37°C. Labeled targets were washed three times before use. CD8 T cells were set up at the indicated ratios with the labeled targets (104 targets/well) at 37°C, and supernatants were collected after 4 h and radioactivity was detected by γ counting. Means and SEs of duplicate cultures are shown. The percentage of cytotoxicity was calculated using the formula: 100 × (cpm experimental − cpm spontaneous) / (cpm total − cpm spontaneous). Spontaneous release was typically 10–15% of the maximum release.
RNase Protection Assay.
Total RNA from 107 Tc1 and Tc2 effectors cells was prepared on day 4 after in vitro primary culture before (t0) and after restimulation with plate-bound anti-CD3 mAb (2C11; 10 μg/ml) for the indicated times using TRIzol Reagent (GIBCO BRL). mRNA levels were then determined using the RNase protection assay kit “RiboQuant Multipurpose Ribonuclease Protection Assay (RPA) System” (PharMingen) and the following probe sets (all from PharMingen): mCK-1, mCK-3 for cytokine mRNA and mCR-5, and mCR-6 for chemokine-R mRNA. The dried gel was placed on film (Eastman Kodak Co.) in a cassette with an intensifying screen and developed at −70°C. Bands were detected using the densitometric feature of the Quantity One software (Bio-Rad Labs.) and normalized against the housekeeping gene L32.
Madine Derby Canine Kidney Virus Plaque Assay.
Viral titers of infected lungs were determined using the Madine Derby canine kidney (MDCK) cell plaque assay modified from the methods described by Lukacher et al. (4). In brief, lungs were snap-frozen in liquid nitrogen at the indicated time points after influenza virus infection and stored at -70°C until ready for titration. MDCK monolayers were grown in DMEM supplemented with 10% FCS, 0.01 mM MEM amino acid solution (GIBCO BLR), 1 mM sodium pyruvate (GIBCO BLR), and PSG (200 IU/ml penicillin, 200 μg/ml streptomycin, and 4 mM glutamine). 10-fold dilutions of the lung homogenates were prepared in DMEM supplemented with 0.2% BSA, 2 mg/ml NaHCO3, 2 mM Hepes, and PSG. 100 μl of each dilution was added to confluent monolayers of MDCK cells in 12-well plates in duplicates for 1 h at 37°C, 7% CO2. Each well received 1 ml of an agar overlay medium containing DMEM, 0.2% BSA, 2 mg/ml NaHCO3, 2 mM Hepes, PSG, 0.5% agar (Sigma Chemical Co.), 0.01% DEAE Dextran, and 0.5 μg/ml trypsin. After a 3-day incubation at 37°C, cells were fixed for at least 20 min with 0.5 ml Carnoy's fixative (3:1, methanol/glacial acetic acid). The agar overlay was then removed and fixed monolayers were stained by adding a 1:10 dilution of 2% crystal violet prepared in 20% ethanol. The results are presented as PFUs/ml = (mean number of plaques/0.1) × (1/dilution factor).
Flow Cytometry.
The following mAbs were used for immunofluorescent staining: Cy-chrome anti-CD8 (PharMingen), FITC anti-CD62L (PharMingen, clone Mel-14), FITC anti-CD44 (PharMingen, clone IM7), FITC anti-Ly6C (PharMingen, clone AL-21), and PE anti-CD90 (Thy 1.2, PharMingen). After staining with the appropriate mAbs, FACS® analysis was carried out on a FACScan® by using the Cell Quest (Becton Dickinson) software. All plots shown were gated on the PI-negative, live population.
Lung Histology.
Lungs were infused with 1 ml O.C.T. medium (VWR, Rochester, NY), and then were frozen in liquid nitrogen and stored at −70°C. 5-μm sections were cryostat cut onto poly-l-lysine-coated (Sigma Chemical Co.) slides and fixed in −20°C acetone for 5 min. Endogenous peroxidases were blocked in 0.3% hydrogen peroxide for 30 min. For further blocking, the avidin/biotin blocking kit No. SP2001 (Vector Labs.) was used and nonspecific binding to Fc receptors was blocked with 2.4G2 mAb. Sections were stained with biotinylated Thy 1.2 mAb (PharMingen) and the Vectastain Elite ABC kit for peroxidase systems (Vector Labs.). The color reaction was developed using the AEC substrate kit for horseradish peroxidase systems (Vector Labs.). Sections were counterstained with hematoxylin (Vector Labs.).
Tissue Sampling.
For bronchoalveolar lavage, mice were anesthetized and bled out from the axilla. The trachea was then exposed and a disposable plastic cannula with a syringe attached was then inserted through an incision immediately posterior to the larynx. The respiratory tract was washed out in a reproducible manner using four separate 1-ml aliquots of PBS containing 3 mM EDTA. Subsequently, the lavaged cells were centrifuged and live cell counts were performed by excluding trypan blue– positive dead cells.
For lung digest, lungs of killed animals were flushed in situ with 20 ml PBS via cannulation of the heart to remove the intravascular blood pool. Minced lung tissue was then incubated on a rocker with collagenase (1 mg/ml; Sigma Chemical Co.) and DNase (50 U/ml; Sigma Chemical Co.) for 1 h and passed through a stainless steel mesh. After centrifugation live cell counts were performed by excluding trypan blue–positive dead cells.
Results
Cytokine Production and Cytokine mRNA Expression of In Vitro–generated HA Peptide–specific Tc1 and Tc2 CD8 T Cell Effectors.
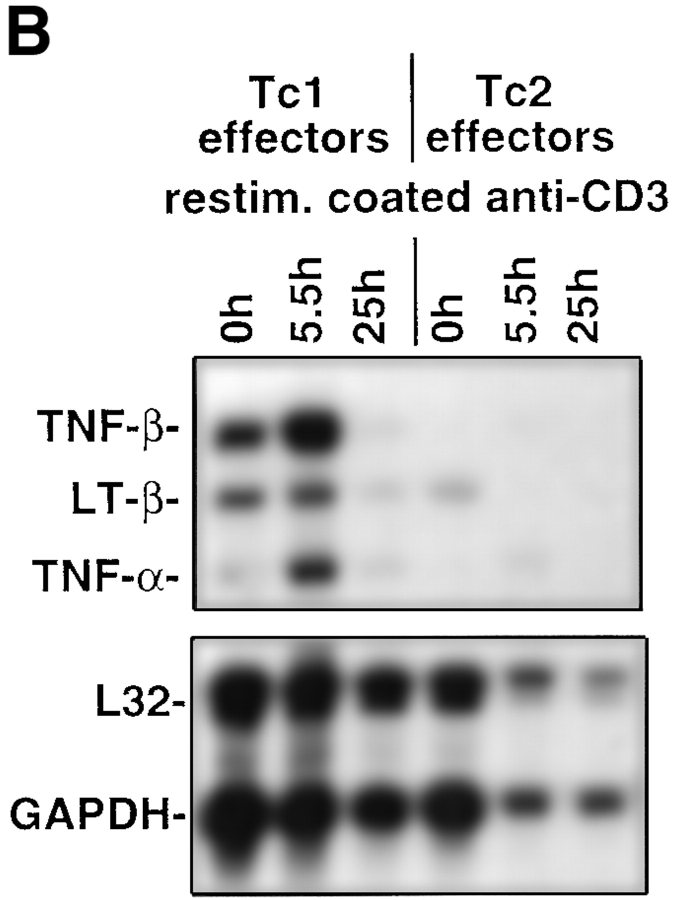
Naive CD8 T cells were isolated from the clone 4 TCR transgenic mice (23) carrying the Vα10/Vβ8.2 TCR specific for a hydrophobic peptide sequence (amino acids 518–528, IYSTVASSL) from the transmembrane region of the HA2 molecule from influenza virus. After enrichment typically >95% of the CD8 cells were naive as assessed by low CD44 expression as previously described (10). CD8 T cell effectors producing type 1 and type 2 cytokines were generated by culturing transgenic CD8 T cells with HA peptide–loaded APCs in the presence of IL-2, IL-12, and anti–IL-4 mAb, or IL-2, IL-4, and anti–IFN-γ mAb, respectively, for 4 d. A representative cytokine profile of the in vitro–generated CD8 effector populations assessed by specific ELISA is shown in Table I. Tc1 effectors produced IFN-γ but no detectable IL-4 or IL-5 upon stimulation with mitomycin-treated HA peptide–loaded P815 cells used as APCs (Table I). No cytokine production was observed with APCs in the absence of HA peptide (data not shown), indicating that the response was highly antigen-specific. In contrast, Tc2 effectors produced IL-4, IL-5, and typically 10-fold lower amounts of IFN-γ as compared with Tc1 effector cells. Additional information about cytokine mRNA expression within CD8 T cell subsets was obtained in RNase protection assays (Fig. 1, A and B) and mRNA was harvested on day 4 of the primary in vitro culture t0 and at the indicated time points after restimulation with plate-bound anti-CD3 mAb. The predominant cytokines expressed in Tc1 effectors were IFN-γ (Fig. 1 A), TNF-β, lymphotoxin-β (LT-β), and TNF-α (Fig. 1 B). Tc1 effectors also expressed low levels of IL-10. No expression of mRNA specific for IL-4, IL-5, or IL-13 was detected with Tc1 effectors. In contrast, high levels of mRNA for IL-13 and IL-10 were found to be expressed with Tc2 effectors even t0. Upon restimulation with plate-bound anti-CD3 mAb, Tc2 effectors expressed mRNA for IL-4, IL-5, and strongly upregulated mRNA for IL-13. With Tc2 effectors a low level of IFN-γ mRNA (27-fold less as compared with the expression with Tc1 effectors at t0 assessed by densitometry) was slightly upregulated upon restimulation (Fig. 1 A), whereas mRNA specific for TNF-β and LT-β was downregulated upon restimulation (Fig. 1 B).
Table I.
Cytokine Production of Tc1 and Tc2 Effectors before Adoptive Transfer Measured by Specific ELISAs
| IFN-γ | IL-4 | IL-5 | ||||
|---|---|---|---|---|---|---|
| ng/ml | ng/ml | U/ml | ||||
| Tc1 effectors | 1,020 ± 29 | – | – | |||
| Tc2 effectors | 95 ± 8 | 7.6 ± 0.09 | 7,963 ± 364 |
Tc1 and Tc2 effectors were generated as described in Materials and Methods. On day 4 of in vitro primary stimulation, Tc1 and Tc2 effectors were washed and restimulated with P815 cells used as APCs loaded with the HA peptide (11 μM). Supernatants were collected after 24 h and the amounts of IFN-γ, IL-4, and IL-5 were measured by specific ELISA. Means ± SE of triplicate wells of 1 representative experiment out of 10 are shown. No cytokine production was detected when Tc1 and Tc2 effectors were restimulated in the absence of the HA peptide (data not shown).
Figure 1.
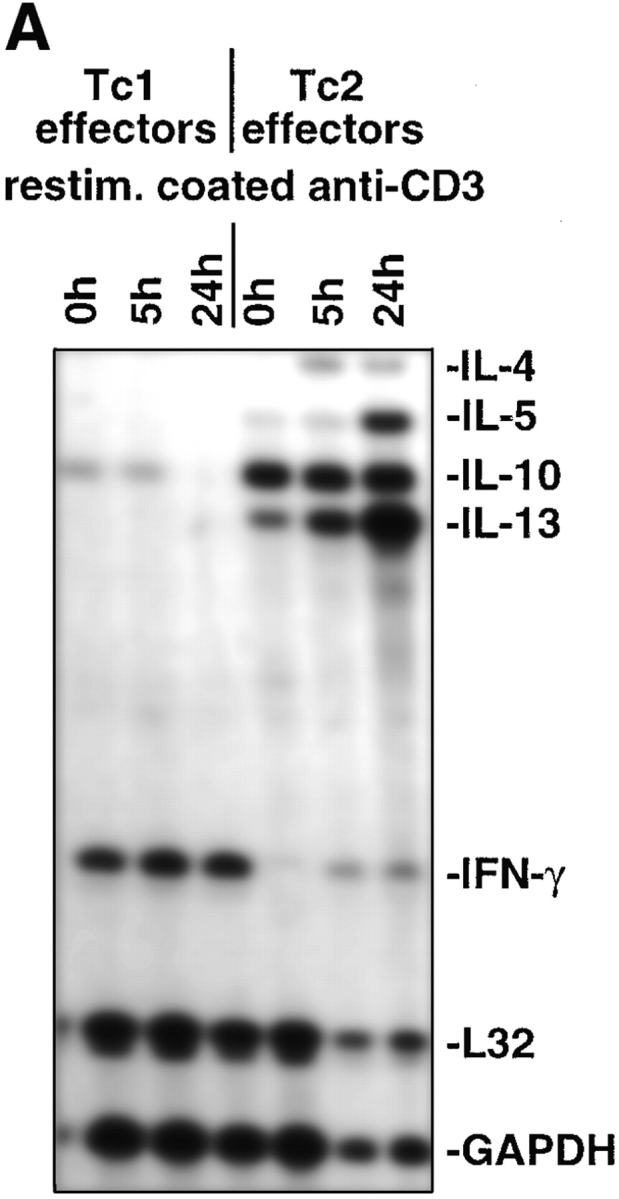
Cytokine mRNA expression within CD8 T cell subsets. On day 4 after in vitro culture RNA from 107 Tc1 and Tc2 effectors was prepared (t0) as described in Materials and Methods. Subsequently, 107 Tc1 or Tc2 effectors were restimulated with plate-bound anti-CD3 mAb (10 μg/ml) and RNA was prepared at the indicated time points. The RiboquantTM Multi-Probe protection assay mCK-1 (A) or mCK-3 (B) was used to detect cytokine mRNA. The results shown are representative for two independently performed experiments.
Both Tc1 and Tc2 Effectors Are Equally Cytolytic before Adoptive Transfer.
We next determined the cytolytic activity of Tc1 and Tc2 effectors before adoptive transfer (Fig. 2). Both Tc1 and Tc2 effectors were highly cytolytic, killing HA peptide–loaded targets but not –unloaded targets. When targets were loaded with titrated doses of the HA peptide (from 1.1 to 1.1 × 10−6 μM), a similar dose response to peptide was seen with Tc1 and Tc2 effectors. The Tc2 effectors were usually slightly more cytotoxic than the Tc1 population, ruling out that the observed cytotoxicity of the effectors generated under type 2–inducing conditions was merely due to the small number of the contaminating Tc1 IFN-γ–producing population (Table I). On day 4 of stimulation, both Tc1 and Tc2 populations were highly activated blast-sized cells with a high forward scatter, expressing high levels of activation markers as previously described (10), and all cells expressed CD8, Thy 1.2, and the transgene Vβ8. No persisting mitomycin-treated APCs were detected on day 4 by FACS® analysis (data not shown).
Figure 2.
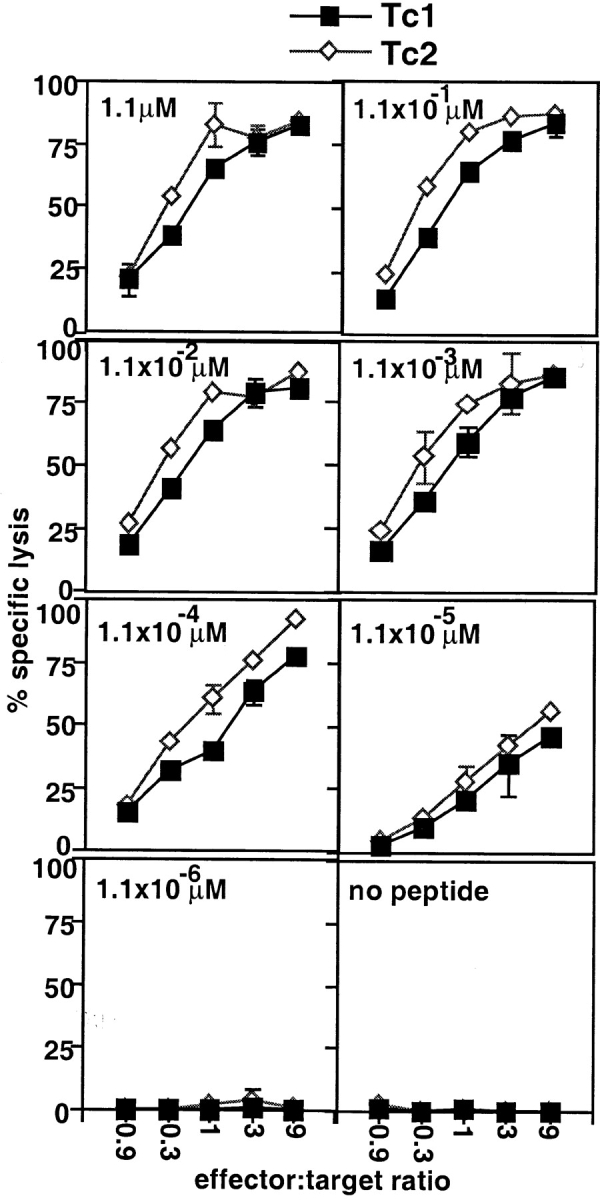
CTL activity of Tc1 and Tc2 effectors. The cytolytic activity of 4 d Tc1 (▪) and Tc2 effectors (⋄) was assayed in a 4-h 51Cr-release assay. P815 cells (104 cells/well) pulsed with titrated amounts of the HA peptide (1.1– 1.1 × 10−6 μM, indicated in the lefthand corners) were used as targets. The results are representative for three independently performed experiments.
Adoptive Transfer of Tc1 Effectors But Not Tc2 Effectors Reduces the Pulmonary Virus Titer on Day 4 after Influenza Virus Infection.
The CD8 T cell–mediated recovery of hosts lethally infected with influenza virus was shown to be associated with a reduction in pulmonary virus titer (4). Thus, we tested the ability of HA peptide–specific Tc1 and Tc2 effectors to reduce the pulmonary virus titer during infection with influenza virus upon adoptive transfer. On day 4 of primary culture 107 HA peptide–specific Tc1 or Tc2 effectors from the clone 4 TCR transgenic mice were adoptively transferred into BALB/c hosts that were infected with 10 LD50 whole influenza virus (A/PR/8/34) ∼1 h before the cell transfer as previously described (4). Infected hosts, which had not received CD8 effector cells, were used as controls. Influenza-infected recipients of Tc1 or Tc2 effectors or lethally infected mice that had not received cells were killed on day 4 after infection, and infectious virus particles in mouse lung extracts were titered by plaque formation on monolayers on MDCK fibroblast cells (Table II). Adoptive transfer of Tc1 effectors resulted in a 1,000-fold reduction of pulmonary virus titer on day 4 after infection and cell transfer as compared with infected mice, which had not been injected with HA peptide–specific effectors. At this time point, only a slight reduction (fivefold) of virus titer was observed with recipients of Tc2 effectors. When kinetics experiments were carried out longer, we found that recipients of Tc1 effectors had almost totally cleared the pulmonary virus titer by day 5 after infection, when the pulmonary viral load of recipients of Tc2 effectors and animals that had not received CD8 T cells was still high (data not shown). On day 7, recipients of Tc1 effectors had completely cleared the virus from their lungs; however, several animals from the other groups were already dead at this time point (data not shown).
Table II.
Adoptive Transfer of Tc1 Effectors Results in Reduction of the Pulmonary Virus Titer (Day 4 after Infection)
| Transfer of | Pulmonary virus titer | |
|---|---|---|
| PFU/ml | ||
| Tc1 effectors | 4 ± 1 × 102 | |
| Tc2 effectors | 8 ± 3 × 104 | |
| no cells | 4 ± 1 × 105 |
107 Tc1 and Tc2 effectors from the HA TCR transgenic mice were adoptively transferred into hosts infected with 10 LD50 influenza virus and the pulmonary virus titer was assessed on day 4 after infection and cell transfer using the MDCK virus plaque assay as described in Materials and Methods. Three individual mice per group were analyzed. The data shown represent the mean ± SE and are representative of three independently performed experiments.
Tc2 Effectors Enter the Lung with Delayed Kinetics.
The results thus far indicate that Tc1 CD8 T cells, but not Tc2 CD8 T cells, reduced the pulmonary viral titer on day 4 after influenza virus infection. Because CD8 T cell homing to the lung is critical for the contact-dependent lysis of the virus-infected airway epithelial cells and subsequently for virus clearance, we determined the total cell counts of Tc1 and Tc2 effector cells from the lung lavage during pulmonary influenza virus infection. We adoptively transferred 107 Tc1 or Tc2 effectors (donor cells were Thy 1.2–positive) into Thy 1.1 BALB/c hosts and assessed percentages of donor cells by CD8/Thy 1.2 staining and subsequent FACS® analysis on days 1, 3, 5, and 7 from the lung lavage (Fig. 3). On day 3 after influenza infection and cell transfer, 70% of cells recovered from the lavage of recipients of Tc1 effectors were donor cells staining positive for CD8 and Thy 1.2, whereas only 10% of the cells recovered from recipients of Tc2 effectors were of donor phenotype. To assess absolute donor cell numbers, total cell counts from the lung lavage (Fig. 4 a), tracheal bronchial lymph node (TBLN) (Fig. 4 b), and spleen (Fig. 4 c) were performed and combined with the percentages of donor cells as determined by FACS® to calculate the total donor cells. Very few cells were seen at any of the sites on day 1. Since the adoptively transferred cells were injected at 4 PM on day zero and the day 1 mice were killed at 7 AM, the first time point is 17 rather than 24 h and the transferred cells were presumably still in the circulation. On day 3 after influenza infection and cell transfer, sixfold more Tc1 effectors were found in the lung lavage as compared with Tc2 effectors and threefold more Tc1 effectors as compared with Tc2 effectors were found in the spleen. Similar numbers of Tc1 and Tc2 effectors were found in the TBLN. However, on day 7 comparable numbers of Tc1 and Tc2 effectors were found in the lung lavage, spleen, and TBLN. Together, these data indicate that Tc2 effectors enter the lung tissue with delayed kinetics.
Figure 3.

Kinetics of migration of Tc1 and Tc2 effectors to the lung. 107 Tc1 and Tc2 effectors expressing Thy 1.2 were adoptively transferred into influenza infected Thy 1.1 BALB/c hosts and cells from lung lavages of recipients of Tc1 (left panels) or Tc2 (right panels) effectors were stained with Thy 1.2 PE and CD8 FITC in order to identify cells of donor origin on days 1, 3, 5, and 7. Dot-plots were gated on PI-negative live cells. The migration kinetics into the lung of one representative animal out of three is shown.
Figure 4.
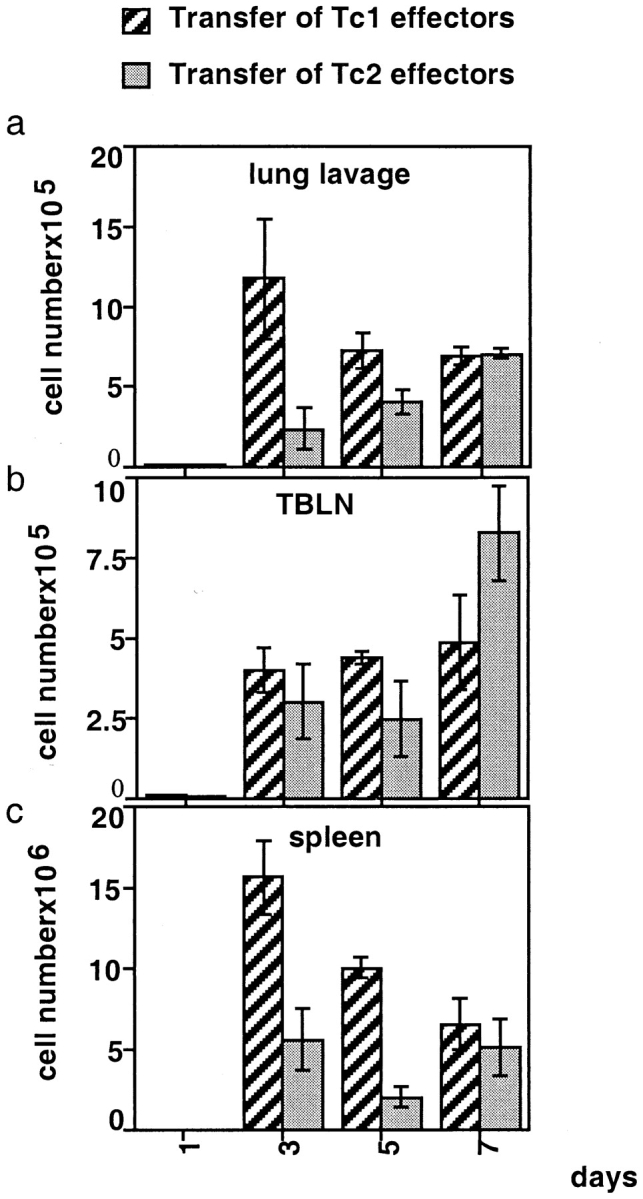
Absolute donor cell numbers recovered from lung lavage, spleen, and TBLN on days 1, 3, 5, and 7 after influenza virus infection and cell transfer. 107 Tc1 and Tc2 effectors expressing Thy 1.2 were adoptively transferred into influenza-infected Thy 1.1 BALB/c hosts as described in Fig. 3. To assess absolute donor cell numbers in the different organs, total cell counts from the isolated organs were performed and were multiplied by the percentages of donor cells determined by FACS® as shown in Fig. 3. Here, three animals per group were analyzed and the average donor cell numbers ± SE isolated from lung lavage (a), TBLN (b), and spleen (c) are shown. It should be noted that the adoptively transferred cells were injected at 4 PM on day 0 and the day 1 mice were killed at 7 AM, an interval of only 15 h.
Tc1 and Tc2 Effectors Localize to Different Sites in the Lung Tissue during Influenza Infection.
The next experiments were designed to visualize the localization of the Tc1 and Tc2 population in the lung tissue by immunohistochemistry. On day 5 after infection with influenza virus and cell transfer, frozen sections of recipient lungs were stained with a Thy 1.2 mAb to identify donor CD8 T cells (Fig. 5). Specific staining for Thy 1.2 was found in both groups, but distinct localization of Tc1 versus Tc2 effectors was observed. Tc1 effectors were found near the basement membrane of the airway (Fig. 5, A and C), whereas Tc2 effectors localized predominantly within large clusters of cellular infiltrates distant from the airway epithelium and also scattered throughout the alveolar air spaces and septa (Fig. 5, B and D). Most of the cells within the cell clusters of the alveolar parenchyma stained positive with the mAb RB-6, which specifically stains neutrophils and eosinophils, and colocalized in the clusters with Tc2 effectors (Cerwenka, A., manuscript in preparation).
Figure 5.
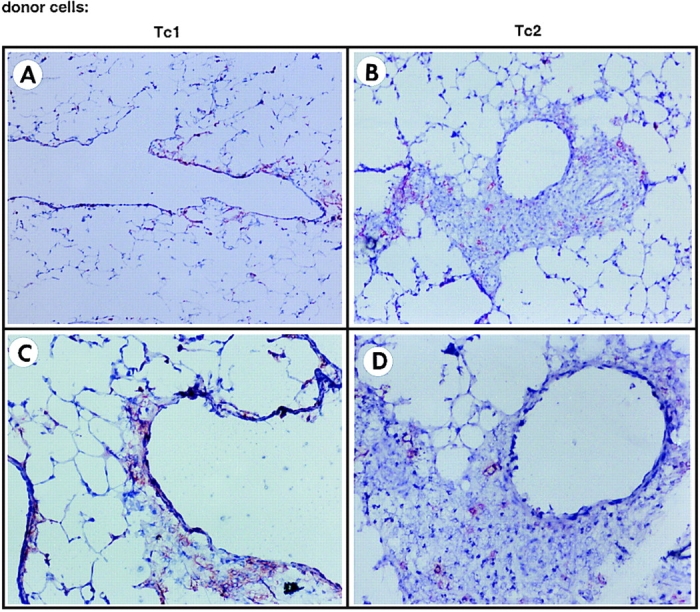
Immunohistochemistry of lungs of recipients of Tc1 and Tc2 effector cells. 107 Tc1 (A and C) and Tc2 effectors (B and D) expressing Thy 1.2 were adoptively transferred into influenza-infected Thy 1.1 BALB/c hosts. On day 5 after infection and cell transfer, lungs were removed and frozen sections were prepared as described in Materials and Methods. Sections were stained with a biotinylated Thy 1.2 mAb or the respective biotinylated isotype control, and the color reaction was developed using a red substrate. The staining for Thy 1.2 (donor cells) was highly specific, because no red stain was detected when slides were incubated with the respective isotype control (data not shown). Original magnification: A and B, ×100; C and D, ×200. The results are representative of four independently performed experiments.
Tc1 and Tc2 Effectors Express Distinct Patterns of Chemokine Receptors.
Chemokines, as well as adhesion molecules, have been shown to be a key component of the leukocyte recruitment process, and human polarized CD4 T cell clones expressing distinct patterns of chemokine receptors were shown to differentially migrate in response to different chemokines in vitro (21, 22). We therefore examined whether Tc1 and Tc2 CD8 T cell effectors differed in the expression of chemokine receptors by RNase protection assay (Fig. 6, A and B). RNA from 4 d in vitro generated Tc1 and Tc2 effectors was extracted t0 and after restimulation for 5 and 24 h with plate-bound anti-CD3 mAb. Expression of CCR1 was selectively found with Tc2 effectors upon stimulation. Tc1 effectors expressed higher levels of CCR5 (7.8-fold difference by densitometry) and CCR2 (8.1-fold difference) as compared with Tc2 effectors t0. At 24 h after restimulation, similar levels of CCR5 and CCR2 were found in the Tc1 and the Tc2 subsets. The chemokine receptor CCR4 was expressed in Tc2 effectors at higher levels than in Tc1 effectors (threefold difference) and this expression remained high in Tc2 effectors upon restimulation. However, in Tc1 effectors, expression of CCR4 was undetectable upon restimulation at 24 h. Both Tc1 and Tc2 effectors expressed CXCR4 and downregulated expression of this chemokine receptor upon restimulation. CXCR2 selectively was expressed within the Tc1 subset and was downregulated upon activation (Fig. 6 B).
Figure 6.
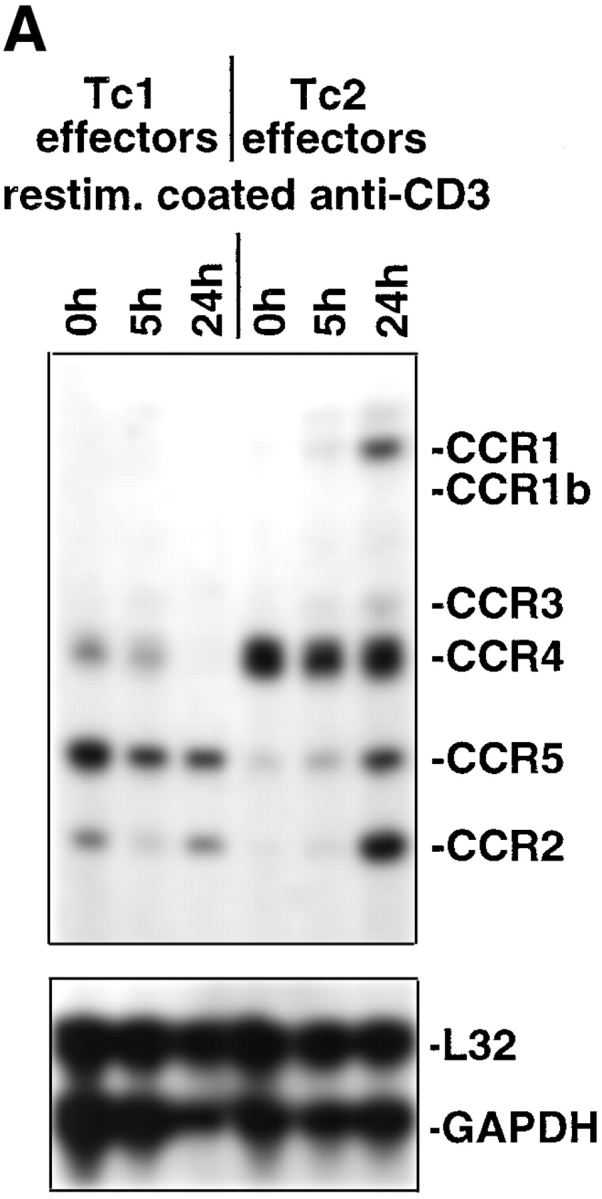

Chemokine receptor mRNA expression within CD8 T cell subsets. On day 4 after in vitro stimulation RNA from 107 Tc1 and Tc2 effectors was prepared (t0) as described in Materials and Methods before adoptive transfers. 107 Tc1 and Tc2 effectors were restimulated with plate-bound anti-CD3 mAb (10 μg/ml), and RNA was prepared at the indicated time points. The RiboQuantTM Multi-Probe protection assay mCR-5 (A) or mCR-6 (B) was used to detect chemokine receptor mRNA. The results shown are representative for three independently performed experiments.
Phenotype of Tc1 and Tc2 Effector Donor Cells on Day 5 after Infection and Cell Transfer.
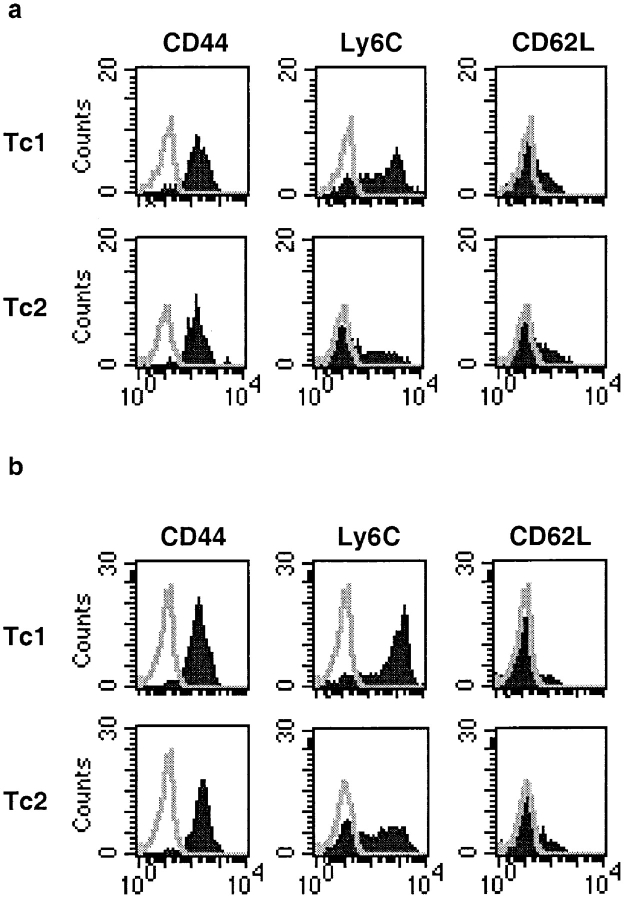
Next, we tested whether Tc1 and Tc2 effectors differed in the expression of surface markers when reisolated from different sites during influenza virus infection. Representative staining profiles of Tc1 and Tc2 effectors isolated from TBLN and lung tissue on day 5 is shown in Fig. 7. Histograms are gated on live Thy 1.2–positive cells and similar results were obtained when donor cells were phenotyped on days 3 and 7 after infection. We have previously described that before adoptive transfer both Tc1 and Tc2 effectors express high levels of the activation markers CD44 and CD25, and a low level of CD62L on day 4 after in vitro primary stimulation (10). On day 5 after adoptive transfer into influenza-infected hosts, both Tc1 and Tc2 donor cells were CD44high and CD62Llow regardless of whether they were isolated from the lymph node or lung lavage. Expression of CD25 was low in both Tc1 and Tc2 effectors isolated on day 5 in vivo (data not shown). Before adoptive transfer on day 4 of primary in vitro stimulation, both Tc1 and Tc2 effectors were Ly6Clow (10). However, on day 5 after adoptive transfer, Tc1 and Tc2 effectors differed profoundly with regard to the expression of the surface marker Ly6C. On day 5 after infection and cell transfer Tc1 effectors had upregulated high levels of Ly6C in all tissues sampled, whereas expression of Ly6C on Tc2 effectors was low in the lymph node and lung lavage. The absence of Ly6C might be a distinct feature of the Tc2 subset at early time points during influenza infection in vivo.
Figure 7.
Phenotype of Tc1 and Tc2 donor cells upon re-isolation from infected hosts. 107 Tc1 and Tc2 effectors expressing Thy 1.2 were adoptively transferred into influenza-infected Thy 1.1 BALB/c hosts. On day 5 after infection and cell transfer, TBLN (a) or lung lavages (b) of recipients of Tc1 or Tc2 effectors were double stained with FITC-conjugated mAbs directed against CD44, Ly6C, and CD62L, respectively and with PE-conjugated Thy 1.2 mAb analyzed on a FACScan®. Histograms were gated on Thy 1.2–positive and PI-negative live cells. Data are representative of three similar experiments performed.
Tc1 and Tc2 Effectors Retain Their Cytokine Polarization and Cytolytic Activity In Vivo.
We have shown previously that the cytokine phenotype of Tc1 and Tc2 effectors is stable and that in the apparent absence of antigen stimulation Tc1 and Tc2 effectors gave rise to Tc1 and Tc2 memory cells, respectively (10). However, in this study we transferred Tc1 and Tc2 effectors into influenza-infected hosts. A virus infection creates a microenvironment where high levels of a variety of cytokines are produced, which might then influence the polarization of the transferred donor CD8 effectors studied. In addition, it was important to establish whether Tc1 and Tc2 effectors retained their polarization pattern after the transfer in vivo, particularly because our Tc2 effectors always produced a low level of IFN-γ (Table I). To this end we isolated CD8 T cells from lung digests from influenza-infected hosts (BALB/c Thy 1.1), which were adoptively transferred with either Tc1 or Tc2 effectors expressing Thy 1.2 on day 4 of infection and cell transfer. CD8 T cells were enriched and similar cell numbers of donor cells were restimulated with HA peptide–loaded P815 cells (Fig. 8 a). When cytokine production of the whole CD8 T cell population was determined, CD8 T cells of hosts that had received Tc1 effectors produced high amounts of IFN-γ and no IL-4 or IL-5. CD8 T cells from hosts that had received Tc2 effectors produced IL-4 and IL-5, but no production of IFN-γ could be observed. When we depleted donor cells from the cell preparation by depleting Thy 1.2–positive cells with mAb and complement before restimulation in vitro, no cytokine production was observed, indicating that at this time point all the polarized cytokine production was due to the presence of the donor cell population (data not shown). Similar patterns of cytokines were observed when donor cells were isolated from the TBLN and the spleen (data not shown). No cytokine production was detected by ELISA when cultures were restimulated with APCs in the absence of the HA peptide (data not shown). When the cytolytic activity was assessed from donor cells isolated from the lung tissue on day 4 after infection, both Tc1 and Tc2 effectors were equally cytolytic on a per cell basis assayed on HA peptide– loaded targets (Fig. 8 b). In this context it is important to note that only half the cell number of Tc2 effectors as compared with Tc1 effectors was found in the lung at this time point (day 4) and thus, if the figure were not corrected for donor cell input number, only half the cytolytic activity of the lung digest recipients of the Tc1 effectors would be found in the lung digest compared with recipients of Tc2 effectors. All the cytolytic activity found in the lung tissue at this time point was due to donor cells because no killing of HA peptide–loaded target cells was found when the effectors were depleted of donor (Thy 1.2–positive) cells before the cytolytic assays were set up.
Figure 8.

(a) Tc1 and Tc2 effectors retain their polarization profile during influenza infection in vivo. Tc1 and Tc2 effectors (107) were adoptively transferred into influenza-infected hosts. On day 4 after infection and cell transfer, CD8 T cells were enriched from the lung digest and similar numbers of Tc1 and Tc2 donor cells (Thy 1.2–positive cells) were restimulated with P815 loaded with the HA peptide (11 μM). Supernatants were collected after 24 h and cytokines were measured by specific ELISAs. No cytokines were detected when Tc1 and Tc2 effectors were restimulated in the absence of the HA peptide (data not shown). No cytokines were detected when the cell cultures were depleted of Thy 1.2 (donor cells)–positive cells, indicating that the observed cytokine production was due to the adoptively transferred Thy 1.2 donor cell population (data not shown). (b) CTL activity of Tc1 and Tc2 effectors upon reisolation from infected lung tissue. 107 Tc1 (▪) and Tc2 effectors (□) were adoptively transferred into influenza-infected hosts. On day 4 after infection and cell transfer, CD8 T cells were enriched from the lung digest and similar numbers of Tc1 and Tc2 donor cells (Thy 1.2– positive cells) were assayed in a 4-h 51Cr-release assay using P815 cells (104 cells/well) pulsed with the HA peptide (11 μM) as targets. Only little CTL activity was detected, and the effectors were depleted of Thy 1.2 (donor cells)–positive cells (♦) in vitro before the CTL assay, indicating that the observed CTL activity was indeed due to the adoptively transferred Thy 1.2 donor cell population.
Survival of Lethally Influenza Virus–infected Recipients of Tc1 and Tc2 Effectors Generated from the Clone 4 TCR Transgenic Mice on the Wild-type or IFN-γ−/− Background.
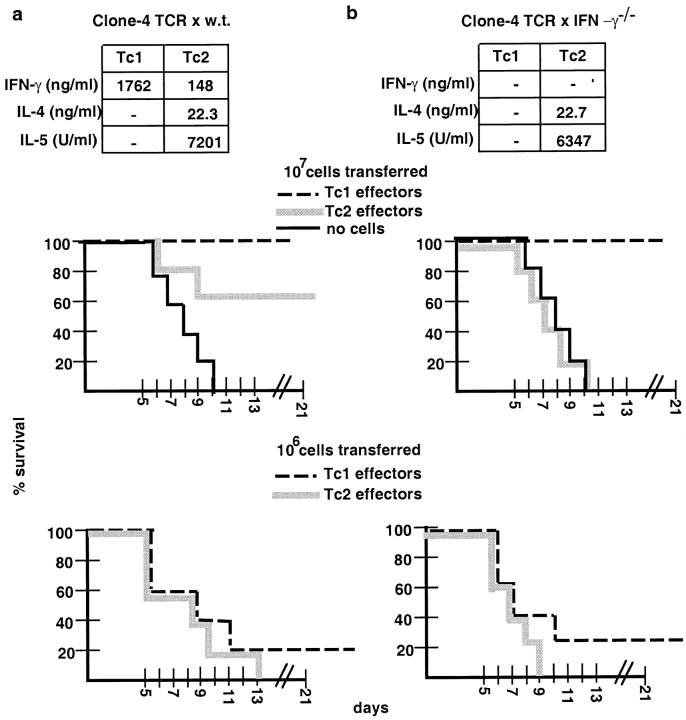
Finally, we tested whether Tc1 and Tc2 effectors differentially promoted host recovery from lethal infection with influenza virus (Fig. 9 a). A high dose (107 effector cells) and a low dose (106 effector cells), respectively, of Tc1 or Tc2 effectors generated from the clone 4 TCR transgenic mice on the wild-type background were adoptively transferred into syngeneic sex- and age-matched recipients that had been infected with 10 LD50 influenza virus intranasally 1 h before the adoptive transfer of effectors (Fig. 9 a). Survival was monitored until day 21 after infection. All animals that had not received HA peptide–specific effector CD8 T cells died between days 6 and 10, indicating that a lethal dose of influenza virus was administered. When a high cell number of Tc1 or Tc2 effector cells (107 cells) was adoptively transferred, all recipients of Tc1 effectors were protected and survived, whereas only 60% of Tc2 effector recipients survived. When lung function studies of these mice were performed, recipients of Tc2 effectors were found to be sicker at all time points as compared with the recipients of Tc1 effectors with a lower level of pO2 in their blood (Cerwenka, A., manuscript in preparation). At 106 Tc1 or Tc2 effector cells transferred, all hosts died except one recipient mouse of Tc1 effectors.
Figure 9.
Recipient survival after adoptive transfer of Tc1 and Tc2 effector cells into lethally influenza-infected hosts. Tc1 (dashed line) and Tc2 effectors (gray line) generated from the clone 4 TCR transgenic mice on the wild-type (a) or on the IFN-γ−/− background (b) were prepared as described in Materials and Methods. Cytokine production of the effector populations before adoptive transfer is indicated. On day 4 of primary stimulation, cells were washed and 107 (center panel) or 106 (bottom panel) effector cells were adoptively transferred into sex- and age-matched BALB/c recipients, which were infected intranasally with 10 LD50 of the influenza virus PR8, approximately 1 h before the cell transfer. As a control no cells were injected into influenza-infected hosts (thin line) and all recipient animals died. The percentage of survival of five animals per group is presented for the time points indicated. Survival was monitored for 21 days. Data presented are representative for 4 (a) or 2 (b) independently performed experiments.
To investigate the role of IFN-γ in the protective value of Tc1 and Tc2 populations against pulmonary influenza virus infection, we performed parallel experiments with effectors generated from the clone 4 TCR transgenic mice crossed to the IFN-γ−/− background on BALB/c (Fig. 9 b). When Tc1 and Tc2 effectors from these mice were characterized with regard to cytokine production, we found that the levels of IL-4 and IL-5 in the Tc2 effectors were comparable to the wild-type, and neither Tc1 and Tc2 effectors produced any detectable IFN-γ (Fig. 9 b). Tc1 effectors from the wild-type and IFN-γ−/− backgrounds were found to be equally cytolytic before transfer (data not shown). Adoptive transfer of Tc1 and Tc2 effectors from clone 4 × IFN-γ−/− mice into influenza-infected mice was performed and survival was monitored. Fig. 9 b shows Tc1 effectors from the clone 4 × IFN-γ−/− mice at 107 cells transferred were still protective, indicating that IFN-γ production was not required. However, all recipients of Tc2 effectors generated from the clone 4 × IFN-γ−/− mice died.
Discussion
In this study we investigated the in vivo function and distribution of HA peptide–specific Tc1 and Tc2 CD8 T cell effectors during pulmonary influenza virus infection. We show that Tc1 effectors, but not Tc2 effectors, very efficiently reduced the pulmonary virus titer (Table II), despite the fact that both populations were highly cytolytic before (Fig. 2) and after (Fig. 8 b) adoptive transfer. Tc1 effectors were found in high numbers in the lung lavage on day 3 after infection and cell transfer. Tc2 effectors, however, entered or accumulated in the lung tissue with delayed kinetics (Figs. 3 and 4). In addition, Tc1 and Tc2 effectors localized to distinct sites within the infected lung tissue (Fig. 5): Tc1 effectors predominately localized near the virus-infected airway epithelium, whereas Tc2 effectors were found more widely scattered in the alveolar parenchyma distant from the infected airway epithelium. Furthermore, we demonstrate that Tc1 and Tc2 effectors differed profoundly in their expression of chemokine receptors, which might account for their different migratory behavior (Fig. 6, A and B).
In general, the effectiveness of a CD8 T cell population to protect a previously unexposed animal against a confined replicating organism is determined by its ability to provide cell-mediated immunity before uncontrolled viral growth has taken over (1, 24). Complex mechanisms could be involved in the observed differential protection of virus- infected hosts after adoptive transfer of Tc1 and Tc2 effector populations.
Protection could be due to the adoptive transfer of a high frequency of virus-specific CD8 T cells and might not directly be the result of the effector potential of the transferred T cell population. In this context, we observed that the adoptive transfer of high numbers of naive unprimed HA peptide–specific CD8 T cells did not result in protection but rather in accelerated death of the recipients (Cerwenka, A., manuscript in preparation). Thus, the protection observed with Tc1, and to a lesser extent with Tc2 effectors, was not simply due to the higher frequencies of influenza virus-specific cells but rather the result of the effector quality of the transferred CD8 T cell population.
We defined the Tc1 and Tc2 effector cell populations with regard to the cytokines produced in vitro and in vivo (Figs. 1 and 8 a). On day 4 after primary in vitro stimulation, the Tc1 effectors made high amounts of IFN-γ and also expressed a high level of mRNA for TNF-β, LT-β, and TNF-α. In contrast, Tc2 effectors made IL-4, IL-5, and little IFN-γ, and expressed high levels of mRNA specific for IL-10 and IL-13. Tc2 effectors expressed lower levels of RNA specific for TNF-β, LT-β, and TNF-α, which almost disappeared upon restimulation. Our data with polarized CD8 T cells showing selective expression of LT-β within the Tc1 subset is in concordance with data recently published for CD4 subsets, suggesting that surface LT-α/β is a surface marker for the Th1 subset (25). In our previous study, we showed that upon adoptive transfer into athymic, bone marrow–reconstituted animals (10) or normal recipients (data not shown), Tc1 or Tc2 effectors gave rise to Tc1 or Tc2 memory cells, respectively. This indicates that the polarized cytokine phenotype is a stable feature of CD8 subsets. In this study, we investigated whether Tc1 and Tc2 populations retained their polarized cytokine profile in virus-infected hosts where presumably high amounts of a variety of cytokines were produced. We also found that in this situation the cytokine-producing phenotype was remarkably stable (Fig. 8 a). Several studies showed that during pulmonary influenza infection a variety of cytokines were expressed within both the CD4 and the CD8 subsets, which could not be clearly categorized into the type 1 or the type 2 pattern (17). This study dissects the role of CD8 T cells as a source of polarized cytokines. Cytokines and soluble factors produced by Tc1 and Tc2 effectors might not only be involved in host protection but might also mediate inflammation in the lung tissue and thus influence the severity of lung immunopathology.
IFN-γ, predominantly produced within the Tc1 subset, has been shown to upregulate MHC class I expression on antigen expressing cells (26), and thus might enhance the expression of viral antigens on lung epithelium cells and thereby accelerate viral clearance. To investigate the role and requirement of IFN-γ in our model, we tested the protective value of Tc1 and Tc2 effectors generated from the clone 4 TCR transgenic mice crossed to the IFN-γ−/− background (Fig. 9, a and b). All recipients of Tc1 effectors, regardless whether they were on the wild-type or on the IFN-γ−/− background, were protected. Pulmonary virus titer in lungs of recipients of Tc1 effectors from either the wild-type and IFN-γ−/− background on day 4 after infection were not significantly different (data not shown). This suggests a minor role of IFN-γ in our experimental model. Our finding is in concordance with the observation that mice lacking IFN-γ successfully cleared pulmonary influenza virus infection (27). We speculate that the Tc1 effectors were more effective than the Tc2 effectors in viral clearance because they arrived at the site of infection earlier in high cell numbers, halting the spread of virus, and homed more efficiently to the infected airway epithelium.
The type 2 cytokines IL-4 and IL-5 have been shown to be mediators of severe lung inflammation in several disease models, including viral (28) and allergy (29) models. Thus far, the in vivo effects of type 2 cytokines have been generally studied with regard to CD4 T cells. Thus, the significance of type 2 cytokine production within the CD8 populations has not previously been defined. Adoptive transfer of influenza-specific CD4 T cell clones into lethally infected hosts resulted in influenza virus clearance when an influenza-specific Th1 clone was adoptively transferred but not when a Th2 clone was transferred (28). This protection was shown to depend on the presence of B cells (30). Adoptive transfer of Th2 effectors caused a higher number of eosinophils in the lung and it was speculated that the lack of protection with the Th2 clone was due to the immunopathology caused by this subset (28). Indeed, we observed infiltrates of inflammatory cells including eosinophils in recipient lungs of Tc2 effectors (Fig. 5 and Cerwenka, A., manuscript in preparation) and we are currently further investigating the immunopathology mediated by type 2 CD8 T cells. As in the study by Graham et al. (28), we transferred Tc1 and Tc2 effectors into normal hosts and it will be interesting to determine whether the transferred Tc1 and Tc2 populations will alter the humoral influenza-specific response of the hosts.
Contact-dependent cytolytic activity appears to be the key effector mechanism used by CD8 T cells during influenza virus infection (5). By using bone-marrow chimeras, Topham et al. (5) demonstrated that perforin-mediated cytotoxicity was the preferred mode, although fas-based cytotoxicity can apparently serve as an alternative mechanism. When we tested the cytolytic activity of Tc1 and Tc2 subsets before adoptive transfer, we found that both subsets were cytolytic regardless of the concentration of the HA peptide loaded onto specific targets (Fig. 2). Our previous study showed that cytotoxicity was mainly perforin-mediated with a small component of fas-mediated cytotoxicity (11). It had been shown that chronic stimulation of IL-4– producing CD8 T cells with PMA and ionomycin resulted in a loss of cytotoxicity (31). It was thus important to establish whether the adoptively transferred Tc1 and Tc2 populations retained cytolytic activity on a per cell basis in vivo. Indeed, both Tc1 and Tc2 effectors reisolated from the lung tissue on day 4 after influenza infection and cell transfer were equally cytolytic on a per cell basis, ruling out the possibility that the in vivo microenvironment had caused a loss of cytolytic activity within the Tc2 subset (Fig. 8 b). To kill virus-infected epithelial cells, the CD8 effector cells have not only to enter the site of infection, i.e., the lung, but also have to migrate to the site of infection, i.e., the virus-infected epithelium within the tissue site. Thus, despite the fact that both Tc1 and Tc2 effectors were equally cytolytic before adoptive transfer and were still equally cytolytic when reisolated from influenza-infected hosts on a per cell basis, Tc2 effectors might be less effective in protecting influenza infected hosts because of their different migration pattern in vivo. First, Tc2 effectors entered the lung with delayed kinetics as compared with Tc1 effectors. On day 3 after influenza infection and cell transfer sixfold more Tc1 effectors as compared with Tc2 effectors were recovered from the lung lavage (Fig. 4, a and b). Second, Tc2 effectors that had entered the lung on day 5 did not localize predominantly at the virus-infected airway epithelium as did the more protective Tc1 effector cells, but rather were found within cellular infiltrates scattered throughout the alveolar air spaces and septa. When frozen sections were stained with an antibody staining specifically for H1N1 influenza virus on day 5 after infection, positive virus staining was detected only within the airway epithelium of recipients of Tc2 effectors, whereas almost all the virus was gone from the recipient lungs of Tc1 effectors (data not shown), which is consistent with the viral titers assessed by viral plaque assays (Table II).
A key finding of our study was a differential accumulation of Tc1 and Tc2 effector cells at the tissue site of a localized virus infection. The Tc1 cells appeared in the lung early (Fig. 4) and were found preferentially adjacent to the airway epithelium (Fig. 5), whereas the Tc2 appeared later and congregated more within clusters of inflammatory cells distant from the epithelium. The accumulation of an adoptively transferred cell population at a particular site in the recipient is the sum of the rate of entry into the tissue and the rate of cell division and cell death both before and after entry into the site. We can only speculate on the relative contributions of each to the present situation. With respect to proliferation, we have shown elsewhere (10) that adoptively transferred Tc1 and Tc2 effectors were equally effective at generating populations of CD8 memory cells when transferred into adult-thymectomized, bone marrow- restored recipients, which persisted at a slowly declining number for many weeks. Tc1 and Tc2 CD8 effector cells labeled with CFSE tracker dye (our unpublished observations) both lose label rapidly on adoptive transfer into Thy1 congenic normal mice, indicating rapid cell division, and there is no indication of any difference in the kinetics between the two populations. There are no significant numbers of cells at any of the sites we monitored at 17 h (lung lavage, TBLN, or spleen, Fig. 4). The number of transferred cells recovered in the spleen at day 3 was threefold smaller for the Tc2 than the Tc1 population (Fig. 4), but the numbers present in the lymph node were similar.
With regards to cell death, we have shown (our unpublished data) that both Tc1 and Tc2 effectors underwent activation-induced cell death when reexposed to antigen in vitro at a relatively slow rate and that there was no difference between the two populations. However, we do not know how these in vitro data relate to cell death of effector populations after the adoptive transfer into virus infected animals and our attempts to assay for cell death immediately ex vivo by annexin V staining remained technically unsatisfying (unpublished observation). Finally, with regard to differential homing, we have no direct evidence other than the observation that the two cell populations do accumulate at different locations (Fig. 5) in the lung and at different rates (Fig. 4). However, we do find that there is a very significant difference both in the expression of several chemokine receptors on the two populations and in the change in expression of these receptors upon restimulation in vitro, as further discussed below.
The regulation of lymphocyte migration is complex and might involve not only the expression of certain adhesion molecules such as selectins and integrins (20) but also chemokines and chemokine receptors. Little is known about the factors controlling migration into the lung or within the lung tissue. Differential expression of chemokine receptors was recently found with human Th1 and Th2 CD4 T cell clones (21, 22), but so far the question of expression of chemokine receptors with CD8 Tc1 and Tc2 subsets has not been addressed. Recent studies with human CD4 T cell clones not only correlated the expression of the chemokine RNA with receptor surface expression but also demonstrated functional significance of the expression of the chemokine receptors by migration assays. For example, macrophage inflammatory protein (MIP)-1β, a CCR5 agonist, or eotaxin, a CCR3 agonist, were shown to have chemotactic activity for Th1 and Th2 clones, respectively (22). Thus, it is likely that the differential expression of chemokine receptors by CD8 subsets is also of functional significance. Thus far, the studies done with human CD4 T cell clones, which represent a population of chronically stimulated cells, only looked at constitutive expression of chemokine receptors. In contrast, we were studying chemokine receptor expression by transgenic CD8 T cells of known specificity and defined stages of differentiation. Thus, we could monitor the expression of chemokine receptors during restimulation of primary effectors. We found that some chemokine receptors were lost upon re-stimulation (e.g., CXCR4 by Tc1 and Tc2) whereas others (e.g., CCR1, CCR5, and CCR2 by Tc2) were acquired at 24 h upon restimulation. The timing of up- and downregulation of chemokine receptors by Tc1 and Tc2 subsets during stimulation has to be tightly regulated to ensure the proper migration along certain chemokine gradients. In this study we analyzed the mRNA expression of five C-C chemokine receptors (CCR1-5) and two C-X-C chemokine receptors (CXCR2 and 4) of in vitro–generated Tc1 and Tc2 CD8 T cell effectors by RNase protection assay (Fig. 6). At t0, Tc1 effectors selectively expressed CCR5, the receptor for the chemokines MIP-1α and MIP-1β. Similar expression patterns were found with human Th1 and Th2 clones (22). CCR2, binding the chemokines monocyte chemoattractant protein (MCP)-1 and MCP-3, was found to be selectively expressed within the Tc1 subset before restimulation which has not yet been demonstrated with CD4 T cells. In addition, studies with CD4 T cells demonstrated that CCR4, binding to the thymus-and-activation–regulated chemokine (TARC), and CCR3, binding to eotaxin, were predominately expressed in the Th2 subset. These expression patterns are similar to those found in Tc1 and Tc2 subsets (Fig. 6, A and B). Studies with CD4 and our study with CD8 T cells show that type 1 and type 2 CD4 and CD8 effectors express similar patterns of chemokine receptors, respectively, and might follow a similar path of migration along certain chemokine gradients. The precise role of che-mokines and chemokine receptors in the migration patterns we have observed have yet to be defined.
In the field of adoptive immunotherapy, the challenge is to target an effective cell population to the right place to fight a tumor or a virus infection. In general, the protective value of a CD8 T cell population against disease might depend on the “effectiveness” to migrate to the “right” place at the right time and to there do the right thing. Our study, suggesting that localization of CD8 effector cells might be crucial for their protective value during influenza virus infection, might help the design for therapeutic intervention during disease involving lung inflammation.
Acknowledgments
The authors thank Drs. Linda Sherman and David Morgan for generously providing the clone 4 TCR transgenic mice and the influenza virus preparation; Joyce Reome for excellent technical help; Nancy Lepak and Dr. Lisa Tsui for helping with the RNase protection assays; and Dr. Susan Swain for critical reading of the manuscript.
Abbreviations used in this paper
- HA
hemagglutinin
- LT-β
lymphotoxin-β
- MDCK cells
Madine Derby canine kidney cells
- t0
before restimulation
- TBLN
tracheal bronchial lymph node
- Tc1/Tc2
CTLs producing type 1/type 2 cytokines
Footnotes
Adelheid Cerwenka was supported by the Schroedinger Stipendium (Project JO111-MED) from the Austrian government. This work was supported in part by National Institutes of Health grants AI7935 and AI36263.
References
- 1.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 2.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 3.Yap KL, Ada GL, McKenzie IFC. Transfer of specific cytotoxic lymphocytes protects mice inoculated with influenza virus. Nature. 1978;238:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 4.Lukacher AE, Braciale VL, Braciale TJ. In vivo function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 6.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 7.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–342. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 8.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 10.Cerwenka A, Carter LL, Reome JB, Swain SL, Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 11.Carter LL, Dutton RW. Relative perforin- and Fas-mediated lysis in T1 and in T2 CD8 effector populations. J Immunol. 1995;155:1028–1031. [PubMed] [Google Scholar]
- 12.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 13.Maggi E, Giudizi MG, Biagiotti R, Annunziato F, Manetti R, Piccinni MP, Parronchi P, Sampognaro S, Giannarini L, Zuccati G, Romagnani S. Th2-like CD8+T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994;180:489–495. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyle AJ, Erard F, Bertrand C, Walti S, Pircher H, Le Gros G. Virus-specific CD8+cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. 1995;181:1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi T, McGhee JR, Coffman RL, Beagley KW, Eldridge JH, Takatsu K, Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990;145:68–77. [PubMed] [Google Scholar]
- 16.Sarawar SR, Doherty PC. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgarth N, Brown L, Jackson D, Kelso A. Novel features of the respiratory tract T cell response to influenza virus infection: lung T cells increase expression of gamma interferon mRNA in vivoand maintain high levels of mRNA expression for interleukin-5 and IL-10. J Virol. 1994;68:7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falchetti R, Lanzilli G, Casalinuovo IA, Gaziano R, Palamara AT, Di Francesco P, Ravagnan G, Garaci E. Splenic CD4+ and CD8+ T cells from influenza immune mice concurrently produce in vitro IL-2, IL-4, and IFN-γ. Cell Immunol. 1996;170:222–229. doi: 10.1006/cimm.1996.0155. [DOI] [PubMed] [Google Scholar]
- 19.Meeusen ENT, Premier RR, Brandon MR. Tissue-specific migration of lymphocytes: a key role for Th1 and Th2 cells? . Immunol Today. 1996;17:421–424. doi: 10.1016/0167-5699(96)10055-4. [DOI] [PubMed] [Google Scholar]
- 20.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan JD, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8+ T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 24.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 25.Croft M, Gramaglia I, Mauri D, Ware CF. Lymphotoxin αβ-a marker for Th1 cells? . FASEB (Fed Am Soc Exp Biol) J. 1998;12:3614. . (Abstr.) [Google Scholar]
- 26.Fellous M, Nir U, Wallach D, Merlin G, Rubinstein M, Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci USA. 1982;79:3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham MB, Braciale VL, Braciale TJ. Influenza specific CD4 T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erard F, Wild MT, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD8−CD4−cells that make TH2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]