Abstract
We have previously reported (Badolato, R., J.M. Wang, W.J. Murphy, A.R. Lloyd, D.F. Michiel, L.L. Bausserman, D.J. Kelvin, and J.J. Oppenheim. 1994. J. Exp. Med. 180:203; Xu, L., R. Badolato, W.J. Murphy, D.L. Longo, M. Anver, S. Hale, J.J. Oppenheim, and J.M. Wang. 1995. J. Immunol. 155:1184.) that the acute phase protein serum amyloid A (SAA) is a potent chemoattractant for human leukocytes in vitro and mouse phagocytes in vivo. To identify the signaling mechanisms, we evaluated patterns of cross-desensitization between SAA and other leukocyte chemoattrctants. We found that the chemotactic bacterial peptide, N-formyl- methionyl-leucyl-phenylalanine (fMLP), was able to specifically attenuate Ca2+ mobilization in human phagocytes induced by SAA, but only at very high concentrations, suggesting that SAA uses a low affinity fMLP receptor. Here we demonstrate that SAA selectively induced Ca2+ mobilization and migration of HEK cells expressing FPRL1, a human seven-transmembrane domain phagocyte receptor with low affinity for fMLP, and high affinity for lipoxin A4. Furthermore, radiolabeled SAA specifically bound to human phagocytes and FPRL1-transfected 293 cells. In contrast, SAA was not a ligand or agonist for FPR, the high affinity fMLP receptor. Thus, SAA is the first chemotactic ligand identified for FPRL1. Our results suggest that FPRL1 mediates phagocyte migration in response to SAA.
Keywords: serum amyloid A, FPRL1, chemotaxis, calcium flux, receptor
Serum amyloid A (SAA),1 an acute phase protein, is normally present in serum at 0.1-μM levels, but increases by 1,000-fold in systemic inflammatory conditions (1–4). It has been proposed that SAA is mainly involved in lipid transportation and metabolism (1–3). Chronic inflammatory conditions with elevated serum SAA may culminate in amyloidosis, characterized by deposition of “amyloid” fibrils in tissues and associated with progressive destruction of organ function (2–4). Although a number of acute phase proteins are known to modulate host immune responses, we recently reported that recombinant human (rh)SAA exhibited considerable chemoattractant activity for human monocytes, neutrophils, and T lymphocytes in vitro (5, 6). rhSAA also induced infiltration of phagocytic cells and T lymphocytes into injection sites in mice (5, 6), suggesting that SAA, when present locally, may play a proinflammatory role by recruiting immune cells.
Since SAA induced significant Ca2+ mobilization in phagocytes (7), and both its chemotactic and Ca2+ mobilizing effects were inhibitable by pretreatment of the leukocytes with pertussis toxin, we proposed that SAA may use seven-transmembrane, G protein–coupled receptor(s) (6, 7). In an effort to identify the receptor(s) for SAA, we carefully evaluated cross-desensitization of Ca2+ mobilization in monocytes and neutrophils induced by SAA and other chemoattractants. Among a number of chemoatrractants tested, only the bacterial chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP), when used at relatively high concentrations (10 μM and more), was able to attenuate a subsequent cell response to SAA, suggesting that SAA may use a receptor on leukocytes for which fMLP has low affinity.
Two receptors that interact with fMLP have been identified and molecularly cloned (for review see references 8, 9). The prototype receptor FPR bound fMLP with high affinity and was activated by low nanomolar concentrations of fMLP. The other, a highly homologous variant of FPR, named FPRL1 (also referred to as FPRH2 and LXA4R), was originally cloned as an orphan receptor (10–14) but was subsequently found to mediate Ca2+ mobilization in response to high concentrations of fMLP (11, 13). Furthermore, a lipid metabolite, lipoxin A4 (LXA4), and its analogues were subsequently found to bind FPRL1 with high affinity and to increase arachidonic acid production and G protein activation in FPRL1-transfected cells (15). LXA4 inhibits proinflammatory neutrophil responses (15–23) as well as the release of the proinflammatory cytokine, IL-8, by epithelial cells (20, 24). These effects of LXA4 have been attributed to the activation of FPRL1 (or LXA4R) in neutrophils and epithelial cells. Another lipid mediator receptor, the leukotriene B4 receptor, is structurally related to FPRL1 (30.7% amino acid sequence identity; reference 25), and was also reported to be a fusion co-factor for HIV-1 (26), similar to various chemokine receptors (for review see reference 27). This activity has not been reported for FPRL1 (9, 27). Because SAA might use a low affinity fMLP receptor on phagocytes, we further investigated whether FPRL1 could be activated by SAA and demonstrate that SAA uses FPRL1 as a functional receptor.
Materials and Methods
Reagents and Cells.
rhSAA was purchased from Pepro Tech Inc. with the sequence as follows: MRSFFSFLGEAFDGARDMWRAYSDM REANYIG SDKYFHAR GNYDAAKRGPGGV-WAAEAISNARENIQRFFGRGAEDSLADQAANEWGRSGK- DPNHFRPAGLPEKY.
This rhSAA corresponds to SAA-1α, one of the major SAA isoforms in the serum, except for the addition of a methionine at the NH2 terminus as well as the substitution of aspartic acid for asparagine at position 60, which appears in the SAA2 isoform (for review see reference 28). rhSAA at concentrations used in the study was negative for endotoxin as assessed by Limulus amebocyte lysate assays (sensitivity: 0.06 IU/ml; BioWhittaker). High density lipoprotein (HDL) was purchased from Sigma Chemical Co. Human peripheral blood enriched in mononuclear cells or neutrophils was obtained from normal donors by leukapheresis (courtesy of the Transfusion Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD). The blood was centrifuged through Ficoll-Hypaque (Sigma Chemical Co.), and PBMCs collected at the interphase were washed with PBS and centrifuged through a 46% isoosmotic Percoll (Pharmacia, Uppsala, Sweden) gradient followed by elutriation to yield monocytes (purity: >90%). Neutrophils were purified by 3% dextran/PBS sedimentation as described elsewhere (5) and were >98% pure. The cells were resuspended in RPMI 1640 medium containing 10% FCS (Hyclone) for future use. The molecular cloning of the receptors for fMLP has been described previously (10, 13, 29, 30). The cDNAs encoding classical formyl peptide receptor FPR and its variant FPRL1 were stably transfected into human embryonic kidney epithelial cell line 293, which was cultured in DMEM in the presence of 800 μg/ml geneticin (G418; GIBCO BRL) to maintain selection. A rat basophil leukemia cell line stably transfected with FPR (ETFR cells) was also used in the study (gift from Drs. H. Ali and R. Snyderman, Duke University Medical Center, Durham, NC).
Chemotaxis.
The migration of human 293 cells expressing FPR (FPR/293) or FPRL1(FPRL1/293) as well as ETFR cells was assessed by a 48-well microchemotaxis chamber technique (31, 32). A 25-μl aliquot of rhSAA or other reagents diluted in chemotaxis medium (RPMI 1640, 1% BSA, 25 mM Hepes) was placed in the wells of the lower compartment, and 50 μl cell suspension (106 cell/ml in chemotaxis medium) were placed in the wells of the upper compartment of the chamber (Neuroprobe, Cabin John, MD). The two compartments were separated by a polycarbonate filter (10 μm pore size, Neuroprobe) coated with 50 μg/ml collagen type I (GIBCO BRL) for 1 h at 37°C. The chamber was incubated at 37°C for 5 h in humidified air with 5% CO2. At the end of the incubation, the filter was removed, fixed and stained with Diff-Quik (Harlew, Gibbstown, NJ). The number of migrated cells in three high-powered fields (400×) was counted by light microscopy after coding the samples. Results are expressed as the mean (± SD) value of the migration in triplicate samples and are representative of at least five experiments performed. For better illustration, chemotaxis indices (CI) reflecting the fold increase of cell migration in response to stimulant over medium are used. Statistical significance of the difference between numbers of cells migrating in response to stimuli versus baseline (migration toward control medium) was calculated with Student's t test and the CI ≥ 2 are statistically significant.
Calcium Mobilization.
Calcium mobilization was assayed by incubating 107/ml of monocytes, neutrophils, or receptor cDNA transfectants in loading buffer containing 138 mM NaCl, 6 mM KCl, 1 mM CaCl2, 10 mM Hepes (pH 7.4), 5 mM glucose, and 0.1% BSA with 5 μM Fura-2 (Sigma Chemical Co.) at 37°C for 30 min. The dye-loaded cells were washed and resuspended in fresh loading buffer. The cells were then transfered into quartz cuvettes (106 cells in 2 ml) that were placed in a luminescence spectrometer LS50 B (Perkin-Elmer Limited). Stimulants at different concentrations were added in a volume of 20 μl to the cuvettes at indicated time points. The ratio of fluorescence at 340 and 380 nm wavelengths was calculated using the FL WinLab (Perkin Elmer) program. The assays were performed at least five times and results from representative experiments are shown.
Ligand Binding Assays.
rhSAA (20 μg) was radio-iodinated on tyrosine residues with the chloramine T method and the specific activity of the labeled SAA was 5.8 mCi/mg (courtesy of J. Dobbs, SAIC Frederick, NCI-FCRDC, Frederick, MD). A constant concentration of 16 nM 125I-labeled SAA was incubated for 20 min at 37°C with human monocytes or 293 cells transfected with chemoattractant receptor cDNAs (1.5–2 × 106/sample, in 200 μl RPMI 1640, 1% BSA, 0.05% NaN3) in the presence of increasing concentrations of unlabeled SAA. After incubation, the cells were washed once with ice-cold PBS then were layered onto a 10% sucrose/PBS cushion in Eppendorf tubes. The cells were centrifuged at 10,000 g for 1 min and the tips of the tubes containing cell pellets were cut and measured for radioactivity in a gamma counter. The binding data were analyzed and plotted with a computer-aided program LIGAND (P. Munson, Division of Computer Research and Technology, NIH, Bethesda, MD). The level of specific binding was determined by subtraction of nonspecific binding (cpm on cells in the presence of 1 μM unlabeled SAA) from the total binding (cpm on cells in the absence of unlabeled SAA). Experiments were performed at least five times, yielding similar results each time.
Results
Assays of Ca2+ mobilization have provided a useful approach to identify ligands for chemoattractant receptors. In primary cells, cross-desensitization of Ca2+ transients is often due to two agonists acting at the same receptor (33). Since SAA induced Ca2+ mobilization in phagocytes (7), we used cross-desensitization to characterize the molecular nature of SAA receptor(s). In a series of cross-desensitization experiments, SAA at 1 μM did not desensitize the Ca2+ flux in monocytes or neutrophils induced by chemokines such as monocyte chemotactic protein (MCP)-1, RANTES, MCP-3, macrophage inflammatory protein (MIP)-1α, IL-8, and stromal cell–derived factor (SDF)-1α (data not shown). Therefore, SAA is unlikely to share a receptor with any of the chemokines tested. SAA also did not attenuate the cell response to the bacterial chemotactic N-formylated peptide fMLP when fMLP was used at 100 nM (10−7 M) (Fig. 1 A). However, in reciprocal tests, fMLP at 100 nM showed a partial desensitizing effect on SAA- induced Ca2+ mobilization in monocytes (Fig. 1 B). Furthermore, the cell response to SAA was completely desensitized by higher concentrations of fMLP (10−3 M = 1 mM, Fig. 1 C), suggesting that SAA might use a receptor(s) for which fMLP has low affinity.
Figure 1.

Cross-desensitization of Ca2+ mobilization in human monocytes between SAA and fMLP. Fura-2–loaded monocytes were sequentially stimulated with SAA and fMLP (A) or vice versa (B and C), and the ratio of fluorescence at 340 and 380 nm wavelengths was recorded and calculated with the FLWinLab program.
Since fMLP is known to induce Ca2+ mobilization in phagocytes through at least two seven-transmembrane, G protein–coupled receptors, FPR and FPRL1 (10, 11, 13, 29), we tested the effect of SAA using cells transfected to express these receptors that originally were not responsive to fMLP stimulation. fMLP in a wide range of concentrations induced Ca2+ mobilization in FPR-transfected rat basophil leukemia cell line (ETFR cells), with an EC50 of 10 pM (data not shown). In contrast, the EC50 for fMLP to induce Ca2+ mobilization in FPRL1 transfected cells (FPRL1/293 cells) was much higher at 10 μM (Fig. 2 A). These results confirmed the previous observation that FPR is a high affinity receptor for fMLP, whereas FPRL1 has a much lower affinity (10, 11, 13, 29). rhSAA induced Ca2+ mobilization in cells transfected with FPRL1 (FPRL1/293 cells; Fig. 2 B), but not in FPR-expressing cells or mock-transfected 293 cells (Fig. 2, C and D). The EC50 of rhSAA on FPRL1 transfected cells was 250 nM, suggesting that SAA activates FPRL1 with higher efficacy than fMLP. This was supported by studies of cross-desensitization of Ca2+ flux between SAA and fMLP in FPRL1/293 cells. As shown in Fig. 2 E, although sequential stimulation of FPRL1/293 cells with SAA and fMLP resulted in bidirectional desensitization, SAA was able to desensitize the cell response to a 100-fold excess of fMLP. In contrast, fMLP at 100-fold excess of SAA only partially desensitized the effect of SAA (Fig. 2 E).
Figure 2.
Calcium mobilization in FPRL1-transfected HEK 293 cells. FPRL1/293 cells were loaded with Fura-2 and were stimulated with various concentrations of fMLP (A) or SAA (B). SAA does not induce Ca2+ mobilization in FPR-expressing 293 cells (C) or mock-transfected 293 cells (D). E shows the sequential stimulation of FPRL1/293 cells with SAA and fMLP or vice versa.
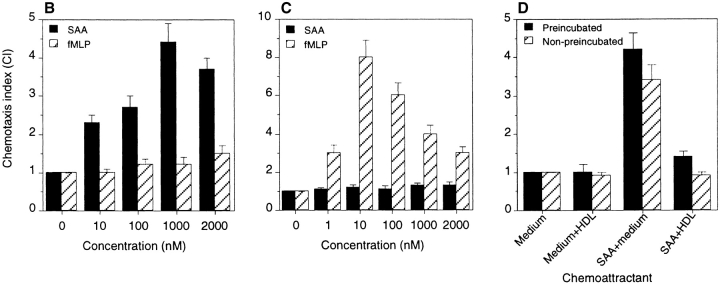
Leukocyte infiltration in vivo is considered to be based on migration of cells toward a gradient of locally produced chemoattractant(s). This process can be emulated by in vitro assays of chemotaxis, which provides a very sensitive and biologically relevant means of evaluating the function of cloned chemoattractant receptors (32–35). Since SAA has been shown in our previous studies to induce leukocyte infiltration in vivo and chemotaxis in vitro (5, 6), we next investigated whether SAA could induce cell migration via FPRL1. FPRL1/293 cells showed a potent migratory response to SAA with an EC50 of 200 nM (Fig. 3 A), but these cells failed to migrate in response to a wide range of concentrations of fMLP (Fig. 3 B). In contrast, fMLP induced migration of ETFR cells at nanomolar range concentrations, whereas the same cells did not migrate in response to SAA (Fig. 3 C). The chemotaxis experiments indicate that fMLP is only a partial agonist for FPRL1 since it did not induce cell migration through FPRL1. On the other hand, SAA showed full agonist activity on FPRL1. Both SAA-induced Ca2+ mobilization and chemotaxis in FPRL1/293 cells were inhibited by pretreatment of the cells with pertussis toxin but not cholera toxin (data not shown) in correlation with the observation in native cells (5–7), suggesting activation of G protein of the Gi type is required for SAA signaling through FPRL1. In addition, since SAA can form complexes with HDL, which acts as a natural inhibitor of SAA (5, 6), we examined the effect of HDL on the chemotactic activity of SAA for FPRL1/293 cells. Fig. 3 D shows that HDL, whether preincubated with SAA or simultaneously added to SAA, completely abolished SAA-induced FPRL1/293 cell migration. In contrast, the same concentration of HDL did not affect migration of FPR-expressing ETFR cells induced by fMLP (data not shown). These results confirmed that HDL specifically inhibited the agonist activity of SAA on FPRL1.
Figure 3.

Chemotactic activity of SAA for human monocytes and cells transfected to express chemoattractant receptors. Different concentrations of SAA were placed in the lower wells of the chemotaxis chamber. Monocytes, FPRL1/293 cells, or FPR-expressing ETFR cells were placed in the upper wells. After incubation, the cells migrated across the polycarbonate filter were counted and photographed (A: SAA 0.8 μM, fMLP 100 nM). The cell migration was expressed as CI representing the fold increase of the cells migrating in response to stimulants over control medium. (B) Migration of FPRL1/293 cells in response to SAA and fMLP. (C) Migration of FPR-expressing ETFR cells in response to SAA and fMLP. (D) Effect of HDL on SAA induced FPRL1/293 cell migration. HDL at 1,000 μg/ml mixed with 0.8 μM SAA was preincubated at 37°C for 4 h. The mixture was then tested for chemotactic activity on FPRL1/293 cells. The HDL/SAA mixture without preincubation was also tested for chemotactic activity and yielded similar results.
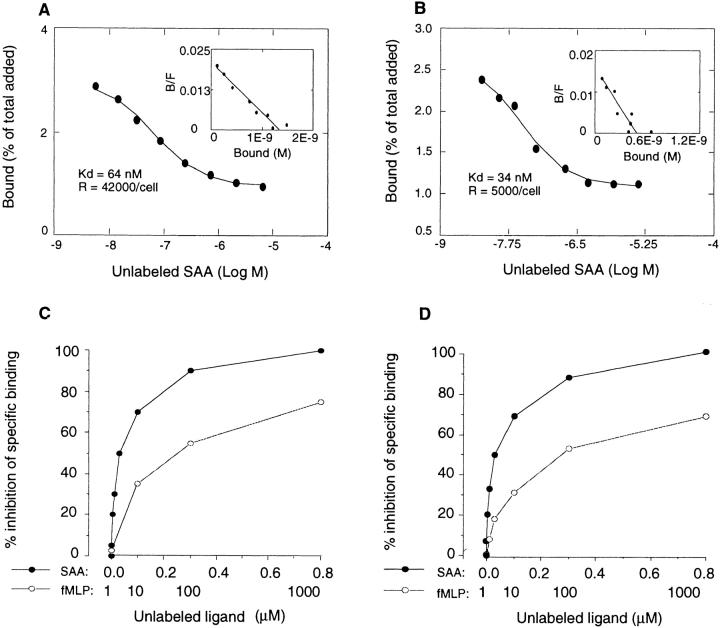
To further verify the usage of FPRL1 by SAA, we performed ligand binding experiments. Fig. 4 shows that radio-iodinated SAA specifically bound to FPRL1/293 cells with an estimated K d at 64 nM and 42,000 binding sites per cell (Fig. 4 A). 125I-labeled SAA also specifically bound to monocytes (Fig. 4 B) and neutrophils (K d = 45 nM, R = 6,700/cell) with K d values comparable to those achieved with FPRL1/293 cells. In the displacement assay, unlabeled SAA in a dose-dependent manner inhibited its own binding to monocytes (Fig. 4 C), neutrophils (data not shown) and FPRL1/293 (Fig. 4 D) with an IC50 at ∼50 nM. In contrast, unlabeled fMLP at high concentrations (≥10 μM) only partially competed with 125I-SAA for binding. These results confirm SAA to be a far more efficient agonist for FPRL1 than fMLP.
Figure 4.
Binding of 125I-labeled SAA to human monocytes and FPRL1/293 cells. rhSAA was radio-iodinated with chloramine T method and the binding of 125I-labeled SAA to FPRL1/293 cells (A) or monocytes (B) was measured by adding a constant concentration of 125I-labeled SAA to the cells in the presence of increasing concentrations of unlabeled SAA. The data was analyzed and plotted with the Macintosh computer aided program LIGAND. C shows the displacement of 125I-labeled SAA binding on monocytes by unlabeled SAA and fMLP. The same results were obtained with neutrophils (data not shown) and FPLR1/293 cells (D).
Discussion
In this study, we demonstrate that SAA uses FPRL1, a seven-transmembrane, G protein–coupled receptor expressed on phagocytes as a chemotactic receptor, suggesting a molecular basis for our previous observations that SAA is a potent chemoattractant and activator for human peripheral blood monocytes and neutrophils (5–7). In addition to SAA, FPRL1 has previously been shown to be a low affinity receptor for fMLP (11, 13) and a high affinity receptor for lipid metabolite LXA4 and its analogues (15–24). Our data suggest that fMLP is a partial agonist incapable of inducing chemotaxis via FPRL1 in this model system. Analysis of LXA4 induction of chemotaxis via FPRL1 has not been reported. Thus, SAA is the first chemotactic agonist identified for FPRL1.
FPRL1 was identified and molecularly cloned from human phagocytic cells by low stringency hybridization of the cDNA library with the FPR sequence and initially was defined as an orphan receptor (10, 11, 13, 14). The cloning of the same receptor termed FPRH2 from a genomic library was described by Bao et al. (12). FPRL1 possesses 69% identity at the amino acid level to FPR, the prototype receptor for synthetic and bacterium-derived formylated peptides (8, 9). Both FPR and FPRL1 are expressed by monocytes and neutrophils and are clustered on human chromosome 19q13 (12, 36). Although fMLP is a high affinity agonist for FPR, it interacts with FPRL1 and transduces signals in response to fMLP only at high concentrations (Fig. 2 and references 11, 13, 36). SAA, on the other hand, selectively bound and activated only FPRL1 at physiologically relevant concentrations, which under inflammatory stimulation could reach 80 μM in the serum (1–4). FPRL1 is mainly expressed in monocytes and neutrophils. However, cells other than phagocytes, such as hepatocytes, have also been shown to express FPRL1 (8). Recently, the expression of this receptor (also termed LXA4R) has been reported to be highly inducible in epithelial cells by specific cytokines (20). Our previous study showed that CD3+ human peripheral blood T lymphocytes were induced by SAA to migrate and adhere to endothelial cell monolayers (6), suggesting that T lymphocytes may also express a receptor(s) for SAA. In fact, we detected specific binding sites for 125I-labeled SAA on human peripheral blood CD3+ T lymphocytes (K d = 300 nM, R = 2,200 sites/cell). However, whether these binding sites on T lymphocytes represent FPRL1 or an additional receptor(s) for SAA is not yet known.
Despite the fact that the chemotactic formyl peptide fMLP has been shown to be a low efficiency agonist for FPRL1, a lipid metabolite LXA4 has been reported to be a high affinity ligand and potent agonist for this receptor (15). LXA4 is an eicosanoid generated during a number of host reactions such as inflammation, thrombosis, and atherosclerosis (22), and was initially discovered as an inhibitor of immune response (for review see reference 37). LXA4 was subsequently reported to inhibit neutrophil chemotaxis (38) and transepithelial migration induced by chemotactic agents (23). A seven-transmembrane, G protein–coupled receptor identical to FPRL1 was recently identified for LXA4 (15, 16, 22). LXA4 bound to CHO cells transfected with this receptor with high affinity and increased GTPase activity and the release of esterified arachidonate (15). Thus, LXA4 has been proposed to be an endogenously produced ligand for FPRL1 (15, 16). Although LXA4 has not been documented to induce Ca2+ mobilization in neutrophils or FPRL1-transfected cells (15), it was reported to induce Ca2+ flux and chemotaxis in monocytes, presumably through FPRL1 (17, 22). Thus, differential activation of second messengers in monocytes versus neutrophils by LXA4 was postulated. In our study, we did not detect significant induction of Ca2+ flux or chemotaxis in FPRL1/ 293 cells by a commercially available LXA4 (Biomol, Plymouth Meeting, PA), nor did we observe inhibition of SAA signaling or binding by this LXA4 in either phagocytes or FPRL1/293 cells. Further study, beyond the scope of this report, will be needed to compare the interaction of FPRL1 with its peptide ligands, SAA and fMLP, versus its lipid ligand, LXA4, to clarify these results.
Our previous studies showed that both SAA-induced leukocyte chemotaxis and activation were inhibited by pertussis toxin (6, 7). This study also showed that the signaling of SAA through FPRL1 was sensitive to pertussis toxin. Thus, although the signal transduction pathways triggered by SAA in FPRL1 requires further investigation, the high level homology of FPRL1 to FPR, its sensitivity to pertussis toxin, and its mediation of potent phagocyte activation by SAA suggest that FPRL1 may share major biochemical events with FPR. It is well known that binding of FPR by bacterium-derived or synthetic peptide agonists results in a G protein–mediated signaling cascade leading to phagocytic cell adhesion, chemotaxis, release of oxygen intermediates, enhanced phagocytosis, and bacterial killing, as well as gene transcription (8, 9). Activation of FPR by its agonists can also result in heterologous desensitization of the subsequent cell response to other G protein receptor ligands (39, 40), including chemokines. This “desensitizing” effect of FPR activation may also be seen with FPRL1, although more studies are needed to elucidate the mechanism(s) involved. For instance, SAA was initially reported as an inhibitor of neutrophil response to fMLP (41). In these experiments, neutrophils preincubated with SAA showed reduced superoxide release in response to fMLP (41). Our previous study also showed that preincubation of monocytes and neutrophils with SAA reduced cell response to a number of chemoattractants, including fMLP and chemokines (7), suggesting that FPRL1 is capable of transducing intracellular biochemical events leading to desensitization of other G protein–coupled receptors.
The pathophysiological significance of use of FPRL1 by SAA requires more in-depth investigation. The optimal concentrations for SAA to induce leukocyte migration, adhesion, and tissue infiltration ranged from 0.8 to 4 μM (5– 7), which are higher than the SAA levels present in normal serum but well below the concentration seen during a systemic acute phase response (1–4). Increased serum levels of SAA have been observed in a number of inflammatory and infectious diseases as well as after organ transplantation (4). The SAA concentrations required for activating FPRL1 are well within the range in which native cells are activated, as shown in this study. The overproduction of SAA by hepatocytes can be induced by inflammatory stimuli such as LPS, IL-1, IL-6, and TNF-α (1–4). Macrophages have also been reported as an extra-hepatic source of SAA during inflammation (42) and may produce relatively high concentrations in microcompartments. The expression of SAA mRNA in human atherosclerotic lesions and the induction of SAA by oxidized low density lipoproteins strengthen the hypothesis that SAA may play an important role in vascular injury and atherosclerosis (4). Under normal conditions, most serum SAA will be associated with HDL, which acts as a natural inhibitor of the chemotactic activity of SAA (references 5, 6 and Fig. 3 D). However, since SAA binds to HDL at equimolar ratios (43), a rapid increase in concentration of locally produced SAA could establish a gradient of free active SAA with consequent recruitment of leukocytes into inflammatory sites. Therefore, it is possible that at local inflammatory sites elevated SAA can attract and activate leukocytes for the clearance of pathogenic agents. This process may also cause tissue injury. Furthermore, signals triggered by activated FPRL1, a functional receptor of SAA, could eventually result in unresponsiveness of leukocytes to additional stimulation, thus immobilizing the cells and limiting the degree of inflammation.
Acknowledgments
The authors thank N. Dunlop for technical assistance. The secretarial assistance by C. Fogle is gratefully acknowledged.
Abbreviations used in this paper
- CI
chemotaxis indexes
- EC
effective concentration
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- HDL
high density lipoprotein
- LXA4
lipoxin A4
- SAA
serum amyloid A
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
S.B. Su and W. Shen are supported in part by a fellowship from the Office of the International Affairs, National Cancer Institute, National Institutes of Health (NCI, NIH). This project has been funded in part with Federal funds from the NCI under contract No. NO1-CO-56000.
References
- 1.Sipe, J.D. 1990. The acute-phase response. In Immunophysiology: The Role of Cells and Cytokines in Immunity and Inflammation. J.J. Oppenheim and E.M. Shevach, editors. Oxford University Press, New York. 259–273.
- 2.Kisilevsky R. Serum amyloid A (SAA), a protein without a function: some suggestions with reference to cholesterol metabolism. Med Hypotheses. 1991;35:337–341. doi: 10.1016/0306-9877(91)90280-c. [DOI] [PubMed] [Google Scholar]
- 3.Skinner M. Protein AA/SAA. J Intern Med. 1992;232:513–514. doi: 10.1111/j.1365-2796.1992.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 5.Badolato R, Wang JM, Murphy WJ, Lloyd AR, Michiel DF, Bausserman LL, Kelvin DJ, Oppenheim JJ. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 1994;180:203–209. doi: 10.1084/jem.180.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Badolato R, Murphy WJ, Longo DL, Anver M, Hale S, Oppenheim JJ, Wang JM. A novel biologic function of serum amyloid A. Induction of T lymphocyte migration and adhesion. J Immunol. 1995;155:1184–1190. [PubMed] [Google Scholar]
- 7.Badolato R, Johnston JA, Wang JM, McVicar D, Xu LL, Oppenheim JJ, Kelvin DJ. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J Immunol. 1995;155:4004–4010. [PubMed] [Google Scholar]
- 8.Prossnitz ER, Ye RD. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 9.Murphy, P.M. 1996. The N-formyl peptide chemotactic receptors. In Chemoattractant Ligands and Their Receptors. CRC Press, Boca Raton, FL. 269 pp.
- 10.Murphy PM, Ozcelik T, Kenney RT, Tiffany HL, McDermott D, Francke U. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J Biol Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- 11.Ye RD, Cavanagh SL, Quehenberger O, Prossnitz ER, Cochrane CG. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem Biophys Res Commun. 1992;184:582–589. doi: 10.1016/0006-291x(92)90629-y. [DOI] [PubMed] [Google Scholar]
- 12.Bao L, Gerard NP, Eddy RL, Jr, Shows TB, Gerard C. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- 13.Gao JL, Murphy PM. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- 14.Nomura H, Nielsen BW, Matsushima K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int Immunol. 1993;5:1239–1249. doi: 10.1093/intimm/5.10.1239. [DOI] [PubMed] [Google Scholar]
- 15.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 18.Fiore S, Romano M, Reardon EM, Serhan CN. Induction of functional lipoxin A4 receptors in HL-60 cells. Blood. 1993;81:3395–3403. [PubMed] [Google Scholar]
- 19.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 20.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon γ and inhibits tumor necrosis factor α–induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen- induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano M, Maddox JF, Serhan CN. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+mobilization. J Immunol. 1994;157:2149–2154. [PubMed] [Google Scholar]
- 23.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 26.Owman C, Garzino-Demo A, Cocchi F, Popovic M, Sabirsh A, Gallo RC. The leukotriene B4 receptor functions as a novel type of coreceptor mediating entry of primary HIV-1 isolates into CD4-positive cells. Proc Natl Acad Sci USA. 1998;95:9530–9534. doi: 10.1073/pnas.95.16.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger EA. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:S3–S16. [PubMed] [Google Scholar]
- 28.Steinkasserer A, Weiss EH, Schwaeble W, Linke RP. Heterogeneity of human serum amyloid A protein. Five different variants from one individual demonstrated by cDNA sequence analysis. Biochem J. 1990;268:187–193. doi: 10.1042/bj2680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy PM, McDermott D. Functional expression of the human formyl peptide receptor in Xenopus oocytesrequires a complementary human factor. J Biol Chem. 1991;266:12560–12567. [PubMed] [Google Scholar]
- 30.Ali H, Sozzani S, Fisher I, Barr AJ, Richardson RM, Haribabu B, Snyderman R. Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase C beta3 phosphorylation by protein kinase A. J Biol Chem. 1998;273:11012–11016. doi: 10.1074/jbc.273.18.11012. [DOI] [PubMed] [Google Scholar]
- 31.Falk W, Goodwin RH, Jr, Leonard EJ. A 48-well microchemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 32.Gong X, Gong W, Kuhns DB, Ben-Baruch A, Howard OM, Wang JM. Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J Biol Chem. 1997;272:11682–11685. doi: 10.1074/jbc.272.18.11682. [DOI] [PubMed] [Google Scholar]
- 33.Wang JM, McVicar DW, Oppenheim JJ, Kelvin DJ. Identification of RANTES receptors on human monocytic cells: competition for binding and desensitization by homologous chemotactic cytokines. J Exp Med. 1993;177:699–705. doi: 10.1084/jem.177.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong W, Howard OM, Turpin JA, Grimm MC, Gray P, Raport CJ, Oppenheim JJ, Wang JM. Monocyte chemotactic protein (MCP)-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J Biol Chem. 1998;273:4289–4292. doi: 10.1074/jbc.273.8.4289. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Baruch A, Xu L, Young PR, Bengali K, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J Biol Chem. 1995;270:22123–22128. doi: 10.1074/jbc.270.38.22123. [DOI] [PubMed] [Google Scholar]
- 36.Durstin M, Gao JL, Tiffany HL, McDermott D, Murphy PM. Differential expression of members of the N-formylpeptide receptor gene cluster in human phagocytes. Biochem Biophys Res Commun. 1994;201:174–179. doi: 10.1006/bbrc.1994.1685. [DOI] [PubMed] [Google Scholar]
- 37.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 38.Lee TH, Lympany P, Crea AE, Spur BW. Inhibition of leukotriene B4-induced neutrophil migration by lipoxin A4: structure-function relationships. Biochem Biophys Res Commun. 1991;180:1416–1421. doi: 10.1016/s0006-291x(05)81354-3. [DOI] [PubMed] [Google Scholar]
- 39.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 40.Ali H, Tomhave ED, Richardson RM, Haribabu B, Snyderman R. Thrombin primes responsiveness of selective chemoattractant receptors at a site distal to G protein activation. J Biol Chem. 1996;271:3200–3206. doi: 10.1074/jbc.271.6.3200. [DOI] [PubMed] [Google Scholar]
- 41.Linke RP, Bock V, Valet G, Rothe G. Inhibition of the oxidative burst response of N-formyl peptide-stimulated neutrophils by serum amyloid-A protein. Biochem Biophys Res Commun. 1991;176:1100–1105. doi: 10.1016/0006-291x(91)90397-p. [DOI] [PubMed] [Google Scholar]
- 42.Steel DM, Donoghue FC, O'Neill RM, Uhlar CM, Whitehead AS. Expression and regulation of constitutive and acute phase serum amyloid A mRNAs in hepatic and non-hepatic cell lines. Scand J Immunol. 1996;44:493–500. doi: 10.1046/j.1365-3083.1996.d01-341.x. [DOI] [PubMed] [Google Scholar]
- 43.Liang J, Sipe JD. Recombinant human serum amyloid A (apoSAAp) binds cholesterol and modulates cholesterol flux. J Lipid Res. 1995;36:37–46. [PubMed] [Google Scholar]