Abstract
The bacterial flagellum is powered by a rotary motor capable of turning the helical flagellar propeller at very high speeds. Energy to drive rotation is derived from the transmembrane electrochemical potential of specific ions. Ions passing through a channel component are thought to generate the force to power rotation. Two kinds of motors, dependent on different coupling ions, have been described: proton-driven and sodium-driven motors. There are four known genes encoding components of the sodium-powered polar flagellar motor in Vibrio parahaemolyticus. Two, which are characterized here, are homologous to genes encoding constituents of the proton-type motor (motA and motB), and two encode components unique to the sodium-type motor (motX and motY). The sodium-channel-blocking drugs phenamil and amiloride inhibit rotation of the polar flagellum and therefore can be used to probe the architecture of the motor. Mutants were isolated that could swim in the presence of phenamil or amiloride. The majority of the mutations conferring phenamil-resistant motility alter nucleotides in the motA or motB genes. The resultant amino acid changes localize to the cytoplasmic face of the torque generator and permit identification of potential sodium-interaction sites. Mutations that confer motility in the presence of amiloride do not alter any known component of the sodium-type flagellar motor. Thus, evidence supports the existence of more than one class of sodium-interaction site at which inhibitors can interfere with sodium-driven motility.
Small but powerful rotary motors propel bacteria by turning semirigid helical propellers, the flagellar filaments (for recent reviews, see refs. 1–4). In Escherichia coli and Salmonella typhimurium, energy to power rotation derives from the proton motive force (5, 6). Somehow, the passage of protons through the torque generator is coupled to rotation of the flagellum (7). Although the molecular mechanism of coupling remains unsolved, the architecture of the proton-driven motor has been studied extensively. The stationary part of the torque generator consists of two cytoplasmic membrane proteins, MotA and MotB. MotA contains four transmembrane domains, and MotB possesses one transmembrane domain (8, 9). Together they form a proton channel (10–13). In addition, MotB contains a C-terminal domain, which is thought to anchor the MotA–MotB complex to the cell wall via an interaction with peptidoglycan (14, 15).
Torque is transmitted from the MotA/B stator to the FliG protein, which acts as part of the rotor (16). FliG is found at the base of the flagellar basal body in a complex with FliM and FliN (17, 18). This complex of interacting proteins, known as the “switch complex,” is essential for torque generation, flagellar assembly, and modulation of the direction of flagellar rotation (19–24).
Other bacteria, including alkalophilic Bacillus and marine Vibrio species, use the transmembrane electrochemical-potential gradient of Na+ to drive flagellar rotation (25). The sodium-driven motor is capable of rotating very fast. For the polarly flagellated Vibrio alginolyticus, rotation rates of as high as 1,700 revolutions per second (rps) have been measured by using laser dark-field microscopy (26). In comparison, the rotation rate for the motor of E. coli has been reported to be 270 rps. (27). In addition to their remarkable rotation rates, sodium-driven flagellar motors are of interest because their function is specifically sensitive to the drugs amiloride and phenamil, which are inhibitors of eukaryotic sodium channels (25, 28). These drugs are potentially powerful probes for sodium channel/motor function.
Vibrio parahaemolyticus possesses two distinct flagellar systems driven by reversible motors, and each is powered differently (29, 30). The systems are adapted for locomotion under different circumstances (31). Proton-powered lateral flagella enable the bacterium to move over solid surfaces or through highly viscous environments (32). In more dilute liquid environments, the sodium-driven polar flagellum propels the organism.
The architecture of the sodium-type motor has yet to be defined. Previous work identified two novel genes, motX and motY, required for torque generation in V. parahaemolyticus (33, 34). Mutants with defects in these genes possess paralyzed flagella. A homolog of motY has also been found in V. alginolyticus (35), as have two other genes, whose products resemble MotA and MotB (36). In this report, we describe the sodium-type motA and motB genes of V. parahaemolyticus. Isolation of mutants able to swim in the presence of phenamil and amiloride allows us to begin to construct a model of the sodium-type flagellar motor.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions.
Vibrio strains were cultured at 30°C. All strains were derived from the wild-type V. parahaemolyticus strain BB22 (37). Strain LM1017 contains a mutation in the lateral-flagellar-hook gene and is unable to swarm on surfaces (32). In addition to strain LM1017, two derivatives of strain LM1017 were used to isolate mutants. LM4316 carries a motX mutation on the chromosome and a motX+ plasmid (motX118/pLM1758; 34), and LM4323 carries a motY mutation on the chromosome and a motY+ plasmid (motY141/pLM1785; 34). These two strains facilitated retrieval of motX or motY for phenotypic and sequence analyses. Strain LM4660 contains the transposon TnphoA in motA and is nonmotile. E. coli strain DFB210 (ΔmotAB) was used to test complementation of V. parahaemolyticus mot genes. HI broth contained 25 g heart infusion broth (Difco) and 15 g NaCl per liter.
Mutant Isolation.
Amiloride was purchased from Sigma. Two sources of phenamil were used. Phenamil was synthesized in and provided by the laboratory of Edmund Bauerlein (Max Planck Institute, Martinsreid, Germany). Phenamil methanesulfonate was purchased from Research Biochemicals, Natick, MA. Drugs were dissolved in dimethyl sulfoxide. Amiloride-resistant motility mutants were isolated in the presence of 2.25 mM amiloride. Phenamil-resistant motility mutants were isolated by using 50 μM phenamil or 40 μM phenamil methanesulfonate as indicated in Table 1. All subsequent characterization was performed by using 40 μM phenamil methanesulfonate, which is referred to simply as phenamil. Motility agar (M agar) contained 10 g/l tryptone and 3.3 g/l Bacto agar (Difco) supplemented with salts, as indicated in figure legends or text. Single colonies were inoculated with toothpicks into M agar and incubated at room temperature. Mutants were purified from the swarms, usually 12–18 days after inoculation, and reexamined for resistance.
Table 1.
Phenamil-resistant mutants
| Strain | Parent | Isolation178 | Defective gene | Mutation |
|---|---|---|---|---|
| LM4467 | LM4316 | 250 mM KCl PMS | motA | D148Y |
| LM4642 | LM1017 | 100 mM LiCl PMS | motA | D148Y |
| LM4616 | LM1017 | 100 mM KCl PMS | motA | D148Y |
| LM4641 | LM1017 | 400 mM KCl PMS | motA | D148V |
| LM4620 | LM4316 | 250 mM KCl P | motA | D148G |
| LM4605 | LM1017 | 250 mM KCl PMS | motA | D148G |
| LM4647 | LM1017 | 200 mM KCl PMS | motA | D148G |
| LM4468† | LM4316 | 250 mM KCl P | motB | A23G |
| LM4613 | LM1017 | 100 mM KCl PMS | motB | A23S |
| LM4615 | LM1017 | 100 mM KCl PMS | motB | A23S |
| LM4621 | LM4316 | 250 mM KCl P | motB | Δ12P-16P |
| LM4606 | LM1017 | 250 mM KCl PMS | motB | Δ13P-16P |
| LM4603 | LM1017 | 250 mM KCl PMS | motB | G20R |
| LM4604 | LM1017 | 250 mM KCl PMS | motB | G20R |
| LM4644 | LM1017 | 100 mM KCl PMS | motB | G20R |
| LM4645 | LM1017 | 100 mM KCl PMS | motB | G20R |
| LM4646 | LM1017 | 200 mM KCl PMS | motB | G20R |
| LM4614 | LM1017 | 100 mM KCl PMS | motB | L15H;G20V |
| LM4470 | LM4323 | 250 mM KCl P | motB | G20V |
| LM4609 | LM1017 | 250 mM KCl PMS | motB | G20V |
| LM4610 | LM1017 | 250 mM KCl PMS | motB | G20V |
| LM4611 | LM1017 | 250 mM KCl PMS | motB | G20V |
| LM4627 | LM1017 | 250 mM KCl P | motB | G20V |
| LM4469 | LM4323 | 250 mM KCl P | Not in motA, B, X, or Y | not identified |
| LM4639 | LM1017 | 250 mM KCl P | Not in motA, B, X, or Y | not identified |
| LM4598 | LM1017 | 250 mM KCl PMS | not in motA, B, X, or Y | not identified |
| LM4649 | LM4316 | 250 mM KCl P | not in motA, B, X, or Y | not identified |
Mutants were isolated in M agar containing KCl or LiCl at the indicated concentration and 50 μM phenamil (P) or 40 μM phenamil methanesulfonate (PMS).
This strain was cured of the plasmid to make the motile strain LM4629 used in Fig. 2.
Retrieval of Clones.
To obtain the motAB locus, two V. parahaemolyticus libraries were probed with a 2.8-kb SacI restriction fragment purified from the V. alginolyticus motAB clone pHK2 (36). One was a pLAFRII cosmid library constructed with wild-type DNA (38), and the second was a library constructed by using DNA isolated from the phenamil-resistant mutant strain LM4467 and the broad-host-range vector pRK415 (39).
Sequence Analysis.
The sequence of the motAB locus was obtained from cosmid pLM2058 and has been deposited with GenBank under accession number AF069391. Clone pLM2059, derived from the phenamil-resistant library, was sequenced by using the same primer sets as for wild-type pLM2058. Other phenamil-resistant mutants were sequenced after purification of PCR products by using the QIAquick system of Qiagen (Chatsworth, CA). Multiple sequence alignment was performed by using the clustal w program (40).
Motility Assays.
Optical densities at 600 nm of overnight cultures grown in Heart Infusion broth were normalized to 2.0, and 2.5 μl of each normalized culture was used to inoculate M agar. Plates were incubated at 30°C for the times indicated and then refrigerated until photographed by using the Eastman Kodak Company Digital Science Imaging System. The average rate of expansion was determined by measuring the diameter of at least three swarms as a function of time. The slope of a best-fit line was determined by plotting diameter in millimeters vs. time in hours, and only lines with an R2 value greater than 0.9 were used to calculate rates. All expansion rates were normalized to the rate of a control strain inoculated in the same plate. Rates were normalized to the parental strain LM1017 in the absence of sodium-channel inhibitor. Rates were normalized to one strain that could swim well in the presence of sodium-channel inhibitor, i.e., phenamil-resistant LM4609 or amiloride-resistant LM4303.
RESULTS
Identification of motA and motB.
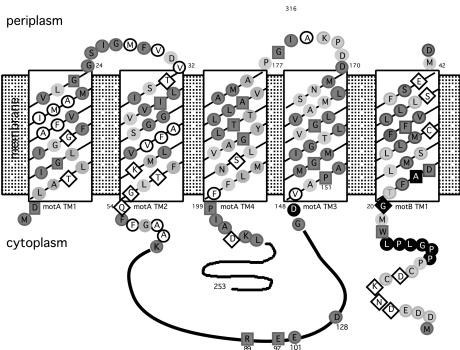
Clones carrying the sodium-type motA and motB genes were identified by hybridization by using DNA from V. alginolyticus. Sequence analysis revealed the predicted products to be 96% identical to their respective sodium-type homologs in V. alginolyticus (36), and similarity with motor components of proton-type motors is significant. Much of the conservation is found in the four transmembrane domains of MotA, the single transmembrane domain of MotB, and the peptidoglycan-binding domain of MotB. This correlation is depicted in Fig. 1, which also shows the topology of MotA and MotB with respect to the cytoplasmic membrane. In E. coli, functionally critical electrostatic interactions have been demonstrated between specific charged residues in FliG and two amino acids in MotA, Arg-90, and Glu-98 (16, 41). These residues are conserved in the V. parahaemolyticus MotA homolog (residues Arg-88 and Glu-96, respectively). Another residue in E. coli MotA, Glu-150, may also have a role in rotor/stator interaction (41), and this charge is also conserved in the V. parahaemolyticus MotA homolog (Asp-128).
Figure 1.
Topology of MotA and MotB, showing conserved residues within the predicted transmembrane (TM) domains and sites conferring phenamil resistance. Shading denotes conservation of amino acids observed in MotA and MotB sequences from E. coli, Rhodobacter sphaeroides, Bacillus subtilis, and V. parahaemolyticus (sequences for lateral and polar Mot proteins). Darkly shaded circles indicate well conserved amino acids (consensus >3), and lightly shaded circles indicate a match of the V. parahaemolyticus residue with at least one other as identified by the clustal w program (40). Invariant residues, conserved among organisms, are designated by the dark boxes. Nonshaded amino acids circled in black are nonconserved and nonpolar, whereas those within diamonds are nonconserved and polar. Filled black symbols indicate amino acids altered in phenamil-resistant mutants.
Homology in sequence does not extend to function. The V. parahaemolyticus lateral proton-type MotA and MotB homologs, LafT and LafU (32), complement the E. coli ΔmotAB deletion strain DFB210 to allow motility. However, the genes encoding the polar sodium-type MotA and MotB homologs do not complement this deletion (data not shown).
Isolation of Phenamil-Resistant Mutants.
Phenamil is a specific inhibitor of the sodium-driven flagellar motor (25, 30). Independent spontaneous mutants were isolated by inoculating single colonies of strain LM1017 (or derivatives of LM1017, as described in Methods) into M agar containing phenamil and different concentrations of various salts. The V. alginolyticus flagellar motor has been shown to use lithium (42), and so it seemed of interest to attempt to isolate phenamil-resistant motility mutants in agar containing alternative salts. A concentration of 50 mM NaCl, KCl, or LiCl will support growth and movement in M agar. The rate of radial expansion increases with increasing salt concentration (although Li+ becomes growth inhibitory at high concentrations).
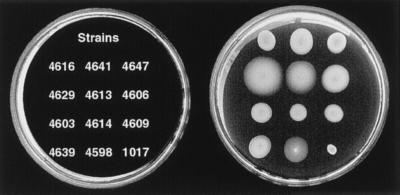
No mutants were isolated in M agar containing 40 or 50 μM phenamil and 50 mM NaCl. Flagellar rotation is not sufficiently inhibited under these conditions, allowing slow swarm expansion that precludes retrieval of mutants. In M agar containing phenamil and KCl or LiCl, radial expansion was restricted, and mutant swarms flared out from the point of inoculation. Isolates purified from the swarms exhibited radial expansion rates in the presence of phenamil and 300 mM NaCl, 300 mM KCl, or 75 mM LiCl that were faster than movement of the wild-type strain. Motility of representative mutants and LM1017 in M agar with 300 mM NaCl and 40 μM phenamil is shown in Fig. 2.
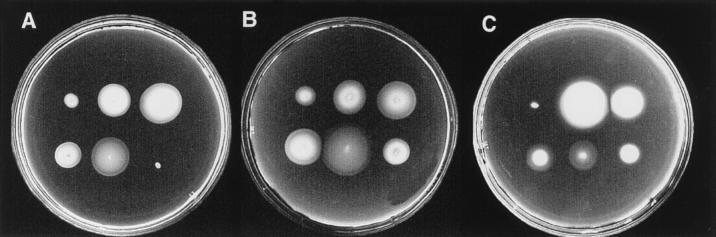
Figure 2.
Motility of wild-type and phenamil-resistant strains in M agar containing 300 mM NaCl and 40 μM phenamil after 10-hr incubation. Relative rates of expansion were normalized to the expansion rate of LM4609. The normalized rates are: LM4616 (0.8 ± .07), LM4641 (1.04 ± .03), LM4647 (0.90 ± .03), LM4629 (1.8 ± .18), LM4613 (1.23 ± .09), LM4606 (1.27 ± .03), LM4603 (1.11 ± .06), LM4614 (.81 ± .05), LM4609 (1.0 ± .05) LM4639 (1.0 ± .07), and LM4598 (1.0 ± .12).
Strain LM1017 contains the transposon mini-Mu lux inserted in the lateral-flagellar hook gene, so the contribution of proton-driven motility to movement in semisolid M agar was eliminated. This strain fails to swarm on solid surfaces and does not synthesize lateral flagella. To rule out the possibility of reversion of the defect in the lateral system, mutants able to swim in the presence of sodium-channel blocking drugs were checked for lateral-flagellar-antigen production. All of the mutants lacked lateral flagella.
The first mutants isolated were obtained with strains carrying motX or motY on plasmids. Plasmids were purified from the mutants and used to transform strain LM1017. None of the plasmids conferred phenamil resistance when transferred to LM1017. The original mutant strains were also cured of their plasmids and transformed with wild-type motX or motY plasmid. In M agar containing phenamil and 300 mM NaCl, movement of the resulting strains was indistinguishable from movement of the original parental mutant strain. Therefore, the mutations conferring phenamil resistance appeared to reside on the chromosome and not in the motX or motY genes carried on the plasmids. To determine the nature of the mutations conferring resistance to the sodium-channel blocker, a library was constructed by using DNA prepared from one phenamil-resistant strain.
Mapping of Phenamil-Interaction Sites in the Motor.
Plasmids pLM2058 and pLM2059 contain the motAB locus derived from the wild-type strain and a phenamil-resistant mutant, respectively. Introduction of either pLM2058 or pLM2059 to nonmotile strain LM4660, a derivative of LM1017 that contains a motA∷Tn phoA, restored swimming motility (Fig. 3A); however, only plasmid pLM2059, and not pLM2058, conferred an ability to swim in the presence of phenamil, and the ability was comparable to that of the parental strain LM4467 from which pLM2059 was derived (Fig. 3B). Thus, the motAB locus was sufficient to determine phenamil-resistant motility.
Figure 3.
Motility conferred by plasmid pLM2059 in M agar with 300 mM NaCl: (A) without phenamil after 6 hr; (B) with 40 μM phenamil after 12 hr. Strains were inoculated in duplicate in each row. From top to bottom: row 1 is LM1017; row 2 is LM4660 (motA∷TnphoA)/pLM2058; row 3 is LM4660 (motA∷TnphoA)/pLM2059; and row 4 is LM4467.
Sequence comparison of the motA and motB genes encoded by the two plasmids revealed a single nucleotide difference in the motA gene that changed Asp-148 to Tyr. The motA and motB genes of 26 additional phenamil-resistant mutants were sequenced. Of the 27 mutants, 23 contained mutations in motA or motB, and four showed no changes from the wild-type sequence (Table 1). Seven mutants contained changes in motA, and all of these altered Asp-148. Three of the changes introduced Tyr, three Gly, and one Val. Sixteen mutants contained changes in motB. All of the mutations mapped in the N terminus of MotB, between residues 12 and 23. Two of the mutants contained deletions, one encompassing amino acids 12 through 16 and the second removing amino acids 13 through 16.
Multiple isolates of identical mutations from independent selections suggested that some of the sites within motA and motB were saturated with respect to spontaneous mutations conferring phenamil resistance. Although mutants were isolated by using a variety of salts and concentrations (indicated in Table 1), there was no apparent specificity in the kind of mutation that permitted motility under differing conditions. To prove that a single lesion in motA or motB was sufficient to confer phenamil-resistant motility, the motAB genes from plasmids pLM2058, pLM2059 (altered motA), and strain LM4468 (altered motB) were cloned by PCR amplification into a broad-host-range plasmid and transferred to nonmotile LM4660. Plasmids containing the mutant alleles conferred phenamil-resistant motility.
The locations of the altered residues conferring phenamil resistance are shown in the topological model for MotA/B in Fig. 1 (denoted by the filled black amino acids). All of these amino acids were located near the cytoplasmic face of the membrane. Asp-148 in MotA precedes transmembrane segment 3, and the majority of the alterations in MotB precede the single transmembrane domain of that molecule. Only one amino acid substitution occurred within the transmembrane region, converting Ala-23 of MotB to Gly or Ser. Ala-23 and the neighboring Asp-24 are conserved residues. Asp-24 corresponds to E. coli Asp-32, a residue critical for motor function and implicated in proton transfer (43). Mutants with substitutions in Ala-23 (e.g., LM4629 and LM4613 in Fig. 2) were among the most resistant to phenamil in terms of rate of radial expansion in M agar containing any of the three salts.
Analysis of motA and motB sequences for four of the phenamil-resistant mutants failed to reveal any nucleotide alterations. The sequences of the motX and motY genes from these four strains were also identical to the respective wild-type genes.
Phenotypic Characterization of Phenamil-Resistant Mutants: Ion Specificity.
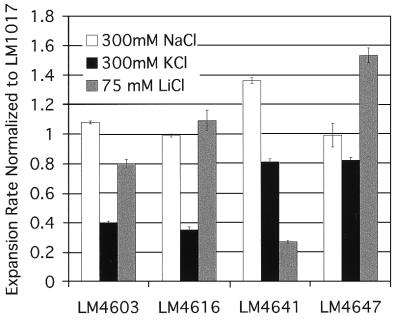
In the absence of phenamil and in M agar with 300 mM NaCl, all of the mutants expanded at rates as great as or greater than the parental strain LM1017. However, differences with respect to radial-expansion rates could be detected among some of the mutants in M agar containing alternate ions in the absence of inhibitor (Fig. 4). For example, the relative expansion rates of strains LM4603 and LM4616 were less than 0.4 the rate of LM1017 in M agar with 300 mM KCl, whereas the rates in 75 mM LiCl and 300 mM NaCl were comparable to the rate of the parental strain. In LiCl-supplemented M agar, the relative expansion of LM4641 was less than 0.3, whereas rates in NaCl and KCl remained similar to those of LM1017. The radial-expansion rate of LM4647 in LiCl was 1.5 the rate of LM1017, and rates in the other two salts were comparable to LM1017.
Figure 4.
Relative expansion rates of selected phenamil-resistant mutants normalized to the expansion rate of LM1017 in M agar supplemented with the indicated salt. Rates are the mean of three determinations, and error bars show standard deviation.
Amiloride-Resistant Mutants.
Multiple classes of mutants resistant to sodium-channel-blocking drugs exist (Table 2). The majority of the phenamil-resistant mutants that were isolated in strain LM1017 and that possessed defects in the motAB locus were unable to swim in M agar containing 2.25 mM amiloride. In contrast, the four unmapped phenamil-resistant mutants displayed motility resistant to both inhibitors. Fifty amiloride-resistant derivatives of strain LM1017 were isolated directly from swarms in M agar containing 2.25 mM amiloride, 50 mM NaCl, and 250 mM KCl. Some of these mutations also conferred some motility in M agar with 40 μM phenamil and 300 mM NaCl. Four of the mutants isolated in amiloride were particularly resistant to phenamil, expanding at rates comparable to the rates of mutants isolated in phenamil. Therefore, there are multiple classes of mutants that display motility in the presence of concentrations of inhibitors that preclude motility of the wild type (Table 2): I, resistant to phenamil; II, resistant to phenamil and amiloride; and III, resistant to amiloride. Class II mutants were isolated in either phenamil or amiloride. Some of the amiloride mutants showed slight radial expansion in the presence of phenamil (e.g., LM4601) and vice versa. Thus, the degrees to which amiloride and phenamil affect motor performance of specific mutant strains remain to be investigated.
Table 2.
Classes of phenamil- and amiloride-resistant mutants
| Strain | Isolation | Amiloride178 | Phenamil178 | Rate in amiloride† | Rate in phenamil‡ | |
|---|---|---|---|---|---|---|
| Parent | LM1017 | S | S | ND§ | 0.54 ± .09 | |
| Class I | LM4609 | phenamil | S | R | ND§ | 1.0 |
| Class II | LM4598 | phenamil | R | R | 0.83 ± .09 | 1.13 ± .03 |
| LM4469 | phenamil | R | R | 1.01 ± .05 | 1.27 ± .08 | |
| LM4300 | amiloride | R | R | 1.15 ± .12 | 1.20 ± .05 | |
| LM4303 | amiloride | R | R | 1.0 | 0.93 ± .07 | |
| Class III | LM4268 | amiloride | R | S | 0.91 ± .02 | 0.57 ± .05 |
| LM4601 | amiloride | R | S | 1.13 ± .04 | 0.68 ± .08 |
Resistance (R) or sensitivity (S) to 2.25 mM amiloride and 40 μM phenamil.
Expansion rate in M agar with 300 mM NaCl and amiloride normalized to the expansion rate of LM4303.
Expansion rate in M agar with 300 mM NaCl and phenamil normalized to the expansion rate of LM4609.
No data were collected because LM1017 and LM4609 failed to move under these conditions.
The motA, motB, motX, and motY genes from eight amiloride-resistant (four Class II and four Class III) mutants were identical to the wild type. Therefore, mutations causing phenamil and/or amiloride resistance can map outside of loci encoding known motor components.
Phenotypic Characterization of Amiloride-Resistant Mutants: Ion Specificity.
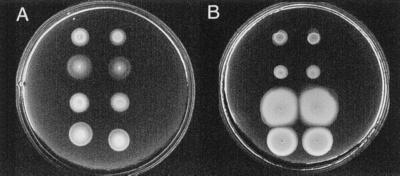
The amiloride-resistant motility of some of the mutants is shown in Fig. 5A. Like mutants isolated in phenamil, mutants retrieved from the amiloride selection displayed differential expansion rates in media supplemented with alternate salts (Fig. 5 B and C). The radial-expansion rate of some strains in 75 mM LiCl was exceptional. For example, the expansion rate of LM4268 in LiCl was 2.63-fold higher than that of LM1017. Thus, not only do the mutations in these strains affect motor function in the presence of amiloride; they can also alter motility in the absence of any sodium-blocking inhibitor.
Figure 5.
Motility of wild-type and amiloride-resistant strains in the presence and absence of amiloride. (A) M agar with 50 mM NaCl, 250 mM KCl, and 2.25 mM amiloride after 12 hr; (B) M agar with 300 mM NaCl after 7 hr; and (C) M agar with 75 mM LiCl after 21 hr. Expansion rates measured in the presence of amiloride were normalized to LM4303 and in the absence of amiloride were normalized to LM1017. Strains (and expansion rates in A, B, and C) are indicated. Top row, from left to right: LM4267 (0.42 ± .06, 0.94 ± .03, 0.76 ± .16), LM4268 (1.09 ± .04, 1.26 ± .07, 2.63 ± .14), and LM4601 (1.56 ± .02, 1.52 ± .08, 2.0 ± .13). Bottom row: LM4303 (1.0, 1.46 ± .13, 1.28 ± .02), LM4300 (1.64 ± .08, 1.91 ± .21, 1.29 ± .06), and LM1017 (ND, 1.0, 1.0).
DISCUSSION
The bacterial flagellar motor couples a transmembrane flux of specific ions to rotation of the flagellum. The sodium-type motor is an attractive object for study. Not only does it spin at amazingly fast rates, but the driving force of the motor, i.e., the sodium motive force, is easily manipulated. In this report, we use sodium-channel inhibitors that prevent motility to define Na+-interacting sites in the energy-coupling unit of the motor.
Two inhibitors of sodium-driven motors have been carefully studied: amiloride and phenamil. Phenamil is a derivative of amiloride and may be more membrane-permeable than amiloride because of a hydrophobic side-group substitution (44). Kinetic data, obtained by using Bacillus firmus RAB, suggest that amiloride inhibits motility by competing with Na+ outside the cell (28), whereas phenamil acts in a noncompetitive manner to prevent rotation (45). In addition, a motility mutant of the alkalophilic Bacillus strain, isolated on a swarm agar plate containing 50 μM phenamil, displayed wild-type sensitivity to 0.25–1.5 mM amiloride with respect to swimming speed (45). These data led Imae and colleagues to propose the existence of two types of Na+-binding sites on the motor, located in the periplasm and cytoplasm (25, 45, 46).
Multiple classes of mutants resistant to sodium-channel-blocking drugs exist. We have isolated more than 75 V. parahaemolyticus mutants able to swim in M agar with concentrations of sodium-channel blockers inhibitory to wild-type motility, i.e., 40 μM phenamil or 2.25 mM amiloride. Phenotypically, the mutants segregate into three classes: phenamil resistant, amiloride resistant, and phenamil and amiloride resistant. The majority of the phenamil-resistant mutants have lesions in genes encoding two cytoplasmic membrane proteins within the torque generator, MotA and MotB, whereas a majority of the mutations conferring amiloride resistance do not alter motA or motB. Thus, molecular evidence supports the existence of more than one class of Na+ interaction that can be targeted by inhibitors of sodium-driven motility.
We describe the identification of the specific alterations in motA or motB that permit motility of 23 mutants in the presence of phenamil. The mutations in these strains alter amino acids clustered in a limited region at the cytoplasmic face of MotA or MotB. Kojima et al. have characterized two phenamil-resistant V. alginolyticus mutants. The mutants were isolated, after ethyl methanesulfonate mutagenesis, from swarms in M agar containing 500 μM phenamil, 50 mM NaCl, and 250 mM KCl (47). The molecular natures of the defects have recently been described (48) and correspond to mutated sites identified in this work (Asp-148 to Tyr in MotA and Pro-16 to Ser in MotB). Therefore, data obtained from two organisms, by using both spontaneous and chemical mutagenesis, reveal a limited number of sites within the sodium-driven flagellar motor that interact with phenamil.
Among the mutations conferring phenamil-resistant motility, only one amino acid within a putative transmembrane region was affected, and it was a residue adjacent to a highly conserved aspartate residue in MotB that has been postulated to play a role in proton conduction (43). Some mutants, isolated in phenamil or amiloride, expanded at rates significantly different than that of the parental strain in the presence of Li+ and absence of inhibitor. Although it is attractive to think that alterations in the Na+-interaction sites could also affect the ion specificity of the torque generator, further analysis is required.
How does the sodium-type flagellar motor work? Four components that may comprise the stator have been identified. Two of these, MotB and MotY, possess domains likely to interact with peptidoglycan and may be the elements responsible for anchoring the force generator. The proton-type motor has only a single component with this function, i.e., MotB. Why are there two proteins with redundant function in the sodium-type motor? Precise orientation of the stator may be critical when the flagellum rotates at high speeds, and two anchoring proteins might ensure proper alignment of the stator complex with respect to the rotating parts of the motor. The truly unusual component of the motor is MotX. When the motX gene is introduced into E. coli, overexpression of MotX renders E. coli sensitive to killing by Na+. Perhaps MotX plays a role analogous to the eukaryotic MinK potassium-channel protein, which modifies ion channel specificity by associating with existing channel-forming proteins (49). In the proton-driven motor, transmembrane domains of MotA and MotB form the proton-conducting channel. In V. parahaemolyticus, phenamil resistance alters sites in MotA and MotB, implicating both proteins in Na+ transfer.
In E. coli and other bacteria, force is transmitted from the stator to FliG, which is part of the rotor, in response to transfer of ions through the torque generator. FliG interacts with FliM and FliN to form the switch complex localized to the base of the flagellum (17–24). The switch complex presumably exists in the sodium-type motor. Specific charged amino acids in E. coli MotA shown to be important in the stator/rotor interaction (16) are fully conserved in the sodium-type motor. Recently, we have identified potential polar flagellar homologs to switch components in V. parahaemolyticus (GenBank accession no. AF069392).
Further dissection of the sodium-driven flagellar motor awaits the characterization of the proteins in the switch complex and identification of the sites altered by the remaining unmapped mutations that confer phenamil and amiloride resistance. Potentially, the unmapped mutations could alter FliG or some other flagellar component. Alternatively, a novel component of the sodium-type motor may remain to be discovered, or motility in the presence of sodium-channel-blocking drugs could be conferred by nonmotor components. Resistance might be determined by modification of a protein participating in the cycling of Na+ in the cell, e.g., the respiratory Na+ pump or Na+/H+ antiporters, or by proteins that determine access of amiloride and/or phenamil to the Na+-interaction sites on the motor, e.g., porins or transporter molecules.
Acknowledgments
We are most appreciative of Edmund Bauerlein, who kindly provided us with phenamil, and Michio Homma for plasmid pHK2. We also thank David Blair for bacterial strains and the DNA Core at the University of Iowa for excellent support. This research was supported by Public Health Service Grant GM43196 from the National Institutes of Health.
ABBREVIATION
- M agar
motility agar
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Database deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF069391).
References
- 1.Berg H C. Biophys J. 1995;68:163s–167s. [PMC free article] [PubMed] [Google Scholar]
- 2.Blair D F. Annu Rev Microbiol. 1995;49:489–522. doi: 10.1146/annurev.mi.49.100195.002421. [DOI] [PubMed] [Google Scholar]
- 3.DeRosier D J. Cell. 1998;93:17–20. doi: 10.1016/s0092-8674(00)81141-1. [DOI] [PubMed] [Google Scholar]
- 4.Macnab R M. In: Escherichia coli and Salmonella typhimurium, Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, Curtiss R, Gross C A, Ingraham J L, Lin E C C, Brooks Low K Jr, Magasanik B, Reznikoff W, Riley M, Schaechter M, et al., editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 123–145. [Google Scholar]
- 5.Larsen S H, Adler J, Gargus J J, Hogg R W. Proc Natl Acad Sci USA. 1974;71:1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manson M D, Tedesco P, Berg H C, Harold F M, van der Drift C. Proc Natl Acad Sci USA. 1977;74:3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meister M, Lowe G, Berg H C. Cell. 1987;49:643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 8.Dean G E, Macnab R M, Stader J, Matsumura P, Burks C. J Bacteriol. 1984;159:991–999. doi: 10.1128/jb.159.3.991-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stader J, Matsumura P, Vacante D, Dean G E, Macnab R M. J Bacteriol. 1986;166:244–252. doi: 10.1128/jb.166.1.244-252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair D F, Berg H C. Cell. 1990;60:439–449. doi: 10.1016/0092-8674(90)90595-6. [DOI] [PubMed] [Google Scholar]
- 11.Blair D F, Berg H C. J Mol Biol. 1991;221:1433–1442. doi: 10.1016/0022-2836(91)90943-z. [DOI] [PubMed] [Google Scholar]
- 12.Garza A G, Harris-Haller L W, Stoebner R A, Manson M D. Proc Natl Acad Sci USA. 1995;92:1970–1974. doi: 10.1073/pnas.92.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stolz B, Berg H C. J Bacteriol. 1991;173:7033–7037. doi: 10.1128/jb.173.21.7033-7037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun S Y, Parkinson J S. Science. 1988;230:276–277. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- 15.DeMot R, Vanderleyden J. Mol Microbiol. 1994;12:333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Lloyd S A, Blair D F. Proc Natl Acad Sci USA. 1998;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis N R, Sosinsky G E, Thomas D, DeRosier D J. J Mol Biol. 1994;235:1261–1270. doi: 10.1006/jmbi.1994.1079. [DOI] [PubMed] [Google Scholar]
- 18.Oosawa K, Ueno T, Aizawa S-I. J Bacteriol. 1994;176:3683–3691. doi: 10.1128/jb.176.12.3683-3691.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irikura V M, Kihara M, Yamaguchi S, Sockett H, Macnab R M. J Bacteriol. 1993;175:802–810. doi: 10.1128/jb.175.3.802-810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marykwas D L, Schmidt S A, Berg H C. J Mol Biol. 1996;256:564–576. doi: 10.1006/jmbi.1996.0109. [DOI] [PubMed] [Google Scholar]
- 21.Sockett H, Yamaguchi S, Kihara M, Irikura V M, Macnab R M. J Bacteriol. 1992;174:793–806. doi: 10.1128/jb.174.3.793-806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toker A S, Macnab R M. J Mol Biol. 1997;273:623–634. doi: 10.1006/jmbi.1997.1335. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi S, Fujita H, Ishihara A, Aizawa S-I, Macnab R M. J Bacteriol. 1986;166:187–193. doi: 10.1128/jb.166.1.187-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi S, Aizawa S-I, Kihara M, Isomura M, Jones C J, Macnab R M. J Bacteriol. 1986;168:1172–1179. doi: 10.1128/jb.168.3.1172-1179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imae Y, Atsumi T. J Bioenerg Biomembr. 1989;21:705–716. doi: 10.1007/BF00762688. [DOI] [PubMed] [Google Scholar]
- 26.Magariyama Y, Sugiyama S, Muramoto K, Maekawa Y, Kawagishi I, Imae Y, Kudo S. Nature (London) 1994;371:752. doi: 10.1038/371752b0. [DOI] [PubMed] [Google Scholar]
- 27.Lowe G, Meister M, Berg H C. Nature (London) 1987;325:637–640. [Google Scholar]
- 28.Sugiyama S, Cragoe E J, Jr, Imae Y. J Biol Chem. 1988;263:8215–8219. [PubMed] [Google Scholar]
- 29.Atsumi T, McCarter L, Imae Y. Nature (London) 1992;355:182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- 30.Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. Mol Microbiol. 1996;20:693–699. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 31.McCarter L L, Silverman M. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 32.McCarter L L, Wright M E. J Bacteriol. 1993;175:3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarter L L. J Bacteriol. 1994;176:4219–4225. doi: 10.1128/jb.176.14.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarter L L. J Bacteriol. 1994;176:5988–5998. doi: 10.1128/jb.176.19.5988-5998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okunishi I, Kawagishi I, Homma M. J Bacteriol. 1996;178:2409–2415. doi: 10.1128/jb.178.8.2409-2415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asai Y, Kojima S, Kato H, Nishioka N, Kawagishi I, Homma M. J Bacteriol. 1997;179:5104–5110. doi: 10.1128/jb.179.16.5104-5110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belas R, Simon M, Silverman M. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarter L L, Silverman M. J Bacteriol. 1987;169:3441–3449. doi: 10.1128/jb.169.8.3441-3449.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 40.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, Blair D F. J Mol Biol. 1997;273:428–439. doi: 10.1006/jmbi.1997.1316. [DOI] [PubMed] [Google Scholar]
- 42.Liu J Z, Dapice M, Khan S. J Bacteriol. 1990;172:5236–5244. doi: 10.1128/jb.172.9.5236-5244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Sharp L L, Tan H L, Lloyd S A, Billings S, Braun T F, Blair D F. J Bacteriol. 1998;180:2729–2735. doi: 10.1128/jb.180.10.2729-2735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleyman T R, Cragoe E J., Jr J Membrane Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- 45.Atsumi T, Sugiyama S, Cragoe E J, Jr, Imae Y. J Bacteriol. 1990;172:1634–1639. doi: 10.1128/jb.172.3.1634-1639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida S, Sugiyama S, Hojo Y, Tokuda H, Imae Y. J Biol Chem. 1990;265:20346–20350. [PubMed] [Google Scholar]
- 47.Kojima S, Atsumi T, Muramoto K, Kudo S, Kawagishi I, Homma M. J Mol Biol. 1997;265:310–318. doi: 10.1006/jmbi.1996.0732. [DOI] [PubMed] [Google Scholar]
- 48.Kojima S, Asai Y, Atsumi T, Kawagishi I, Homma M. J Mol Biol. 1999;285:1537–1547. doi: 10.1006/jmbi.1998.2377. [DOI] [PubMed] [Google Scholar]
- 49.Kaczmarek L K, Blumenthal E M. Physiol Rev. 1997;77:627–641. doi: 10.1152/physrev.1997.77.3.627. [DOI] [PubMed] [Google Scholar]