Abstract
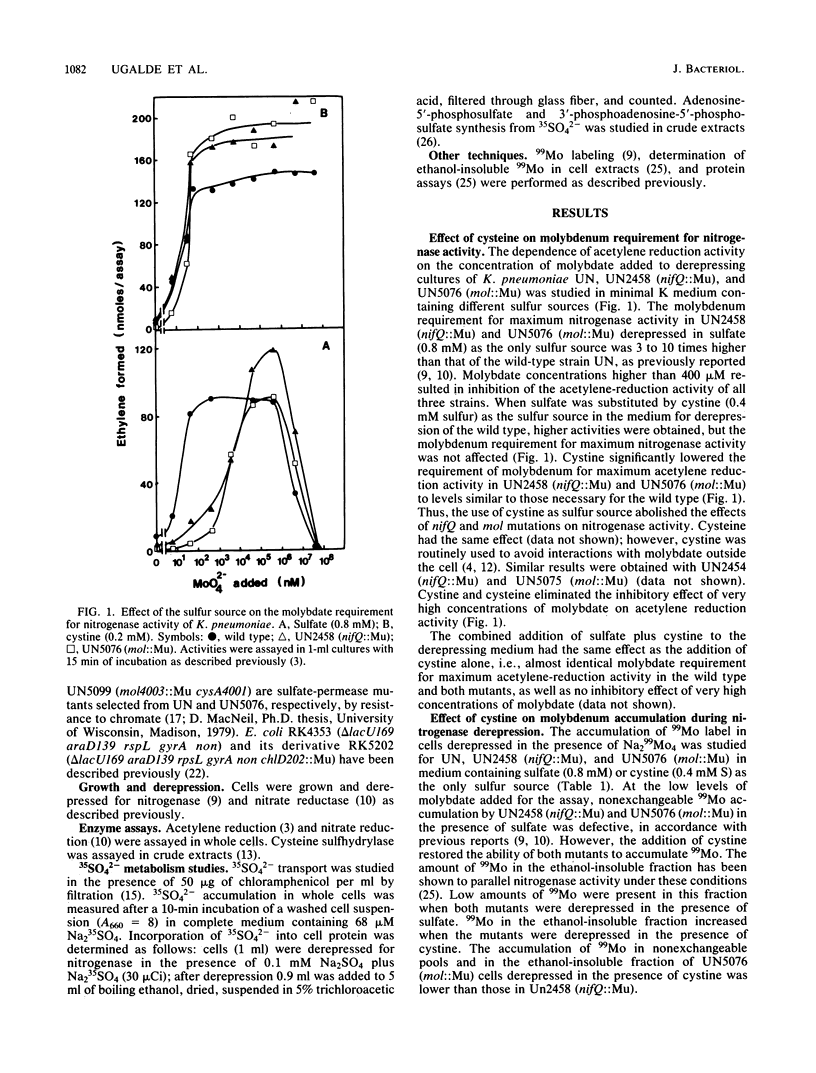
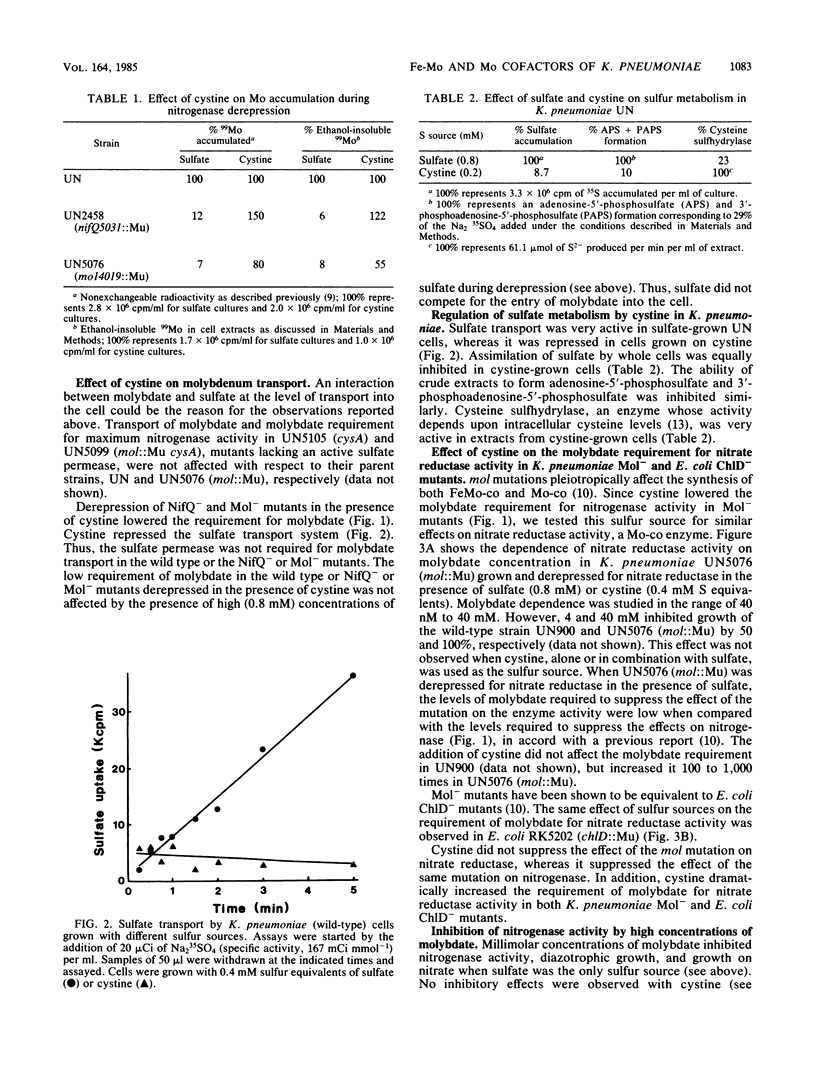
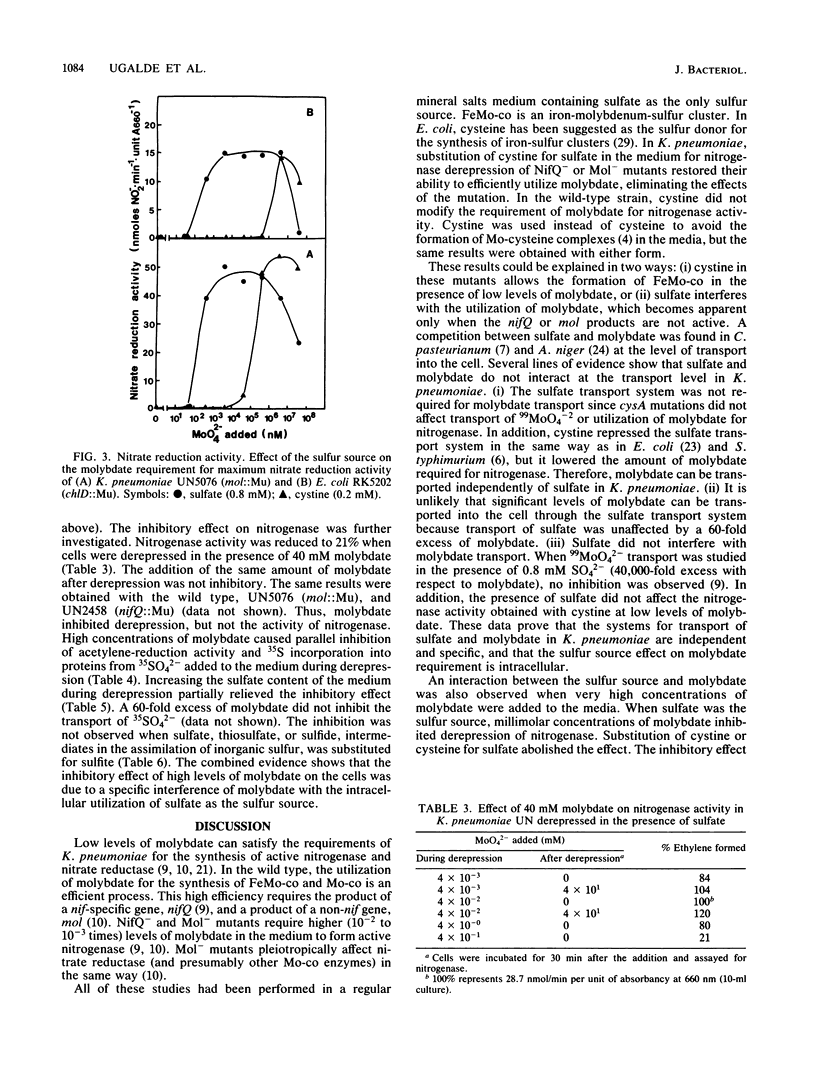
NifQ- and Mol- mutants of Klebsiella pneumoniae show an elevated molybdenum requirement for nitrogen fixation. Substitution of cystine for sulfate as the sulfur source in the medium reduced the molybdenum requirement of these mutants to levels required by the wild type. Cystine also increased the intracellular molybdenum accumulation of NifQ- and Mol- mutants. Cystine did not affect the molybdenum requirement or accumulation in wild-type K. pneumoniae. Sulfate transport and metabolism in K. pneumoniae were repressed by cystine. However, the effect of cystine on the molybdenum requirement could not be explained by an interaction between sulfate and molybdate at the transport level. Cystine increased the molybdenum requirement of Mol- mutants for nitrate reductase activity by at least 100-fold. Cystine had the same effect on the molybdenum requirement for nitrate reductase activity in Escherichia coli ChlD- mutants. This shows that cystine does not have a generalized effect on molybdenum metabolism. Millimolar concentrations of molybdate inhibited nitrogenase and nitrate reductase derepression with sulfate as the sulfur source, but not with cystine. The inhibition was the result of a specific antagonism of sulfate metabolism by molybdate. The effects of nifQ and mol mutations on nitrogenase could be suppressed either by the addition of cystine or by high concentrations of molybdate. This suggests that a sulfur donor and molybdenum interact at an early step in the biosynthesis of the iron-molybdenum cofactor. This interaction might occur nonenzymatically when the levels of the reactants are high.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi J. M., Campbell L. L. STUDIES ON THERMOPHILIC SULFATE-REDUCING BACTERIA III. : Adenosine Triphosphate-sulfurylase of Clostridium nigrificans and Desulfovibrio desulfuricans. J Bacteriol. 1962 Dec;84(6):1194–1201. doi: 10.1128/jb.84.6.1194-1201.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J., Steiner A. L., Shah V. K. Effect of molybdenum starvation and tungsten on the synthesis of nitrogenase components in Klebsiella pneumonia. J Bacteriol. 1974 Jun;118(3):986–989. doi: 10.1128/jb.118.3.986-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis G. E., Wentworth R. A. Tungsten vs. Molybdenum in models for biological systems. Bioinorg Chem. 1977;7(1):57–70. doi: 10.1016/s0006-3061(00)80128-3. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Elliott B. B., Mortenson L. E. Transport of molybdate by Clostridium pasteurianum. J Bacteriol. 1975 Dec;124(3):1295–1301. doi: 10.1128/jb.124.3.1295-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Mol- mutants of Klebsiella pneumoniae requiring high levels of molybdate for nitrogenase activity. J Bacteriol. 1985 Sep;163(3):1285–1287. doi: 10.1128/jb.163.3.1285-1287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Role of the nifQ gene product in the incorporation of molybdenum into nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1984 Apr;158(1):187–194. doi: 10.1128/jb.158.1.187-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Ugalde R. A., Imperial J., Brill W. J. Molybdenum in nitrogenase. Annu Rev Biochem. 1984;53:231–257. doi: 10.1146/annurev.bi.53.070184.001311. [DOI] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. Specificity of transport processes for sulfur, selenium, and molybdenum anions by filamentous fungi. Biochim Biophys Acta. 1970 Jan 6;196(1):95–106. doi: 10.1016/0005-2736(70)90170-7. [DOI] [PubMed] [Google Scholar]
- Ugalde R. A., Imperial J., Shah V. K., Brill W. J. Biosynthesis of iron-molybdenum cofactor in the absence of nitrogenase. J Bacteriol. 1984 Sep;159(3):888–893. doi: 10.1128/jb.159.3.888-893.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A. K., Nicholas D. J. Metabolism of 35 S-sulphate and properties of APS-kinase and PAPS-reductase in Nitrobacter agilis. Arch Mikrobiol. 1971;78(2):99–117. doi: 10.1007/BF00424867. [DOI] [PubMed] [Google Scholar]
- WHELDRAKE J. F., PASTERNAK C. A. THE CONTROL OF SULPHATE ACTIVATION IN BACTERIA. Biochem J. 1965 Jul;96:276–280. doi: 10.1042/bj0960276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wheldrake J. F. Intracellular concentration of cysteine in Escherichia coli and its relation to repression of the sulphate-activating enzymes. Biochem J. 1967 Nov;105(2):697–699. doi: 10.1042/bj1050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H. Origin of the labile sulfide in the iron-sulfur proteins of Escherichia coli. Biochem Biophys Res Commun. 1983 Apr 15;112(1):66–72. doi: 10.1016/0006-291x(83)91798-9. [DOI] [PubMed] [Google Scholar]


