Abstract
Experimental leishmaniasis offers a well characterized model of T helper type 1 cell (Th1)-mediated control of infection by an intracellular organism. Susceptible BALB/c mice aberrantly develop Th2 cells in response to infection and are unable to control parasite dissemination. The early CD4+ T cell response in these mice is oligoclonal and reflects the expansion of Vβ4/ Vα8-bearing T cells in response to a single epitope from the parasite Leishmania homologue of mammalian RACK1 (LACK) antigen. Interleukin 4 (IL-4) generated by these cells is believed to direct the subsequent Th2 response. We used T cells from T cell receptor–transgenic mice expressing such a Vβ4/Vα8 receptor to characterize altered peptide ligands with similar affinity for I-Ad. Such altered ligands failed to activate IL-4 production from transgenic LACK-specific T cells or following injection into BALB/c mice. Pretreatment of susceptible mice with altered peptide ligands substantially altered the course of subsequent infection. The ability to confer a healer phenotype on otherwise susceptible mice using altered peptides that differed by a single amino acid suggests limited diversity in the endogenous T cell repertoire recognizing this antigen.
Keywords: Leishmania major, CD4+ T cell subsets, altered peptide ligands, LACK antigen
L eishmania major is a complex intracellular protozoan parasite of macrophages in the vertebrate host that requires a robust Th1 response for control of infection (reviewed in 1). Requirements for MHC class II molecules, T cells, IFN-γ, IL-12, and NOS2 have delineated a pathway by which CD4+ T cells differentiate into Th1 effector cells that activate macrophages and confer resistance to the disease. Among mice with I-Ad class II molecules, the CD4+ T cell immune response is focused dominantly on a single epitope from the parasite LACK1 antigen, which results in oligoclonal expansion of cells bearing Vβ4/Vα8 heterodimeric TCR in the regional lymph nodes (2, 3). The dominant nature of this antigen was demonstrated using TCR-transgenic mice that expressed a monoclonal αβ T cell repertoire to this epitope; such mice displayed remarkable control over the organism, despite the absence of T cells that recognize any parasite-specific antigens other than the single epitope from LACK (4).
Unlike resistant strains of mice, BALB/c mice develop aberrant Th2 responses after challenge with L. major and sustain fatal disseminated disease. The early CD4+ T cell response is also dominated by expansion of LACK-specific T cells that express Vβ4/Vα8 TCR. However, activation of these cells results in the rapid production of IL-4 that drives the subsequent Th2 response (5, 6). Deletion of LACK- reactive cells, either through thymic expression of LACK as a transgene (7) or superantigen-mediated deletion of Vβ4-expressing CD4+ T cells (5), attenuated the early production of IL-4 and promoted the differentiation of Th1 effector cells that controlled disease in infected BALB/c mice. These and other data have suggested a model whereby susceptibility to L. major in BALB strain mice is driven by a confluence of factors involving the inherent propensity to produce IL-4 after activation in the setting of an appropriately sized precursor pool of LACK-reactive CD4+ T cells (8).
The L. major model provides an opportunity to test the plasticity of the wild-type CD4+ T cell repertoire in susceptible mice. The conserved charge and length of the peptide-interacting complementarity determining region 3 (CDR3) domain from LACK-reactive T cell hybridomas suggested the observation that a single parasite antigen served as the focus of their recognition (2). The constraints on the entire LACK-reactive repertoire in the intact animal remain unknown, however. We decided to indirectly test such constraints by attempting to abrogate activation of these cells in vivo using altered ligands. We demonstrate substantial protection against disease in this system, suggesting that identification of dominant antigens from organisms can be used to target pathogenic T cells that mediate progressive disease in a highly specific manner.
Materials and Methods
Reagents.
The LACK156–173 peptide (ICFSPSLEHPIVVSGSWD), LACK-N164 and LACK-K164 mutant peptides with the designated alterations of H at position 164, and the OVA323–336 peptide (ISQAVHAAHAEINE) were synthesized on an Advanced Chemtech Multiple Peptide Synthesizer. Peptides were purified by reverse phase HPLC and their identities confirmed by analysis with an LCQ mass spectrometer (Finnigan MAT).
The full length rLACK sequence was ligated downstream of a hemagglutinin epitope, six histidine residues, and factor X cleavage site in the pET3a-δ9 vector as described (5). A 41–amino acid deletion construct (rLACKΔ41) that excised the LACK156–173 epitope was created and expressed from the same vector as described (5). rLACK-N164 and rLACK-K164 were produced by site-directed mutagenesis of the nucleotide triplet at bp 684–686 from CAC (H) to AAC (N) or AAG (K), respectively, based on codon usage in Leishmania (9). The XbaI/HindIII fragment of rLACK (1,134 bp) was cloned into the pGEM11Zf(−) vector (Promega). Mutations were generated with the PCR-based QuikChange™ site-directed mutagenesis kit (Stratagene) using the primers 5′-CGTCGCTGGAGAACCCGATCGTG-3′ (N) or 5′-CGTCGCTGGAGAAGCCGATCGTG-3′ (K) according to the manufacturer's protocol. The mutated rLACK proteins were sequenced to confirm their identities and cloned using the XbaI and HindIII sites into pET3a-δ9. Escherichia coli BL21 (DE3)plysS (Novagen) were transformed, and the expressed proteins were purified using [Ni]nitrilotriacetic acid chromatography as described (5).
Mice.
Female BALB/c, C57BL/6 (The Jackson Laboratory or IFFA Credo), and B10.D2 mice (The Jackson Laboratory) were housed in the University of California San Francisco or University of Lausanne pathogen-free animal facilities and used at 8–10 wk of age. Designated mice were thymectomized at 5 wk of age using standard methods. LACK T cell receptor– specific transgenic (ABLE) mice are TCR-transgenic mice that express a Vβ4/Vα8 TCR that recognizes a peptide epitope comprising amino acids 156–173 from the Leishmania LACK antigen in the context of MHC class II I-Ad molecules. The generation and characterization of the ABLE mice are described elsewhere (4). ABLE mice were backcrossed 10 generations onto the BALB/c background and, where indicated, onto backcrossed BALB/c TCR constant region α chain deletion mutants (TCR-Cα0; 10) to create BALB/c ABLE TCR-Cα0 mice as described (4).
Parasites and Infection.
L. major strains WHOM/IR/−/173 (designated IR/173) and MRHO/Sv/59/P (designated LV39 were passaged and maintained as described (4, 5). Groups of 4–10 mice were infected in the hind footpads with purified metacyclic (4 × 105) or stationary phase (2 × 106) promastigotes as previously described (4, 5, 11). After inoculation, disease progression was monitored using a metric caliper to quantitate footpad size. Animals were killed at the designated times, and the popliteal lymph nodes were harvested for the evaluation of cell types and cytokine production as described (5). Serum was collected terminally for quantitation of IgE by ELISA as described (11), and the footpads and spleens were used to quantitate the parasite burden by limiting dilution (12).
MHC Binding Affinities.
I-Ad molecules were affinity purified from cell lysates of A20 lymphoma cells using anti–I-Ad mAb MKD-6 (American Type Culture Collection [ATCC]). Peptides were tested for binding to I-Ad as measured by their capacity to inhibit the binding of 125I-radiolabeled OVA323–336 as previously described (13).
Stimulation of ABLE T Cells In Vitro.
Spleen and lymph nodes were harvested from BALB/c ABLE TCR-Cα0 mice and used to produce single-cell suspensions after disruption through a 0.75-μm nylon mesh filter. Cells were washed, the red cells were lysed, and the resulting populations of ABLE T cells and APC were distributed to duplicate wells of round-bottom microtiter plates (106 cells/well) in cell culture medium (RPMI 1640 with 10% heat-inactivated FCS [Hyclone], 2 mM l-glutamine, 0.1 mM sodium pyruvate, 50 μM β2-ME, and 100 U/ml each of penicillin and streptomycin) with the indicated concentrations of synthetic peptides in a final volume of 0.2 ml. Supernatants were harvested after 48 h for cytokine analysis by ELISA. The wells were pulsed at 48 h with 1 μCi [3H]thymidine, and cell proliferation was assessed 18 h later.
T Cell Antagonism Assay.
CD4+ T cells from BALB/c ABLE TCR-Cα0 mice were enriched from spleen and lymph node cell suspensions by antibody- and complement-mediated lysis of B cells, MHC class II-, and CD8-expressing cells using mAbs J11d, BP107, and 3155 (ATCC), respectively, and low-toxicity rabbit and guinea pig complement (Cedarlane Labs., Ltd.). The resulting populations were 80% Vβ4+ cells, of which 35–40% were CD4+ and the remainder CD4−CD8− as previously described (14). Irradiated spleen cell populations from BALB/c TCR-Cα0 mice were used as APC. Antagonism was quantitated using the method of DeMagistris et al. (13), with slight modifications. APC (107 cells/ml) were prepulsed with suboptimal concentrations of the wild-type LACK peptide (0.008–0.2 μM as established in preliminary experiments) in culture medium for 2 h at 37°C. The APC were washed, irradiated, and distributed to 96-well round-bottom microtiter plates (2 × 106 cells/well) and further incubated with varying concentrations of the designated peptides (0.01–100 μM) for 2 h at 37°C. The plates were washed, and enriched ABLE T cells were added using 1.6 × 105 T cells/well in 0.2 ml medium. After 48 h, the supernatants were collected and analyzed for cytokines by ELISA. Proliferation was assessed at hour 66, after pulsing for the final 18 h with 1 μCi [3H]thymidine/well.
Immune Response in Mice Injected with rLACK Proteins.
Groups of BALB/c mice were injected in the hind footpads with 5, 25, or 50 μg of the designated rLACK protein or 25 μg chicken egg OVA in 50 μl buffer. At various time points, the popliteal lymph nodes were harvested and mRNA purified for analysis of IL-4 transcripts using a semiquantitative reverse transcriptase (RT)-PCR assay as previously described (15). Groups of these treated control or adult thymectomized mice were challenged either 24 h or 10, 20, or 30 d later with either designated rLACK proteins or viable L. major and analyzed by similar methods.
BALB/c ABLE-Cα0 mice were immunized in both footpads with 25 μg of the purified rLACK proteins or chicken egg OVA in 50 μl buffer and, 24 h later, the popliteal lymph nodes were collected and single-cell suspensions prepared. Cells (2 × 105) were analyzed by flow cytometry (FACSVantage™; Becton Dickinson) after incubation with a combination of fluorescein isothiocyanate–conjugated anti-Vβ4, PE-conjugated anti-CD4, and biotinylated anti-CD69 mAbs, followed by streptavidin-tricolor (all from Caltag Labs.).
Immunization with Altered rLACK Proteins.
Nonthymectomized or adult thymectomized BALB/c mice were immunized in the hind footpad with 25 μg purified rLACK proteins or chicken egg OVA in 50 μl of 50 mM Tris/100 mM NaCl, pH 8.0. Mice were infected 24 h later with the designated strains of L. major promastigotes in the left footpad and the course of infection monitored as described above.
Cytokine Analysis and Serum IgE Determination.
IL-4 and IFN-γ were measured using sandwich ELISA with mAbs 11B11 and biotinylated BVD6 for IL-4 detection and R46A2 and biotinylated XMG1.2 for IFN-γ detection as described (11). Samples were normalized to standard recombinant controls. The limits of detection in these assays were 50 pg/ml for IL-4 and 1 ng/ml for IFN-γ. Cytokine production by individual lymphocytes was assessed by ELISPOT assay as described (11). Total serum IgE was measured using ELISA with mAbs B.IE.3 and biotinylated EM-95 and normalized to concurrently analyzed standards (11). Cytokine mRNA transcript abundance was quantitated using RT-PCR with the competitor plasmid pPQRS as described (5, 15). In brief, cDNA samples were first normalized for expression of a constitutively expressed gene, hypoxanthine phosphoribosyltransferase (HPRT), and then quantitated for expression of IL-4 and IFN-γ as compared with competitor pseudogene transcripts amplified within the same reaction. The ratio of the authentic and competitive amplicons was quantitated using densitometry.
Results
Construction of Analogue Peptides of the LACK Antigenic Determinant.
Using overlapping synthetic peptides and a panel of T cell hybridomas generated from BALB/c mice immunized with the recombinant protein, a single I-Ad– restricted epitope in LACK was localized to amino acids 156–173, comprising the sequence ICFSPSLEHPIVVSGSWD (data not shown). Almost all hybridomas reactive to LACK expressed a Vβ4/Vα8 heterodimeric TCR, although considerable junctional diversity was apparent. The putative CDR3 peptide contact domain, however, was generally conserved in length and charge, with a negatively charged QE or QD motif in the TCR β chain of each of the LACK-reactive hybridomas (3). Similarly, hybridomas established from the lymph node cells of infected BALB/c mice that expressed the Vβ4 TCR contained the QE motif in the CDR3; one had a charged WD motif at the same position (2). Such features suggested that a positively charged amino acid within the LACK antigenic determinant represented a critical TCR contact residue. Based on the use of histidine and other charged residues at TCR contact points among peptides binding to I-Ad (16), we mutated the histidine at position 164 in the wild-type peptide (LACK) to asparagine or lysine, thus creating peptides LACK-N164 and LACK-K164, respectively.
Binding Affinities of LACK Analogue Peptides to I-Ad.
The relative affinities for MHC class II molecules by LACK and the LACK analogues were tested by assaying their capacities to compete with an I-Ad ligand of known affinity, chicken egg OVA peptide323–336. By this assay, each of the LACK-derived peptides displayed binding affinities for I-Ad in the same nanomolar range as the OVA323–336 reference peptide; if anything, they showed slightly stronger affinities (Table I). Substitution of H164 in the wild-type LACK determinant by N or K did not, therefore, affect its binding affinity for MHC class II molecules.
Table I.
Affinity of LACK Analogue Peptides for I-Ad MHC Class II Molecules
| Peptide | Amino acid sequence | I-Ad | ||
|---|---|---|---|---|
| nM | ||||
| LACK156–173 | ICFSPSLEHPIVVSGSWD | 63 | ||
| LACK-K164 | ICFSPSLEKPIVVSGSWD | 68 | ||
| LACK-N164 | ICFSPSLENPIVVSGSWD | 100 | ||
| OVA323–336 | ISQAVHAAHAEINE | 150 |
Analogue LACK Peptides Do Not Activate LACK-reactive T Cells from TCR-transgenic Mice.
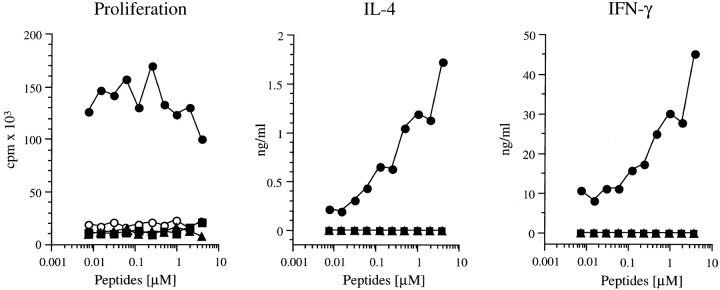
ABLE mice express a transgenic TCR derived from a LACK-reactive Vβ4/Vα8 T cell clone that is activated by the LACK156–173 peptide in the context of I-Ad (4). These mice have been crossed to BALB/c TCR-Cα0 mice, thus creating BALB/c ABLE TCR-Cα0 mice. These mice express a monoclonal αβ T cell repertoire consisting exclusively of the LACK-reactive TCR transgene and were used as a source of T cells, designated ABLE T cells. ABLE T cells proliferated in response to the LACK wild-type peptide at low concentrations (7 nM) but not after stimulation with the LACK-N164 or LACK-K164 analogues, even at concentrations up to 4 μM (Fig. 1). Although ABLE T cells generated both IFN-γ and IL-4 in culture supernatants after incubation with the LACK peptide, neither cytokine was detected after incubation with the two analogue peptides nor with the irrelevant OVA peptide that also binds I-Ad (Fig. 1). Thus, a single amino acid substitution at position 164 in the LACK T cell epitope substantially altered reactivity of the transgenic T cells, indicating that this amino acid position is likely to be a critical TCR contact residue.
Figure 1.
Response of LACK-specific transgenic T cells to altered LACK peptides. Spleen cells (106) from ABLE TCR-Cα0 mice were incubated with increasing amounts of the designated peptides for 48 h. Supernatants were collected from duplicate wells and assayed for IL-4 (center) and IFN-γ (right) by ELISA. Additional duplicate wells were pulsed with [3H]thymidine and incubated an additional 18 h before harvesting to assess proliferation by incorporation of radioactivity (left). No proliferation occurred in the absence of LACK peptide. Cells from TCR-Cα0 mice generated no signals in these assays (not shown). Results were comparable in four separate experiments. •, LACK; ▴, LACK-N164; ▪, LACK-K164; ○, OVA.
LACK Analogue Peptides Act as TCR Antagonists In Vitro.
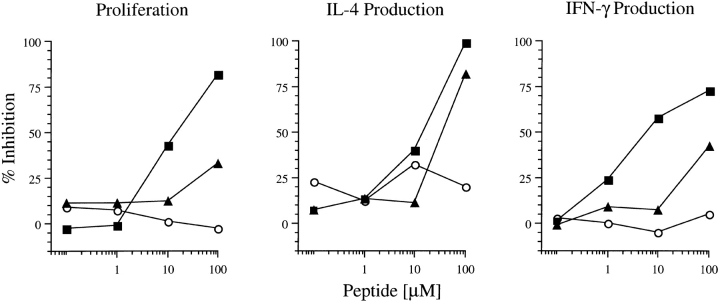
The LACK-derived peptides were next analyzed in an antagonism assay that was developed to avoid peptide competition at the level of MHC occupancy and thus allow measurements of events mediated by the TCR (13). Spleen cells from BALB/c TCR-Cα0 mice were used as APC and were preincubated with a suboptimal concentration (0.2 μM) of the wild-type LACK peptide. Washed and irradiated APC were then incubated with increasing concentrations (0.1–100 μM) of the LACK analogue peptides or the OVA323–336 peptide before ABLE T cells were added and assessed for their capacity to proliferate and produce cytokines.
LACK-K164 showed dose-dependent inhibition of proliferation to the wild-type peptide; 50% inhibition occurred at a concentration of 10 μM of the analogue peptide (Fig. 2). At similar concentrations, the peptide also inhibited IL-4 and IFN-γ production. LACK-N164 inhibited the proliferation of ABLE T cells only at very high concentrations (>100 μM). The production of IL-4 was inhibited comparably to the LACK-K164 peptide, but IFN-γ production was inhibited consistently less by LACK-N164 in multiple assays. The unrelated OVA323–336 peptide displayed no inhibitory activity. Thus, in the presence of otherwise stimulatory amounts of the wild-type LACK peptide, the two analogue peptides behaved as TCR antagonists. Of the TCR-mediated functions tested, LACK-N164 preferentially inhibited IL-4 production by ABLE T cells, whereas the capacity to proliferate and produce IFN-γ was less affected; LACK-K164 was more global in its inhibitory capacities.
Figure 2.
Antagonism of LACK-specific transgenic T cells by altered LACK peptides. APC prepared from BALB/c TCR-Cα0 mice were prepulsed with 0.2 μM LACK peptide for 2 h, washed and irradiated, and distributed to wells for incubation with varying concentrations of either the LACK-N164 (▴), LACK-K164 (▪), or OVA323–336 (○) peptides. After 2 h, wells were washed and LACK-specific ABLE T cells were added. Proliferation and cytokine production were assayed as described in the Fig. 1 legend. Results depict the percent inhibition as compared with the T cell responses to the LACK-prepulsed APC in the absence of added peptides. Comparable results were obtained in three independent experiments.
LACK Analogues Fail to Activate LACK-specific T Cells In Vivo.
The same amino acid substitutions were introduced into the full length rLACK protein by site-directed mutagenesis, creating rLACK-N164 and rLACK-K164 altered proteins. When tested in vitro for its capacity to stimulate ABLE T cells, the rLACK protein stimulated proliferation and IL-4 and IFN-γ production at molar concentrations comparable to those of the wild-type LACK peptide. In contrast, the altered rLACK proteins, as their peptide counterparts, did not stimulate proliferation or measurable cytokine production over a wide range of concentrations (data not shown).
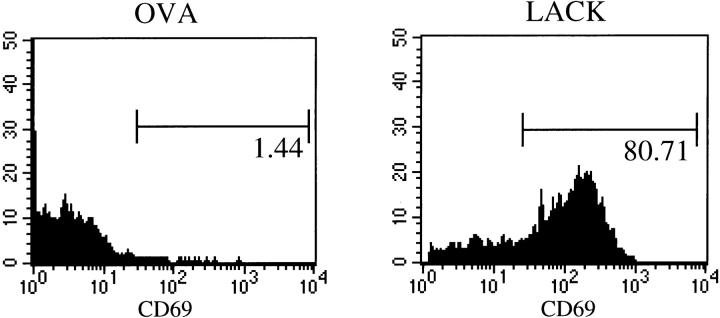
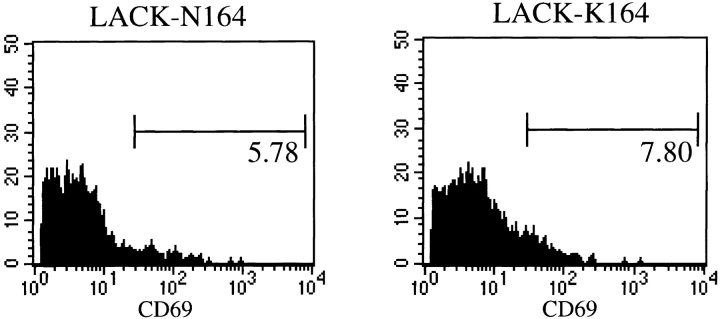
To assess the activity of the rLACK proteins in vivo, ABLE-Cα0 mice were injected in the hind footpads with 25 μg of purified rLACK, rLACK-N164, rLACK-K164, or OVA. After 24 h, the popliteal lymph node cells were recovered and analyzed using flow cytometry for activation, as assessed by expression of CD69 and enlargement by light-scattering characteristics. Inoculation of rLACK effectively targeted the transgenic T cells: 80% of Vβ4+ cells expressed CD69 (Fig. 3) and forward/side scattering increased significantly (data not shown). The total number of transgenic T cells actually decreased in the draining lymph nodes (from 2.9 × 105 after OVA to 1.3 × 105 after rLACK), consistent with antigen-mediated deletion as previously described in other TCR-transgenic mice (17, 18). In contrast, Vβ4+ T cells collected from animals injected with the rLACK analogues showed CD69 induction and forward/side scattering indices that were only modestly greater than those from cells collected from animals injected with the control protein, OVA (Fig. 3). Furthermore, the total number of transgenic T cells in these mice was not significantly different from the number of transgenic T cells in mice that received OVA (data not shown). Thus, as assessed by these criteria, the rLACK analogues did not activate ABLE T cells in vivo in a manner comparable to the cognate LACK protein containing the wild-type epitope.
Figure 3.
Activation of LACK-specific transgenic T cells in vivo by altered LACK proteins. Popliteal lymph node cells were collected from ABLE TCR-Cα0 mice that had been injected 24 h previously with 25 μg rLACK, LACK-N164, LACK-K164, or OVA proteins. Activation of Vβ4+ T cells was quantitated by the presence of the CD69 surface activation marker after gating on Vβ4+ T cells. Data are representative mice from three comparable experiments.
LACK Analogue Proteins Block Activation of the LACK-specific Response in Wild-type Mice.
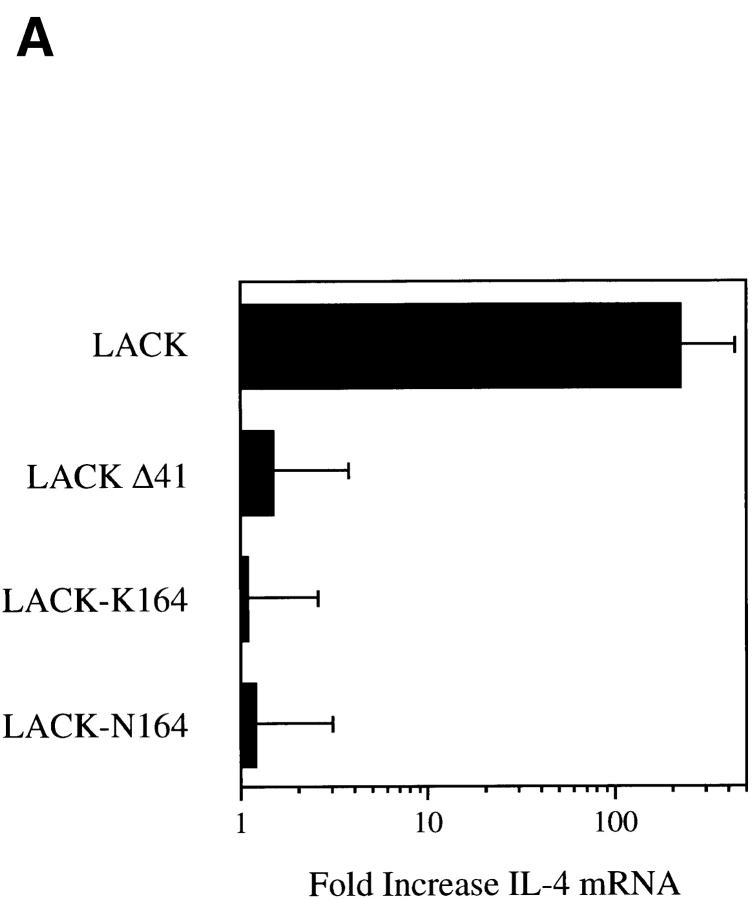
Prior experiments demonstrated that the LACK antigen induced prominent IL-4 expression in Vβ4/Vα8 CD4+ T cells after injection into BALB/c mice that reached levels 30–100-fold greater than after injection of a construct with the I-Ad epitope deleted (5). Over a 5–50 μg range of rLACK, IL-4 mRNA was induced 100-fold, whereas no IL-4 mRNA was induced by either the 41–amino acid LACK deletion mutant or rLACK-K164 (Fig. 4 A and data not shown). Although a 10-fold induction of IL-4 mRNA was seen after injection of 5 μg rLACK-N164, no IL-4 mRNA was induced after injection of 25- or 50-μg doses. None of the LACK derivatives caused induction of IFN-γ mRNA under the conditions used. After immunization with CFA, however, each of the constructs, rLACK, rLACK-K164, and rLACK-N164, was capable of inducing a proliferative response from subsequently isolated popliteal lymph node CD4+ T cells in response to their respective LACK156–173 epitopes (stimulation indices increased 10–18-fold; data not shown). No proliferation was induced by any of the LACK epitopes after immunization with the LACK deletion mutant or OVA. The 25-μg protein dose was selected for use in subsequent experiments.
Figure 4.
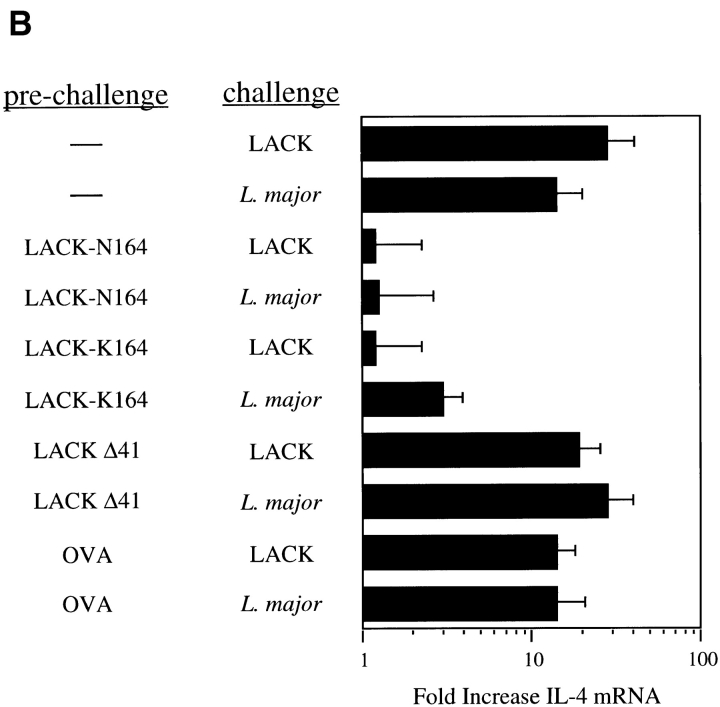
LACK analogue proteins fail to activate IL-4 mRNA in vivo. (A) Popliteal lymph node cells were collected 16 h after injection of 25 μg rLACK, LACK-K164, LACK-N164, or LACKΔ41 with the I-Ad epitope deleted. RNA was purified and used to template a semiquantitative RT-PCR assay for the amounts of IL-4 transcripts. Results are expressed as the fold increase in IL-4 mRNA as compared with uninjected mice. Results shown represent comparable findings from three experiments. (B) BALB/c mice were injected in the hind footpads with 25 μg rLACK, LACK-K164, LACK-N164, LACKΔ41, or OVA (prechallenge) and then again 24 h later with either 25 μg LACK antigen or 3 × 106 L. major promastigotes (challenge). After 16 h, the popliteal lymph node cells were collected, RNA was isolated, and the relative IL-4 mRNA levels were determined using RT-PCR as described in Materials and Methods. Results depict fold increases in IL-4 transcripts as compared with mice immunized with the same peptides but not challenged in the secondary experiment and are representative of three comparable experiments.
To assess the capacity to alter the response of the endogenous LACK-reactive repertoire, mice were first injected with 25 μg of the LACK analogue proteins and then, 24 h later, with either the authentic LACK protein or viable L. major promastigotes, both shown previously to activate a brisk IL-4 mRNA response in BALB/c CD4+ T cells (5). In three separate experiments, prior injection of either analogue protein substantially decreased the subsequent activation of the IL-4 response, consistent with an alteration of the normal Vβ4/Vα8 CD4+ T cell response (Fig. 4 B). Prior injection of the LACK construct with the deleted I-Ad epitope or an unrelated I-Ad epitope (OVA) did not affect the subsequent IL-4 response. Kinetic analysis, in which the second injection of recombinant LACK was delayed 10, 20, or 30 d after the initial immunization, revealed that IL-4 nonresponsiveness was maintained for 10–20 d in mice that had been injected with the mutated LACK analogues but then subsequently recovered. Recovery of IL-4 responsiveness to LACK or L. major injection was ablated by prior thymectomy (data not shown).
Protection of Susceptible BALB/c Mice by Immunization with LACK Analogue Proteins.
Based on the capacity of LACK analogue proteins to abrogate the early IL-4 response in BALB/c mice, we assessed the ability of immunization to render these mice resistant to progressive disease. Cohorts of mice were immunized once with 25 μg of the various recombinant proteins in the footpad and challenged 24 h later with a lethal infectious dose of wild-type L. major promastigotes of either the IR/173 or LV39 strain.
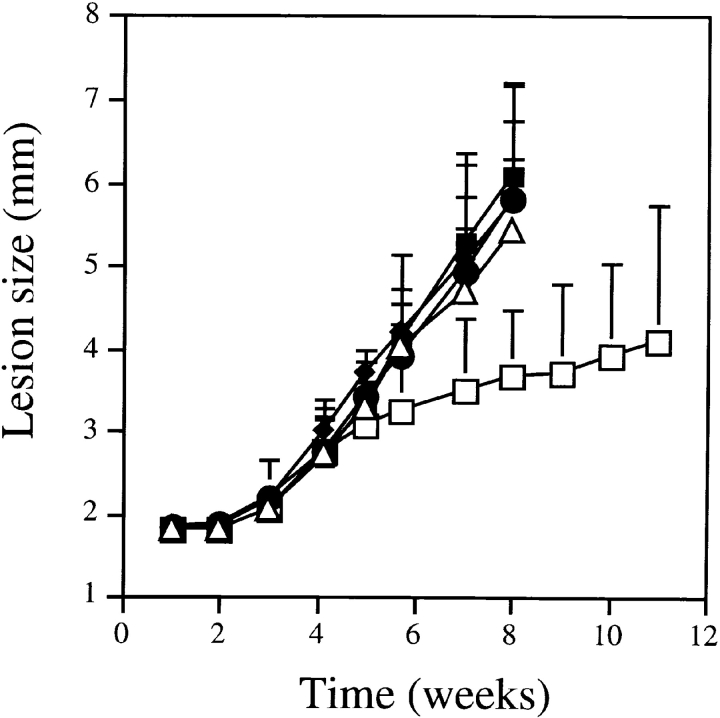
In three separate experiments with the IR/173 parasite, the course of disease was significantly attenuated in animals that received rLACK-N164; no attenuation was seen in animals that received rLACK-K164 or any of the control proteins, including the wild-type LACK protein (Fig. 5). Whereas animals in all of the other groups had to be killed by week 8, mice that received rLACK-N164 controlled disease up to 12 wk after inoculation, when the experiment was terminated. Parasitologic control was confirmed by limiting dilution of tissues that demonstrated a 2–4-log reduction in parasite numbers. Immunologic analysis revealed a threefold reduction in the number of IL-4–producing cells in the draining lymph nodes and in serum IgE levels, whereas the number of IFN-γ–producing cells was similar in all groups.
Figure 5.
Course of L. major IR/173 infection in BALB/c mice immunized with altered LACK ligands. Groups of 5–10 mice were inoculated in the hind footpads with 25 μg rLACK (▵), LACK-K164 (•), LACK-N164 (□), OVA (♦), or vehicle control (▪). The next day, animals were infected with L. major IR/173 metacyclic promastigotes, and the course of infection was followed by quantitating the size of the local lesions using a metric caliper. Mice were killed at the final time point and analyzed for tissue parasite burdens, the lymphocyte cytokine response, and serum IgE as described in Materials and Methods.
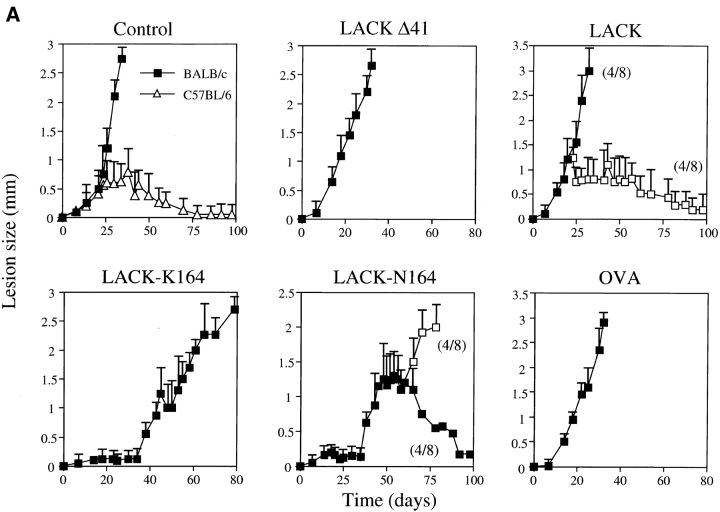
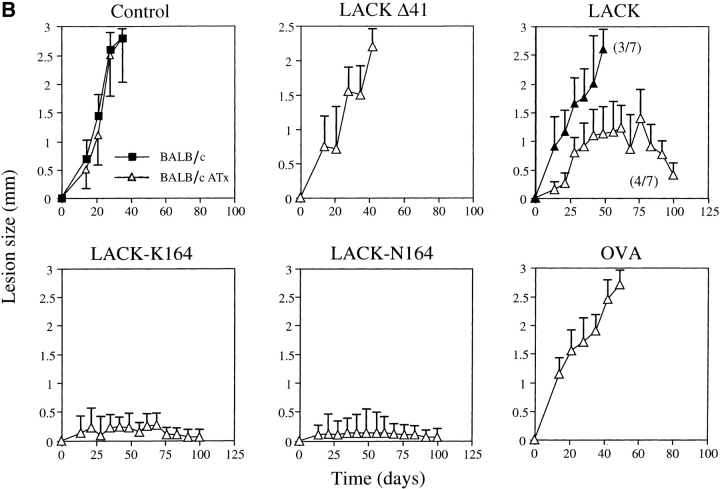
Protection was more dramatic using the LV39 strain. Similar to the results using the IR/173 strain, rLACK-N164 but not rLACK-K164 provided lasting protection in a subgroup of nonthymectomized mice (Fig. 6 A). The LACK protein itself provided protection in approximately half of both wild-type and thymectomized BALB/c mice (Fig. 6, A and B). Strikingly, either of the altered LACK proteins, in contrast to the LACK deletion mutant or OVA controls, provided a complete protection in thymectomized mice that was sustained over 100 d (Fig. 6 B). When studied at the conclusion of these experiments, the cure phenotype was associated with attenuation of IL-4 production and control of parasite growth in the footpads that was entirely concordant with the lesion phenotype (data not shown).
Figure 6.
Course of L. major LV39 infection in BALB/c mice immunized with altered LACK ligands. (A) Groups of eight nonthymectomized BALB/c mice (▪) were immunized in the hind footpads with either vehicle control or 25 μg recombinant LACK, LACK-K164, LACK-N164, LACKΔ41, or OVA. Mice were infected in one footpad the following day with L. major LV39 stationary phase promastigotes and the size of the local lesion quantitated as infected minus noninfected footpads using a metric caliper. A cohort of age-matched, resistant C57BL/6 mice was included as shown (▵). Subgroups of resistant and susceptible mice occurred after immunization with LACK and LACK-N164. Results were comparable in two independent infections. (B) Groups of 7–8 BALB/c mice were thymectomized (▵) at 8 wk of age and immunized and infected with L. major LV39 promastigotes as above. Control adult thymectomized BALB/c mice (BALB/c ATx) remained as susceptible as nonthymectomized BALB/c mice (▪), as shown in the first panel. Subgroups of resistant and susceptible mice occurred after immunization with LACK. Results were comparable in two independent infections.
Discussion
L. major includes a heterogeneous group of protozoa strains that express some 10,000 proteins from a 35.5-megabase genome (19). Despite this complexity, the early immune response is highly focused on a single epitope from the parasite LACK antigen in mice that express I-Ad MHC class II molecules. As shown here, targeting T cells that recognize this epitope using ligands that differed by a single amino acid from the natural epitope was capable of redirecting an otherwise ineffective immune response with a fatal outcome to a completely protective response with long-term cure. The specificity of the immune intervention suggests limited plasticity in the innate LACK-reactive repertoire in I-Ad–bearing mice, as well as limited ability of Leishmania parasites to mediate progressive infection in such mice in the absence of exuberant LACK recognition.
The mechanisms underlying the dominant recognition of the LACK epitope remain unclear. Recognition does not seem related simply to the abundance of the LACK protein. As compared with other parasite molecules like the major surface protease gp 63 or the major surface glycolipid LPG, which are present in ∼5 × 105 and 3–5 × 106 molecules per organism (20, 21), respectively, LACK was less abundantly expressed. Quantitation against recombinant standards showed that LACK comprised only ∼0.03% of total cellular protein or ∼30,000 molecules per organism (Pingel, S., and R. Locksley, unpublished data). The LACK epitope displayed in vitro affinity for I-Ad that was comparable with endogenously eluted I-Ad peptides (22) and contained a centrally disposed histidine residue, creating a charged element that has been noted in other peptides that bind this MHC molecule. Presumably, the dominance of the epitope must result from some confluence of stability and processing of the peptide, the efficiency of targeting to MHC class II molecules, and/or the size of the responding T cell repertoire (23). Equally perplexing is the dominant nature of the Vβ4/Vα8 TCR response to the I-Ad/LACK peptide complex. The convergence of the immune response on the LACK epitope through use of a dominant Vα/Vβ-paired TCR has been reported using other immunogenic peptides (24), suggesting that other antigens or adjuvant-like molecules from live parasites do not affect this clonal affinity maturation process.
Earlier studies reported the ability to vaccinate susceptible BALB/c mice against L. major using LACK antigen administered in a manner such that LACK-specific Th1 cells were generated (3, 25). Indeed, LACK-specific Th1 cells were alone sufficient to establish substantial control over infection with L. major, demonstrating that immunoreactive LACK peptide is expressed in vivo at physiologically important levels (4). Despite the capacity of LACK-specific T cells to control infection with the parasite in vivo, such T cells are not required. Thus, BALB/c mice rendered deficient in CD4+ T cells that recognize this dominant epitope, either through thymic expression and central deletion or by superantigen-mediated deletion of all Vβ4+CD4+ T cells, were capable of containing L. major infection (3, 5). These experiments suggested that LACK recognition was required for establishing the susceptible state of BALB/c mice, although neither method directly targeted epitope-specific T cells. Thus, overexpression of LACK antigen in the thymus might affect the T cell repertoire in ways other than deletion of LACK-reactive T cells. Similarly, deletion of all Vβ4+ CD4+ T cells targets cells of additional specificities but unknown contributions to defense against Leishmania.
The ability of peptide ligands that differed at a single amino acid residue to affect the subsequent course of disease in susceptible BALB/c mice argues strongly for highly conserved specificity to the Th2-mediating repertoire. Altered ligands are presumed to anergize or functionally alter discrete populations of T cells by their ability to establish incomplete signaling through the TCR complex (reviewed in 26). Modulation of cytokine patterns by altered ligands has been previously demonstrated (27), although application of this technology to an acute infectious process has been infrequently examined. Redirection of Th subset differentiation, or immune deviation, has been reported by either variations in antigen dose (28, 29) or through use of altered peptide ligands (30). For a variety of reasons, we consider it unlikely that immune deviation can account for the protection mediated by the altered LACK proteins. First, over a wide dose range, the LACK peptide caused no shift in the production of IL-4 and IFN-γ relative to each other by the TCR-transgenic ABLE T cells. Second, the altered LACK peptides induced neither proliferation nor cytokine production by ABLE T cells. Third, the massive activation of ABLE T cells after injection of LACK was absent after injection of the altered LACK antigens. Finally, injections of lower doses of LACK or the altered LACK proteins into BALB/c mice did not induce early production of IFN-γ, rather than IL-4, mRNA. We could, therefore, find no evidence for the establishment of a LACK-specific Th1 response that could account for the protection mediated by the altered LACK analogues.
More likely, protection of susceptible mice was accomplished through tolerance or deletion of LACK-reactive T cells, a mechanism consistent with previous experimental findings (5, 7). Both altered LACK proteins antagonized IL-4 production by transgenic T cells in response to LACK. When used to immunize BALB/c mice, they abrogated the early IL-4 response to L. major parasites. Treated mice were able to control parasite multiplication of the LV39 strain up to 5 wk; over prolonged periods, and with both the LV39 and IR/173 strains, mice pretreated with the rLACK-N164 protein demonstrated persistent immunity. Thymectomized BALB/c mice immunized with either rLACK-N164 or rLACK-K164 were completely protected, a finding consistent with the ability of the altered LACK proteins to abrogate IL-4 production by Vβ4/Vα8 CD4+ T cells in these mice. Presumably, the delayed yet progressive disease in nonthymectomized LV39-infected mice was dependent on new thymic emigrants. Immunization with the LACK protein itself conferred protection to some mice. As demonstrated using the TCR-transgenic mice, this presumably relates to the capacity of the cognate ligand to mediate peripheral deletion of high-affinity LACK-specific T cells. The observed differences in the overall grade of protection between the two strains of L. major were surprising, but such differences in virulence have been previously described (31, 32), as have subset responses within cohorts of identically treated mice, as seen here among rLACK- and rLACK-N164–treated mice (33–35). Boosting or otherwise optimizing the immunization schedule might have enhanced protection against the IR/173 strain. The rLACK-N164 ligand, however, conferred protection against both L. major strains, suggesting that identification of dominant antigens from pathogens can be used to target disease-producing T cells in a highly sequence-specific manner.
Previous studies have documented that different T cell clonotypes can respond to the same antigen within a given T cell repertoire. Furthermore, considerable cross-reactivity is an essential feature of the T cell receptor, which assures that pathogenic peptides are efficiently recognized (36). Our results suggest that the endogenous LACK-specific repertoire is highly constrained in its CDR3 recognition domain. Limited plasticity of the endogenous T cell repertoire has been previously noted with certain peptide antigens (37), suggesting that infectious diseases may have contributed to the evolutionary divergence of V region genes. Expression of dominant epitopes by parasites, together with the diversity and size of the responding host T cell repertoire, might greatly affect the outcome of such infections and thus contribute to the highly diverse clinical manifestations of leishmaniasis in human populations.
L. major contains two LACK genes expressed in tandem from the same chromosome. Apart from what is known regarding their mammalian homologues, little is known regarding the biochemical action of these proteins. Aside from the potential vaccine use of this antigen (3, 25), additional study promises to shed much light on the coevolution of host and parasite within the context of the immune system, MHC recognition, and the T cell repertoire. Such studies may have great implications for our understanding of the basis for susceptibility and resistance to infectious diseases.
Acknowledgments
The authors thank Z.-E. Wang, L. Stowring, C. McArthur, and E. Weider for technical assistance.
This work was supported by grants from the National Institutes of Health (AI26918), the Howard Hughes Medical Institute, the Swiss National Science Foundation, the World Health Organization, and the European Union. D.J. Fowell was supported by a Juvenile Diabetes Foundation International Fellowship.
Abbreviations used in this paper
- ABLE
LACK T cell receptor–specific transgenic
- CDR3
complementarity determining region 3
- LACK
Leishmania homologue of mammalian RACK1
- RT
reverse transcriptase
- TCR-Cα0
T cell receptor constant region-α–deficient
Footnotes
S. Pingel and P. Launois contributed equally to this work.
References
- 1.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. . Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 2.Reiner SL, Wang Z-E, Hatam F, Scott P, Locksley RM. Th1 and Th2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 3.Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang Z-E, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:56–66. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 4.Reiner SL, Fowell DJ, Moskowitz NH, Swier K, Brown DR, Brown CR, Turck CW, Scott PA, Killeen N, Locksley RM. Control of Leishmania majorby a monoclonal αβ T cell repertoire. J Immunol. 1998;160:88–89. [PubMed] [Google Scholar]
- 5.Launois P, Maillard I, Pingel S, Swihart KG, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley RM, MacDonald HR, Louis JA. IL-4 rapidly produced by Vβ4 Vα8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania majorin BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 6.Launois P, Swihart KG, Milon G, Louis JA. Early production of IL-4 in susceptible mice infected with Leishmania majorrapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 7.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania majorinduced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 8.Fowell, D.J., and R.M. Locksley. 1999. Leishmania major infection of inbred mice: unmasking genetic determinants of infectious diseases. BioEssays. In press. [DOI] [PubMed]
- 9.Langford CK, Ullman B, Landfear S. Leishmania: codon utilization of nuclear genes. Exp Parasitol. 1992;74:360–361. doi: 10.1016/0014-4894(92)90161-3. [DOI] [PubMed] [Google Scholar]
- 10.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini I, Itohara S, Lafaille JJ, Wang L, Ickikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes α and β block thymocyte differentiation at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 11.Brown DR, Fowell DJ, Corry DB, Wynn TA, Moskowitz NH, Cheever AW, Locksley RM, Reiner SL. β2-microglobulin–dependent NK1.1+T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantitating Leishmania majorin tissues of infected mice. Parasit Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 13.DeMagistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FCA, Grey HM, Sette A. Antigen analog-major histocompatibility complexes act as agonists of the T cell receptor. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 14.Fowell DJ, Magram J, Turck CW, Killeen N, Locksley RM. Impaired Th2 subset development in the absence of CD4. Immunity. 1997;6:559–569. doi: 10.1016/s1074-7613(00)80344-1. [DOI] [PubMed] [Google Scholar]
- 15.Reiner SL, Zheng S, Wang Z-E, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sette A, Buus S, Colon S, Smith JA, Miles C, Grey HM. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. Nature. 1987;328:395–399. doi: 10.1038/328395a0. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KE, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+ TCRlothymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 18.Liblau RS, Tisch R, Shokat K, Yang X-D, Dumont N, Goodnow CC, McDevitt HO. Intravenous injection of soluble antigen induces thymic and peripheral T cell apoptosis. Proc Natl Acad Sci USA. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravel C, Debussay P, Blackwell JM, Ivens AC, Bastien P. The complete chromosomal organization of the reference strain of the Leishmania genome project, L. major‘Friedlin'. Parasitol Today. 1998;14:301–303. doi: 10.1016/s0169-4758(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 20.McConville MJ, Bacic A. The glycoinositolphospholipid profiles of two Leishmania majorstrains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990;38:57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- 21.Bouvier J, Etges RJ, Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmaniapromastigotes. J Biol Chem. 1985;260:15504–15509. [PubMed] [Google Scholar]
- 22.Hunt DF, Michel H, Dickinson TA, Shabanowitz J, Cox AL, Sakaguchi K, Appella E, Grey HM, Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad . Science. 1992;256:1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 23.Bousso P, Casrouge A, Altman JD, Haury M, Kanellopoulos J, Abastado J-P, Kourilsky P. Individual variations in the murine T cell response to a specific peptide reflect variability in naïve repertoires. Immunity. 1998;9:169–178. doi: 10.1016/s1074-7613(00)80599-3. [DOI] [PubMed] [Google Scholar]
- 24.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 25.Gurunathan S, Sacks DL, Brown DR, Reiner SL, Charest H, Glaichenhaus N, Seder RA. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. . J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 27.Windhagen A, Scholz C, Hollsberg P, Fukaura H, Sette A, Hafler DA. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity. 1995;2:373–380. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 28.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+T helper cell phenotype development in a T cell receptor–αβ-transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naïve CD4+T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopf M, Brombacher F, Kohler G, Kienzle G, Widman KH, Lefrang K, Humborg C, Lederman B, Solbach W. IL-4–deficient BALB/c mice resist infection with Leishmania major. . J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania majorinfection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell GF. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. . J Immunogenet. 1983;10:395–412. doi: 10.1111/j.1744-313x.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell GF, Handman E, Spithill TW. Examination of variables in the vaccination of mice against cutaneous leishmaniasis using living avirulent cloned lines and killed promastigotes of Leishmania major. . Int J Parasitol. 1985;15:677–684. doi: 10.1016/0020-7519(85)90015-3. [DOI] [PubMed] [Google Scholar]
- 35.Varkila K, Chatelain R, Leal LM, Coffman RL. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type-1 response and renders them capable of healing Leishmania majorinfection. Eur J Immunol. 1993;23:262–268. doi: 10.1002/eji.1830230141. [DOI] [PubMed] [Google Scholar]
- 36.Mason D. A very high level of cross-reactivity is an essential feature of the T cell receptor. Immunol Today. 1998;19:395–403. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 37.Nanda NK, Apple R, Sercarz E. Limitations in plasticity of the T-cell receptor repertoire. Proc Natl Acad Sci USA. 1991;88:9503–9507. doi: 10.1073/pnas.88.21.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]