Abstract
Fas antigen (Apo-1/CD95) is an apoptosis-signaling cell surface receptor belonging to the tumor necrosis factor receptor superfamily. Adult T cell leukemia (ATL) cells express Fas antigen and show apoptosis after treatment with an anti-Fas monoclonal antibody. We established the ATL cell line KOB, which showed resistance to Fas-mediated apoptosis, and found that KOB expressed two forms of Fas mRNA, the normal form and a truncated form. The truncated transcript lacked 20 base pairs at exon 9, resulting in a frame shift and the generation of a premature stop codon at amino acid 239. The same mutation was detected in primary ascitic cells and peripheral blood cells. The mutation was not detected in lymph node cells, however, although all of the primary ATL cells were of the same clonal origin. A retroviral-mediated gene transfer of the truncated Fas to Jurkat cells rendered the cells resistant to Fas-mediated apoptosis, suggesting a dominant negative interference mechanism. These results indicate that an ATL subclone acquires a Fas mutation in the lymph nodes, enabling the subclone to escape from apoptosis mediated by the Fas/Fas ligand system and proliferate in the body. Mutation of the Fas gene may be one of the mechanisms underlying the progression of ATL.
Keywords: human T lymphotropic virus type I, adult T cell leukemia, apoptosis, death domain, CD95
The Fas antigen (Apo-1/CD95) is a 45-kD transmembrane protein that is a member of the TNF receptor superfamily that can induce programmed cell death, i.e., apoptosis, when cross-linked by natural ligand or specific antibodies. The human Fas antigen consists of 325 amino acids with a single transmembrane domain, including signal peptides. The extracellular domain is rich in cysteine residues and shows similarity to domains of human TNF receptor, human nerve growth factor receptor, and human B cell antigen CD40 (1). The cytoplasmic domain (∼70 amino acids), designated a death-signaling domain, is highly conserved between Fas and TNF receptor and is necessary for the transduction of the apoptotic signal. A deletion of 15 amino acids from the COOH terminus enhances the Fas antibody–induced killing activity, whereas a further deletion abolishes its activity (2). Fas can exist as both a cell surface and a soluble protein, both of which are generated by alternative splicing. While the cell surface Fas anchored by a transmembrane domain induces apoptosis, soluble Fas protects cells from apoptosis (3–5).
The Fas/Fas ligand system of apoptosis appears to play an important role in the normal development of T lymphocytes in the thymus by eliminating self-reactive lymphocytes (6, 7). Mutations in the Fas-encoding gene are responsible for the lymphoproliferative disorder in lpr and lprcg mice, which are models of autoimmune disease. These mice fail to eliminate self-reactive T lymphocytes, resulting in the accumulation of a large number of nonmalignant CD4− CD8− T lymphocytes in the lymph nodes and spleen (8– 10). Similarly, several recent studies have identified function-ablating mutations of the Fas antigen in patients with a human lupus–like syndrome, designated congenital autoimmune lymphoproliferative syndrome (ALPS),1 which is identical to the lpr mouse phenotype and characterized by lymphadenopathy, hypergammaglobulinemia, and autoimmunity. Interestingly, such mutations of the Fas gene inhibited the Fas-mediated apoptosis by a dominant negative mechanism (11–14).
Adult T cell leukemia (ATL) is a mature helper T cell– derived lymphoma (15, 16) caused by human T lymphotropic virus type I (HTLV-I; 17–19). ATL is endemic to the southwest area of Japan, the Caribbean Basin, West Africa, and the southern United States. Because the clinical features of ATL patients are quite diverse, ATL is usually classified into four subtypes—acute, lymphoma, chronic, and smoldering—according to the criteria of Shimoyama (20). Compared with non-Hodgkin's lymphoma patients, ATL patients show significantly shorter terms of survival; the median survival time is only 6.2 mo for an acute ATL patient and 10.2 mo for patients with the lymphoma type of ATL (21). The mechanism of chemoresistance of ATL cells is not yet well understood. ATL cells express abundant Fas antigen on their surfaces and undergo apoptosis induced by an IgM anti-Fas mAb in vitro (22–25), but ATL cells do not show apparent apoptosis in vivo.
Several Fas gene mutations have been reported in primary samples of myeloma, T cell lymphoblastic leukemia, and ATL in patients other than our present patient (26–28), which suggests that Fas resistance contributes to the development of neoplasms. However, a functional analysis of the detected mutant Fas gene in these diseases has not, to our knowledge, been performed. In this study, we found that the ATL cell line KOB carried a heterozygous Fas gene mutation in exon 9 lacking a death-signaling domain and acquired Fas resistance. The same mutation was detected in the primary samples, ascites, and peripheral blood but not in the lymph node. Moreover, by transfer of the mutant Fas gene to Fas-sensitive Jurkat cells, we observed that the aberrant Fas antigen functioned as a dominant negative interfering Fas signal.
Materials and Methods
Patient.
A 72-yr-old man visited our hospital with complaints of low grade fever and systemic lymphadenopathy in April 1992. His white blood cell count was 1.2 × 1010/liter, with 8% abnormal lymphocytes. His lactic dehydrogenase (LDH) level and serum calcium level were 4,400 IU/liter and 11 mg/dl, respectively. The diagnosis of acute type ATL was made hematocytologically and confirmed by the demonstration of monoclonal integration of HTLV-I proviral DNA in the peripheral blood and lymph node mononuclear cells. The patient was administered 10 cycles of combination chemotherapy and discharged with a partial response. However, only 3 mo after the discontinuation of chemotherapy, the patient was readmitted to our hospital with massive ascites and an elevated LDH level (4312 IU/liter). Although combination chemotherapy was started again, the effect was transient and the patient died of a systemic invasion of ATL cells 3 mo after his second hospital admission.
Clinical Samples and Cell Lines.
Peripheral blood was drawn several times during the patient's hospital stays. A lymph node was biopsied during his first admission, and ascites were obtained during his second admission. All materials were obtained after informed consent. Mononuclear cells were separated from the samples by centrifugation over a Ficoll gradient and cryopreserved until use. The ATL cell line KOB was established from the patient's ascitic ATL cells. The other three ATL cell lines (KK1, ST1, and SO4) used for the present study were also of primary ATL cell origin established from the respective ATL patients (29, 30). All ATL cell lines were dependent on exogenously added IL-2 and were maintained in RPMI 1640 supplemented with 10% FBS and 0.5 U/ml IL-2.
Flow Cytometric Analysis for Membrane Fas.
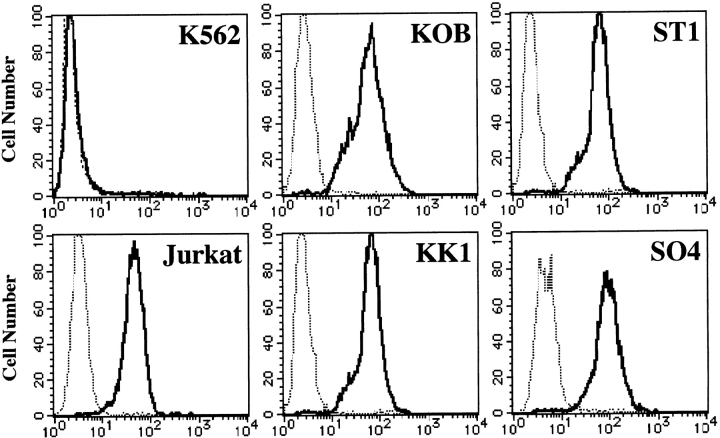
Membrane Fas was examined by flow cytometry as described previously using a Cytron flow cytometer (Ortho; 31). The resulting histograms (see Fig. 1) correspond to the cell number (y-axis) versus fluorescence intensity (x-axis) plotted on a logarithmic scale.
Figure 1.
Flow cytometric analysis of Fas antigen. All cell lines but one (K562) expressed the Fas antigen on their surfaces. Cells were incubated with IgG anti-Fas mAb (solid line) or control mAb (dashed line).
RNA Extraction and Reverse Transcriptase (RT)-PCR.
Total RNA was prepared from the cell lines and clinical samples using ISOGEN (Wako). After the removal of DNA contamination using a MassageClean™ kit (GenHunter Corp.), cDNA was made from 1 μg of total RNA using an RNA PCR core kit (Perkin Elmer) according to the manufacturer's protocol. Oligo dT primers were used to prime the first strand synthesis for each of the reactions. Four oligonucleotide primer pairs were designed to amplify the full length coding region of Fas according to the method of Cheng et al. (3). The reaction conditions were 35 cycles of 94°C for 70 s, 60°C for 30 s, and 72°C for 60 s (for full length Fas) or 30 cycles of 95°C for 60 s, 54°C for 60 s, and 72°C for 60 s (for Fas I, Fas II, and Fas III; Table I). These amplified products were electrophoretically separated and visualized on an ethidium bromide–stained 2% agarose gel or a silver-stained 12.5% acrylamide gel using a GenePhor electrophoresis unit (Pharmacia Biotech).
Table I.
Fas Primers
| Gene segment | Primer sequence | PCR product | ||
|---|---|---|---|---|
| Fas full length | Sense: 5′-CACTTCGGAGGATTGCTCAACA | |||
| Antisense: 5′-TATGTTGGCTCTTCAGCGCTA | 1,167 bp (nt. 170–1336) | |||
| Fas I | Sense: 5′-ATGCTGGGCATCTGGACCCT | |||
| Antisense: 5′-GCCATGTCCTTCATCACACAA | 336 bp (nt. 195–530) | |||
| Fas II | Sense: 5′-CATGGCTTAGAAGTGGAAAT | |||
| Antisense: 5′-ATTTATTGCCACTGTTTCAGG | 339 bp (nt. 525–863) | |||
| Fas III | Sense: 5′-AAATTTATCTGATGTTGACT | |||
| Antisense: 5′-TCTAGACCAAGCTTTGGATTTC | 344 bp (nt. 860–1203) |
cDNA nucleotide numbers correspond to those reported by Itoh et al. (1).
Subcloning and Sequencing.
We cloned the RT-PCR products of full length Fas and Fas III derived from KOB or clinical samples into the pCR™II vector using a TA cloning kit (Invitrogen Corp.). All sequencing experiments were performed using an AutoRead™ sequencing kit designed for an ALF™ DNA sequencer (Pharmacia Biotech).
Construction of Retroviral Vectors and Transfection.
The truncated full length Fas cDNA of KOB origin was inserted into the retroviral vector LXSN. This vector contains Moloney murine sarcoma virus–derived long terminal repeats and a neomycin resistance gene under the control of simian virus 40 early promoter. The recombinant retroviral vector LdelSN was purified using an EndoFree Plasmid Maxiprep kit (Qiagen) and was transfected into the ecotropic packaging cell line ψ2 using FuGENE™6 transfection reagent (Boehringer Mannheim). After a 48-h culture in DMEM, the transient retroviral supernatant was used to transduce the amphotropic packaging cell line PA317. The PA317 cells infected with recombinant retroviruses were cultured in DMEM for 48 h and selected with 0.8 mg/ml G-418. Supernatants of PA317 cells producing amphotropic retroviruses were used to transduce Jurkat cells. The transduced Jurkat cells were selected in RPMI 1640 containing 1 mg/ml G-418 and were cloned by the colony formation method on methylcellulose-containing culture plates.
Detection of HTLV-I Proviral DNA and TCR Gene Rearrangement Profile.
The integration site(s) of HTLV-I proviral DNA and TCR gene rearrangement were examined with a Southern blot hybridization assay using probes for the pX region of HTLV-I and TCR Cβ1 according to the described method (24, 32). In brief, the high molecular DNA samples (10 μg) extracted from the cell lines and clinical samples were digested with restriction enzymes and size fractionated on 0.7% agarose gels. They were then denatured and transferred onto positively charged nitrocellulose membranes (Boehringer Mannheim) and hybridized to digoxigenin-labeled probes. Thereafter, the blots were washed at appropriate stringency and treated with an anti-digoxigenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim), and the alkaline phosphatase was changed to a light signal by CDP-Star™ (Boehringer Mannheim) and exposed to film. The restriction enzymes used were EcoRI and PstI for HTLV-I proviral DNA and EcoRV and BamHI for TCR β chain gene rearrangement.
Detection of Apoptosis.
In the process of early apoptosis, cells express phosphatidylserine on the outer leaflet of the cell surface membrane and consequently bind annexin V. Cell lines cultured with or without IgM anti-Fas mAb at a final concentration of 1 μg/ml were examined for annexin V binding by flow cytometry at several time points after culture using an annexin V–FITC kit (Takara Shuzo Co.) according to the manufacturer's protocol. A DNA fragmentation analysis was also performed using the same samples and an apoptosis ladder detection kit (Wako).
Cell Proliferation Assay.
First, 105 cells/100 μl RPMI 1640 supplemented with 10% FCS were cultured with or without 1 μg/ml of IgM anti-Fas mAb in 96-well tissue culture plates for 7, 16, 24, or 32 h, and proliferation status was estimated by measuring the conversion of MTS (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt) into water-soluble formazan (CellTiter 96TMAQueous; Promega) according to the manufacturer's protocol. All experiments were performed in triplicate.
Results
Expression of Fas Antigen on Cell Lines and Response to IgM anti-Fas mAb.
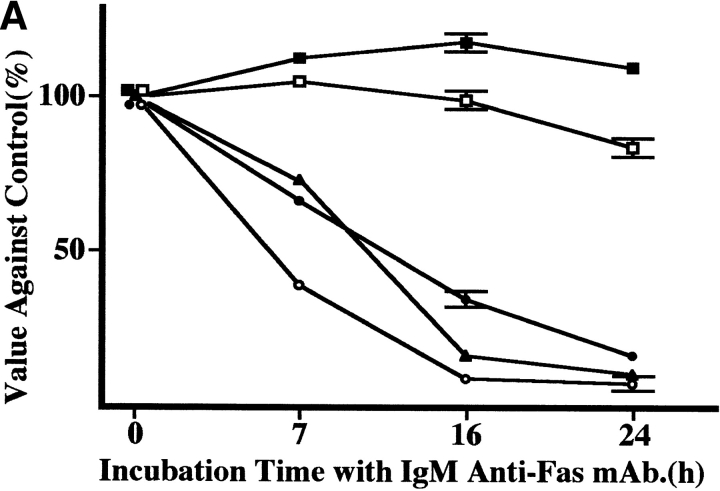
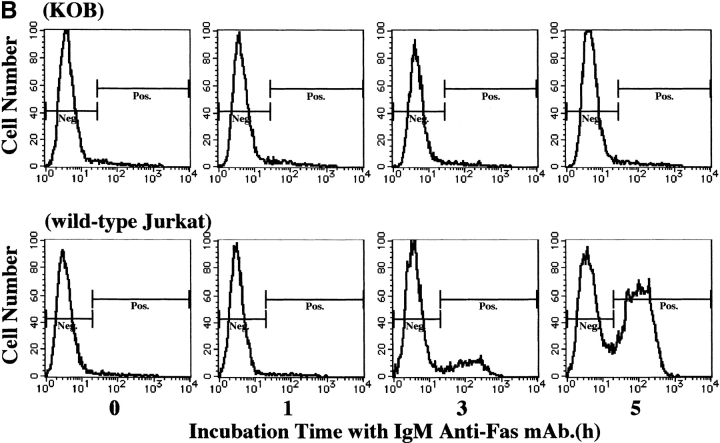
Four ATL cell lines were analyzed for the expression of Fas antigen (Apo-1/CD95) by flow cytometry using an IgG anti-Fas mAb. As shown in Fig. 1, all four ATL cell lines (KK1, KOB, ST1, and SO4) and Jurkat cells expressed Fas antigen, whereas the cell line K562 used as a negative control did not. These cell lines were cultured with 1 μg/ml IgM anti-Fas mAb, and cell proliferation status was examined. The results are expressed as a value against the control cells cultured without mAb. As shown in Fig. 2 A, after a 24-h culture with the mAb, the proliferation of KK1, SO4, and ST1 cells was significantly suppressed: to 16.8, 7.7, and 10.8% of the control level, respectively. In contrast, the KOB and K562 cells were not affected. To confirm that suppression was due to apoptosis, we performed a flow cytometric analysis of annexin V binding and a DNA fragmentation assay for the early and late phases of apoptosis, respectively. In Jurkat cells, an annexin V–binding cell population appeared 3 h after treatment with IgM anti-Fas mAb (14%), and the population was further increased at 5 h (50.1%). In contrast, no annexin V–binding cell population appeared in KOB cells (Fig. 2 B). Likewise, the other ATL cell lines (KK1, SO4, and ST1) exhibited annexin V binding but K562 cells did not (data not shown). Supporting the results of annexin V binding, DNA ladder formation appeared in the three ATL cell lines (KK1, SO4, and ST1) but not in KOB and K562 cells after 3 h of culture (data not shown). These results prompted us to examine the mechanism of Fas resistance in KOB cells.
Figure 2.
Response to IgM anti-Fas mAb. (A) Proliferation status after treatment with IgM anti-Fas mAb. Results are expressed as values against the control cells cultured without mAb. The proliferation of three ATL cell lines, SO4 (open circle), KK1 (filled circle), and ST1 (filled triangles) were strongly suppressed to 7.7, 16.8, and 10.8% of the control level, respectively, whereas KOB (open square) and K562 (filled square) cells were not affected. Data are the mean ± SD from three independent experiments. (B) Flow cytometric detection of annexin V binding. Jurkat cells clearly exhibited annexin V binding 3 h after treatment with IgM anti-Fas mAb (14%), and the binding population was further increased at 5 h (50.1%), whereas no annexin V binding was observed in KOB cells.
RT-PCR and Sequencing of Fas Gene.
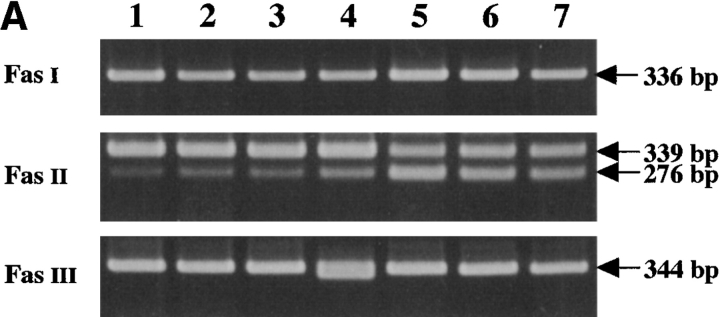
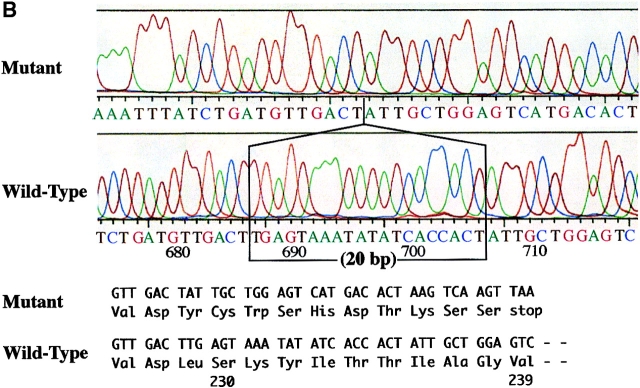
To investigate the Fas mRNA, four primer pairs were designed to cover its full length coding region. These PCR reactions were named Fas I, Fas II, and Fas III (Table I). The expected size of the bands in Fas I and Fas III was 336 and 344 bp, respectively. Fas II was expected to show two bands (339 and 276 bp) generated by alternative splicing; the larger PCR product corresponds to the membrane form, and the smaller product corresponds to the soluble form of Fas mRNA, which is 63 bp shorter than the membrane form. Irregardless of the samples, only the expected bands were observed in the Fas I and Fas II reactions. In contrast, in the Fas III reaction of KOB cells, a distinct smaller product was observed in addition to the expected 344-bp band (Fig. 3 A). The sequencing analysis of full length RT-PCR products of KOB cells showed two transcripts, the normal form and a truncated form. The truncated transcript lacked 20 bp (from nucleotide [nt] 686 to nt 705, nucleotide numbers that correspond to those reported by Itoh et al. [1]) at exon 9 within the cytoplasmic death-signaling domain. The frame shift caused by the deletion generated a premature stop codon at amino acid 239, resulting in the production of aberrant Fas antigen which lacked the entire death-signaling domain (Fig. 3 B).
Figure 3.
Detection of Fas gene mutation. (A) RT-PCR analysis of human Fas mRNA. The expected bands for Fas I and Fas III were 336 and 344 bp, respectively. Due to alternative splicing, two expected bands (339 bp and 276 bp) were detected in Fas II analysis. No difference in Fas I and Fas II was observed between samples. For Fas III, in addition to the expected 344-bp band, a distinct smaller product was observed in lane 4, which corresponds to KOB cells. Lanes 1–4 correspond to the ATL cell lines SO4, ST1, KK1, and KOB, respectively. Lanes 5 and 6 correspond to freshly isolated ATL cells. Lane 7 is a normal control. (B) Sequencing analysis of RT-PCR products of KOB cells. The aberrant transcript that lacks 20 bp (from nucleotide 686 to nucleotide 705, numbers that correspond to those reported by Itoh et al. [1]) at exon 9 was detected. The frame shift caused by this deletion generates a premature stop codon at amino acid 239.
Origin of KOB Cell Line.
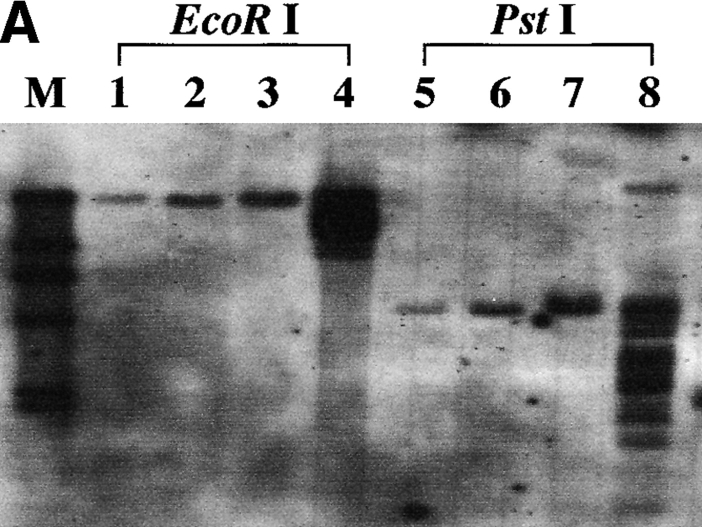
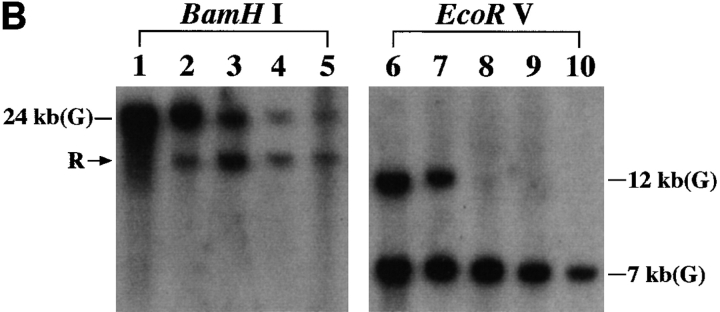
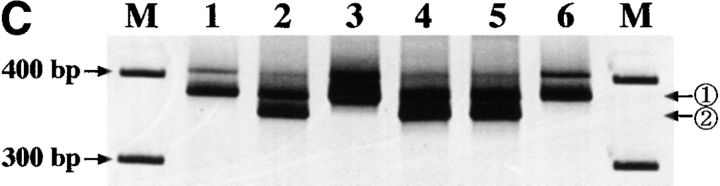
Hematocytologically, characteristic ATL cells with deeply indented or lobulated nuclei and relatively scanty basophilic cytoplasm were observed in the peripheral blood, lymph node, and ascites of our patient. We examined the integration pattern of HTLV-I proviral DNA and TCR β chain gene rearrangement profiles by Southern blot analysis to determine the origin of KOB cells. As shown in Fig. 4 A, one monoclonal band was observed at the same position in all primary samples when cellular DNAs was digested with EcoRI or PstI. In addition, multiple bands other than the original band of the primary samples were observed in KOB cells, suggesting the superinfection of HTLV-I in vitro. In the TCR β chain gene rearrangement profile, the same rearranged band (Fig. 4 B, arrowhead R) was observed in all samples including KOB when the cellular DNA was digested with BamHI. The loss of the germline band at 12 kb was observed in all but one sample (peripheral blood) when the cellular DNA was digested with EcoRV, indicating the deletion of the TCR Cβ 1 locus in this clone (Fig. 4 B). The germline band at 12 kb observed in peripheral blood indicates the presence of normal non-T cells. These results suggest that KOB cells originated from the primary ATL cells. We therefore investigated whether the primary samples also expressed the truncated Fas gene. We performed an RT-PCR analysis of Fas III in all primary samples. Interestingly, although the same smaller product observed in KOB cells was detected in peripheral blood ATL cells and ascitic ATL cells, no such product was detected in lymph node ATL cells (Fig. 4 C). The sequence analysis of these products disclosed that the clinical samples from peripheral blood and ascites had the same Fas gene mutation observed in KOB cells. No such mutation was observed in the lymph node cells (data not shown).
Figure 4.
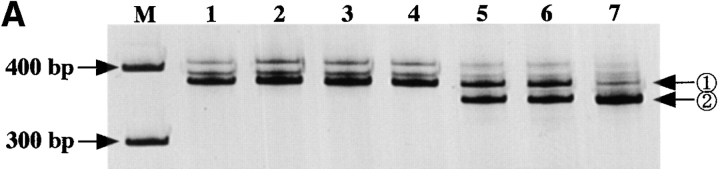
Origin of KOB cells. (A) Southern blot analysis of HTLV-I proviral DNA. A monoclonal band is observed at the same position in all primary samples. Multiple extra bands are observed in lanes 4 and 8, which correspond to KOB cells, suggesting the superinfection of HTLV-I in vitro. Lanes 1 and 5, peripheral blood ATL cells; lanes 2 and 6, lymph node ATL cells; lanes 3 and 7, ascitic ATL cells; lanes 4 and 8, KOB cells; M, size marker. (B) TCR Cβ gene rearrangement profile. The same rearranged bands (arrowhead R) were observed in all samples digested with BamHI. With EcoRV digestion, the loss of the germline band at 12 kb is observed in lanes 8–10. The germline band at 12 kb in lane 8 indicates the presence of some non-T cells in the peripheral blood. Lanes 1 and 7, human placenta; lanes 2 and 8, peripheral blood ATL cells; lanes 3 and 9, lymph node ATL cells; lanes 4 and 10, ascitic ATL cells; lanes 5, 6, 11, and 12, KOB cells; G, germline bands. (C) RT-PCR analysis of Fas III reaction. In addition to the normal 344-bp band (arrow 1), the same distinct smaller products (arrow 2) are observed in lanes 2, 4, and 5. Lane 1, Jurkat cells; lane 2, KOB cells; lane 3, lymph node ATL cells; lane 4, peripheral blood ATL cells; lane 5, ascitic ATL cells; lane 6, peripheral blood mononuclear cells from a healthy volunteer. M, size marker.
Retroviral-mediated Transfer of the Mutant Fas Gene.
To confirm that the mutant Fas gene is responsible for Fas resistance in KOB cells, we constructed the retroviral vector LdelSN containing the truncated Fas cDNA and transferred it into Jurkat cells. The successful transfer of the gene in G418-resistant cells was confirmed by RT-PCR of Fas III and neomycin resistance gene–specific sequences and by sequencing analysis. RT-PCR analysis of Fas III showed two transcripts in all LdelSN-transduced Jurkat cell clones (RJ-9, RJ-11, and RJ-14) but only normal transcript in wild-type and LXSN-transduced Jurkat cell clones (LX-1, LX-2, and LX-3; Fig. 5 A). We performed a sequencing analysis of the RT-PCR products from transduced Jurkat cells and verified the expression of transferred Fas gene (data not shown).
Figure 5.
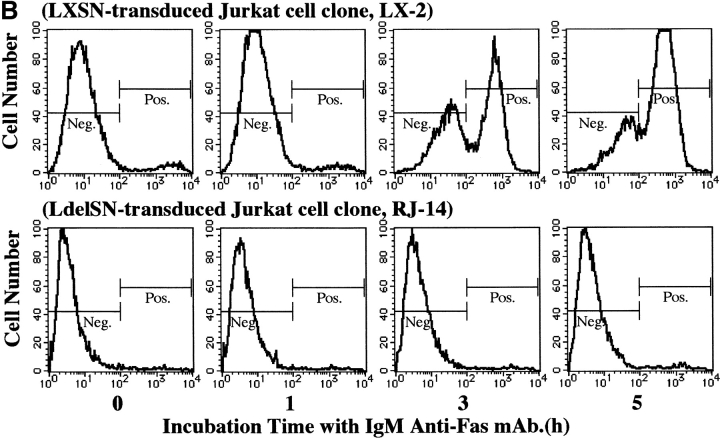
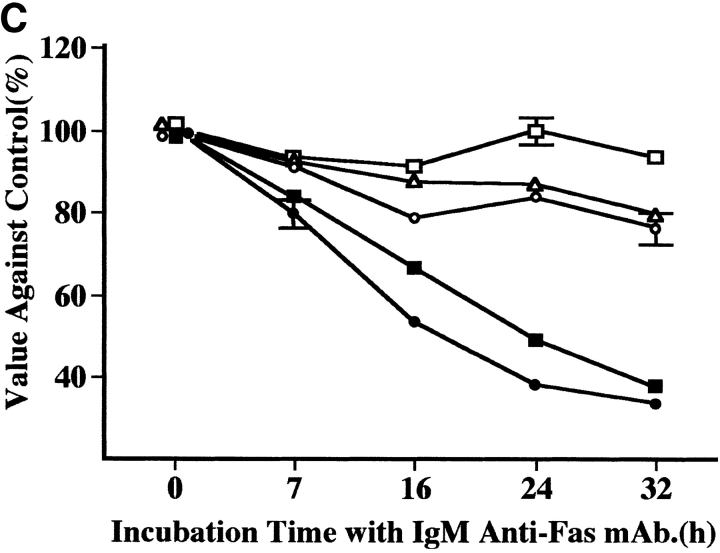
Retroviral-mediated gene transfer and response to IgM anti-Fas mAb. (A) RT-PCR analysis in Fas III reaction. All LdelSN-transduced Jurkat cell clones (lane 5, RJ-9; lane 6, RJ-11; lane 7, RJ-14) expressed two transcripts corresponding to the normal Fas (arrow 1) and truncated Fas (arrow 2), but only the normal transcript was detected in wild-type (lane 1) and all LXSN-transduced Jurkat cell clones (lane 2, LX-1; lane 3, LX-2; lane 4, LX-3); M, size marker. (B) Flow cytometric detection of annexin V binding. A marked increase in the annexin V–binding cell population was observed in the LXSN-transduced Jurkat cell clone LX-2 after a 3-h culture with mAb (3.3% at 0 h, 3.1% at 1 h, 56.8% at 3 h, and 77.2% at 5 h). No increase was detected in the LdelSN-transduced Jurkat cell clone RJ-14. (C) Evaluation of proliferation status. The proliferation of LXSN-transduced Jurkat cell clones LX-1 (filled square) and LX-3 (filled circle) was significantly suppressed to 37.6 and 33.4% of the control level, respectively, but the LdelSN-transduced Jurkat cell clones RJ-9 (open circle), RJ-11 (open triangle), and RJ-14 (open square) were not affected by mAb. Data are the mean ± SD from three independent experiments.
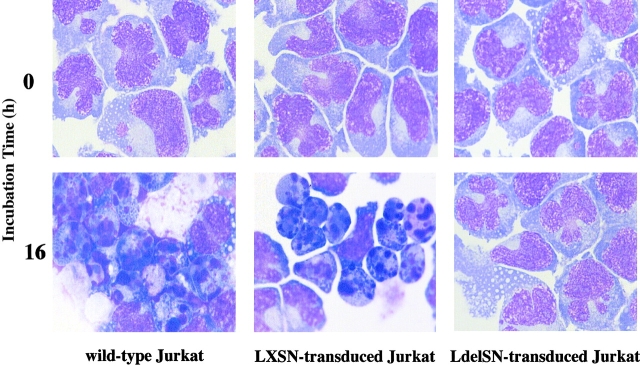
The LXSN- and LdelSN-transduced Jurkat cells were assayed for Fas sensitivity. The flow cytometric analysis of annexin V binding showed that the LXSN-transduced Jurkat cell clone LX-2 demonstrated a marked increase of the annexin V–binding cell population of 3.3% at 0 h to 56.8% at 3 h after treatment with IgM anti-Fas mAb, and the population increased further to 77.2% at 5 h. In contrast, the LdelSN-transduced Jurkat cell clone RJ-14 showed no such increase (Fig. 5 B). Using CellTiter 96TMAQueous, the proliferation status was evaluated at several time points of culture with 1 μg/ml of IgM anti-Fas mAb. The proliferation of the LXSN-transduced Jurkat cell clones LX-1 and LX-3 was significantly suppressed to 37.6 and 33.4% of the control level, respectively, but the LdelSN-transduced Jurkat cell clones RJ-9, RJ-11, and RJ-14 were not affected (Fig. 5 C). The characteristic features of apoptosis, i.e., reduction of cell size, condensation and aggregation of nuclear chromatin, and nuclear fractionation were observed in wild-type Jurkat cells and LXSN-transduced Jurkat cell clones but not in LdelSN-transduced Jurkat cell clones (Fig. 6).
Figure 6.
Morphological changes after treatment with mAb. May-Grünwald Giemsa staining at an original magnification of 1000 is shown. The characteristic features of apoptosis, including the reduction of cell size, condensation and aggregation of nuclear chromatin, and nuclear fractionation, were observed in wild-type Jurkat cells and LXSN-transduced Jurkat cell clones; no morphological change was observed in the LdelSN-transduced Jurkat cell clone.
Discussion
Most ATL cells express abundant Fas antigen on their surfaces and show apoptosis in a short term culture with IgM anti-Fas mAb (22, 23, 25, 33). We reported that ATL cells from all but 2 of 33 patients expressed Fas antigen, and the Fas− ATL cells were resistant to apoptosis (34). Differences in Fas expression seem to be correlated with subtypes of ATL; the level of Fas expression was higher in chronic type than in acute type ATL patients and was inversely correlated with serum LDH activity (24, 25). These results may indicate that the Fas/Fas ligand system plays a critical role in elimination of ATL cells.
Fas gene mutations were recently reported in some malignancies. 7 cases of malignancy with Fas gene mutations were detected in 48 multiple myeloma patients by the RT-PCR method and a single-stranded conformation polymorphism (SSCP) analysis. All of these mutations were point mutations in the cytoplasmic region, and two of them were silent mutations (26). 2 cases of Fas gene mutations were found among 81 childhood T-lineage acute lymphoblastic leukemia patients using PCR and single-stranded conformation polymorphism analysis as the screening methods; one case was a heterozygous point mutation in exon 3, and another case was a homozygous alteration in the promoter region (27). The 3 cases of Fas gene mutations detected among 47 ATL patients by an RNase protection assay were a 5-bp deletion in exon 2, a 1-bp insertion in exon 2, and a loss of exon 4. Two silent point mutations were also observed (28). Rearrangement or allelic loss of Fas gene were also reported in 5 of 70 patients with non-Hodgkin's lymphoma: 3 cases of allelic loss and 2 cases of rearrangement (35). According to these reports, it is likely that Fas gene mutations, including silent mutations, occur at relatively low but diverse frequencies, ∼2.5–14.6%, and are consistently detected in lymphoid malignancies, suggesting an association between Fas mutation and disease progression in lymphoid malignancies. However, a functional analysis of the aberrant Fas was not performed in any of the above studies.
In many receptor and ligand systems such as epidermal growth factor, fibroblast growth factor, and platelet-derived growth factor β, dimerizations of receptor subunits appear to be essential for signal transduction. The truncated forms of these receptors suppress the response of wild-type receptor by heterodimerization (36–41). Analysis of the crystal structure of TNF receptor–TNF-β complex demonstrated that three TNF receptor molecules symmetrically bound to TNF-β trimer (42). The human soluble Fas ligand also forms homotrimers (43). It has been reported that the trimerization of the death-signaling domain within the cytoplasmic region of Fas is essential for signal transduction in the process of Fas-mediated apoptosis (44). Therefore, it is likely that KOB cells prevented apoptosis by an expression of function-ablating Fas antigen on their surfaces similar to the expression on lymphocytes of ALPS patients. The truncated Fas antigen, which lacks the entire death-signaling domain, may behave as a decoy receptor and interfere with the trimerization of normal Fas in a competitive manner, resulting in the impairment of signal transduction.
To test this hypothesis, we constructed the retroviral vector LdelSN containing the truncated Fas cDNA and transferred it into Jurkat cells, which are sensitive to IgM anti-Fas mAb. The resulting Jurkat cells became almost completely resistant to IgM anti-Fas mAb, suggesting that heterotrimers between the truncated Fas and normal Fas, which was endogenously expressed in Jurkat cells, did not transduce the apoptotic signal. Although the pathological role of aberrant Fas antigen has been demonstrated in ALPS by the method of transient gene transfer, no such trial has been performed in neoplasms. The present study is, to our knowledge, the first that has successfully demonstrated the dominant negative inhibition of apoptosis by a stably transfected cell line.
More interestingly, the mutated Fas gene observed in KOB cells was not detected in lymph node ATL cells, although a Southern blot analysis indicated that all of the primary ATL cells and KOB cells were of the same cell origin. These results indicate that ATL cells developed in the lymph nodes have acquired Fas gene mutation, and the subclone with the mutation spread to the whole body, escaping elimination by the Fas/Fas ligand system. It has been suspected that HTLV-I–infected T lymphocytes proliferate in lymph nodes at an early phase of malignant alteration and gradually progress from the carrier type to the smoldering, chronic, and acute types of ATL. Based on a statistical analysis of the age distribution of ATL patients, the lymphomagenesis of ATL was proposed to consist of at least five steps (45). Although abnormalities in tumor suppressor genes such as p53, p15, or p16 are supposed to be one of the steps for progression of ATL (46–48), the precise mechanism of lymphomagenesis remains unknown. Function-ablating Fas mutations might be involved in the multistep lymphomagenesis of ATL.
It is, however, unlikely that the Fas gene mutation alone leads to a malignant transformation. Fas-deficient lpr mice do not develop malignancies, and patients with ALPS have rarely developed malignancies. Interestingly, lpr mice, which constitutively express the L-myc transgene in the lymphoid lineage, show an accelerated formation of T and B cell lymphomas, but the L-myc transgene alone did not produce such an acceleration in normal mice (49). It has been reported that mice deficient in both T cells and Fas gene develop B cell lymphoma (50). These reports suggest that lymphomagenesis requires other events besides the loss of Fas receptor function. In the present investigation, it seems feasible that lymph node ATL cells with some genetic abnormalities that are crucial for the development of ATL progressed to a more aggressive form by acquiring a Fas gene mutation.
In conclusion, we found a function-ablating Fas gene mutation in an ATL cell line (KOB) and its primary ATL cells. By retrovirus-mediated gene transfer, we verified that the aberrant Fas interfered with apoptosis by a dominant negative mechanism. The absence of the Fas gene mutation in lymph node ATL cells suggests that this mutation is a late event that occurs after the development of ATL and that the ATL subclone with Fas mutation escaped from the apoptosis mediated by the Fas/Fas ligand system and spread to the entire body of the patient. Abnormality of Fas function may be one of the important steps in the progression of ATL.
Acknowledgments
We are grateful to Dr. T. Fujimoto, Mr. K. Nohda, Miss T. Hayashi, and Miss N. Dateki for their excellent technical assistance and to Dr. A. Mizogami, Dr. D. Imanishi, and Dr. Y. Imaizumi for their critical advice. IL-2 was kindly provided by Takeda Pharmaceutical Co.
This work was supported by research grants from the Japanese Ministry of Education (Y. Yamada, 10670956 and 07671214; and S. Kazuyuki, 06280117).
Abbreviations used in this paper
- ALPS
autoimmune lymphoproliferative syndrome
- ATL
adult T cell leukemia
- HTLV-I
human T lymphotrophic virus type I
- LDH
lactic dehydrogenase
- nt
nucleotide
- RT
reverse transcriptase
References
- 1.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 2.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 3.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 4.Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis- inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–2713. [PubMed] [Google Scholar]
- 5.Papoff G, Cascino I, Eramo A, Starace G, Lynch DH, Ruberti G. An N-terminal domain shared by Fas/ Apo-1 (CD95) soluble variants prevents cell death in vitro. J Immunol. 1996;156:4622–4630. [PubMed] [Google Scholar]
- 6.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 7.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 9.Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA. 1993;90:1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 11.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 12.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 13.Bettinardi A, Brugnoni D, Quiros-Roldan E, Malagoli A, La Grutta S, Correra A, Notarangelo LD. Missense mutations in the Fas gene resulting in autoimmune lymphoproliferative syndrome: a molecular and immunological analysis. Blood. 1997;89:902–909. [PubMed] [Google Scholar]
- 14.Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med. 1996;335:1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 16.Yamada Y. Phenotypic and functional analysis of leukemic cells from 16 patients with adult T-cell leukemia/lymphoma. Blood. 1983;61:192–199. [PubMed] [Google Scholar]
- 17.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87) Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 21.Shimoyama M, Ota K, Kikuchi M, Yunoki K, Konda S, Takatsuki K, Ichimaru M, Ogawa M, Kimura I, Tominaga S, et al. Chemotherapeutic results and prognostic factors of patients with advanced non-Hodgkin's lymphoma treated with VEPA or VEPA-M. J Clin Oncol. 1988;6:128–141. doi: 10.1200/JCO.1988.6.1.128. [DOI] [PubMed] [Google Scholar]
- 22.Debatin KM, Goldmann CK, Bamford R, Waldmann TA, Krammer PH. Monoclonal-antibody- mediated apoptosis in adult T cell leukaemia. Lancet. 1990;335:497–500. doi: 10.1016/0140-6736(90)90735-n. [DOI] [PubMed] [Google Scholar]
- 23.Debatin KM, Goldman CK, Waldmann TA, Krammer PH. APO-1-induced apoptosis of leukemia cells from patients with adult T-cell leukemia. Blood. 1993;81:2972–2977. [Google Scholar]
- 24.Sugahara K, Yamada Y, Hiragata Y, Matsuo Y, Tsuruda K, Tomonaga M, Maeda T, Atogami S, Tsukasaki K, Kamihira S. Soluble and membrane isoforms of Fas/CD95 in fresh adult T-cell leukemia (ATL) cells and ATL-cell lines. Int J Cancer. 1997;72:128–132. doi: 10.1002/(sici)1097-0215(19970703)72:1<128::aid-ijc18>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Kamihira S, Yamada Y, Hirakata Y, Tsuruda K, Sugahara K, Tomonaga M, Maeda T, Tsukasaki K, Atogami S, Kobayashi N. Quantitative characterization and potential function of membrane Fas/APO-1 (CD95) receptors on leukaemic cells from chronic B and T lymphoid leukaemias. Br J Haematol. 1997;99:858–865. doi: 10.1046/j.1365-2141.1997.4963301.x. [DOI] [PubMed] [Google Scholar]
- 26.Landowski TH, Qu N, Buyuksal I, Painter JS, Dalton WS. Mutations in the Fas antigen in patients with multiple myeloma. Blood. 1997;90:4266–4270. [PubMed] [Google Scholar]
- 27.Beltinger C, Kurz E, Bohler T, Schrappe M, Ludwig WD, Debatin KM. CD95 (APO-1/Fas) mutations in childhood T-lineage acute lymphoblastic leukemia. Blood. 1998;91:3943–3951. [PubMed] [Google Scholar]
- 28.Tamiya S, Etoh K, Suzushima H, Takatsuki K, Matsuoka M. Mutation of CD95 (Fas/Apo-1) gene in adult T-cell leukemia cells. Blood. 1998;91:3935–3942. [PubMed] [Google Scholar]
- 29.Yamada Y, Fujita M, Suzuki H, Atogami S, Sohda H, Murata K, Tsukasaki K, Momita S, Kohno T, Maeda T, et al. Established IL-2-dependent double-negative (CD4− CD8−) TCR alpha beta/CD3+ ATL cells: induction of CD4 expression. Br J Haematol. 1994;88:234–241. doi: 10.1111/j.1365-2141.1994.tb05012.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Ohmoto Y, Hata T, Yamamura M, Murata K, Tsukasaki K, Kohno T, Chen Y, Kamihira S, Tomonaga M. Features of the cytokines secreted by adult T cell leukemia (ATL) cells. Leuk Lymphoma. 1996;21:443–447. doi: 10.3109/10428199609093442. [DOI] [PubMed] [Google Scholar]
- 31.Kamihira S, Sohda H, Atogami S, Toriya K, Yamada Y, Tsukazaki K, Momita S, Ikeda S, Kusano M, Amagasaki T, et al. Phenotypic diversity and prognosis of adult T-cell leukemia. Leuk Res. 1992;16:435–441. doi: 10.1016/0145-2126(92)90168-7. [DOI] [PubMed] [Google Scholar]
- 32.Tsukasaki K, Ikeda S, Murata K, Maeda T, Atogami S, Sohda H, Momita S, Jubashi T, Yamada Y, Mine M, et al. Characteristics of chemotherapy-induced clinical remission in long survivors with aggressive adult T-cell leukemia/lymphoma. Leuk Res. 1993;17:157–166. doi: 10.1016/0145-2126(93)90061-o. [DOI] [PubMed] [Google Scholar]
- 33.Kotani T, Aratake Y, Kondo S, Tamura K, Ohtaki S. Expression of functional Fas antigen on adult T-cell leukemia. Leuk Res. 1994;18:305–310. doi: 10.1016/0145-2126(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 34.Kamihira S, Yamada Y, Hiragata Y, Yamaguchi T, Izumikawa K, Matsuo Y, Sugahara K, Tsuruta K, Atogami S, Tsukasaki K, et al. Serum levels of soluble Fas/APO-1 receptor in human retroviral infection and associated diseases. Intern Med. 1997;36:166–170. doi: 10.2169/internalmedicine.36.166. [DOI] [PubMed] [Google Scholar]
- 35.Xerri L, Carbuccia N, Parc P, Birg F. Search for rearrangements and/or allelic loss of the fas/APO-1 gene in 101 human lymphomas. Am J Clin Pathol. 1995;104:424–430. doi: 10.1093/ajcp/104.4.424. [DOI] [PubMed] [Google Scholar]
- 36.Basu A, Raghunath M, Bishayee S, Das M. Inhibition of tyrosine kinase activity of the epidermal growth factor (EGF) receptor by a truncated receptor form that binds to EGF: role for interreceptor interaction in kinase regulation. Mol Cell Biol. 1989;9:671–677. doi: 10.1128/mcb.9.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashles O, Yarden Y, Fischer R, Ullrich A, Schlessinger J. A dominant negative mutation suppresses the function of normal epidermal growth factor receptors by heterodimerization. Mol Cell Biol. 1991;11:1454–1463. doi: 10.1128/mcb.11.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopusembryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 39.Ueno H, Gunn M, Dell K, Tseng A, Jr, Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J Biol Chem. 1992;267:1470–1476. [PubMed] [Google Scholar]
- 40.Li Y, Basilico C, Mansukhani A. Cell transformation by fibroblast growth factors can be suppressed by truncated fibroblast growth factor receptors. Mol Cell Biol. 1994;14:7660–7669. doi: 10.1128/mcb.14.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueno H, Colbert H, Escobedo JA, Williams LT. Inhibition of PDGF beta receptor signal transduction by coexpression of a truncated receptor. Science. 1991;252:844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- 42.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor- human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO 1(Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto T, Ohno Y, Tsugane S, Watanabe S, Shimoyama M, Tajima K, Miwa M, Shimotohno K. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191–195. doi: 10.1111/j.1349-7006.1989.tb02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakashita A, Hattori T, Miller CW, Suzushima H, Asou N, Takatsuki K, Koeffler HP. Mutations of the p53 gene in adult T cell leukemia. Blood. 1992;79:477–480. [PubMed] [Google Scholar]
- 47.Hatta Y, Hirama T, Miller CW, Yamada Y, Tomonaga M, Koeffler HP. Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood. 1995;85:2699–2704. [PubMed] [Google Scholar]
- 48.Yamada Y, Hatta Y, Murata K, Sugawara K, Ikeda S, Mine M, Maeda T, Hirakata Y, Kamihira S, Tsukasaki K, et al. Deletions of p15 and/or p16 genes as a poor-prognosis factor in adult T-cell leukemia. J Clin Oncol. 1997;15:1778–1785. doi: 10.1200/JCO.1997.15.5.1778. [DOI] [PubMed] [Google Scholar]
- 49.Zornig M, Grzeschiczek A, Kowalski MB, Hartmann KU, Moroy T. Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in E mu L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene. 1995;10:2397–2401. [PubMed] [Google Scholar]
- 50.Peng SL, Robert ME, Hayday AC, Craft J. A tumor-suppressor function for Fas (CD95) revealed in T cell– deficient mice. J Exp Med. 1996;184:1149–1154. doi: 10.1084/jem.184.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]