Abstract
Interleukin (IL)-18 is functionally similar to IL-12 in mediating T helper cell type 1 (Th1) response and natural killer (NK) cell activity but is related to IL-1 in protein structure and signaling, including recruitment of IL-1 receptor–associated kinase (IRAK) to the receptor and activation of c-Jun NH2-terminal kinase (JNK) and nuclear factor (NF)-κB. The role of IRAK in IL-18–induced responses was studied in IRAK-deficient mice. Significant defects in JNK induction and partial impairment in NF-κB activation were found in IRAK-deficient Th1 cells, resulting in a dramatic decrease in interferon (IFN)-γ mRNA expression. In vivo Th1 response to Propionibacterium acnes and lipopolysaccharide in IFN-γ production and induction of NK cytotoxicity by IL-18 were severely impaired in IRAK-deficient mice. IFN-γ production by activated NK cells in an acute murine cytomegalovirus infection was significantly reduced despite normal induction of NK cytotoxicity. These results demonstrate that IRAK plays an important role in IL-18–induced signaling and function.
Keywords: c-Jun NH2-terminal kinase, inhibitor of nuclear factor κB, nuclear factor κB, interferon γ, murine cytomegalovirus
Interleukin (IL)-18 (previously known as IFN-γ–inducing factor, or IGIF) was cloned from the liver of mice primed with Propionibacterium acnes, followed by challenge with LPS (1). IL-18 induces production of IFN-γ from Th1 cells and NK cells (2–5). In addition, IL-18 enhances NK cell cytotoxicity and also synergizes with IL-12 in potentiating IFN-γ production and NK cell cytotoxicity (1, 3, 4). Thus, cellular responses to IL-18 are similar to the biological functions known to be elicited by IL-12 (6).
Despite functional similarities, IL-12 and IL-18 differ in their protein structure and exert these overlapping and synergistic biological effects via different mechanisms. IL-12 mediates Th1 responses and IFN-γ production via activation of Janus kinases (JAKs),1 JAK2 and TYK2 (7), and transcription factors called signal transducer and activator of transcription (STAT), STAT3 and STAT4 (8). STAT4-deficient mice are defective in mounting Th1 responses and IFN-γ production (9, 10). Stimulation with IL-18 results in activation of c-Jun NH2-terminal kinase (JNK) and transcription factor nuclear factor (NF)-κB (2, 4).
IL-18 is similar in tertiary structure to proteins of the IL-1 family (11). Similar to IL-1β, IL-18 is synthesized as an inactive precursor protein and is cleaved to the active form by IL-1β–converting enzyme (ICE, also named caspase 1; references 12, 13). Cleavage of IL-18 by ICE is essential for the biological effects of IL-18. ICE-deficient mice exhibit defects similar to those observed in IL-18−/− mice, such as reduced IFN-γ production in response to LPS injection (3, 12, 13). The IL-18 receptor was originally identified as IL-1 receptor–related protein (IL-1Rrp; references 14, 15). Receptors for IL-1, IL-18, and the recently identified mammalian Toll-like receptors (16) are evolutionarily conserved and homologous to the Drosophila protein Toll (17). Toll mediates activation of Dorsal, an NF-κB–like molecule, via the serine threonine kinase Pelle and the adapter protein Tube (17, 18). The IL-1 signaling pathway in mammals is similar to the Toll pathway. NF-κB activation by IL-1 requires the interaction of the Pelle-like kinase IL-1 receptor– associated kinase (IRAK) with the IL-1 receptor complex via the adapter protein MyD88 (19–21). IL-18 also triggers phosphorylation of IRAK and its recruitment to the IL-18 receptor complex (4, 22). However, IL-1 and IL-18 act on different cell types and lead to divergent cellular responses. For example, IL-18 has been implicated primarily in inducing IFN-γ from NK and Th1 cells (1–5), whereas IL-1 is a potent inducer of IL-6 from fibroblasts and macrophages during inflammation (23–25). We have recently demonstrated that IRAK is required for optimal induction of IL-1 signaling, including JNK, p38, and NF-κB activation (25). Defective IL-1 signaling in IRAK-deficient fibroblasts results in impaired IL-6 induction (25). Although IL-18 signaling is known to involve activation of JNK and NF-κB (2, 4), details of IL-18 signaling have not been well characterized. It is also unclear whether IL-18 uses IRAK in pathways similar to IL-1 signaling to elicit distinct cellular responses. To determine the role of IRAK in IL-18–mediated responses, we analyzed IL-18–induced signaling and function in IRAK-deficient mice.
In this report we showed that IRAK was essential for IL-18–mediated activation of JNK and was also involved in NF-κB activation. Signaling defects in IRAK-deficient Th1 cells resulted in a dramatic decrease in IFN-γ expression. Serum IFN-γ increase in response to P. acnes and LPS treatment was severely impaired. IRAK-deficient mice also exhibited defects in NK IFN-γ production in an acute murine cytomegalovirus (MCMV) infection. NK cell cytotoxicity induced by IL-18 was defective, although its induction was normal in MCMV infection. These results suggest that IRAK plays an important role in IL-18–mediated signaling and function.
Materials and Methods
Generation of IRAK-deficient Mice.
The mouse IRAK gene in embryonic stem (ES) cells was disrupted by homologous recombination as described in our previous report (25). In brief, the mouse IRAK gene was disrupted by replacement of a 940-bp region covering exons 5–7 of the gene with a neomycin resistance gene. Chimeric mice were generated from embryos injected with ES cells. Germline mice were obtained from breeding of chimeric male mice with C57BL/6J females. Because the IRAK gene is on X chromosome (sequence data available from EMBL/GenBank/ DDBJ under accession No. U52112) and the ES cell line was derived from a male embryo, all the germline female mice were heterozygous for the disrupted IRAK gene. IRAK-deficient male mice carrying only the disrupted IRAK gene were obtained from breeding of heterozygous female mice with wild-type littermates. IRAK-deficient female mice were obtained from breeding of heterozygous females with IRAK-deficient males.
Phenotypic Analysis of T Cells.
Thymocytes and splenocytes were stained with CD4- and CD8-specific antibodies (PharMingen). Enriched CD4+ T cells before and after 5 d of differentiation were stained with antibodies specific for CD25 and CD44 (PharMingen). Cells after antibody staining were analyzed using a FACScan™ (Becton Dickinson).
Preparation of Th1/Th2 Cells.
CD4+ T cells were purified from lymph node and spleen cells by depletion of B cells and CD8+ T cells using guinea pig and rabbit complements and a combination of antibodies from hybridoma lines J11d, 28-16-8s, and 3-168. Purity of CD4+ T cells in different preparations was ∼90%. Enriched CD4+ T cells were activated with immobilized anti-CD3 (PharMingen), which was coated overnight onto 6-well plates at 5 μg/ml. Differentiation of T cells towards Th1 cells was triggered by addition of 5 ng/ml IL-12 (R&D Systems) and 5 μg/ml anti–IL-4 (PharMingen) in RPMI medium with 10% FCS. Th2 cell differentiation was driven by supplementing culture medium with 5 ng/ml IL-4 (R&D Systems) and 5 μg/ml of anti–IFN-γ plus anti–IL-12 (PharMingen). The cytokine profile of Th1 or Th2 cells was determined by plating the cells at 2 × 105 cell/well in 96-well plates that were precoated overnight with 5 μg/ml anti-CD3. Culture supernatants were collected 24 h later for cytokine detection by ELISA.
Cell Stimulation.
After 5 d of differentiation, Th1 cells were washed once with serum-free RPMI medium and starved for 3 h before stimulation. Cells were treated with different stimuli at 37°C. The different stimuli used in the assays include: IL-18 (PeproTech, Inc.), IL-12 (R&D Systems); IL-1β (R&D Systems); TNF-α (Genzyme Corp.); PMA and ionomycin (Sigma Chemical Co.). After stimulation, cells were lysed for 20 min at 4°C with lysis buffer (100 μl/5 × 106 cells) containing 10 mM Hepes, pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM Na3VO4, 10% glycerol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM PMSF, and 1 mM EDTA. Cell lysates for kinase assays and Western blots were collected after centrifugation of samples at 14,000 rpm for 10 min at 4°C.
In Vitro Kinase Assay.
Cell lysates (5 × 106 cells in 100 μl of lysis buffer) were immunoprecipitated with JNK1 kinase antibody (Santa Cruz Biotechnology). An in vitro kinase assay was performed using glutathione S-transferase (GST)–c-Jun (Santa Cruz Biotechnology) as a substrate in a reaction buffer containing 25 mM Hepes, pH 7.4, 25 μM ATP, 10 mM MgCl2, 1 μg GST– c-Jun, and 10 μCi [γ-32P]ATP for 30 min at 37°C. Samples after kinase assays were separated on 10% SDS-PAGE, transferred to nitrocellulose filters (MSI), and then subjected to autoradiography. Radioactivity of the phosphorylated c-Jun bands were quantitated by PhosphorImager (Molecular Dynamics Inc.). JNK1 protein on blotted filters was detected with a JNK1-specific antibody (Santa Cruz Biotechnology).
Western Blotting.
Western blot analyses were carried out as previously described (26). For detection of IκB-α protein levels, cell lysates (105 cells/sample) were separated on 10% SDS-PAGE (Novex) and transferred to nitrocellulose filters. Filters were immunoblotted with an IκB-α–specific rabbit antibody (Santa Cruz Biotechnology) and detected with horseradish peroxidase–conjugated rabbit IgG and enhanced chemiluminescence (Amersham Pharmacia Biotech). Filters were then stripped by soaking in 0.1 M glycine/HCl, pH 2.6, for 30 min and reprobed with an ERK-2– specific antibody (Santa Cruz Biotechnology) for normalization.
NF-κB Mobility Shift Assay.
Cells after treatment with different stimuli were pelleted and taken up in 500 μl ice-cold buffer A (10 mM Hepes, pH 7.9, 10 mM KCl, 100 μM EDTA, 100 μM EGTA, 1 mM dithiothreitol, 500 μM PMSF) and kept on ice for 15 min. Samples were then added with 30 μl of 10% NP-40 followed by centrifugation at 14,000 rpm for 30 s. Nuclear pellets were washed once with buffer A and lysed in 50 μl buffer B (20 mM Hepes, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM PMSF) for 30 min at 4°C. Nuclear extracts were collected after centrifugation of the samples at 14,000 rpm for 10 min. Protein concentration was determined by the BCA method (Pierce Chemical Co.). The consensus double-stranded oligonucleotide for NF-κB binding (Santa Cruz Biotechnology) was labeled using [γ-32P]ATP and T4 polynucleotide kinase. Nuclear binding reactions were carried out for 30 min at room temperature in a 20 μl mixture containing 10 μg nuclear extract and 0.5 ng 32P-labeled oligonucleotide probe (100,000 cpm) in buffer C (5 mM Hepes, pH 7.9, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, and 1 μg/ml poly[dI-dC]). Oligonucleotide–protein complexes were separated on 6% polyacrylamide/ 0.5× TBE gels and detected by autoradiography.
Northern Blot Analysis.
Total RNA was extracted from cells or spleens with RNAzol (Tel-Test Inc.). RNA was separated on 1% agarose-formaldehyde gels and transferred to nylon membranes (Amersham Pharmacia Biotech). Filters were hybridized to cDNA probes specific for IFN-γ (Clontech) and IL-18 (Research Genetics, Inc.). cDNA probes were labeled with α-[32P]dCTP (Amersham Pharmacia Biotech) by random priming (Boehringer Mannheim). Radioactive signals were detected by autoradiography. Filters were then stripped by boiling in 0.1% SDS and rehybridized with glycerol-3-phosphate dehydrogenase cDNA probe (Clontech) for normalization.
Cytokine Detection.
IFN-γ and IL-4 in serum samples and culture supernatants were determined by using commercially available ELISA kits (Genzyme) and recombinant cytokines were used as standards provided by the manufacturer.
Cell Proliferation Assay.
Th1 cells were plated in 96-well plates at 105/well and treated with different cytokines for 24 h. [3H]thymidine (1 μCi/well) was then added for 16 h, and radioactivity incorporated in dividing cells was measured using a Topcount Microplate Scintillation Counter (Packard).
In Vivo Th1 Response.
Mice were injected intraperitoneally with PBS as controls or PBS with 2 mg of heat-killed P. acnes (Van Kempen Group, Inc.). 7 d later, control mice were injected intravenously with PBS, whereas P. acnes–primed mice were injected with 1 μg LPS (Sigma Chemical Co.). Mice were bled 6 h after LPS challenge, and serum IFN-γ levels were measured by ELISA (Genzyme). Total RNA was extracted from spleen samples for Northern blot analysis.
NK Cytotoxic Assay.
Mice were injected intraperitoneally daily with PBS alone as controls or PBS containing 1 μg IL-18 or 200 μg poly(I):poly(C) (Sigma Chemical Co.) for 2 d. Spleen cells prepared from these mice were incubated with 51Cr-labeled YAC-1 target cells for 4 h at 37°C at different E/T ratios. After 4 h of incubation, 51Cr released from target cells was counted using a gamma counter (Packard). Specific lysis was calculated as: (measured 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release) × 100. Maximum release was obtained by counting acid-lysed target cells. Spontaneous release was obtained by incubating target cells in the absence of effector cells.
MCMV Infections.
The Smith strain of MCMV was obtained from the American Type Culture Collection (VR-1399). MCMV stocks were prepared in NIH 3T3 murine fibroblasts, and determination of viral titers was carried out by a standard plaque assay (27). Mice were intraperitoneally injected with 108 PFU of tissue culture–propagated MCMV in 400 μl DMEM. Animals were killed on day 3 after inoculation. Blood sera for cytokine detection were collected by bleeding mice daily starting from day 0 before viral inoculation. Spleens were collected after 71 h of infection for NK cytotoxic assays and RNA extraction.
Results
Normal Differentiation of Th1 and Th2 Cells in the Absence of IRAK.
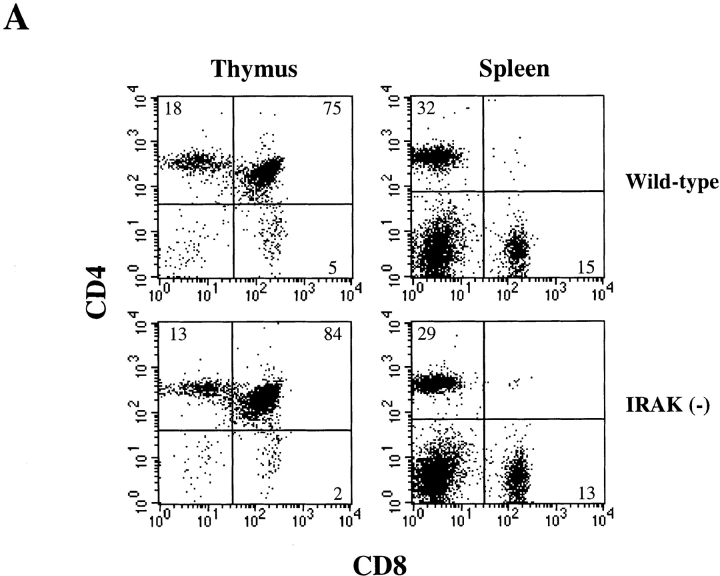
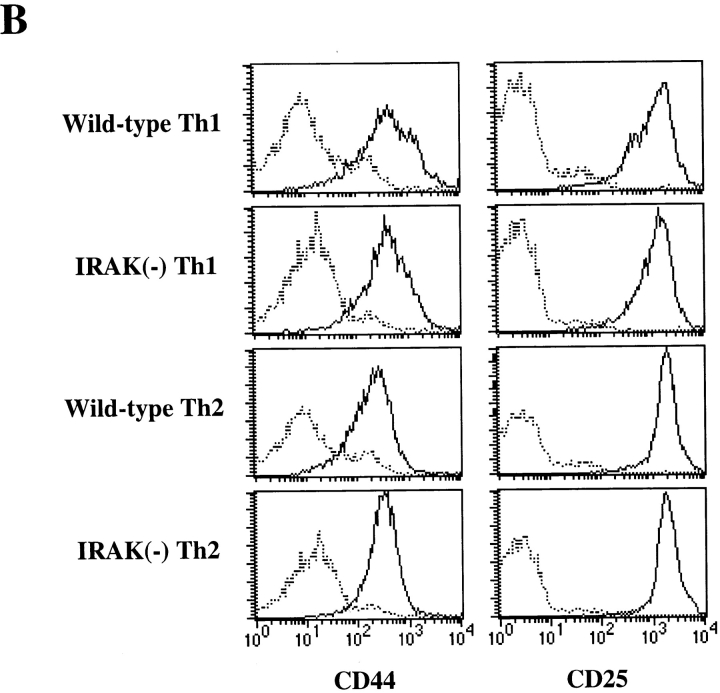
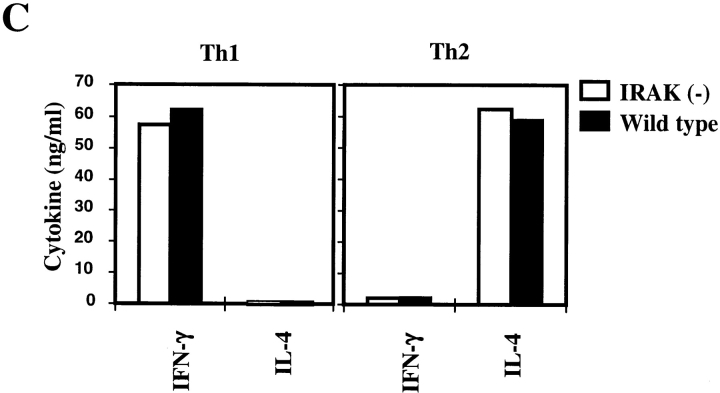
IL-18 by itself has no effect on Th1 differentiation but can synergize IL-12–driven Th1 development (4). In differentiated helper T cells, IL-18 exerts its biological effects only on Th1 cells but not on Th2 cells (4). Since IRAK is involved in IL-18 signaling (4, 22), we examined helper T cell development and phenotype in IRAK-deficient mice. Development of T cells in the thymus and distribution of mature CD4+ and CD8+ T cells in the primary and secondary lymphoid organs of IRAK-deficient mice appeared to be normal (Fig. 1 A). The vast majority of CD4+ T cells were characterized as CD25loCD44lo naive phenotype and were comparable to the cells harvested from control wild-type mice (Fig. 1 B). Th1 and Th2 cells were prepared by in vitro differentiation of CD4+ T cells from IRAK-deficient and wild-type mice. CD4+ T cells were enriched from lymph node cells and splenocytes by antibody and complement depletion as described in Material and Methods. Enriched CD4+ T cells were activated with immobilized anti-CD3 and differentiation towards Th1 cells was triggered by coculture with IL-12 and anti–IL-4. Th2 cell differentiation was driven by IL-4 and anti–IFN-γ plus anti–IL-12. Activation of IRAK-deficient CD4+ T cells appeared to be normal, as indicated by the upregulation of the activation marker CD25 and the memory T cell marker CD44 (Fig. 1 B). Numbers of blasting T cells after 5 d of differentiation were similar between IRAK-deficient and wild-type T cells (data not shown), suggesting normal proliferation of activated IRAK-deficient T cells. Cytokines expressed by T cells after 5 d of differentiation were characterized by ELISA. Similar to wild-type Th1 cells, IRAK-deficient Th1 cells secreted predominantly IFN-γ, whereas IL-4 was undetectable (Fig. 1 C). IRAK-deficient Th2 cells also showed a typical Th2 cytokine profile similar to that of wild-type Th2 cells, with high amounts of IL-4 but minimal levels of IFN-γ (Fig. 1 C). Our data suggest that IRAK-deficient T cells can differentiate into Th1 or Th2 effector cells.
Figure 1.
IRAK is dispensable for T cell development and Th1/Th2 differentiation. (A) CD4+ and CD8+ T cell populations in the thymuses and the spleens of IRAK-deficient [IRAK(−)] and wild-type mice were analyzed in flow cytometry. Immature CD4+ CD8+ double positive T cells in thymuses are also shown. (B) CD4+ T cells purified from the spleens and lymph nodes of IRAK-deficient [IRAK(−)] and wild-type mice were stimulated in anti-CD3 coated plates in the presence of IL-12 and anti–IL-4 for Th1 differentiation or IL-4 and anti–IFN-γ plus anti–IL-12 for Th2 differentiation. Enriched CD4+ T cells before (broken line) and after (solid line) 5 d of stimulation were stained with antibodies specific for CD25 and CD44 for flow cytometry analysis. (C) After 5 d of Th1 or Th2 differentiation, cells were washed and restimulated overnight with anti-CD3 antibody. IFN-γ and IL-4 levels in culture supernatants were determined by ELISA.
Impaired JNK Activation by IL-18 in IRAK-deficient Cells.
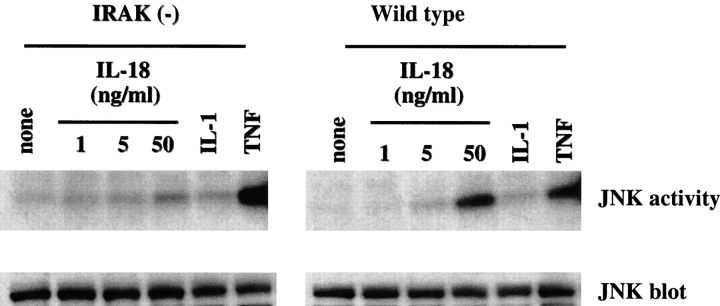
The stress-activated protein kinase (SAPK) family of mitogen-activated protein (MAP) kinases JNK and p38 are rapidly activated by proinflammatory cytokines such as IL-1 and TNF-α (24, 25, 28). IL-18 stimulation of Th1 cells also results in JNK activation (2). JNK plays a role in induction of activator protein (AP)-1–dependent genes via phosphorylation of the transcription factor c-Jun (28, 29). We have shown previously that IRAK-deficient fibroblasts are defective in IL-1–mediated JNK activation (25). To determine the role of IRAK in IL-18–induced JNK activation, JNK activity in Th1 cells was studied by immunoprecipitating JNK1 with a specific antibody followed by an in vitro kinase assay using the GST–c-Jun protein as a substrate. Activation of JNK was minimal in IL-18–treated IRAK-deficient Th1 cells as opposed to the significant JNK activation observed in wild-type cells (Fig. 2). In contrast, TNF-α induced similar levels of JNK activity in both wild-type and IRAK-deficient Th1 cells, whereas IL-1β did not have any effects on either samples (Fig. 2). IL-18 and IL-1 have been suggested to act on Th1 and Th2 cells, respectively, to induce signaling and cellular responses (4). In our studies, Th1 cells do not respond to IL-1β in JNK activation and this is consistent with a previous observation (4), which suggested that IL-1 does not induce signaling in Th1 cells due to the lack of IL-1 receptor expression. Our results indicate that the defect in JNK activation observed in IRAK-deficient Th1 cells is IL-18 specific.
Figure 2.
Impaired IL-18–mediated JNK activation in IRAK-deficient Th1 cells. IRAK-deficient [IRAK(−)] and wild-type Th1 cells were stimulated with medium alone, IL-1β (10 ng/ml), TNF-α (50 ng/ml), or different concentrations of IL-18 as indicated for 15 min. Cell lysates were immunoprecipitated with a JNK-specific antibody. In vitro JNK kinase assay was performed using GST–c-Jun as a substrate. Amounts of JNK protein in the immune complexes were detected by Western blotting using a JNK-specific antibody.
IRAK Is Dispensable for NF-κB Activation by IL-18.
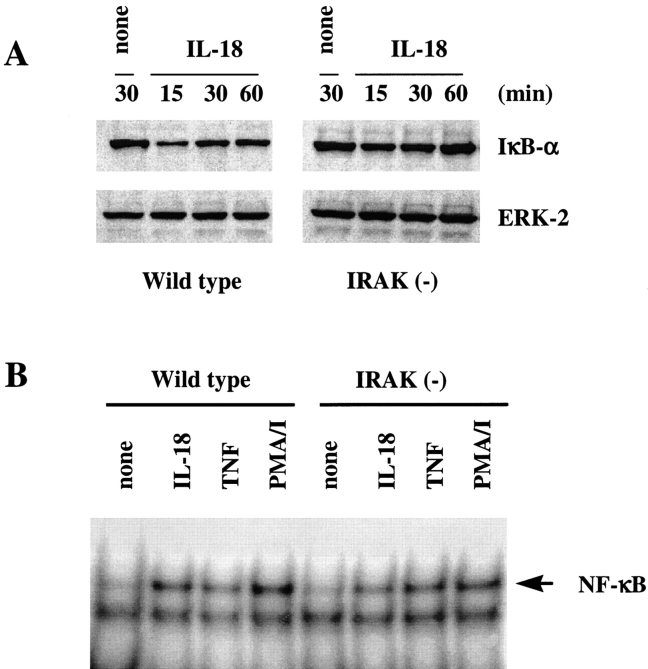
IL-18 binding to its receptor mediates activation of NF-κB (4, 22). In unstimulated cells, NF-κB is present in the cytoplasm as an inactive complex sequestered by its inhibitory partners, IκB (30). Upon activation, IκB proteins are phosphorylated by IκB kinases IKK1 and IKK2, and subsequently degraded by proteosomes to allow nuclear translocation and activation of NF-κB (30–32). To determine the role of IRAK in IL-18–induced NF-κB activation, IκB-α protein levels were determined in Th1 cells stimulated with IL-18 for different time courses (Fig. 3 A). Reduced levels of IκB-α due to protein degradation were observed in both wild-type and IRAK-deficient cells after IL-18 treatment (Fig. 3 A). Maximum degradation of IκB protein occurred 15 min after stimulation and returned to 80% of original levels within 60 min of stimulation. The extent of IκB-α protein degradation mediated by IL-18 appeared to be less in IRAK-deficient Th1 cells as compared with that in wild-type cells. Similar results were observed when wild-type and IRAK-deficient Th1 cells were stimulated with different concentrations of IL-18 (data not shown). This result was further confirmed by NF-κB DNA binding activity in nuclear extracts determined by mobility shift assay. IL-18–induced NF-κB activation in both IRAK-deficient and wild-type cells, but NF-κB DNA binding activity was slightly lower in IRAK-deficient cells than in wild-type cells (Fig. 3 B). Stimulation with TNF-α or phorbol ester plus ionomycin induced comparable levels of NF-κB activation in both cell types, indicating that the partial defect in NF-κB activation observed in IRAK-deficient cells is restricted to IL-18 stimulation. These results suggest that involvement of IRAK in IL-18–mediated NF-κB activation is dispensable.
Figure 3.
Reduced NF-κB activation by IL-18 in IRAK-deficient Th1 cells. (A) Reduced degradation of IκB-α in IRAK-deficient cells. IRAK-deficient [IRAK(−)] and wild-type cells were stimulated with IL-18 (50 ng/ml) for different time courses as indicated. Cell lysates were prepared and separated on SDS-PAGE. IκB-α was detected by Western blot analysis using an IκB-α–specific antibody. Amounts of protein loading were determined by probing the same filter with antibody specific for ERK-2. (B) Reduced NF-κB activation by IL-18 in IRAK-deficient cells. Cells were stimulated with IL-18 (50 ng/ml), TNF-α (50 ng/ml), or PMA (50 ng/ml) plus ionomycin (0.125 μM) for 30 min. Nuclear extracts were prepared and incubated with 32P-labeled NF-κB specific double-stranded oligonucleotide. The NF-κB DNA complex was separated on 6% polyacrylamide gel in 0.5% Tris-borate-EDTA.
IRAK Is Required for IL-18–induced IFN-γ Production.
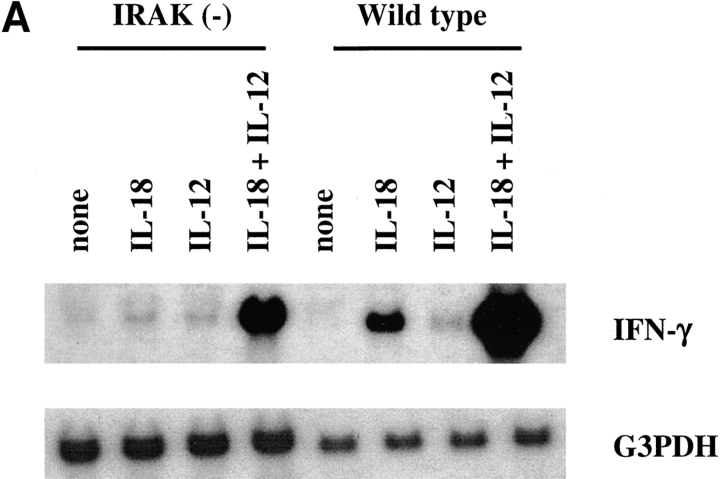
IL-18 induces IFN-γ production from Th1 cells (1, 5). Stimulation of Th1 cells with a combination of IL-18 and IL-12 results in synergistic induction of IFN-γ production (4). To determine whether IRAK is required for induction of IFN-γ by IL-18 itself or in combination with IL-12, IFN-γ mRNA expression was determined by Northern blot analysis. IFN-γ was significantly induced by IL-18 in wild-type cells but its induction in IRAK-deficient cells was minimal (Fig. 4 A). A suboptimal dose of IL-12 was used in our experiments, which induced minimal amounts of IFN-γ, and no difference was observed between IRAK-deficient and wild-type cells. Synergistic induction of IFN-γ expression by a combination of IL-18 and IL-12 was substantially decreased in IRAK-deficient cells as compared with wild-type cells. This result shows that IRAK is required for optimal induction of IFN-γ by IL-18.
Figure 4.
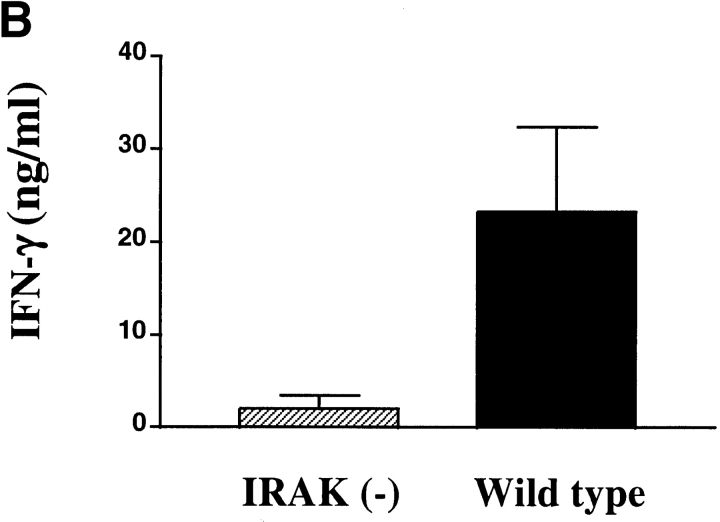
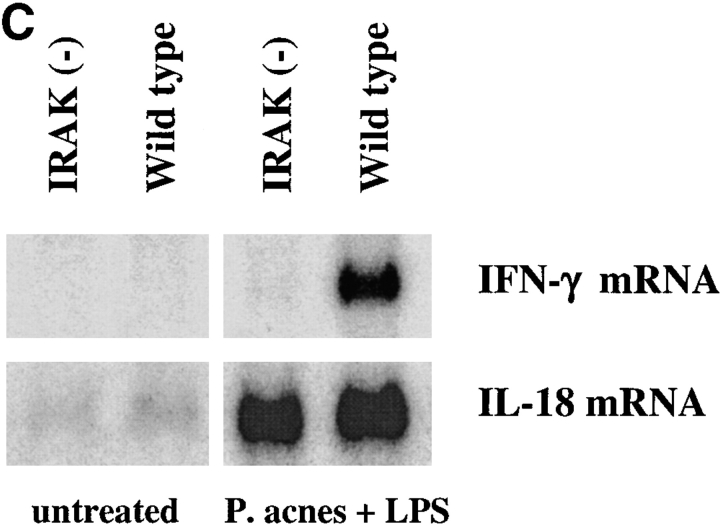
Defective induction of IFN-γ production in IRAK-deficient mice. (A) Defective IFN-γ mRNA expression induced by IL-18 in IRAK-deficient Th1 cells. IRAK-deficient [IRAK(−)] and wild-type Th1 cells were stimulated with IL-18 (50 ng/ml), IL-12 (10 ng/ml), or a combination of IL-18 and IL-12 for 2 h. Total RNA was prepared from the cells and IFN-γ expression was determined by Northern blot analysis using 32P-labeled IFN-γ cDNA probe. The blots were stripped and hybridized to glycerol-3-phosphate dehydrogenase (G3PDH) cDNA for normalization. (B) Reduced induction of IFN-γ production in P. acnes–primed and LPS-challenged IRAK-deficient mice. IRAK-deficient [IRAK(−)] and wild-type mice were intraperitoneally injected with 2 mg of heat-killed P. acnes. Mice were intravenously injected with 1 μg LPS on day 7. Serum samples were collected 6 h after LPS challenge and levels of IFN-γ in sera were determined by ELISA. (C) Total spleen RNA was prepared from P. acnes– and LPS–treated mice. IFN-γ expression was determined by Northern blot hybridization using 32P-labeled IFN-γ cDNA probe. The blot was stripped and hybridized with IL-18 cDNA probe.
It has been demonstrated that treatment with LPS in P. acnes–sensitized mice results in a significant increase in serum IFN-γ (33). IL-18–deficient mice exhibited a minimal increase in serum IFN-γ under these conditions (3). To determine the role of IRAK in IL-18–dependent IFN-γ production in vivo, IRAK-deficient mice were tested in this experimental system. Mice were injected intraperitoneally with heat-killed P. acnes and 7 d later were injected intravenously with LPS. IFN-γ in the serum was detected by ELISA 6 h after LPS treatment. Serum IFN-γ levels were significantly lower in IRAK-deficient mice as compared with wild-type animals (Fig. 4 B). Consistent with these data, IFN-γ mRNA expression in the spleen was substantially reduced in IRAK-deficient mice. In contrast, induction of IL-18 mRNA expression was comparable between wild-type and IRAK-deficient animals (Fig. 4 C). These results suggest that the reduced IFN-γ production in IRAK-deficient mice is not due to a change in IL-18 levels but rather originated from defects in IL-18 signaling.
Decreased Proliferation of IRAK-deficient Th1 Cells.
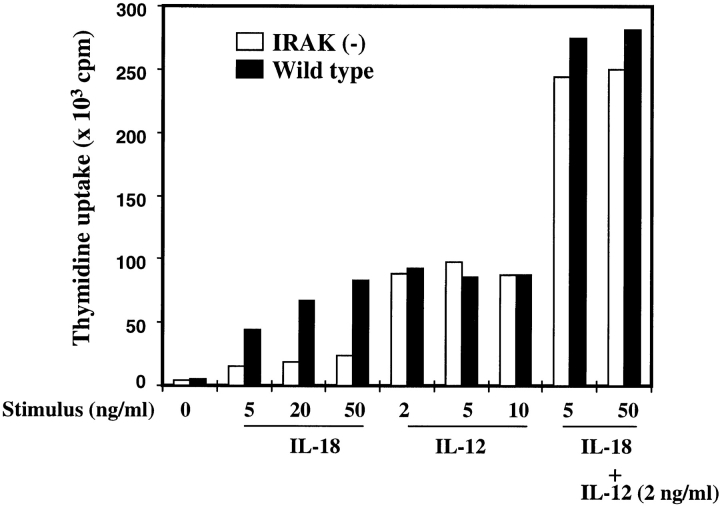
Similar to induction in IFN-γ production, proliferation of Th1 cells was also enhanced by IL-18 or IL-12, and synergized by the combination of both (4). The effect of IL-18 and its synergism with IL-12 on proliferation of IRAK-deficient Th1 cells was studied. Wild-type and IRAK-deficient Th1 cells were treated with different concentrations of IL-18, IL-12, or IL-18 plus IL-12. Proliferation of Th1 cells after 24 h of stimulation was determined by [3H]thymidine uptake. As shown in Fig. 5, proliferation of wild-type Th1 cells was significantly enhanced by IL-18 in a dose-dependent manner, whereas the effect of IL-18 on proliferation of IRAK-deficient cells was minimal. At concentrations as low as 2 ng/ml, IL-12 stimulated proliferation of both wild-type and IRAK-deficient cells to maximal extent. Combination of IL-18 and IL-12 resulted in a synergistic proliferative response in both cell types, and no significant difference was observed. Consistent with the defects shown in IFN-γ expression, IRAK-deficient cells are also impaired in proliferative response to IL-18, confirming the role of IRAK in IL-18–mediated cellular responses.
Figure 5.
Defective IL-18–induced proliferation of IRAK-deficient Th1 cells. Th1 cells (105 cells /well) were plated on a 96-well plate and cultured in RPMI medium with the indicated concentrations of IL-18, IL-12, or IL-18 plus IL-12 for 24 h. Cell proliferation was determined by [3H]thymidine incorporation.
Defective Induction of NK Cell Activity by IL-18 in IRAK-deficient Mice.
IL-18 has been shown to enhance NK cell cytotoxicity (1). Reduced NK activity was reported in IL-18– deficient mice (3). To determine whether IRAK is involved in IL-18–induced NK activity, mice were injected intraperitoneally with IL-18 for 2 d consecutively, and NK activity in splenocytes was assayed using 51Cr-labeled YAC-1 as target cells. Basal NK cell activities in PBS injected wild-type and IRAK-deficient mice were comparable (Fig. 6). IL-18 injection resulted in significant increase in NK activity in wild-type animals but its effect in IRAK-deficient mice was minimal (Fig. 6). However, injection of the double-stranded RNA poly(I):poly(C), an inducer of IFNs, resulted in a pronounced NK activity in both IRAK-deficient and wild-type animals (Fig. 6), indicating that IRAK is required specifically for IL-18–mediated induction of NK cytotoxic activity.
Figure 6.

Impaired IL-18–induced NK cytotoxicity in IRAK-deficient mice. IRAK-deficient [IRAK(−)] and wild-type mice were injected intraperitoneally with PBS, 1 μg IL-18, or 200 μg poly(I):poly(C) for 2 d consecutively. Spleen cells were assayed for NK cytotoxic activity against 51Cr-labeled YAC-1 target cells.
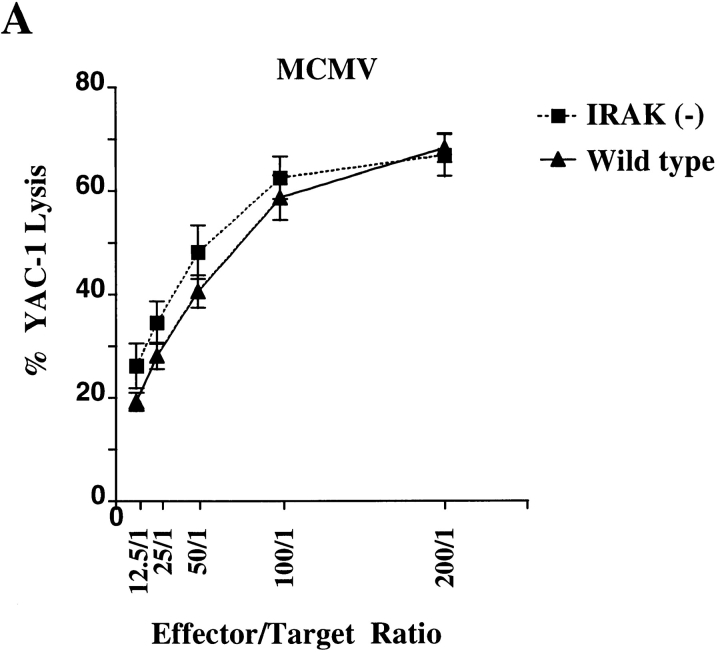
IRAK Is Essential for MCMV-induced IFN-γ Production but Not for NK Cell Cytotoxicity.
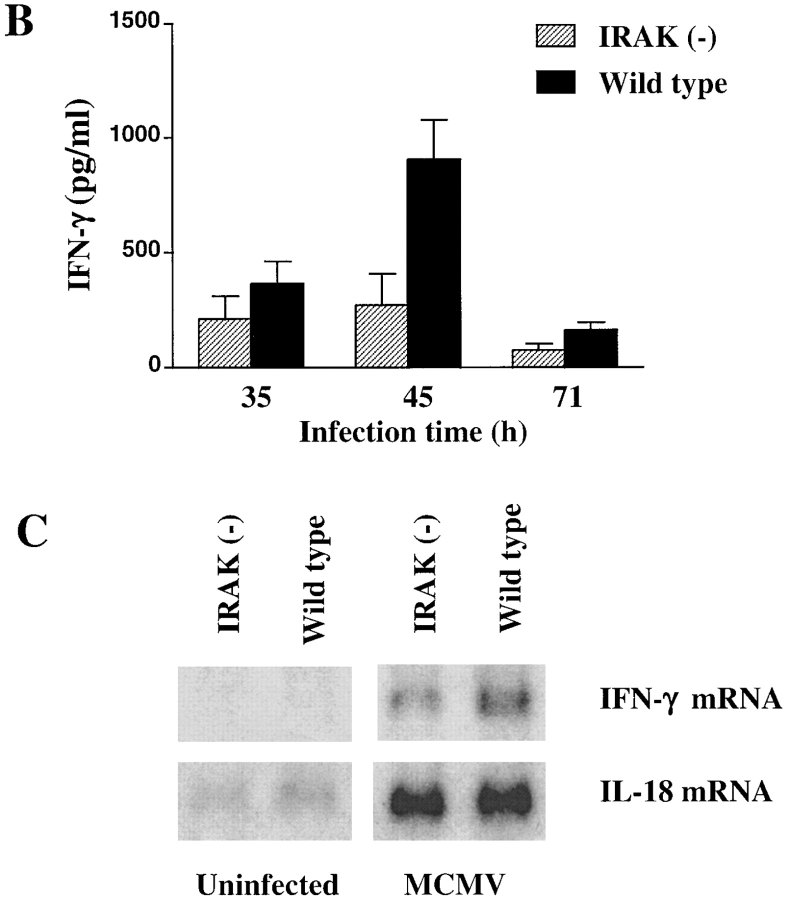
NK cells are the major effector cells in the early defense against viral infections. Both NK cytotoxicity and IFN-γ production by NK cells are induced in mice upon MCMV infection (34). IL-12 has been shown to be responsible for induction of IFN-γ in NK cells (35), whereas possible roles of IL-18 in NK activities during MCMV infection have not been reported. IRAK-deficient mice were infected with MCMV to study the involvement of IRAK in NK activity during viral infection. Splenocytes obtained from mice on day 3 of MCMV infection were assayed for NK cytolytic activity using 51Cr- labeled YAC-1 cells as targets. Dramatic increase in NK cytotoxicity was observed in both MCMV-infected wild-type and IRAK-deficient mice and no significant difference was found between the two types of mice (Fig. 7 A). IFN-γ produced by NK cells during MCMV infection can be detected as an increase in serum IFN-γ levels. Kinetics of IFN-γ increase in sera during the first 3 d of MCMV infections was studied. In both wild-type and IRAK-deficient mice, IFN-γ levels peaked at 45 h of infection (Fig. 7 B). However, maximal IFN-γ levels in IRAK-deficient mice were significantly lower than those in wild-type control mice (Fig. 7 B). Similar results were obtained in studies of IFN-γ mRNA expression. IFN-γ mRNA in the spleens was undetectable in uninfected mice but was induced significantly in MCMV infected mice (Fig. 7 C). IFN-γ mRNA induced by MCMV was less significant in IRAK-deficient mice than that in wild-type mice. IL-18 expression in response to MCMV infection was also studied. IL-18 mRNA was expressed at low levels in the spleens of uninfected mice and was induced strongly on day 3 of MCMV infection (Fig. 7 C). IL-18 expression in IRAK-deficient mice was comparable to that in wild-type mice under healthy and viral-infected conditions (Fig. 7 C). Thus, impairment in viral-induced IFN-γ production in IRAK-deficient mice is not due to the levels of IL-18 expression, suggesting that other mechanisms, such as IL-18 signaling, may be responsible for the defects.
Figure 7.
Reduced MCMV-induced IFN-γ production but normal NK cell cytotoxicity in IRAK-deficient mice. IRAK-deficient [IRAK(−)] and wild-type mice were injected intraperitoneally with 108 PFU of MCMV. (A) Spleen cells from mice after 71 h of infection were assayed for NK cytotoxicity against 51Cr-labeled YAC-1 target cells. (B) IFN-γ levels in sera collected at 35, 45, and 71 h of MCMV infection were determined by ELISA. (C) Expression of IFN-γ mRNA in the spleens of infected mice after 71 h of MCMV injection was determined by Northern blot hybridization using 32P- labeled specific IFN-γ cDNA probe.
Discussion
Role of IRAK in IL-18 Signaling Pathways.
We have demonstrated IL-18 signaling defects in IRAK-deficient Th1 cells. Impairment is significant in JNK activation but much less obvious in NF-κB induction. The partial activation of NF-κB suggests that other mechanisms can compensate for the function of IRAK. However, the role of IRAK in JNK activation is essential. We have previously reported that IRAK-deficient fibroblasts are defective in both NF-κB and JNK/p38 pathways induced by IL-1 (25). The defects in IL-18 signaling that we observed here are similar to the defects in IL-1 signaling (25). Impairment in NF-κB activation can be overcome by high concentrations of IL-1, but similar treatment cannot correct defects in JNK activation (25). Taken together, our results suggest that IRAK is used similarly by both IL-18 and IL-1 in mediating intracellular signaling.
IL-1 signaling leading to NF-κB activation has been relatively well characterized. Upon IL-1 binding to its receptor, IRAK is rapidly recruited to the receptor complex via MyD88 (20). Activated IRAK interacts with TNF receptor–associated factor (TRAF)6, which in turn activates NF-κB–inducing kinase (NIK) (36, 37). NIK is involved in the NF-κB pathway by activating IκB kinases (IKKs) (31, 32, 38, 39). Activated IKKs phosphorylate IκB for degradation, allowing nuclear translocation of NF-κB for gene induction (30). However, details of IL-1–mediated signaling leading to JNK activation are still unclear. It has been reported that only MyD88 but not TRAF6 is essential for JNK activation (40). Since IRAK is positioned between MyD88 and TRAF6 in the signaling cascade, IRAK could be the bifurcating molecule for both NF-κB and JNK pathways. IL-18 stimulation also induces the stress-activated MAP kinase JNK (2). The defects in JNK activation in IRAK-deficient cells indicate that IRAK is essential for JNK activation but intermediary molecules linking IRAK to JNK have not been identified.
Recent reports on IL-1 signaling also suggest additional complexity and divergence in this pathway. In addition to IRAK, other proteins, including IRAK homologue IRAK2, are reported to interact with the IL-1 receptor (19, 41). IRAK2 also interacts with MyD88 and TRAF6 (19). A dominant negative form of IRAK2 mutant blocks MyD88-induced NF-κB activation (19). Although overexpression of IRAK in transfection studies has been reported to activate NF-κB but not JNK (42), our studies demonstrate that IRAK is essential for IL-18–mediated activation of JNK but its role in NF-κB pathway is less critical. In the absence of IRAK, NF-κB activation can still occur, possibly mediated by other related kinases such as IRAK2. The relative role of IRAK and IRAK2 in inducing JNK and NF-κB downstream of MyD88 is still unclear and the involvement of IRAK2 in IL-18 signaling remains to be elucidated.
Defect in IL-18–mediated Functions in the Absence of IRAK.
IL-18 alone does not support Th1 differentiation, but it can potentiate Th1 development driven by IL-12 (4). In differentiated Th1 cells, IFN-γ production can be induced by IL-18 or IL-12 alone, and this effect can be further synergized by a combination of both cytokines (4). We observed no defect in Th1 differentiation of IRAK-deficient cells mediated by IL-12, confirming that IL-18 signaling does not play an essential role in Th1 development. IL-18–induced IFN-γ expression in IRAK-deficient Th1 cells is significantly decreased, although NF-κB activation is only partially impaired. Increase in serum IFN-γ levels as a result of a Th1 response to P. acnes and LPS treatment, or an NK response in the early phase of MCMV infection, was also reduced significantly in IRAK-deficient mice. The results suggest that optimal gene induction by IL-18 requires proper activation of multiple signaling pathways including NF-κB and JNK. Dramatic decrease in IFN-γ production in IRAK-deficient cells may have resulted from the impairment in JNK activation even though NF-κB activity was not severely affected. We also observed similar phenomena in our previous studies; IL-6 induction in IRAK-deficient fibroblasts in response to IL-1 treatment was significantly reduced due to defects in JNK/p38 activation despite minimal impacts on NF-κB activation (25). The involvement of both JNK and NF-κB pathways may imply that optimal gene expression depends on the binding of multiple transcription factors including NF-κB and AP-1 onto the promoter regions of the target genes. It is also possible that the transactivation potential of NF-κB is modulated by the kinase activity of JNK and the other MAP-related kinase p38, as suggested in studies of TNF-induced IL-6 gene expression (43).
IL-18 and IL-12 share many biological properties, although different signaling pathways are used by the two cytokines. Transcription factor STAT4 is activated by IL-12 whereas NF-κB and AP-1 are activated by IL-18. The binding sites of these transcription factors within the IFN-γ promoter region have been identified (44). IL-18 alone can directly induce IFN-γ promoter activity via AP-1, whereas IL-12– mediated induction of the promoter activity requires both AP-1 and STAT4 (44). Synergistic expression of IFN-γ by IL-18 and IL-12 probably results from the interplay of multiple transcription factors in differential regulation of IFN-γ promoter activity. A strong synergistic effect in IFN-γ induction and cell proliferation was observed in IRAK-deficient Th1 cells when treated with combination of IL-12 and IL-18. The results suggest that minimal activation of NF-κB and JNK by IL-18 in IRAK-deficient cells is sufficient to function synergistically with IL-12 to achieve a significant synergistic response.
MCMV Infection in IRAK-deficient Mice.
MCMV infection in mice results in a strong NK response in the early phase of infection before the onset of T and B cell responses (34). Induction of NK cell IFN-γ production and cytotoxicity peaks on day 2–5 of infection (34, 45). IFN-γ production by NK cells is the major defense mechanism in controlling MCMV replication (34). It has been well characterized that in MCMV infection IL-12 plays a major role in NK cell IFN-γ production, whereas induction of NK cytotoxicity is regulated by IFN-α/β (35, 46). Although IL-18 is known to be involved in NK cell function, the role of IL-18 in NK responses during MCMV infection has not been investigated. We have undertaken this study to understand the importance of IRAK in IL-18–mediated responses in a viral infection. MCMV-infected IRAK-deficient mice have a normal induction of IL-18 expression but exhibited a significant decrease in IFN-γ induction and in serum IFN-γ. The results in this study suggest that IL-18 plays a distinct role in IFN-γ production during the NK response to MCMV infection and that its function cannot be overcome completely by IL-12 or other mechanisms. However, we cannot rule out the possibility that IRAK may also play a role in viral infections via its involvement in other related receptors in addition to the IL-18 receptor. Although we have demonstrated a defect in IL-18–induced NK cytotoxicity in IRAK-deficient mice, MCMV infection or poly(I): poly(C) injection triggers a normal NK cytotoxicity in these mice. Our results suggest that the role of IL-18 in NK cytotoxicity can be compensated by IFN-α/β or other mechanisms during MCMV infection. Studies are underway in our laboratory to understand the mechanisms of MCMV-induced immune responses in IRAK-deficient mice.
Acknowledgments
We thank Julie Culver and Michelle Courtney for their excellent technical assistance, and Lars Karlsson for critical review of the manuscript.
This work was supported in part by grants from the National Institutes of Health to P. Ghazal (CA66167 and AI30627). P. Ghazal is a Scholar of the Leukemia Society of America. A. Angulo is a Fellow from the University of California Universitywide AIDS research program.
Abbreviations used in this paper
- AP-1
activator protein 1
- ES
embryonic stem
- GST
glutathione S-transferase
- ICE
IL-1β converting enzyme
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- IRAK
IL-1 receptor–associated kinase
- JAK
Janus kinase
- JNK
c-Jun NH2-terminal kinase
- MAP
mitogen-activated protein
- MCMV
murine cytomegalovirus
- NF
nuclear factor
- STAT
signal transducer and activator of transcription
- TRAF
TNF receptor–associated factor
References
- 1.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 2.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 4.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NF-κB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 5.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 6.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 7.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O'Shea JJ, Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schriber RD, Darnell JE, Jr, Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DAA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH, Sun Y, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 11.Bazan JF, Timans JC, Kastelein RA. A newly defined interleukin-1? . Nature. 1996;379:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Activation of interferon-γ-inducing factor mediated by interleukin-1 β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 13.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Caspase-1 processes IFN-γ inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 14.Parnet P, Garka KE, Bonnert TP, Dower SK, Sims JE. IL-1Rrp is a novel receptor-like molecule similar to the type 1 interleukin-1 receptor and its homologues T1/ ST2 and IL-1RAcp. J Biol Chem. 1996;271:3967–3970. doi: 10.1074/jbc.271.8.3967. [DOI] [PubMed] [Google Scholar]
- 15.Torigoe K, Ushino S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 16.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to DrosophilaToll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophilatoll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 18.Shelton CA, Wasserman SA. Pelle encodes a protein kinase required to establish dorsoventral polarity in the drosophila embryo. Cell. 1993;72:515–525. doi: 10.1016/0092-8674(93)90071-w. [DOI] [PubMed] [Google Scholar]
- 19.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 20.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 21.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 22.Kojima H, Takeuchi M, Ohta T, Nishida Y, Arai N, Ikeda M, Ikegami H, Kurimoto M. Interleukin-18 activates the IRAK-TRAF6 pathway in mouse EL-4 cells. Biochem Biophy Res Commun. 1998;244:183–186. doi: 10.1006/bbrc.1998.8236. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Kishimoto T. IL-6 and NF-IL-6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 24.Bankers-Fulbright JL, Kalli KR, McKean DJ. Interleukin-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 25.Kanakaraj P, Schafer PH, Cavender DE, Wu Y, Ngo K, Grealish PF, Wadsworth SA, Peterson PA, Siekierka JJ, Harris CA, Fung-Leung W-P. Interleukin (IL)-1 receptor–associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187:2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanakaraj P, Duckworth B, Azzoni L, Kamoun M, Cantley LC, Perussia B. Phosphatidylinositol-3 kinase activation induced upon FCγRIIIA–ligand interaction. J Exp Med. 1994;179:551–558. doi: 10.1084/jem.179.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brautigam AR, Dutko FJ, Olding LB, Oldstone MBA. Pathogenesis of murine cytomegalovirus infection: the macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J Gen Virol. 1979;44:349–359. doi: 10.1099/0022-1317-44-2-349. [DOI] [PubMed] [Google Scholar]
- 28.Westwick JK, Weitzel C, Minden A, Karin M, Brenner DA. Tumor necrosis factor-α stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 29.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 31.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 32.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li JW, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 33.Okamura H, Kawaguchi K, Shoji K, Kawade Y. High level induction of gamma interferon with various mitogens in mice pretreated with Propionibacterium acnes. Infect. . Immun. 1982;38:440–443. doi: 10.1128/iai.38.2.440-443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 35.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and anti-viral defense. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 36.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 37.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 38.Natoli G, Costanzo A, Moretti F, Fulco M, Balsano C, Levrero M. Tumor necrosis factor (TNF) receptor 1 signaling downstream of TNF receptor–associated factor 2. Nuclear factor κB (NF-κB)–inducing kinase requirement for activation of activating protein 1 and NF-κB but not of c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem. 1997;271:26079–26082. doi: 10.1074/jbc.272.42.26079. [DOI] [PubMed] [Google Scholar]
- 39.Woronicz D, Gao X, Cao Z, Rothe M, Goeddel DV. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 40.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human Toll signaling pathway: divergence of nuclear factor κB and JNK/SAPK activation upstream of tumor necrosis factor receptor–associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh R, Huang S, Guth T, Konieczkowski M, Sedor JR. Cytosolic domain of the type 1 interleukin-1 receptor spontaneously recruits signaling molecules to activate a proinflammatory gene. J Clin Invest. 1997;100:419–428. doi: 10.1172/JCI119549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knop J, Wesche H, Lang D, Martin MU. Effects of overexpression of IL-1 receptor associated kinase on NF-kB activation, IL-2 production and stress-activated protein kinses in the murine T cell line EL4. Eur J Immunol. 1998;28:1–10. doi: 10.1002/(SICI)1521-4141(199810)28:10<3100::AID-IMMU3100>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Van den Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 44.Barbulescu K, Becker C, Schlaak JF, Schmitt E, Meyer zum Buschenfelde KH, Neurath MF. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-γ promoter in primary CD4+T lymphocytes. J Immunol. 1998;160:3642–3647. [PubMed] [Google Scholar]
- 45.Welsh RM, Brubaker JO, Vargas-Cortes M, O'Donnell CL. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell–dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orange JS, Biron CA. Characterization of early IL-12, IFNαβ and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]