Abstract
The natural killer T (NKT) cell ligand α-galactosylceramide (α-GalCer) exhibits profound antitumor activities in vivo that resemble interleukin (IL)-12–mediated antitumor activities. Because of these similarities between the activities of α-GalCer and IL-12, we investigated the involvement of IL-12 in the activation of NKT cells by α-GalCer. We first established, using purified subsets of various lymphocyte populations, that α-GalCer selectively activates NKT cells for production of interferon (IFN)-γ. Production of IFN-γ by NKT cells in response to α-GalCer required IL-12 produced by dendritic cells (DCs) and direct contact between NKT cells and DCs through CD40/CD40 ligand interactions. Moreover, α-GalCer strongly induced the expression of IL-12 receptor on NKT cells from wild-type but not CD1−/− or Vα14−/− mice. This effect of α-GalCer required the production of IFN-γ by NKT cells and production of IL-12 by DCs. Finally, we showed that treatment of mice with suboptimal doses of α-GalCer together with suboptimal doses of IL-12 resulted in strongly enhanced natural killing activity and IFN-γ production. Collectively, these findings indicate an important role for DC-produced IL-12 in the activation of NKT cells by α-GalCer and suggest that NKT cells may be able to condition DCs for subsequent immune responses. Our results also suggest a novel approach for immunotherapy of cancer.
Keywords: natural killer T cells, dendritic cells, α-galactosylceramide, interleukin 12, interleukin 12 receptor
Natural killer T (NKT)1 cells represent a novel lymphoid lineage distinct from mainstream T cells, B cells, and NK cells. NKT cells are characterized by the expression of an invariant TCR encoded by Vα14 and Jα281 gene segments and Vβ8, 7, or 2 gene segments (1, 2). It was demonstrated recently that NKT cells are strongly stimulated by the glycolipid α-galactosylceramide (α-GalCer), a potent inducer of antitumor immunity in mice (3–5). Recognition of α-GalCer by NKT cells appeared to depend on the interaction of the invariant TCR of these cells with α-GalCer presented by the nonclassical MHC molecule CD1d on APCs (6). Stimulation of NKT cells by α-GalCer resulted in the production of large amounts of IFN-γ and some IL-4, and the development of a cytotoxic phenotype (7).
The in vivo antitumor activity of α-GalCer strongly resembles the antitumor activity mediated by the cytokine IL-12 (8, 9). Moreover, both α-GalCer and IL-12 are strong inducers of NKT cell activity and exert their antitumor activities through activation of these cells (8, 9). Because of these striking similarities between α-GalCer and IL-12 for activation of NKT cells, we decided to investigate whether α-GalCer activation of NKT cells involves regulation by IL-12. First, we demonstrated that NKT cells are the main, if not the only, target for activation by α-GalCer in spleen cell populations of mice. Second, we showed that endogenous IL-12 produced by dendritic cells (DCs) is critically important for the activation of NKT cells by α-GalCer and that the interaction between DCs and NKT cells involves CD40 and its ligand. Third, α-GalCer induced the expression of IL-12R on NKT cells, which required the production of IFN-γ by NKT cells. Fourth, α-GalCer acted synergistically with IL-12 in the activation of natural killing activity and IFN-γ production in vivo. Collectively, these findings indicate that α-GalCer exerts its function through IL-12 and suggest a novel approach for therapeutic intervention in cancer and other disease processes.
Materials and Methods
Mice.
C57BL/6 mice were purchased from Charles River Japan. Vα14 NKT cell–deficient (Jα281−/−) and CD1d−/− mice were established by specific deletion of the Jα281 and CD1d gene segment, respectively (3, 10). All mice used in this study were at 5–8 wk of age and were maintained in specific pathogen– free conditions.
α-GalCer.
α-GalCer [(2S,3S,4R)-1-O-(α-d-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol] used for this study was provided by Dr. Y. Koezuka (Kirin Brewery Co., Ltd., Gunma, Japan [4, 5]). The stock solution of α-GalCer (220 μg/ml) was diluted in 0.5% polysorbate 20 (Nikko Chemical) in 0.9% NaCl solution. This stock solution was further diluted into an appropriate concentration with saline and used for the experiments. A vehicle control solution was prepared from a solution of 0.5% polysorbate 20 in 0.9% NaCl solution. The vehicle control was used in all experiments.
Isolation of Lymphoid Cell Subsets by FACS®.
Spleen cells were incubated on nylon wool columns for 45 min, and the nonadherent cells were used for the isolation of NKT cells, NK cells, CD4+ T cells, and CD8+ T cells by cell sorting using a FACS Vantage™ instrument (Becton Dickinson). All mAbs used in these experiments (mAbs against NK1.1, CD4, CD8, and TCR-α/β) were purchased from PharMingen. Unless noted otherwise, NK1.1+TCR-α/β+ cells were used as purified NKT cells. The stained cells were isolated using the FACS Vantage™. The purity of the sorted cells was >98%. The details of the staining and sorting have been described previously (11).
Coculture of DCs and NKT Cells.
DCs were prepared according to the method of Steinman et al. (12) with some modifications. In brief, spleen cells were incubated in 10-cm plastic dishes (Falcon; Becton Dickinson) for 2 h, and the nonadherent cells were removed from the culture. The adherent cells were further incubated overnight and the nonadherent cells were harvested. Then, CD11c+B220−CD4−CD8− cells were isolated from the nonadherent populations by cell sorting and used as the source of DCs. Generally, DCs (105) were cocultured with purified NK1.1+TCR-α/β+ NKT cells (2 × 105) in the presence of 50 ng/ml of α-GalCer in 96-well U-bottomed plates (Costar Corp.). After incubation for 36 h, the culture supernatants were harvested to detect cytokine levels.
Detection of Cytokine Activity.
IL-4 or IFN-γ activity in culture supernatants was determined using the Biotrac™ mouse IL-4 or Biotrac™ mouse IFN-γ ELISA system (Nycomed Amersham plc). Serum samples were obtained from C57BL/6 mice 24 h after injection of α-GalCer (200 ng/mouse) and/or IL-12 (200 U/mouse; donated by Genetics Institute, Inc., Cambridge, MA), and cytokine levels were measured using ELISA kits (Nycomed Amersham plc). IL-12 p70 activity in culture supernatants was measured using Intertest-12X™ ELISA kits (Genzyme Corp.).
Cytotoxicity Assay.
The natural killing activity of spleen cells was determined by 4-h 51Cr-release assays using YAC-1 cells as target. 1 lytic unit (LU) was defined as the number of effector cells required to cause 25% lysis of 2,500 target cells as described previously (13).
Measurement of the Synergistic Effect of α-GalCer and IL-12 In Vivo.
Wild-type C57BL/6 mice received an injection of α-GalCer (200 ng/mouse i.v.), and 6 h later mice were injected with recombinant IL-12 (200 U/mouse i.p.) or saline. 1 d after treatment with IL-12, IFN-γ production in the serum and NK activity of spleen cells were determined. Control mice were treated with vehicle only.
Quantitative Reverse Transcription PCR Assay for IL-12R mRNA Measurement.
C57BL/6, CD1d−/−, and Vα14 NKT cell–deficient mice were injected with α-GalCer (2 μg/mouse i.v.) or vehicle. At different time points (0–6 h) after treatment, mice were killed and spleen cells were isolated. TaqMan™ real-time quantitative reverse transcription (RT)-PCR assay was carried out for the detection of IL-12R mRNA expression by these cells according to published methods (14). In brief, total RNA extracts from the cells were added to the master mixture. To detect the amount of the IL-12R mRNA RT-PCR amplificon, target (IL-12Rβ1, IL-12Rβ2) and control (glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) hybridization probes were mixed with target and control PCR primers, respectively. This mixture was transferred to a set of thermocycler tubes and transcribed at 42°C for 30 min, followed by 40 cycles of amplification at 95°C for 15 s and 60°C for 1.5 min, and analyzed using an ABI PRISM 7700 sequence detector (Applied Biosystems). IL-12Rβ1 and IL-12Rβ2 mRNA expression were estimated from the ratio of fluorescence intensity to GAPDH. IL-12R expression induced by α-GalCer is indicated in the figures as induction index, calculated as follows: induction index = IL-12R expression of α-GalCer–stimulated sample/IL-12R expression of unstimulated sample.
TaqMan™ probes used for these analyses are as follows: IL-12Rβ1 mRNA-605T, 5′-CGGATGCCCACAACGAATTGGA-3′; IL-12Rβ2 mRNA-551T, 5′-AGCCACCTCAAAACATATCATGTGTCCAGG-3′; GAPDH-542T, 5′-CCTGGCCAAGGTCATCCATGACAACTTT-3′.
PCR primers used for these analyses are as follows: IL-12β1 mRNA, forward primer (-563F) 5′-AATGTGTCTGAAGAGGCCGGT-3′ and reverse primer (-657R) 5′-GAGTTAACCTGAGGTCCGCAGT-3′; IL-12Rβ2 mRNA, forward primer (-529F) 5′-ATCTCAGTTGGTGTTGCTCCA-3′ and reverse primer (-602R) 5′-GCCACAGTTCCATTTTCTCCT-3′; GAPDH, forward primer (-368F) 5′-CTTCACCACCATGGAGAAGGC-3′ and reverse primer (-605R) 5′-GGCATGGACTGTGGTCATGAG-3′.
Blocking of IL-12R Induction by Anti–IFN-γ mAb.
Wild-type C57BL/6 mice were injected with 500 μg i.p. anti–IFN-γ mAb (R4-6A2; PharMingen) or IL-12 (C15.1 and C15.6, donated by Dr. G. Trinchieri, Wistar Institute of Anatomy and Biology, Philadelphia, PA) at 0 and 1 d before priming with α-GalCer. As a control, the same amount of rat IgG1 (PharMingen) was injected intraperitoneally into control mice before injection of α-GalCer.
Results
α-GalCer Selectively Activates NK1.1+TCR-α/β+ NKT Cells.
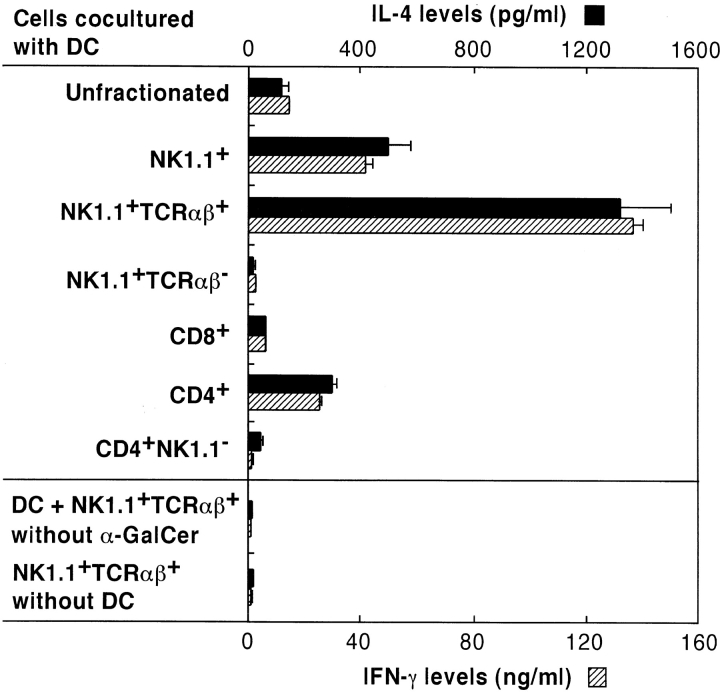
To provide direct evidence that NKT cells are the only target cells for activation by α-GalCer, various lymphoid subsets were isolated from mouse spleen cell suspensions by flow cytometry and cocultured with DCs in the presence of α-GalCer. After 36 h of culture, the supernatants were harvested and their IL-4 and IFN-γ contents were measured by ELISA. Fig. 1 shows that purified NK1.1+ T cells produce higher levels of IL-4 and IFN-γ than unfractionated spleen cells. The IFN-γ produced in these cultures was not derived from classical NK cells, because enrichment of NK1.1+TCR-α/β− NK cells showed no significant cytokine production. In contrast, NK1.1+ TCR-α/β+ cells, which represent the NKT cell population, revealed markedly high levels of IL-4 and IFN-γ production. Although CD4+ T cells produced higher levels of cytokines compared with unfractionated spleen cells, this appeared to be due to the presence of CD4+NK1.1+ NKT cells, because CD4+NK1.1− cells produced neither IL-4 nor IFN-γ in response to α-GalCer. Culture of NK1.1+ TCR-α/β+ NKT cells alone or with DCs in the absence of α-GalCer caused no significant production of IFN-γ or IL-4, indicating that DCs are essential for the stimulation of cytokine production by NKT cells.
Figure 1.
α-GalCer selectively activates NK1.1+TCR-α/β+ NKT cells. Spleen cells from C57BL/6 mice were separated into a variety of lymphoid cell subsets by cell sorting as described in Materials and Methods. Their responsiveness to α-GalCer in the presence of DCs was then determined by measuring IL-4 (▪) and IFN-γ () levels in the culture supernatants using ELISA. As a control, NK1.1+TCR-α/β+ NKT cells were cultured alone or with DCs in the absence of α-GalCer. The bars represent mean ± SE of triplicate samples.
Endogenous IL-12 Production by DCs Is Essential for the Triggering of NKT Cells.
Fig. 2 A shows that coculture of DCs and NKT cells in the presence of α-GalCer results in high levels of IFN-γ production. However, addition of anti–IL-12 mAb into these cultures caused a marked inhibition of IFN-γ production. Such inhibition was not observed when control anti-CD8 rat IgG mAb was added. Therefore, these results indicated that endogenously produced IL-12 by DCs was essential for the early activation of NKT cells by α-GalCer. The effect of mAbs against CD40 and CD40L on the activation of NKT cells by α-GalCer was also investigated (Fig. 2 B). Both anti-CD40 mAb and anti-CD40L mAb greatly inhibited the production of IFN-γ by NKT cells in response to α-GalCer. These findings suggested that direct contact between DCs and NKT cells through CD40/CD40L interactions is critically important for the activation of NKT cells by α-GalCer. To study the requirements for IL-12 production by DCs in these cultures in further detail, IL-12 p70 activity in culture supernatants was measured by ELISA. As shown in Fig. 2 C, DCs produced IL-12 p70 when cultured with NKT cells and α-GalCer. However, DCs did not produce IL-12 p70 when cultured with α-GalCer alone or when cultured with α-GalCer and NK (NK1.1+TCR-α/β−) cells.
Figure 2.
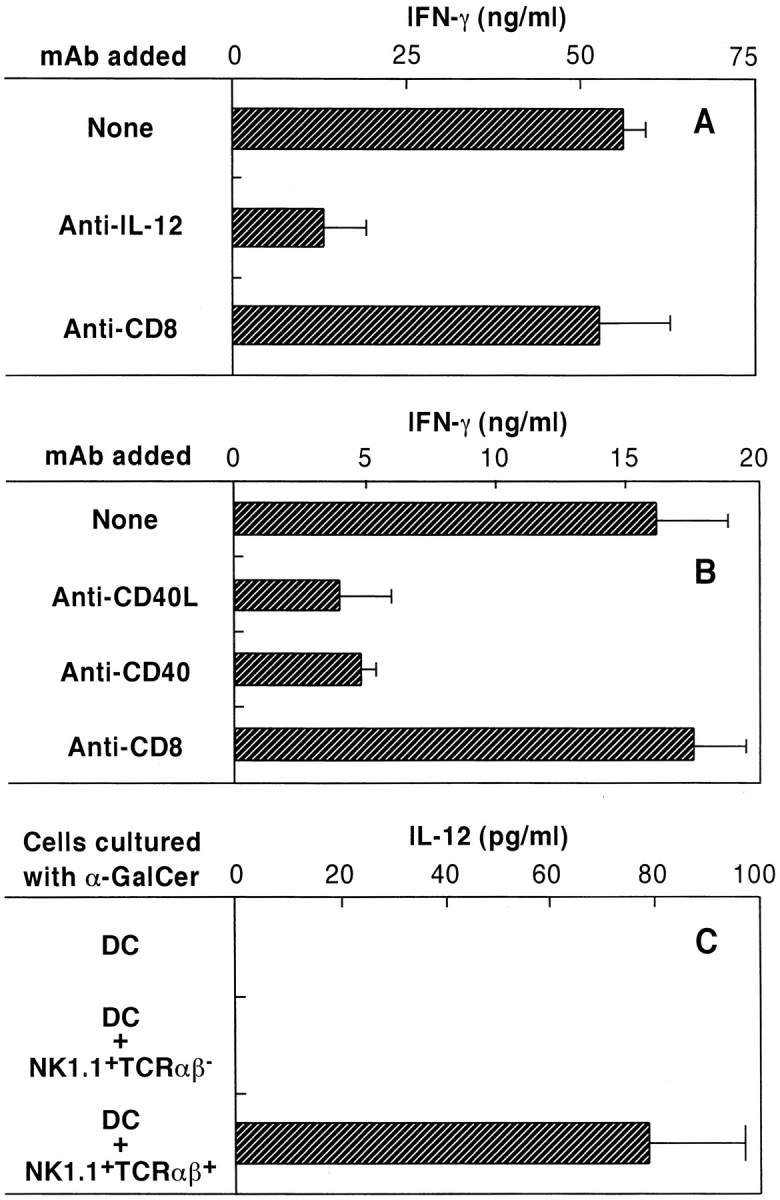
Endogenously produced IL-12 and CD40/CD40L interaction during coculture of DCs and NKT cells is essential for NKT cell activation by α-GalCer. Purified NKT cells were cocultured with DCs in the presence of α-GalCer for 36 h. The IFN-γ levels in culture supernatants were then determined by ELISA. (A) The ability of anti–IL-12 mAb to block NKT cell activation by α-GalCer. Anti-CD8 mAb was used as control rat IgG Ab. (B) The ability of anti-CD40 mAb and anti-CD40L mAb to block NKT cell activation by α-GalCer. As a control, rat anti-CD8 IgG mAb was added to the culture. (C) IL-12 production by DCs cultured with α-GalCer and NKT cells. DCs (5 × 105) were activated with 50 ng/ml of α-GalCer for 8 h in the presence or absence of NK1.1+TCR-α/β− NK cells (105) or NK1.1+TCR-α/β+ NKT cells (105). The bars represent mean ± SE of triplicate samples.
α-GalCer Induces IL-12R Expression on NKT Cells.
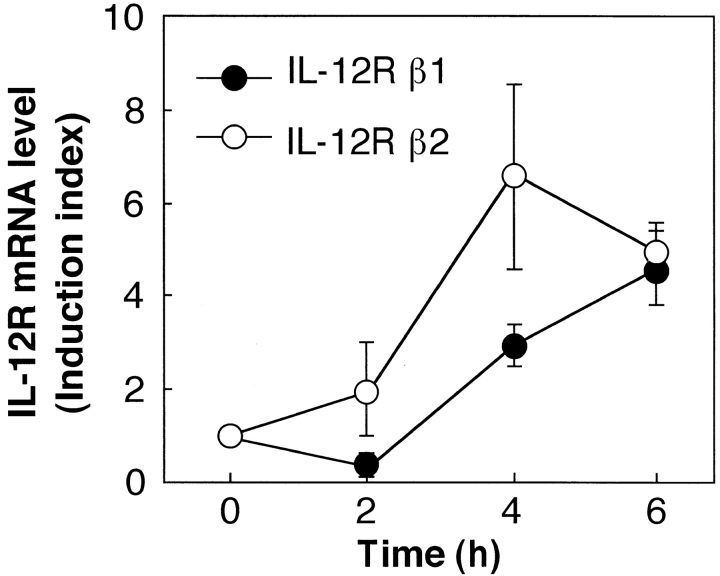
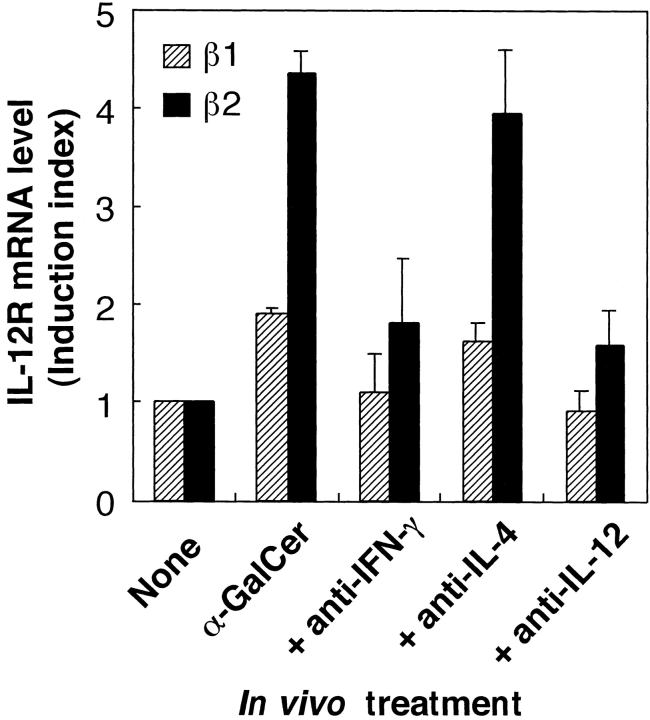
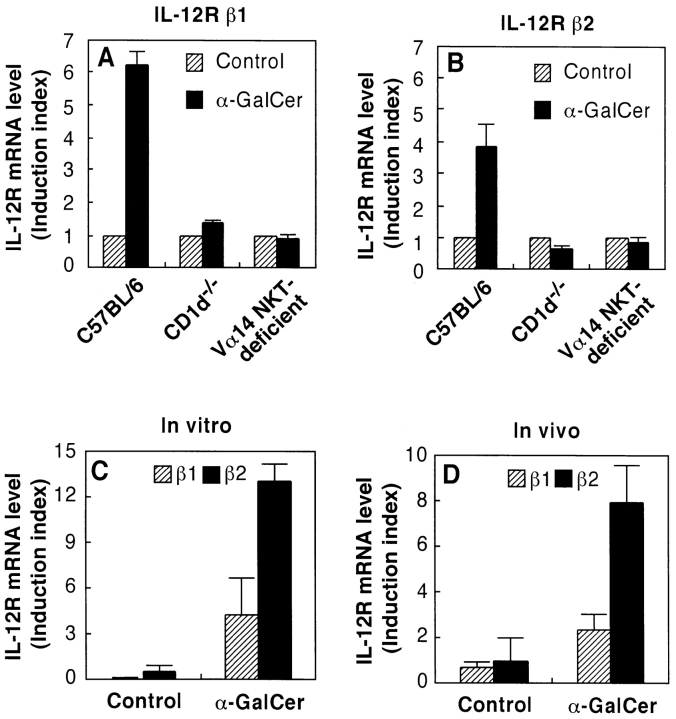
The effect of α-GalCer on the induction of IL-12R mRNA expression in spleen cells was examined by RT-PCR. As shown in Fig. 3, intravenous injection of α-GalCer into C57BL/6 mice caused the induction of mRNA for both IL-12Rβ1 and IL-12Rβ2 in spleen cells within 4 h. This upregulation of IL-12R was strongly blocked by administration of anti–IL-12 mAb or anti–IFN-γ mAb before injection of α-GalCer (Fig. 4). Moreover, the IL-12R induction by α-GalCer was almost completely abolished in both CD1d−/− and Vα14 NKT cell–deficient mice (Fig. 5, A and B). Thus, these results suggested that CD1d-dependent α-GalCer–induced IFN-γ production by NKT cells may be critically important for the upregulation of IL-12R on NKT cells. To provide direct evidence for this hypothesis, we measured the expression of IL-12R on purified NKT cells that were previously activated in the presence of DCs and α-GalCer, either in vitro or in vivo. Fig. 5 C shows that in vitro activation of spleen cells by DCs plus α-GalCer strongly induced the expression of IL-12R on NKT cells. Similar findings were made when mice were injected in vivo with α-GalCer (Fig. 5 D).
Figure 3.
Upregulation of IL-12R expression in the spleen upon in vivo administration of α-GalCer. C57BL/6 mice were injected intravenously with α-GalCer. Various times (0, 2, 4, and 6 h) after the treatment, spleen cells were prepared and their expression of IL-12Rβ1 (•) and IL-12Rβ2 (○) mRNA was measured by quantitative RT-PCR. The IL-12R mRNA levels are represented as an induction index, as described in Materials and Methods. The bars represent mean ± SE of triplicate samples.
Figure 4.
Role of IL-12 and IFN-γ in the induction of IL-12R expression in the spleen. C57BL/6 mice were injected intravenously with α-GalCer. 4 h after the injection, spleen cells were prepared and their expression of IL-12Rβ1 () and IL-12Rβ2 (▪) mRNA was measured by quantitative RT-PCR. The IL-12R mRNA levels are presented as an induction index, as described in Materials and Methods. The blocking effect of anti–IL-12 mAb and anti–IFN-γ mAb was determined by injection of these mAbs (500 μg/mouse i.p.) into the mice 1 and 0 d before the treatment with α-GalCer. The bars represent mean ± SE of triplicate samples.
Figure 5.
α-GalCer induces IL-12R expression on NKT cells. C57BL/6 wild-type mice, CD1d−/− mice, and NKT-deficient mice were injected intravenously with vehicle () or α-GalCer (▪). 4 h after the injection, spleen cells were prepared and their expression of IL-12Rβ1 (A) and IL-12Rβ2 (B) mRNA was measured by quantitative RT-PCR. (C) Spleen cells from wild-type mice were cultured with DCs plus α-GalCer or vehicle for 8 h, NK1.1+TCR-α/β+ NKT cells were purified by cell sorting, and the expression of IL-12Rβ1 () and IL-12Rβ2 (▪) was examined by quantitative RT-PCR. (D) Wild-type mice were injected intravenously with α-GalCer or vehicle; after 6 h the spleens from these mice were isolated, and NK1.1+TCR-α/β+ NKT cells were purified by cell sorting, and expression of IL-12Rβ1 () and IL-12Rβ2 (▪) was examined by quantitative RT-PCR. The IL-12R mRNA levels are presented as an induction index, as described in Materials and Methods. The bars represent mean ± SE of triplicate samples.
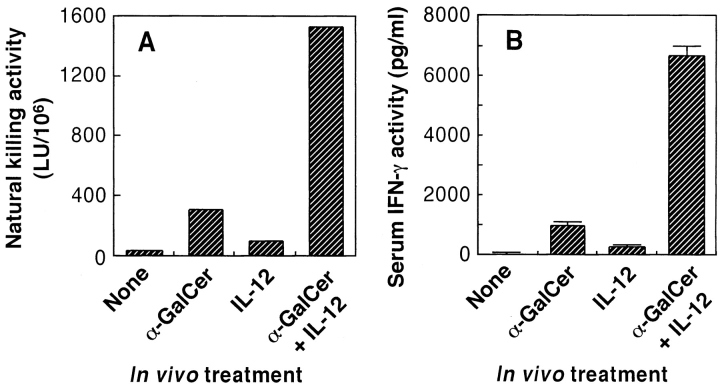
α-GalCer Synergistically Acts with Exogenously Administered IL-12 in the Activation of Natural Killing Activity and IFN-γ Production In Vivo.
C57BL/6 mice were injected intravenously with α-GalCer, and their splenic natural killing activity against YAC-1 cells was determined 24 h later. As shown in Fig. 6 A, a suboptimal dose of neither α-GalCer nor IL-12 was able to activate natural killing activity in vivo. However, combined administration of α-GalCer and IL-12 at a suboptimal dose caused a marked augmentation of natural killing.
Figure 6.
Synergistic effect of α-GalCer and IL-12 in vivo. C57BL/6 mice were injected with a suboptimal dose of α-GalCer (200 ng/mouse i.v.) and 6 h later, mice were injected with a suboptimal dose of IL-12 (200 U/mouse i.p.). 1 d after the treatment with IL-12, the mice were killed and splenic natural killing activity (A) and serum IFN-γ levels (B) were determined as described in Materials and Methods. The bars represent mean ± SE of triplicate samples.
A similar synergistic effect of α-GalCer and IL-12 was demonstrated for the elevation of serum IFN-γ production. As shown in Fig. 6 B, the administration of α-GalCer plus IL-12 resulted in a strong enhancement of serum IFN-γ levels in C57BL/6 mice compared with mice treated with α-GalCer or IL-12 only.
Discussion
The finding that NKT cells recognize α-GalCer presented by DCs in a CD1d-dependent manner represents a novel recognition mechanism in the immune system (15). NKT cells, which can produce both IFN-γ and IL-4 (16, 17), play an important role in immunoregulation and have been considered to play a central role as innate effector cells involved in both the protection and the onset of immune diseases (18). The NKT cell ligand α-GalCer has a strong immunopotentiating effect in vivo, and this chemical mediates strong antitumor activity (3–5, 9). Therefore, it is important to dissect the mechanism by which α-GalCer activates NKT cells.
The previous finding (3) that NKT-deficient mice did not respond to α-GalCer strongly suggested that NKT cells may be the primary target cells to α-GalCer. However, it still remained unclear whether only NKT cells responded to α-GalCer. To answer this question, we used highly purified splenic NK cells, NKT cells, CD4+ T cells, and CD8+ T cells and determined their responsiveness to α-GalCer in the presence of DCs. The data illustrated in Fig. 1 clearly demonstrate that NKT cells are the only cells that respond to α-GalCer (3). It is surprising that neither classical NK cells nor mainstream CD4+ T cells or CD8+ T cells revealed a significant response to α-GalCer even in the presence of DCs. Together with previous findings (3), the present data indicate that α-GalCer selectively stimulates NKT cells in the presence of DCs.
Recently, the mechanisms of activation of naive CD4+ T cells through interaction with DCs have been examined (12, 19–22). Cell–cell adhesion between CD4+ T cells and DCs through CD40/CD40L and B7.1/CD28 resulted in the activation of both DCs and T cells, which triggered the production of IL-12 by DCs and IFN-γ by Th1 cells (12, 19, 20, 23–25). Such conditioned DCs were able to prime cytotoxic T cells (22, 26, 27). This recognition system has resemblance to that discussed here. As shown in Fig. 2, IL-12 production by DCs appears to be essential for NKT cell activation by α-GalCer, because neutralization of endogenously produced IL-12 by anti–IL-12 mAb caused a strong inhibition of IFN-γ production by NKT cells. The important role of CD40/CD40L for the production of IFN-γ in the cocultures of DCs and NKT cells with α-GalCer is also apparent from these experiments (Fig. 2 B). As demonstrated in Fig. 2 C, DCs produce IL-12 only when they are cultured with α-GalCer in the presence of NKT cells, indicating that direct contact between α-GalCer–bound DCs and NKT cells may be essential for IL-12 production by DCs. This interaction may be required for the production of IFN-γ by IL-12–activated NKT cells, because mAbs directed against CD40/CD40L greatly inhibited IFN-γ production by NKT cells (Fig. 2). These findings indicate that the interaction of NKT cells with DCs may be very similar to the interaction of helper T cells with DCs (22, 26, 27). Since the interactions between DCs and NKT cells occur very quickly after administration of α-GalCer, NKT cells may be able to condition DCs very early in an immune response, and affect subsequent adaptive responses.
In this paper, we also demonstrate that α-GalCer upregulates IL-12R expression in vivo (Fig. 3). IL-12R upregulation is blocked by mAbs against IL-12 or IFN-γ and is absent in CD1d−/− and NKT-deficient mice (Figs. 4 and 5). Moreover, activation of NKT cells in vitro and in vivo results in a strong induction of IL-12Rβ1 and IL-12Rβ2 on these cells (Fig. 5, C and D). Therefore, we speculate that the following series of events is induced upon culture of α-GalCer with DCs and NKT cells: (a) α-GalCer first binds to CD1d molecules on DCs; (b) NKT cells recognize α-GalCer–bound DCs via their TCRs and also interact with DCs via CD40/CD40L; (c) during this interaction, DCs produce IL-12; (d) the endogenously produced IL-12 stimulates IFN-γ production by NKT cells; and (e) IFN-γ produced by NKT cells upregulates IL-12R on NKT cells in an autocrine manner. The dramatic synergistic effect of suboptimal α-GalCer and exogenously administered IL-12 indicates that expression of IL-12Rβ1 and β2, detected by quantitative RT-PCR, is functionally upregulated in vivo. Moreover, since this synergistic effect of α-GalCer and IL-12 was not demonstrated in NKT-deficient mice, we conclude that in wild-type mice coadministration of α-GalCer and IL-12 leads to upregulation of IL-12R on CD1-dependent NKT cells.
Both α-GalCer and IL-12 have been demonstrated to exhibit potent antitumor activity in vivo. IL-12 has multiple effects on the immune system that are beneficial for the induction of antitumor immunity in vivo (28–30). However, the unexpected severe side effects of IL-12 have made it difficult to use this cytokine in clinical trials (31). We demonstrated that α-GalCer synergistically acts with small doses of IL-12 in vivo to activate NKT cells and to induce IFN-γ production (Fig. 6). These findings suggest that coadministration of α-GalCer with IL-12 could be used as a new approach for tumor immunotherapy.
Recent studies have demonstrated that Th1 immunity regulated by IL-12 and IFN-γ plays a critical role in the induction of protective immunity against tumors and infectious agents (32, 33). Although NKT cells are involved in both Th1 and Th2 immunity through IFN-γ or IL-4 production, the immunomodulating protocol using α-GalCer and IL-12 preferentially induces NKT cells that produce large amounts of IFN-γ (34). These NKT cells may facilitate the development of Th1-dominant cellular immunity essential for the induction of protective immunity against tumors and some infectious agents. Recently, it was demonstrated that α-GalCer can stimulate human NKT cells in a CD1d-dependent manner (35, 36), indicating that our proposed immunotherapy protocol using α-GalCer and IL-12 will be useful for the application to human immune diseases, including cancer.
Acknowledgments
We would like to thank Dr. S.H. Herrmann and Dr. M. Kobayashi (Genetics Institute, Inc.) for their kind gift of IL-12. We also thank Dr. G. Trinchieri for his kind gift of anti–IL-12 mAbs, and Dr. Y. Koezuka for providing α-GalCer.
This work was supported in part by a Grant-in-Aid from The Science Frontier Program and a Grant-in-Aid for Scientific Research on Priority Areas, both from the Ministry of Education, Science, Sports and Culture, a Grant-in-Aid from the Ministry of Health and Welfare for Cancer Control, and a Grant-in-Aid for the IL-12 Project of Tokai University School of Medicine.
Abbreviations used in this paper
- α-GalCer
α-galactosylceramide
- DC
dendritic cell
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- NKT
natural killer T
- RT
reverse transcription
References
- 1.Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M. Homogenous junctional sequence of the Vα14+T-cell antigen receptor α chain expanded in unprimed mice. Proc Natl Acad Sci USA. 1990;87:5248–5252. doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, Pardoll DM. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly express a single Vβ gene family. Nature. 1987;329:251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 5.Kazuhiko M, Maeda K, Ueno H, Kobayashi E, Uchida T, Fukushima H, Koezuka Y. Antitumor activities of combined treatment with a novel immunomodulator, (2S,3S,4R)-1-O-(α-d-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol (KRN7000), and radiotherapy in tumor-bearing mice. Oncol Res. 1996;8:155–162. [PubMed] [Google Scholar]
- 6.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 7.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 8.Cui J, Tahiro S, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 9.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura T, Santa K, Yahata T, Sato N, Ohta A, Ohmi Y, Sato T, Hozumi K, Habu S. Involvement of IL-4-producing Vβ8.2+ CD4+ CD62L− CD45RB−T cells in non-MHC gene-controlled predisposition toward skewing into T helper type-2 immunity in BALB/c mice. J Immunol. 1997;158:5698–5706. [PubMed] [Google Scholar]
- 12.Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979;149:1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato N, Yahata T, Santa K, Ohta A, Ohmi Y, Habu S, Nishimura T. Functional characterization of NK1.1+ Ly-6C+cells. Immunol Lett. 1996;54:5–9. doi: 10.1016/s0165-2478(96)02632-6. [DOI] [PubMed] [Google Scholar]
- 14.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 15.Porcelli SA, Segelke BW, Sugita M, Wilson IA, Brenner MB. The CD1 family of lipid antigen-presenting molecules. Immunol Today. 1998;19:362–368. doi: 10.1016/s0167-5699(98)01289-4. [DOI] [PubMed] [Google Scholar]
- 16.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Paul WE. CD4+, NK1.1+T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimoto T, Bendelac A, Watoson C, Hu-Li J, Paul WE. Role of NK1.1+T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 19.Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980;151:1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba K, Granelli-Piperno A, Steinman RM. Dendritic cells induce T lymphocytes to release B cell–stimulating factors by an interleukin 2–dependent mechanism. J Exp Med. 1983;158:2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 22.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Nagase H, Ishida T, Inoue J, Nariuchi H. Induction of interleukin-12 p40 transcript by CD40 ligation via activation of nuclear factor-κB. Eur J Immunol. 1997;27:3461–3470. doi: 10.1002/eji.1830271247. [DOI] [PubMed] [Google Scholar]
- 24.Maruo S, Oh M -hora, H.J. Ahn, S. Ono, M. Wysocka, Y. Kaneko, H. Yagita, K. Okumura, H. Kikutani, T. Kishimoto, et al. B cells regulate CD40 ligand-induced IL-12 production in antigen-presenting cells (APC) during T cell/ APC interactions. J Immunol. 1997;158:120–126. [PubMed] [Google Scholar]
- 25.Kawamura T, Takeda K, Mendiratta SK, Kawamura H, Van Kaer L, Yagita H, Abo T, Okumura K. Critical role of NK1+T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160:16–19. [PubMed] [Google Scholar]
- 26.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;4:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 27.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;4:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 29.Hashimoto W, Takada K, Anzai R, Ogasawara K, Sakihara H, Sugiura K, Seki S, Kumagai K. Cytotoxic NK1.1 Ag+αβ T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–4340. [PubMed] [Google Scholar]
- 30.Yahata T, Ando K, Watanabe K, Mori T, Ohta A, Ohmi Y, Iwakabe K, Kuge S, Nakui M, Ito M, et al. Reconstitution of immune systems in RAG2−/−mice by transfer with interleukin-12-induced splenic hematopoietic progenitor cells. Immunol Lett. 1998;62:165–170. doi: 10.1016/s0165-2478(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 31.Ryffel B. Interleukin-12: role of interferon-γ in IL-12 adverse effects. Clin Immunol Immunopathol. 1997;83:18–20. doi: 10.1006/clin.1996.4306. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura T, Watanabe K, Yahata T, Ushaku L, Ando K, Kimura M, Saiki I, Uede T, Habu S. Application of interleukin 12 to antitumor cytokine and gene therapy. Cancer Chemother Pharmacol. 1996;38:27–34. doi: 10.1007/s002800051033. [DOI] [PubMed] [Google Scholar]
- 33.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. . Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 34.Leite-De-Moraes MC, Moreau G, Arnould A, Machavoine F, Garcia C, Papiernik M, Dy M. IL-4-producing NK T cells are biased towards IFN-γ production by IL-12. Influence of the microenvironment on the functional capacities of NK T cells. Eur J Immunol. 1998;28:1507–1515. doi: 10.1002/(SICI)1521-4141(199805)28:05<1507::AID-IMMU1507>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorad G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spada FM, Koezuka Y, Porcelli SA. CD1d- restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]