Abstract
As a result of interaction with epithelial cells in the thymic cortex, immature CD4+8+ (double positive, DP) thymocytes express relatively few T cell receptors (TCRs) and contain diminished numbers of coreceptor-associated p56lck (lck) PTK molecules. As a result, TCR signal transduction in DP thymocytes is significantly impaired, despite its importance for repertoire selection. We report here that, in DP thymocytes, tyrosine phosphorylation of TCR signaling motifs (ITAMs) by lck, an early event in TCR signal transduction, is dependent upon ZAP-70 protein independent of ZAP-70's kinase activity. Furthermore, the dependence on ZAP-70 protein for ITAM phosphorylation diminishes as available lck increases. Importantly, ZAP-70's role in ITAM phosphorylation in DP thymocytes is not limited to protecting phosphorylated ITAMs from dephosphorylation. Rather, this study indicates that ZAP-70 protein augments ITAM phosphorylation in DP thymocytes and so compensates in part for the relative deficiency of coreceptor-associated lck.
Keywords: T cell receptor, development, signaling, thymus, phosphorylation
Tcell development progresses in the thymus via an ordered series of differentiation events that are best characterized by changes in expression of CD4 and CD8 coreceptors. Early thymocytes are CD4−8− (double negative, DN) and differentiate into CD4+8+ (double positive, DP) thymocytes, which in turn differentiate into CD4+8− and CD4−8+ (single positive, SP) T cells or, alternatively, are induced to undergo cell death. Each developmental transition is induced by signals transduced by surface TCR components. Despite the importance of TCR signals for development, TCR expression on immature DP thymocytes is low, with only ∼10% the number of surface TCR as on mature T cells; and coreceptor-associated p56lck (lck) is diminished in DP thymocytes mostly, but not entirely, because their surface CD4 molecules are chronically engaged by the high concentrations of MHC class II (MHC II) present on thymic cortical epithelium (1–3). Consequently, it remains unclear how TCR signals are transduced in immature DP thymocytes.
It is known that TCR signal transduction requires activation of ZAP-70 protein tyrosine kinase (PTK) for all downstream signaling events (4). Indeed, enzymatic activation of ZAP-70 PTK is necessary for immature murine DP thymocytes to differentiate into mature SP T cells (5, 6). However, the molecular interactions linking TCR engagements on the cell surface with ZAP-70 activation inside the cell are complex, involving multiple protein–protein interactions (7). TCR signal transduction is initiated primarily by the src family PTK lck, which tyrosine phosphorylates conserved signaling motifs referred to as ITAMs (immunoreceptor tyrosine-based activation motifs) that are present in the cytosolic tails of TCR-associated ζ and CD3γ/δ/ε chains. Tyrosine phosphorylated ITAMs are bound by ZAP-70 PTK molecules whose enzymatic activity is subsequently elicited by lck-induced phosphorylation of ZAP-70's catalytic domain (7). Thus, tyrosine phosphorylation of ZAP-70's catalytic domain is the target of proximal TCR signaling events.
Because of the diminished numbers of surface TCR complexes and coreceptor-associated lck molecules in immature DP thymocytes compared with mature T cells, TCR signal transduction may not proceed in DP thymocytes precisely as it does in mature T cells. Indeed, it is conceivable that TCR signal transduction in DP thymocytes may require signal amplification mechanisms that are not critical for TCR signal transduction in mature T cells. In this report, we demonstrate that ITAM phosphorylation by lck, an early event in TCR signal transduction, is significantly enhanced in DP thymocytes by ZAP-70 protein and so represents a molecular amplification mechanism that compensates in part for the relative deficiency of coreceptor-associated lck in DP thymocytes.
Materials and Methods
Mice.
Normal C57BL/6 mice were obtained from The Jackson Laboratory and were designated ZAP+/+. ZAP-70 knockout mice were provided by Dennis Loh (Roche Research Institute, Nutley, NJ) and Izumi Negishi (Nippon Roche Research Center, Kanagawa, Japan; reference 5), were bred in our own animal colony, and were designated ZAP−/−. F1 offspring between B6 and ZAP-70 knockout mice were designated ZAP+/−. ST mice are homozygous for a spontaneously arising point mutation in ZAP-70's kinase domain, which renders them kinase dead (6). Double knockout mice lacking both ZAP-70 and MHC II expression were generated by crossing ZAP-70 knockout and MHC II knockout mice together, and screening the F2 generation for animals designated as ZAP−/−II−/−. The care of experimental animals was in accordance with National Institutes of Health guidelines.
DP Thymocytes.
Upon their removal from the thymus, thymocytes were kept strictly at 4°C in all experiments unless otherwise indicated, in order to avoid the biochemical alterations that occur in DP thymocytes upon removal from their intrathymic signaling environment (3, 8). DP thymocyte populations (>96% pure) were obtained by panning whole thymocytes on anti-CD8 plates and collecting the adherent fraction.
Antibodies.
Antibodies used for immunoprecipitation and/or immunoblotting in this study were specific for ζ (serum No. 551), ZAP-70, CD4 (RM4.5, PharMingen), phosphotyrosine (4G10, Upstate Biotechnology, Inc.), or lck (serum No. 688). Biotinylated antibodies used for TCR and coreceptor cross-linking were specific for TCR-β (H57-597) or CD4 (GK1.5).
Signal Generation.
DP thymocytes were coated with biotinylated anti-TCR and/or biotinylated anti-CD4 mAbs for 10 min at 4°C, after which the cells were warmed to 37°C and exposed to streptavidin for the indicated time (usually 5 min). Where indicated, cells were also treated with pervanadate (0.3 mM H2O2 and 0.1 mM Na3VO4) for 5 min at 37°C (2, 3).
Immunoprecipitation and Immunoblotting.
DP thymocytes were lysed in 1% triton and the lysates immunoprecipitated with the indicated antibodies and resolved by SDS-page under reducing conditions (5 × 107 cells per sample). The gels were transferred onto immobilon PVDF membranes (Millipore), blotted with the indicated antibodies, and visualized by chemiluminescence.
Immune Complex Kinase Assay.
DP thymocytes were lysed at 108 cells/ml in lysis buffer containing 1 mM vanadate (a potent inhibitor of protein tyrosine phosphatases) and 1% Triton X-100; and the lysates immunoprecipitated with the indicated antibodies. Immune complexes were incubated at ambient temperature for 3 min in kinase buffer containing 15 μCi/sample of γ-[32P]ATP, after which the immune complexes were resolved by SDS-PAGE and visualized by autoradiography. Radiolabeled proteins in the immune complex kinase assay reflect transfer of 32P by an activated PTK molecule present in the immunoprecipitate (3).
Results
Greater than 50% of surface TCR complexes on immature DP thymocytes in the thymic cortex contain ‘constitutively' tyrosine phosphorylated ζ ITAMs (3, 9), in contrast to <5% of TCR on mature T cells in the periphery (3, 10). Constitutive ITAM phosphorylation in DP thymocytes results from lck signals generated by interactions between DP thymocytes and thymic cortical epithelium that are mediated primarily, but not exclusively, by CD4–MHC II interactions. Aggregation of surface CD4 molecules on DP thymocytes by engagement of MHC II on cortical thymic epithelium activates CD4-associated lck to phosphorylate ζ ITAMs, after which the activated lck molecules are degraded (2, 3, 8–11). The tyrosine phosphorylated ITAMs then recruit ZAP-70 molecules that remain enzymatically inactive (3, 10), perhaps because the remaining pool of activated lck available to the TCR in DP thymocytes is insufficient to induce ZAP-70 activation. Importantly, the lck that is available to the TCR complex in DP thymocytes appears to be primarily the lck that is associated with coreceptor molecules and for which coreceptor molecules compete for binding (2, 3). However, additional factors may also influence the availability of lck to the TCR. For example, transfection experiments in nonlymphoid cells have found that ITAM phosphorylation by lck is increased by ZAP-70, an effect ascribed to ZAP-70's protection of phospho-ITAMs from dephosphorylation (12).
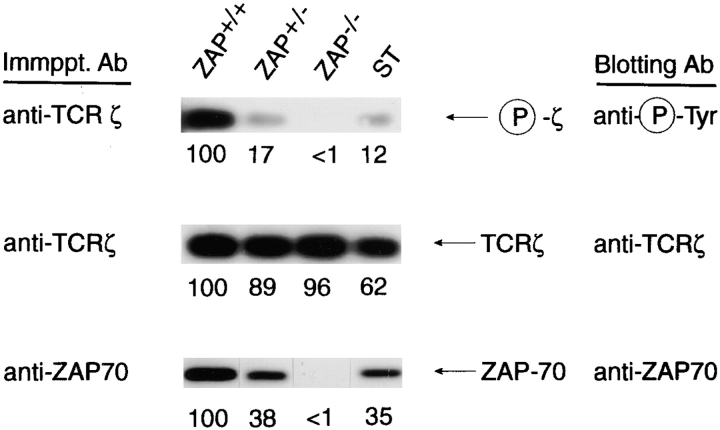
To assess a possible role for ZAP-70 in ITAM phosphorylation in DP thymocytes, we examined purified DP thymocyte populations from mice expressing different quantities of ZAP-70. Remarkably, we found that the extent of ζ phosphorylation was proportional to the amount of ZAP-70 protein expressed (Fig. 1, columns 1–3). Importantly, all three DP thymocyte populations (ZAP+/+, ZAP+/−, and ZAP−/−) contained comparable levels of ζ protein (Fig. 1, center row). To determine if the relationship between ZAP-70 expression and ζ phosphorylation required ZAP-70 kinase activity, we examined ζ phosphorylation in DP thymocytes from mutant ST mice that express only kinase-dead ZAP-70 proteins. Mutant ZAP-70 proteins are less stable than their wild-type counterparts so that DP thymocytes from mutant ST mice contained reduced steady state levels of ZAP-70 protein, comparable with the levels of ZAP-70 protein expressed in ZAP+/− DP (Fig. 1, bottom row). Interestingly, tyrosine phosphorylation of ζ ITAMs from mutant ST and ZAP+/− mice were also essentially equivalent (Fig. 1, compare columns 2 and 4), especially considering that the ST lane contained somewhat less ζ protein. Thus, ST thymocytes contained phosphorylated ζ ITAMs in amounts that were roughly concordant with their reduced level of ZAP-70 protein, indicating that ζ ITAM phosphorylation is quantitatively dependent upon ZAP-70 protein but is independent of ZAP-70's enzymatic activity.
Figure 1.
Constitutive tyrosine phosphorylation of ζ ITAMs in DP thymocytes is dependent upon ZAP-70 protein, but not ZAP-70 kinase activity. Freshly explanted DP thymocytes were obtained from mice expressing different amounts of ZAP-70 protein (5). ST thymocytes express only a kinase-dead ZAP-70 molecule (6). Detergent (1% triton)-solubilized cell lysates were immunoprecipitated, resolved by SDS-PAGE, and immunoblotted as indicated in the figure. The extent of ζ phosphorylation in DP thymocytes correlated with the amount of ZAP-70 protein present, but not with its enzymatic capability.
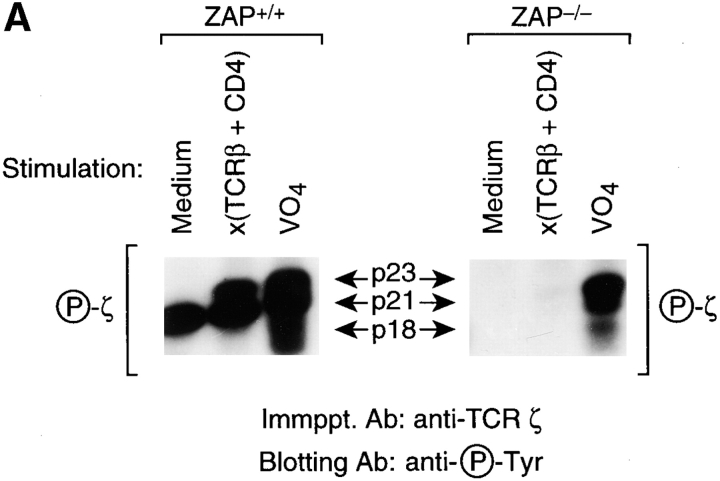
We next wished to determine if the requirement for ZAP-70 in coreceptor-induced ITAM phosphorylation could be overcome by coengagement of CD4 with the TCR. Although TCR plus CD4 co-engagement did induce ζ ITAM phosphorylation in DP thymocytes from ZAP+/+ mice, it failed to do so in DP thymocytes from ZAP−/− mice (Fig. 2 A, right). The intrinsic ability of ζ ITAMs to be tyrosine phosphorylated was not obviously altered in ZAP−/− thymocytes, as indicated by treatment of DP thymocytes with pervanadate, which inhibits intracellular tyrosine phosphatases and so indiscriminately activates intracellular PTK (13).
Figure 2.
TCR plus CD4–signaled tyrosine phosphorylation of TCR ITAMs in DP thymocytes is dependent upon ZAP-70 both in vivo and in vitro. Freshly explanted DP thymocytes were signaled for 5 min at 37°C with medium, anti–TCR-β plus anti-CD4, or pervanadate (VO4) (13), and the cell lysates immunoprecipitated with anti-ζ. Note that with the exception of the 5 min of signaling, DP thymocytes were strictly maintained at 4°C to prevent the molecular changes that occur upon removal of DP thymocytes from their intrathymic signaling environment (2, 3, 8, 9). Detergent (1% triton) solubilized cell lysates were immunoprecipitated with anti-ζ plus protein A beads which also precipitated the anti–TCR-β and anti-CD4 mAbs used for signaling. In A, immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine to detect phospho-ζ. In B, immunoprecipitates were subjected to an immune complex kinase assay in which in vitro phosphorylation of target TCR ITAMs is indicated by phosphorylation of TCR-ζ and CD3ε chains. Enolase is an exogenously added lck substrate that is tyrosine phosphorylated by lck's enzymatic activity. Note that the immune complex kinase assay was performed in the presence of vanadate, a potent inhibitor of protein tyrosine phosphatases, to prevent tyrosine dephosphorylation. RF, running front.
To further examine TCR ITAM phosphorylation in TCR plus CD4–signaled DP thymocytes, we assessed DP thymocyte lysates in an immune complex kinase assay for their ability to tyrosine phosphorylate TCR ITAMs in vitro (Fig. 2 B). Concordant with our observation in intact DP thymocytes, in vitro phosphorylation of TCR ITAMs (ε and ζ) was markedly reduced >10-fold in ZAP−/− relative to ZAP+/+ anti-ζ immunoprecipitates from TCR plus CD4–signaled DP thymocytes (Fig. 2 B). Because dephosphorylation of TCR ITAMs is already inhibited in in vitro kinase assays by exogenously added vanadate, a potent tyrosine phosphatase inhibitor, the requirement for ZAP-70 protein in ITAM phosphorylation indicates that ZAP-70's role is not limited to protecting phosphorylated ITAMs from dephosphorylation. Rather, this experiment reveals that ZAP-70 promotes tyrosine phosphorylation of TCR ITAMs in DP thymocytes.
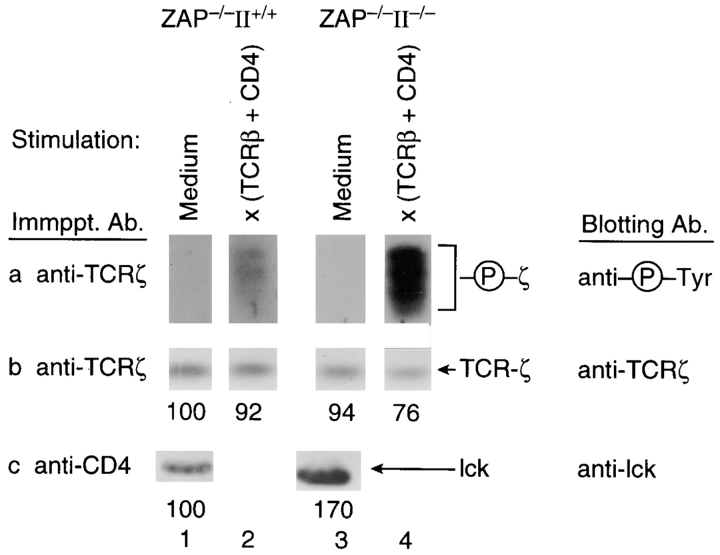
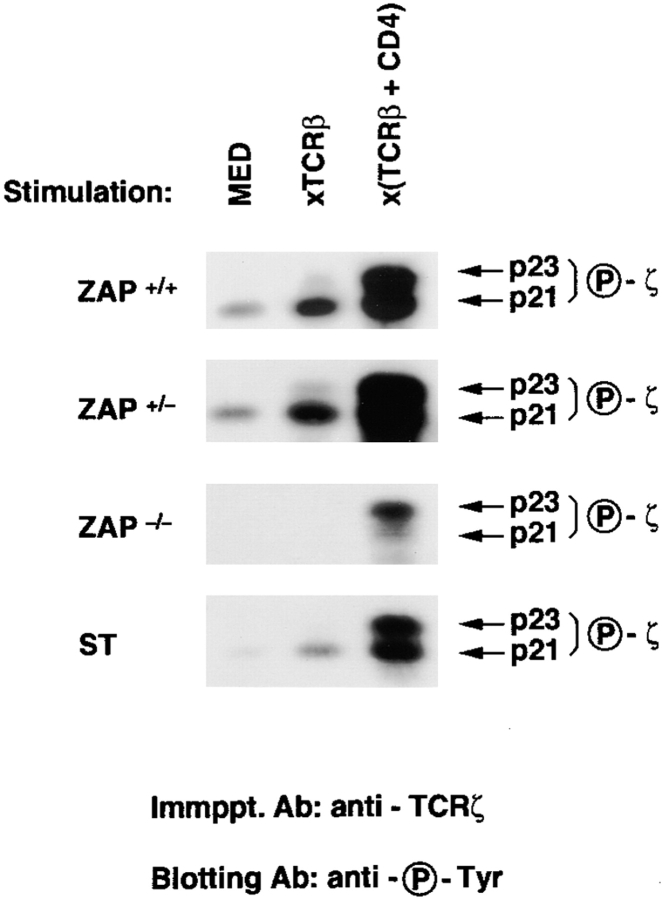
Because ITAM phosphorylation is mediated primarily by lck, we next considered that in vivo ITAM phosphorylation might be less dependent upon ZAP-70 in DP thymocytes containing increased amounts of TCR-accessible lck. Consequently, we examined signaling in DP thymocytes that lacked ZAP-70 but contained increased CD4-associated lck, because their thymi did not express MHC II (Fig. 3). In the absence of MHC II engagement in the thymus, CD4 molecules on DP thymocytes are not aggregated and, consequently, are not depleted of associated lck molecules (2). Indeed, ZAP−/− DP thymocytes from II−/− mice contained more CD4-associated lck than did ZAP−/− DP thymocytes from II+/+ mice (Fig. 3). More importantly, cocross-linking of TCR plus CD4 surface molecules on DP thymocytes from ZAP−/−II−/− mice induced significant ITAM phosphorylation despite the absence of ZAP-70 protein (Fig. 3 a), indicating that the requirement for ZAP-70 diminishes with increasing amounts of coreceptor-associated lck. The pool of CD4-associated lck in DP thymocytes can also be increased by removing thymocytes from their thymic microenvironment and culturing them as single cell suspensions in the absence of MHC II+ cells. In such cultures, intrathymic engagements are disrupted, permitting newly synthesized CD4 and lck molecules to repopulate the depleted pool of coreceptor-associated lck (2). It is clear that TCR plus CD4 cocross-linking increased ITAM phosphorylation in these cultured DP thymocytes whether or not ZAP-70 protein was present (Fig. 4, compare columns 1 and 3). Thus, ITAM phosphorylation is less dependent upon ZAP-70 in DP thymocytes containing increased amounts of coreceptor-associated lck.
Figure 3.
TCR-induced ζ ITAM phosphorylation in ZAP-70−/− DP thymocytes containing different amounts of coreceptor-associated lck. Freshly explanted ZAP−/− DP thymocytes were obtained from mice differing in MHC II expression and were stimulated in vitro for 5 min. Detergent (1% triton) solubilized cell lysates were immunoprecipitated with either anti-ζ or anti-CD4, resolved by SDS-PAGE, and immunoblotted with the indicated antibodies. DP thymocytes from mice lacking MHC II expression contained increased amounts of CD4-associated lck (row c) and increased ζ phosphorylation upon TCR plus CD4 cocross-linking (row a).
Figure 4.
TCR-induced ζ phosphorylation in cultured DP thymocytes. DP thymocytes that had been cultured overnight in single cell suspension at 37°C were signaled for 5 min. DP thymocytes that have been removed from their intrathymic signaling environment and cultured overnight contain significantly increased amounts of coreceptor-associated lck (2, 3) and exhibit decreased dependence on ZAP-70 for ζ phosphorylation.
A more stringent assessment of the role of ZAP-70 in regulating ITAM phosphorylation is the one signaled by engagement of the TCR alone, as opposed to ITAM phosphorylation signaled by the forcible coengagement of TCR with CD4 (Fig. 4, compare columns 2 and 3). Signaling by isolated TCR cross-linking requires that lck be captured within TCR aggregates, which is an inefficient method of bringing lck into contact with TCR ITAMs compared with direct TCR plus CD4 cocross-linking. Nevertheless, isolated TCR cross-linking did signal ITAM phosphorylation in cultured DP thymocytes that contained ZAP-70 protein (even enzymatically inactive ZAP-70 protein in mutant ST thymocytes), but failed to signal ζ ITAM phosphorylation in cultured DP thymocytes that lacked ZAP-70 protein (Fig. 4, compare columns 1 and 2). The inability of isolated TCR cross-linking to induce ITAM phosphorylation in cultured DP thymocytes lacking ZAP-70 protein indicates that ZAP-70 is specifically required for ITAM phosphorylation when the amount of lck available to TCR aggregates is limiting.
Discussion
Current models of TCR signaling are derived primarily from experiments in mature T cells and cell lines, and do not include a role for ZAP-70 in early TCR signaling events such as ITAM phosphorylation (7). In contrast, our study found that in immature DP thymocytes ZAP-70 protein is required for ITAM phosphorylation, and that the requirement for ZAP-70 protein decreases as the amount of lck available to the TCR quantitatively increases. ZAP-70's role in ITAM phosphorylation in DP thymocytes was not limited to protecting phosphorylated ITAMs from dephosphorylation, as ZAP-70 was required for in vitro ITAM phosphorylation by DP thymocyte lysates even though tyrosine dephosphorylation was already inhibited by the pharmacologic agent vanadate. Rather, our study indicates that ZAP-70 in DP thymocytes promotes lck's phosphorylation of TCR ITAMs, a function that is especially significant in immature DP thymocytes with limiting lck.
Our understanding of ZAP-70's role in promoting ITAM phosphorylation by lck in DP thymocytes builds on the key finding that lck can bind to ZAP-70 via an SH2 domain interaction (14, 15). We suggest that ITAM phosphorylation in DP thymocytes occurs in two phases. In the first phase of ITAM phosphorylation, a chance encounter between an activated lck molecule and a TCR ITAM induces initial ITAM phosphorylation without any involvement of ZAP-70. The number of TCR ITAMs initially phosphorylated by such chance encounters depends upon the number of activated lck molecules available to the TCR. In immature DP thymocytes, the amount of lck available to the TCR is limiting, so that only a few TCR ITAMs are phosphorylated in this initial ZAP-70–independent phase, perhaps only a single ITAM in a TCR aggregate. In the second phase of ITAM phosphorylation, the initially phosphorylated ITAM is bound by a ZAP-70 molecule whose backbone tyrosine residue is phosphorylated and then bound by activated lck. The activated ZAP-70–bound lck molecule is now within the TCR complex and so can efficiently phosphorylate the remaining ITAMs in the TCR aggregate. (In fact, being bound to ZAP-70 via its SH2 domain might prolong lck's enzymatic activity because lck's SH2 domain would not be available to bind lck's regulatory phosphotyrosine, interfering with downregulation of lck's kinase activity.) The newly phosphorylated ITAMs are subsequently bound by additional ZAP-70 molecules and it is these additional ZAP-70 molecules that make up the majority of ZAP-70 molecules constitutively associated with TCR in DP thymocytes. These ZAP-70 molecules are only recruited into the TCR complex after the second phase of ITAM phosphorylation and they are not themselves tyrosine phosphorylated in DP thymocytes, presumably because the activated lck has already disappeared from the TCR complex. However, these TCR-bound ZAP-70 molecules can be efficiently activated by subsequent TCR plus coreceptor engagements of intrathymic ligands that bring newly activated lck molecules into the TCR complex. Thus, ZAP-70's promotion of ITAM phosphorylation represents a signal amplification mechanism in DP thymocytes.
In conclusion, our study demonstrates that ZAP-70 promotes ITAM phosphorylation in DP thymocytes in response to TCR and/or coreceptor engagement, and that DP thymocytes are particularly dependent upon ZAP-70 for ITAM phosphorylation because their pool of available lck is limiting.
Acknowledgments
We gratefully acknowledge Dennis Loh and Izumi Negishi for ZAP-70−/− mice; and Larry Samelson and Ron Wange (both from NIH, Bethesda, MD) for anti–ZAP-70 and anti-lck antibodies.
References
- 1.Van Ewijk W, Rouse RV, Weissman IL. Distribution of H-2 microenvironments in the mouse thymus. Immunoelectron microscopic identification of I-A and H-2K bearing cells. J Histochem Cytochem. 1980;28:1089–1099. doi: 10.1177/28.10.6999083. [DOI] [PubMed] [Google Scholar]
- 2.Wiest DL, Yuan L, Jefferson J, Benveniste P, Tsokos M, Klausner RD, Glimcher LH, Samelson LE, Singer A. Regulation of T cell receptor expression in immature CD4+CD8+ thymocytes by p56lcktyrosine kinase: basis for differential signaling by CD4 and CD8 in immature thymocytes expressing both coreceptor molecules. J Exp Med. 1993;178:1701–1712. doi: 10.1084/jem.178.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiest DL, Ashe JM, Abe R, Bolen JB, Singer A. TCR activation of ZAP70 is impaired in CD4+CD8+ thymocytes as a consequence of intrathymic interactions that diminish available p56lck . Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
- 4.Qian D, Mollenauer MN, Weiss A. Dominant-negative ζ-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan AC, Loh DY. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 6.Wiest DL, Ashe JM, Howcroft TK, Lee HM, Kemper DM, Negishi I, Singer DS, Singer A, Abe R. A spontaneously arising mutation in the DLAARN motif of murine ZAP-70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997;6:663–671. doi: 10.1016/s1074-7613(00)80442-2. [DOI] [PubMed] [Google Scholar]
- 7.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama T, June CH, Munitz TI, Sheard M, McCarthy SA, Sharrow SO, Samelson LE, Singer A. Inhibition of T cell receptor expression and function in immature CD4+CD8+cells by CD4. Science. 1990;249:1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama T, Singer A, Hsi ED, Samelson LE. Intrathymic signalling in immature CD4+CD8+thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- 10.van Oers NS, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 11.van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 13.Secrist JP, Burns LA, Karnitz L, Koretzky GA, Abraham RT. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- 14.Duplay P, Thome M, Herve F, Acuto O. p56lckinteracts via its src homology 2 domain with the ZAP-70 kinase. J Exp Med. 1994;179:1163–1172. doi: 10.1084/jem.179.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straus DB, Chan AC, Patai B, Weiss A. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J Biol Chem. 1996;271:9976–9981. doi: 10.1074/jbc.271.17.9976. [DOI] [PubMed] [Google Scholar]