Abstract
The identification of molecules that regulate human hematopoietic stem cells has focused mainly on cytokines, of which very few are known to act directly on stem cells. Recent studies in lower organisms and the mouse have suggested that bone morphogenetic proteins (BMPs) may play a critical role in the specification of hematopoietic tissue from the mesodermal germ layer. Here we report that BMPs regulate the proliferation and differentiation of highly purified primitive human hematopoietic cells from adult and neonatal sources. Populations of rare CD34+CD38−Lin− stem cells were isolated from human hematopoietic tissue and were found to express the BMP type I receptors activin-like kinase (ALK)-3 and ALK-6, and their downstream transducers SMAD-1, -4, and -5. Treatment of isolated stem cell populations with soluble BMP-2, -4, and -7 induced dose-dependent changes in proliferation, clonogenicity, cell surface phenotype, and multilineage repopulation capacity after transplantation in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Similar to transforming growth factor β, treatment of purified cells with BMP-2 or -7 at high concentrations inhibited proliferation yet maintained the primitive CD34+CD38− phenotype and repopulation capacity. In contrast, low concentrations of BMP-4 induced proliferation and differentiation of CD34+ CD38−Lin− cells, whereas at higher concentrations BMP-4 extended the length of time that repopulation capacity could be maintained in ex vivo culture, indicating a direct effect on stem cell survival. The discovery that BMPs are capable of regulating repopulating cells provides a new pathway for controlling human stem cell development and a powerful model system for studying the biological mechanism of BMP action using primary human cells.
Keywords: hematopoiesis, stem cells, bone morphogenetic proteins, ex vivo, xenotransplantation
Knowledge of specific factors that regulate adult murine and human blood stem cells is poor and has been mainly limited to cytokines (1–3). Recent work using developmental systems has shown that mesodermal precursors can be induced to differentiate into hematopoietic cells, thereby giving rise to the earliest blood stem cells (4–9). For example, cells purified from murine tissue that differentiate from the ventral mesoderm, such as the aorta- gonad-mesonephros and fetal liver, have been isolated and shown to contain stem cells capable of multilineage hematopoietic repopulation (10, 11). These circulating stem cells seed the embryonic rudiments of blood-forming tissue to establish hematopoiesis. Analysis using developmental models of lower organisms have identified soluble and/or paracrine growth factors (GFs)1 that induce hematopoietic tissue from ventral mesoderm (12, 13). The majority of these factors are members of the TGF-β superfamily of secreted polypeptide GFs (14, 15).
TGF-β itself is a potent inhibitor of cell cycle progression of murine blood stem cells and primitive human long-term culture initiating cells (LTC-ICs) detected using in vitro assays (16, 17). Treatment of quiescent LTC-ICs with neutralizing antibody against TGF-β can induce cell cycle entry (18). Moreover, TGF-β is also secreted by immature human hematopoietic cells, suggesting that a TGF-β autoregulatory loop is an important component of regulation in these primitive cells (19, 20). However, TGF-β is the prototype of a large family of cytokines that includes the TGF-βs, activins, inhibins, and bone morphogenetic proteins (BMPs) (21). This family exerts a wide range of biological responses such as cell growth, apoptosis, and differentiation in a variety of cell types, including potent responses in patterning during embryonic development (22). One member of the BMP subfamily, BMP-4, has been shown to be a potent ventralizing factor and can induce hematopoietic tissue in Xenopus and differentiation of mouse embryonic stem cells into hematopoietic lineages (23). BMP-4 and the transcription factor GATA-2 can function in two adjacent germ layers, mesoderm and ectoderm, respectively, to participate in blood cell formation during embryogenesis (9, 13). This observation suggests that this subfamily of BMPs may play a role in the development of primitive hematopoiesis. In humans, BMPs are expressed in adult human bone marrow (BM) and are essential in bone remodeling and growth (24). However, the ability of BMPs to continue to play a role in regulating blood stem cells once the tissue has committed to the hematopoietic lineage, or to play any direct role in adult and neonatal human stem cells, is unknown.
The TGF-β family of molecules signal through two serine kinase receptors and a family of intracellular signal transducers termed SMADs (25, 26). Soluble TGF-β and related molecules induce formation of heterodimeric complexes of type II and type I transmembrane kinase receptors (27). Within this complex, the type II kinase transphosphorylates the type I receptor, which transmits downstream signals to receptor-related SMADs, thereby specifying the nature of the biological response to ligand. Phosphorylation of receptor-regulated SMADs induces association in the cytoplasm with a common mediator of SMADs, called SMAD-4 (28, 29). This heteromeric complex then moves into the nucleus to regulate gene expression (26). Introduction of homologous SMAD proteins from human or mouse into frog embryos mimics the effects of TGF-β activation in the induction of mesodermal specification, illustrating the phylogenetic conservation of these molecules (25, 30). Moreover, recent evidence in humans indicates that disregulation of the SMAD molecules can affect normal growth, leading to neoplastic hematopoiesis (31).
Most of the studies aimed at understanding the potential biological role of BMPs in hematopoietic tissue have relied almost exclusively on model systems involving lower organisms and in vitro systems (32, 33). Therefore, there is a great need for a more biologically relevant model system to determine the role and function of BMPs in human hematopoietic development. In this study, to ascertain whether BMPs are capable of regulating primitive blood cells, we have used a highly purified fraction of human hematopoietic tissue enriched for human repopulating stem cells (34, 35). These human repopulating cells can be assayed by transplantation into nonobese diabetic (NOD)/SCID mice (36, 37). This repopulating cell, termed SCID-repopulating cell (SRC), is capable of extensive proliferation and multilineage engraftment (34, 36). Cell purification and retroviral gene-marking studies demonstrated that the SRCs are the most primitive cell type detected in the human stem cell hierarchy and are biologically distinct from most cells detected using in vitro assays, including colony-forming cells (CFCs) and LTC-ICs (34, 35, 38). Here, we report that BMP receptors activin-like kinase (ALK)-3 and -6 together with the signal transducers SMAD-1, -4, and -5 are expressed by highly purified CD34+CD38−Lin− cells isolated from various human hematopoietic tissues. The addition of human BMP-2, -4, and -7 into previously designed serum-free ex vivo cultures (38) resulted in alterations in the proliferation, differentiation, and number of clonogenic progenitors within the CD34+CD38−Lin− population. Among these ligands, BMP-4 had a regulatory function distinct from BMP-2 and -7, and was capable of acting on rare repopulating SRCs. These data demonstrate that BMPs modulate the developmental program of human stem cells and provide a novel model system to further understand the mechanism of BMP action within primary human cells.
Materials and Methods
Human Cells.
Samples of human cord blood (CB) were obtained from placental and umbilical tissues and diluted (1:3) in IMDM (GIBCO BRL). The mononuclear cells were collected by centrifugation on Ficoll-paque (Amersham Pharmacia Biotech).
Cell Purification.
CD34+CD38−Lin− cells were collected using our standard protocol (22, 23). CB cells were first enriched for CD34+ cells by negative selection using a cocktail of lineage (Lin) antibodies and the StemSep device as described by the manufacturer (Stem Cell Technologies, Inc.). These cell fractions were then stained with anti–human CD34-FITC and anti–human CD38–PE (Becton Dickinson Immunocytometry Systems), analyzed, and sorted on a FACStarPlus™ (Becton Dickinson). The sorting gates used were similar to those shown previously (34, 38). Data acquisition and analysis were performed using CELLQuest™ software (Becton Dickinson).
Reverse Transcription PCR Analysis.
Purified cells were collected after sorting in 500-μl tubes, and mRNA was extracted from 1,000 cells for each PCR reaction using a purification kit (Amersham Pharmacia Biotech). The mRNA was reverse transcribed into cDNA by standard methods using Superscript II (GIBCO BRL) as the reverse transcriptional enzyme. PCR was performed for the detection of transcripts using a Perkin-Elmer 9700 cycler with the indicated specific primers for 40 cycles. Primer sequences used for transcript detection for SMADs and ALK receptor were as follows: SMAD-1F, 5′-CGAATGCCTTAGTGACAG-3′, and SMAD-1R, 5′-GAGGTGAACCCATTTGAG-3′; SMAD-4F, 5′-AGGTGAAGGTGATGTTTG-3′, and SMAD-4R, 5′-GCTATTCCACCTACTGAT-3′; SMAD-5F, 5′-TGTTGGTGGAGAGGTGTA-3′, and SMAD-5R, 5′-AGATATGGGGTTCAGAGG-3′; ALK-3F, 5′-ACCATCGGAGGAGAAACT-3′, and ALK-3R, 5′-CTGCTGCGCTCATTTATC-3′; ALK-6F, 5′-AAGTTACGCCCCTCATTC-3′, and ALK-6R, 5′-TGATGTCTTTTGCTCTGC-3′.
Clonogenic Progenitor Assays.
Human clonogenic progenitors were assayed under standard conditions as shown previously, which included the addition of 10% 5637 conditioned medium as a source of cytokines (38). In brief, 100–500 purified cells were plated in methylcellulose cultures aliquoted in 1-ml vol in 35-mm suspension culture dishes and incubated at 37°C. After 10–14 d, clonogenic progenitors were scored according to standard criteria (38).
Liquid Suspension Cultures.
CD34+CD38−Lin− cells were incubated in serum-free conditioned medium shown previously to maintain primitive human populations (38). In brief, conditioned medium is comprised of 50 μl of IMDM supplemented with 1% BSA (Stem Cell Technologies, Inc.), 5 μg/ml of human insulin (Humulin R; Eli Lilly and Co.), 100 μg/ml of human transferrin (GIBCO BRL), 10−4 M β-mercaptoethanol, and GFs. GF cocktail was used at final concentrations of 300 ng/ml of stem cell factor (SCF; Amgen) and Flt-3 (Immunex), 50 ng/ml of G-CSF (Amgen), and 10 ng/ml of IL-3 (Amgen) and IL-6 (Amgen). Cells were cultured in flat-bottomed suspension wells of 96-well plates (Nunc), incubated for the appropriate times as indicated, at 37°C and 5% CO2, and 50 μl of fresh GF cocktail was added to each well every other day. Mesodermal factors were added to obtain final concentrations as indicated. Individual factors were obtained from the following sources: TGF-β1 and TGF-β1–3 neutralizing antibody (R&D Systems), BMP-2 (gift from Dr. Vicki Rosen, Genetics Institute, Cambridge, MA), BMP-4 (gift from Dr. Steve Neben, Genetics Institute), and BMP-7 (gift from Dr. Kuber Sampath, Creative Biomolecules, Inc., Boston, MA).
Transplantation of Purified Cells into NOD/SCID Mice.
Cells were transplanted by tail vein injection into sublethally irradiated NOD/LtSz-scid/scid (NOD/SCID) mice (375-cGy 137Cs) according to our standard protocol (36, 39). In all cases, cells were cotransplanted with irradiated nonrepopulating CD34−Lin+ cells as accessory cells (34, 38). Mice were killed 8 wk after transplantation, and BM cells were collected from femurs, tibiae, and iliac crests.
Analysis of Human Cell Engraftment.
High molecular weight DNA was isolated from the BM of transplanted mice, and the percentage of human cells was determined by probing with a human chromosome 17–specific α-satellite probe as described previously (36, 39). The level of human cell engraftment was quantified by visual inspection of film developed from Southern blot by comparing the characteristic 2.7-kb band with human/mouse DNA mixture controls (limit of detection, 0.05% human DNA) that provided a linear signal response. In some cases, BM of transplanted mice was analyzed by staining with human panleukocyte marker CD45 to detect the presence of human hematopoietic cells using the FACScan® as described previously (34, 36, 38, 39). Two- or three-color flow cytometric analysis was performed as shown previously to ensure that human engrafting cells contained multiple lineages (data not shown).
Statistical Analysis.
The data were analyzed by the unpaired, two-tailed Student's t test assuming a Gaussian distribution (parametric test) using Prism® software, version 2.0 (GraphPad).
Results
Expression of SMAD Signaling Molecules and BMP Receptors in Primitive Human Hematopoietic Populations.
TGF- β receptors have previously been shown to be expressed on highly purified primitive hematopoietic populations in which soluble TGF-β is capable of regulating proliferation and progenitor cell content (19, 20). Our previous studies demonstrated that highly purified SRCs derived from both human CB and BM were found in the fraction of CD34+ CD38−Lin− cells (34, 35, 38). The type I BMP receptors ALK-3 and ALK-6 are capable of binding BMPs in the absence of type II receptors, and it has been suggested that these receptors may be capable of ligand selection and may be specific for BMPs (15, 40). Reverse transcription (RT)-PCR analysis demonstrated that both ALK-3 and -6 are expressed in primitive CD34+CD38−Lin− cells isolated from human CB and BM tissue (Fig. 1 A). Detection of ALK-3 and -6 expression in BM samples was more difficult compared with CB-derived primitive populations at similar RT-PCR conditions, suggesting lower expression in BM versus CB. SMAD-1 and -5 are restricted for BMP signaling, whereas SMAD-4 is a shared mediator of TGF-β signaling, and acts as a common partner with pathway-specific SMADs (25, 26). Purified CD34+CD38−Lin− cells isolated from human CB, BM, mobilized peripheral blood (M-PB), and human BM-derived stroma express SMAD-1 and -5 transcripts (Fig. 1 B). SMAD-4 expression was found in all sources of primitive cells, but was more easily detected in BM (n = 2) than in CB samples (n = 4) and was barely detectable in M-PB (n = 2). In summary, transducers of the BMP signaling pathway are expressed in primitive subfractions of both embryonic and adult human hematopoietic tissue, suggesting that candidate human stem cell populations have the capability of responding to BMPs.
Figure 1.
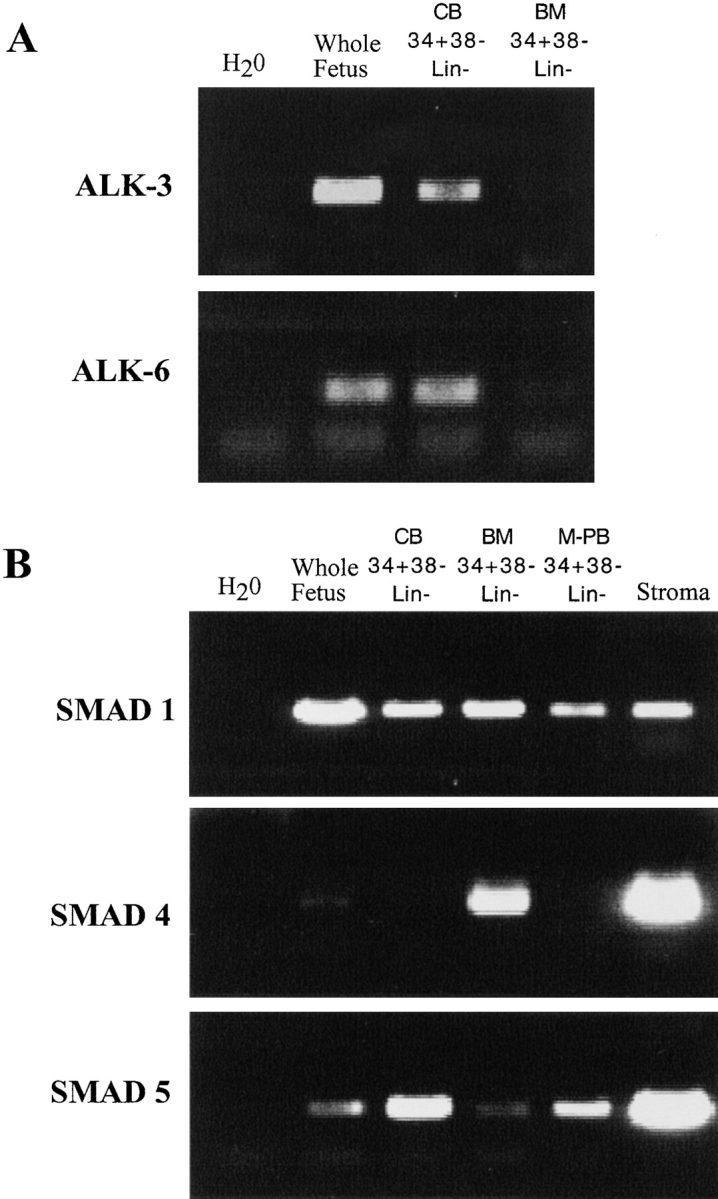
Expression of BMP receptors and SMADs in primitive hematopoietic tissue derived from human sources. RT-PCR reactions were performed on human CD34+CD38−Lin− cells from CB (n = 4), BM (n = 2), M-PB (n = 2), and stromal cells (n = 2) as indicated for (A) BMP receptors ALK-3 and -6, and (B) human SMAD-1, -4, and -5. RT-PCR was performed on whole human fetus sample as a positive control for the reaction.
Effect of BMPs on the Proliferation and Differentiation of Human CD34+CD38−Lin− Cells.
The proliferative response of CD34+CD38−Lin− cells in response to BMP treatment was determined by comparing the number of cells obtained after 3 d of culture in the presence or absence of BMPs to the number originally seeded at day 0 (Fig. 2 A). Cells were seeded in ex vivo culture conditions with serum-free media (control SF, which we had previously designed to allow for the expansion of primitive human blood cells [38]) and were compared with cultures in which TGF-β, BMP-2, -4, -7, or 5% serum was added (Fig. 2 A). The addition of TGF-β inhibited the proliferative response seen in control SF conditions by 80% but did maintain the number of cells initially incubated, whereas TGF-β neutralizing antibody had no additional effect on total cell number compared with control conditions (Fig. 2 A). Consistent with effects on cell growth, treatment with TGF-β maintained the number of progenitors capable of producing CFCs after 3 d of culture, whereas TGF-β antibody had no effect on CFC capacity (Fig. 2 B). BMP-2 and -7 had a modest effect on cell growth at concentrations of 5 ng/ml; however, at higher concentrations (50 ng/ml) both BMP-2 and -7 treatment inhibited cell proliferation similar to TGF-β. The inhibitory growth effect of BMP-2 and -7 is consistent with decreased CFC content of treated cultures. BMP-2 and -7 decreased the CFC capacity of CD34+CD38−Lin− cells in a dose-responsive manner; however, at 5 ng/ml BMP-7 was less effective than BMP-2 (Fig. 2 B). In the cases of TGF-β, BMP-2, and BMP-7, changes in total cell number correlated with changes in CFC content but did not selectively alter the specific type of progenitor detected, demonstrating that BMPs do not affect lineage commitment. This suggests that BMP-2 and -7 are capable of modulating proliferation of primitive CD34+CD38− Lin− cells in a manner that does not alter the developmental program and differentiation capacity of primitive cell populations. Addition of 5% serum caused a massive proliferative response (Fig. 2 A) along with a dramatic decrease in the number of cells capable of producing progenitors, indicative of differentiation induction (Fig. 2 B).
Figure 2.
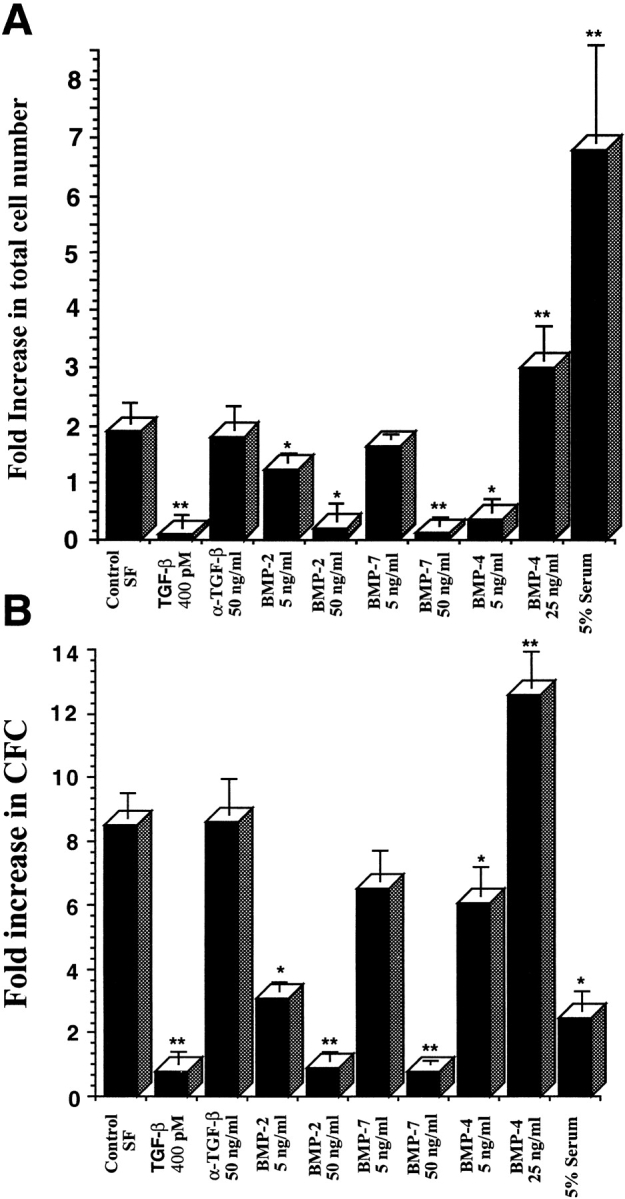
Effect of ex vivo culture on the total cell number and number of clonogenic progenitors present after in vitro culture of CD34+CD38−Lin− cells in the presence of BMPs. (A) Purified CD34+CD38−Lin− cells were counted and seeded (700–1,000) in wells containing serum-free media or with the addition of factors indicated at day 0. Cells were harvested from individual wells after 3 d of culture and counted, and the mean fold increase in absolute cell number was calculated (n = 4). (B) An aliquot of 100–300 CD34+CD38−Lin− cells was plated in progenitor cell assays at the initiation of ex vivo cultures (day 0), and the frequency of progenitors was calculated. Similar cell doses were plated from wells harvested after 3 d of cultures containing the various factors indicated, and the mean fold increase in number of CFCs was calculated compared with day 0 (n = 3). Values are the mean ± SEM of determinations in four and three separate culture samples for cell number and clonogenic progenitors, respectively. *P < 0.05, **P < 0.01 indicate statistically significant differences from controls.
Treatment of CD34+CD38−Lin− cells with BMP-4 invoked a unique response compared with TGF-β, BMP-2, and BMP-7. The addition of BMP-4 at 5 ng/ml inhibited the cell growth of CD34+CD38−Lin− cells, in contrast to the effects observed using BMP-2 and -7 at similar concentrations (Fig. 2 A). Increasing BMP-4 concentrations to 50 ng/ml was toxic to CD34+CD38−Lin− cells (data not shown). Concentrations of 25 ng/ml of BMP-4 did not alter cell viability and were capable of inducing an increase in cell number over control SF conditions (Fig. 2 A). The ability of CD34+CD38−Lin− cells to produce CFCs in response to BMP-4 was also dose dependent. Although 5 ng/ml of BMP-4 inhibited proliferation of CD34+CD38−Lin− cells, CFC content was only slightly decreased compared with control, resulting in increased frequency. CD34+ CD38−Lin− cells treated with 25 ng/ml of BMP-4 dramatically expanded CFCs compared with control SF conditions (Fig. 2 B). These data indicate that members of the TGF-β family, including those in the BMP subfamily, are capable of modulating proliferation and differentiation of primitive human blood cells and that the effects are specific to the dose and subtype of BMP ligand.
Phenotypic Analysis of Human CD34+CD38−Lin− Cells Treated with BMPs.
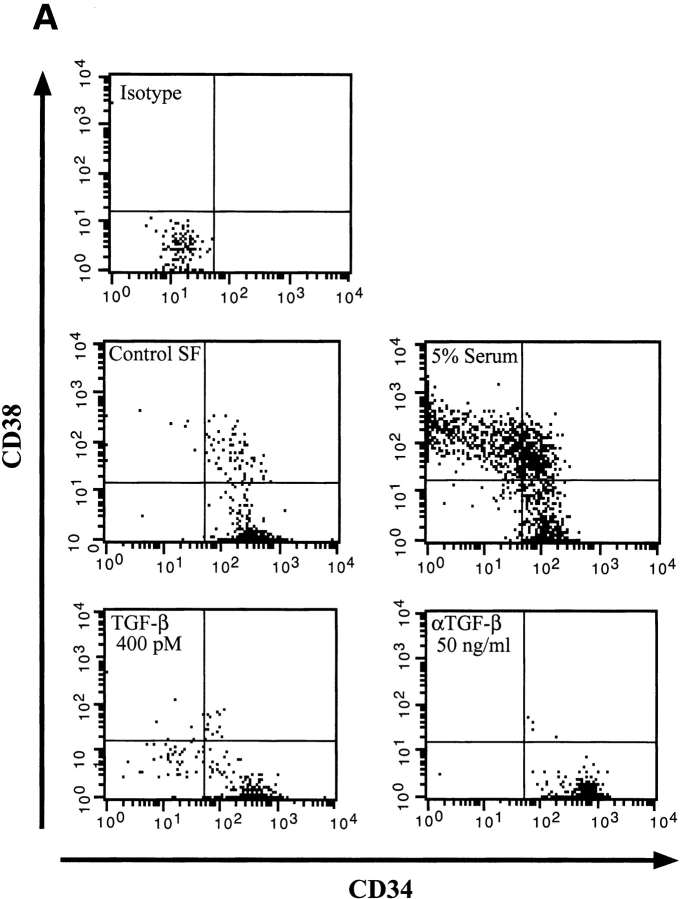
To determine whether BMP treatment affected the differentiation program of primitive human CD34+CD38−Lin− cells, cultures were analyzed by flow cytometry for changes in CD34 and CD38 expression (Fig. 3). Similar to that shown previously (38), control SF cultures induced modest differentiation of CD34+CD38− Lin− cells into CD34+CD38+ cells (Fig. 3 A), whereas the addition of 5% serum induced a differentiation response as demonstrated by the acquisition of CD38 and loss of CD34 expression. Both TGF-β and TGF-β neutralizing antibody had little effect on the CD34+CD38− phenotype and remained similar to control cultures (Fig. 3 A).
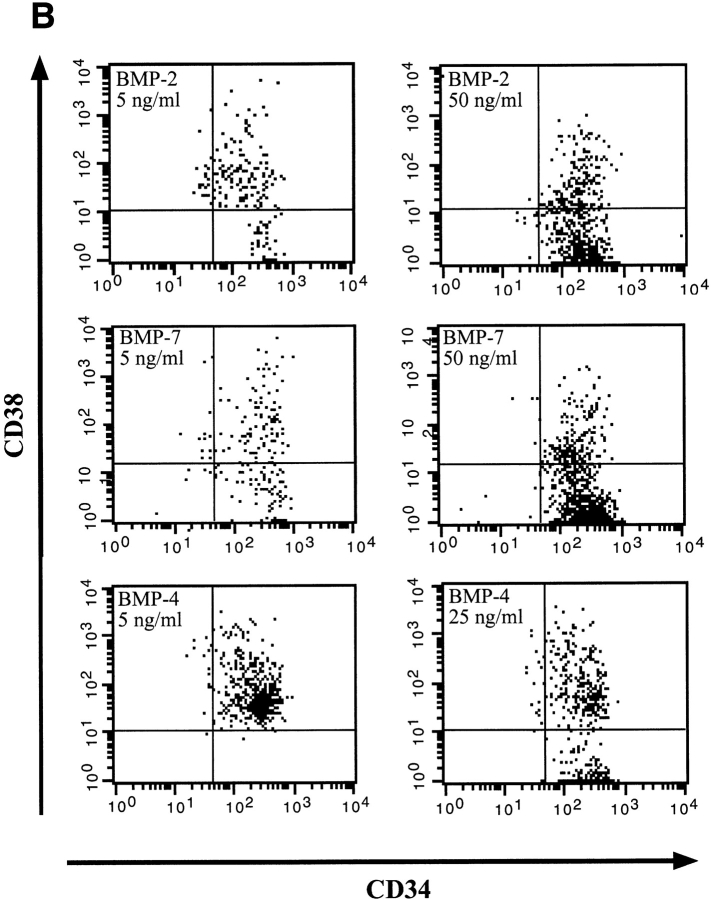
Figure 3.
Comparative analysis of CD34 and CD38 expression of highly purified CD34+CD38−Lin− cells after 4 d of culture in the presence of BMPs. A representative experiment (n = 3) of CD34 and CD38 cell surface expression performed on initially purified CD34+CD38−Lin− cells after 4 d of culture in serum-free conditions or with the addition of factors as indicated. The entire contents of individual wells were collected at 4 d, stained with mAbs, and analyzed using flow cytometric analysis.
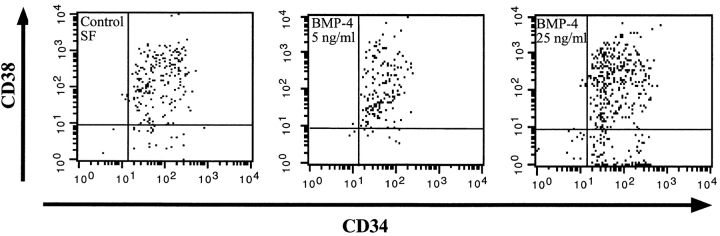
Members of the BMP subfamily caused changes in CD34+CD38− phenotype compared with control SF conditions (Fig. 3 A). At concentrations of 5 ng/ml, both BMP-2 and -7 had a potent differentiation effect on CD34+CD38−Lin− cells as demonstrated by the acquisition of CD38; however, the population remained relatively immature since the cells were all CD34 positive. At higher concentrations of 50 ng/ml, both BMP-2 and -7 maintained equivalent numbers of CD34+CD38− cells compared with control SF conditions (Fig. 3 B). Phenotypic analysis of CD34+CD38−Lin− cells after treatment with BMP-4 was distinct from that observed for BMP-2 and -7. At low doses, BMP-4 induced a complete differentiation of CD34+CD38− cells into CD34+CD38+ cells, whereas at higher doses two populations developed: one that had differentiated and one that maintained a primitive phenotype (Fig. 3 B). To further investigate the unique response of CD34+CD38−Lin− cells to BMP-4, phenotypic analysis was extended to 6 d of ex vivo culture (Fig. 4). Parallel cultures of CD34+CD38−Lin− cells incubated in control SF conditions for 6 d differentiated into CD34+CD38+ cells, similar to the differentiation response seen when low concentrations of 5 ng/ml of BMP-4 were added (Fig. 4). In contrast, the high concentration of BMP-4 treatment resulted in the maintenance of a significant proportion of primitive CD34+CD38− cells. These changes in the phenotype of CD34+CD38−Lin− cells in response to BMP treatment illustrate that these factors are capable of modulating the developmental program of primitive subsets of CD34+ cells. Consistent with effects on proliferation and CFC capacity, BMP-4 is capable of inducing distinct dose-dependent effects on the differentiation program of human CD34+CD38−Lin− cells compared with other members of the TGF-β superfamily tested.
Figure 4.
Analysis of CD34 and CD38 expression of highly purified CD34+ CD38−Lin− cells after 6 d of culture in the presence of BMP-4. A representative experiment (n = 3) of CD34 and CD38 cell surface expression performed on initially purified CD34+CD38−Lin− cells after 6 d of culture in serum-free conditions or with the addition of BMP-4 at 5 or 25 ng/ml. The entire contents of individual wells were collected at 6 d, stained with mAbs, and analyzed using flow cytometric analysis.
Role of BMPs on Human Hematopoietic Cells Capable of Repopulation (SRCs).
Using the SRC assay, we have previously demonstrated that serum-free ex vivo cultures were the only conditions capable of maintaining pluripotent repopulating cells for as long as 4 d before transplantation (38). However, even under these conditions, no SRCs were present after 9 d although CFCs and CD34+ cells expanded enormously during this time period. These previous results indicated that even these optimized cultures had limited ability to expand or even maintain SRCs. Accordingly, we have assessed the effect of BMP treatment on SRC maintenance and expansion. Wells were seeded initially with 700–1,000 CD34+CD38−Lin− cells in control SF conditions representing 1 or 2 SRCs. BMPs were added as indicated, and the entire contents of each well were then transplanted into NOD/SCID mice at 2, 4, and 6 d. Human cell engraftment was determined 8 wk after transplant, and results are summarized in Table I.
Table I.
Analysis of SRCs after 2, 4, and 6 d of Ex Vivo Culture in the Presence of BMPs
| Treatments | Day 2 | Day 4 | Day 6 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. pos | No. neg | % positive | No. pos | No. neg | % positive | No. pos | No. neg | % positive | ||||||||||
| Control SF | 7 | 0 | 100 | 10 | 1 | 100 | 0 | 9 | 0 | |||||||||
| α–TGF-β (50 ng/ml) | 1 | 1 | 50 | 2 | 0 | 100 | 1 | 3 | 25 | |||||||||
| TGF-β (400 pM) | 3 | 0 | 100 | 3 | 7 | 30 | 0 | 2 | 0 | |||||||||
| BMP-2 (5 ng/ml) | 7 | 0 | 100 | 6 | 0 | 100 | 1 | 3 | 25 | |||||||||
| BMP-2 (50 ng/ml) | ND | 0 | 4 | 4 | ND | |||||||||||||
| BMP-7 (5 ng/ml) | 6 | 0 | 100 | 7 | 0 | 100 | 0 | 5 | 0 | |||||||||
| BMP-7 (50 ng/ml) | ND | 1 | 3 | 25 | 0 | 4 | 0 | |||||||||||
| BMP-4 (5 ng/ml) | ND | 1 | 2 | 33 | 0 | 4 | 0 | |||||||||||
| BMP-4 (25 ng/ml) | 1 | 0 | 100 | 7 | 0 | 100 | 5 | 1 | 83 | |||||||||
| 5% FCS | 0 | 4 | 0 | 0 | 3 | 0 | ND | |||||||||||
Summary of the frequency of engrafted mice after transplantation of CD34+CD38−Lin− cells after ex vivo culture. The human cell engraftment in the BM of 121 mice transplanted with CD34+CD38−Lin− cells expanded for 2, 4, and 6 d was determined by DNA extraction 8 wk after transplant and hybridized with a human chromosome 17–specific α-satellite probe as shown previously. The percentage of human engraftment in the BM of positive mice ranged between 0.1 and 1%. pos, positive; neg, negative.
The frequency of mice engrafted after transplantation of cells cultured for 2 d in ex vivo cultures was similar in all treatment groups, with the exception of cultures containing 5% serum, which were unable to sustain SRCs after 2 and 4 d (Table I). The addition of serum caused the majority of CD34+CD38− cells to acquire CD38 and/or lose CD34 cell surface expression after 4 d (Fig. 2). This differentiation shift suggests that the SRCs have most likely undergone differentiation into more mature cells that no longer repopulate. However, since primitive CD34+CD38− cells were present in serum-containing cultures, the loss of SRCs is most likely associated with maturation that occurred independent of CD38 acquisition. Similar results have also been obtained in a separate study examining the loss of SRCs during culture for retroviral transduction, and demonstrate that there is dissociation between phenotype and function as a consequence of downregulation of CD38 in differentiated cells after culture in serum (our unpublished data). All mice transplanted with purified cells cultured for 4 d in control SF conditions were repopulated (100%). However, cultures containing ligands at concentrations having inhibitory effects on cell growth and CFC capacity, such as TGF-β, 50 ng/ml of BMP-2 and -7, and 5 ng/ml of BMP-4 (Fig. 2, A and B), all resulted in a decrease in the frequency of engrafted animals, suggesting a loss of SRCs during culture (Table I). In contrast, but consistent with mitogenic responses detected by cell growth and CFC capacity, the addition of TGF-β neutralizing antibody, 5 ng/ml of BMP-2 and -7, or 25 ng/ml of BMP-4 allowed for the maintenance of SRCs after 4 d of culture as indicated by 100% engraftment frequency in transplanted animals.
Since all mice transplanted with control SF cultures at 4 d of culture were also engrafted, it was difficult to determine whether the addition of BMPs was affecting human repopulating cells. By using similar techniques of limiting dilution analysis employed in our previous studies (34, 38), we compared cultures treated with BMPs or TGF-β to assess effects on the number of SRCs at day 4; no significant differences in the frequency of SRCs were found (data not shown). To determine whether BMPs were capable of affecting the survival of SRCs, cultures were extended for up to 6 d. After 6 d of ex vivo culture, SRCs could not be detected under SF conditions. In contrast, one out of four cultures containing TGF-β neutralizing antibody or 5 ng/ml of BMP-2 contained repopulating cells. The most dramatic effect was seen in cultures containing 25 ng/ml of BMP-4, where as few as 700 CD34+CD38−Lin− cells cultured under these conditions for 6 d were capable of engrafting 5 out of 6 mice (83%; Table I). The percentage of human chimerism in the BM of all positive mice shown in Table I ranged between 0.1 and 1%. These results are consistent with our previous studies in which transplantation of one SRC enriched in purified CD34+CD38− cells at limiting dose allowed for similar levels of human engraftment (34, 38). Further extension of ex vivo cultures containing 25 ng/ml of BMP-4 to 8 d resulted in the loss of SRCs (data not shown). These data demonstrate the novel role of BMPs in regulating the repopulating function of primitive human hematopoietic cells and demonstrate that BMP-4 acts as a survival factor for candidate human stem cells.
Discussion
The development of serum-free ex vivo culture systems together within xenogenic transplant systems that are capable of detecting human repopulating cells has provided a method for the further identification of factors regulating the developmental program of candidate human stem cells (SRCs [38]). In this report, we provide the first evidence for the role of BMPs in the modulation of the developmental program of primitive subfractions of CD34+CD38− Lin− cells that contain SRCs. Treatment with BMP-2, -4, and -7 resulted in dose-dependent effects on the growth, differentiation, and repopulating function of CD34+ CD38−Lin− cells. Evidence of the regulatory role of BMPs was best demonstrated by BMP-4 treatment. At low concentrations of BMP-4, rapid differentiation was seen as well as loss of SRCs. At high doses, some differentiation occurred, but a significant proportion of primitive CD34+ CD38−Lin− cells remained and more importantly SRCs were present. BMP-4 was capable of extending the duration of stem cell activity in culture for an additional 2 d in comparison with all other previously optimized conditions using cytokines believed to act on primitive blood cells. Thus, BMP-4 is acting as a survival factor, preserving stem cell function under conditions that normally lead to stem cell loss.
The expression of pathway-restricted SMAD-1 and -5, together with expression of BMP receptors ALK-3 and -6, suggest that the mechanism of BMP action is due to the specific activation of the BMP pathway that is distinct from TGF-β signaling. In addition, BMP-2 and -4 normally induce similar cellular responses, and therefore the differential effects of BMP-2 compared with BMP-4 on primitive hematopoietic tissue shown here are unique, and remain to be tested in other species and tissue types using similar in vivo model systems for primary tissue. This unique response of human blood stem cells to BMP-4 ligand may be due to a previously unreported receptor and/or inhibitory molecule mediating BMP-4 signals that is expressed by this population of rare blood cells. Alternatively, the divergent effect of BMP-2 and -4 may not be at the receptor level and may be due to the synergistic effects of BMP-4 with other cytokines used in this ex vivo culture system, which do not have overlapping effects on BMP-2–specific pathways. The differential response of BMP-2 and -4 may not diverge at the level of intercellular signaling in individual cells, but could be due to an intrinsic heterogeneity of BMP receptor expression within the cells that comprise this population. At day 3 and 6 of ex vivo culture, BMP-4 was capable of inducing a differentiation response shown by the acquisition of CD38, but also of maintaining the primitive phenotype of a potentially distinct subset of CD34+CD38− cells. These results are most easily interpreted by the existence of two differentially responsive populations that are heterogeneous at the level of BMP binding proteins. Evidence from other gene transfer and cell purification studies has already suggested that CD34+CD38−Lin− cells are heterogeneous and that there is a hierarchy of stem cells within this cell fraction (35). To address this possibility, it would be necessary to develop flow cytometric methods to detect subpopulations within CD34+CD38−Lin− fractions using fluorochrome-conjugated BMP ligands or antibodies to BMP receptors. Reagents to perform these experiments are currently being developed.
The results reported here, together with studies using other developmental systems, underscore the role of BMP-4 in primitive hematopoietic tissue and demonstrate that BMPs continue to regulate blood development well after tissue specification (13, 41). Thus, an entirely new avenue remains to be explored to identify this new biological mechanism of stem cell regulation. Moreover, ex vivo culture of human hematopoietic cells is a crucial component of many therapeutic applications, including gene therapy, tumor cell purging, and stem/progenitor cell expansion (42), and therefore the identification of this novel class of stem cell regulatory molecules opens the way to developing these clinical applications. Since current ex vivo culture systems for human blood cells are limited in their ability to maintain stem cells in vitro (38, 42), the ability to extend the period in which repopulating cells can be maintained in culture with the addition of factors such as BMP-4 represents a significant advance in these systems. Based on the novel role of this family of molecules in the regulation of primitive hematopoietic tissue, this study establishes the foundation for the use of these and other mesodermal regulators in the manipulation of human stem cells in a clinical setting.
Acknowledgments
We thank Amgen, Inc. for cytokines, Vicki Rosen and Steve Neben at Genetics Institute for BMP-2 and BMP-4, respectively, Kuber Sampath at Creative Biomolecules for BMP-7, and L. McWhirter and M. Watson for providing CB specimens.
Supported by grants to M. Bhatia from the Medical Research Council of Canada (MRC) and Bayer Inc. Research Fund; to J.E. Dick from the MRC, the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society, the Bayer/Red Cross Research Fund, the Canadian Genetic Diseases Network of the National Centers of Excellence, and an MRC Scientist award; and by postdoctoral fellowships to D. Bonnet from the Human Frontier Science Organization Program and the French Cancer Research Association.
Abbreviations used in this paper
- ALK
activin-like kinase
- BM
bone marrow
- BMP
bone morphogenetic protein
- CB
cord blood
- CFC
colony-forming cell
- GF
growth factor
- Lin
lineage
- LTC-IC
long-term culture initiating cell
- M-PB
mobilized peripheral blood
- NOD
nonobese diabetic
- RT
reverse transcription
- SRC
SCID-repopulating cell
Footnotes
D. Bonnet's present address is Coriell Institute for Medical Research, Camden, NJ 08103.
M. Bhatia and D. Bonnet contributed equally to this work.
References
- 1.Metcalf D. Hematopoietic regulators: redundancy or subtlety? . Blood. 1993;82:3515–3523. [PubMed] [Google Scholar]
- 2.Metcalf D. Lineage commitment and maturation in hematopoietic cells: the case for extrinsic regulation. Blood. 1998;92:345–347. [PubMed] [Google Scholar]
- 3.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 4.Huber TL, Zon LI. Transcriptional regulation of blood formation during Xenopusdevelopment. Semin Immunol. 1998;10:103–109. doi: 10.1006/smim.1998.0111. [DOI] [PubMed] [Google Scholar]
- 5.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development (Camb) 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 6.Yoder MC, Papaioannou VE, Breitfeld PP, Williams DA. Murine yolk sac endoderm- and mesoderm-derived cell lines support in vitro growth and differentiation of hematopoietic cells. Blood. 1994;83:2436–2443. [PubMed] [Google Scholar]
- 7.Rollins-Smith LA, Blair P. Contribution of ventral blood island mesoderm to hematopoiesis in postmetamorphic and metamorphosis-inhibited Xenopus laevis. . Dev Biol. 1990;142:178–183. doi: 10.1016/0012-1606(90)90161-b. [DOI] [PubMed] [Google Scholar]
- 8.Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- 9.Kelley C, Yee K, Harland R, Zon LI. Ventral expression of GATA-1 and GATA-2 in the Xenopusembryo defines induction of hematopoietic mesoderm. Dev Biol. 1994;165:193–205. doi: 10.1006/dbio.1994.1246. [DOI] [PubMed] [Google Scholar]
- 10.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 11.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development (Camb) 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 13.Maeno M, Mead PE, Kelley C, Xu RH, Kung HF, Suzuki A, Ueno N, Zon LI. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. . Blood. 1996;88:1965–1972. [PubMed] [Google Scholar]
- 14.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 15.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 16.Sitnicka E, Ruscetti FW, Priestley GV, Wolf NS, Bartelmez SH. Transforming growth factor beta 1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood. 1996;88:82–88. [PubMed] [Google Scholar]
- 17.Garbe A, Spyridonidis A, Mobest D, Schmoor C, Mertelsmann R, Henschler R. Transforming growth factor-beta 1 delays formation of granulocyte-macrophage colony-forming cells, but spares more primitive progenitors during ex vivo expansion of CD34+haemopoietic progenitor cells. Br J Haematol. 1997;99:951–958. doi: 10.1046/j.1365-2141.1997.4893291.x. [DOI] [PubMed] [Google Scholar]
- 18.Imbert AM, Bagnis C, Galindo R, Chabannon C, Mannoni P. A neutralizing anti-TGF-β1 antibody promotes proliferation of CD34+Thy-1+peripheral blood progenitors and increases the number of transduced progenitors. Exp Hematol. 1998;26:374–381. [PubMed] [Google Scholar]
- 19.Hatzfeld J, Li ML, Brown EL, Sookdeo H, Levesque JP, O'Toole T, Gurney C, Clark SC, Hatzfeld A. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor β1 or Rb oligonucleotides. J Exp Med. 1991;174:925–929. doi: 10.1084/jem.174.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatzfeld J, Batard P, Cardoso AA, Li ML, Panterne B, Sansilvestri P, Ginsbourg M, Levesque JP, Hatzfeld A. Purification and release from quiescence of umbilical cord blood early progenitors reveal their potential to engraft adults. Blood Cells. 1994;20:430–435. [PubMed] [Google Scholar]
- 21.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 22.Whitman M. Smads and early developmental signaling by the TGFβ superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- 23.Wiles, M.V., and B.M. Johansson. 1997. Analysis of factors controlling primary germ layer formation and early hematopoiesis using embryonic stem cell in vitro differentiation. Leukemia. 11(Suppl. 3):454–456. [PubMed]
- 24.Harland RM. The transforming growth factor beta family and induction of the vertebrate mesoderm: bone morphogenetic proteins are ventral inducers. Proc Natl Acad Sci USA. 1994;91:10243–10246. doi: 10.1073/pnas.91.22.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attisano L, Wrana JL. Mads and Smads in TGF beta signalling. Curr Opin Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- 26.Wrana J, Pawson T. Signal transduction. Mad about SMADs. Nature. 1997;388:28–29. doi: 10.1038/40290. [DOI] [PubMed] [Google Scholar]
- 27.Wrana JL, Attisano L. MAD-related proteins in TGF-beta signalling. Trends Genet. 1996;12:493–496. doi: 10.1016/s0168-9525(96)30109-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Buckhaults P, Zawel L, Bunz F, Riggins G, Le Dai J, Kern SE, Kinzler KW, Vogelstein B. Targeted deletion of Smad4 shows it is required for transforming growth factor beta and activin signaling in colorectal cancer cells. Proc Natl Acad Sci USA. 1998;95:2412–2416. doi: 10.1073/pnas.95.5.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- 30.Hoodless PA, Wrana JL. Mechanism and function of signaling by the TGF beta superfamily. Curr Top Microbiol Immunol. 1998;228:235–272. doi: 10.1007/978-3-642-80481-6_10. [DOI] [PubMed] [Google Scholar]
- 31.Zavadil J, Brezinova J, Svoboda P, Zemanova Z, Michalova K. Smad5, a tumor suppressor candidate at 5q31.1, is hemizygously lost and not mutated in the retained allele in human leukemia cell line HL60. Leukemia. 1997;11:1187–1192. doi: 10.1038/sj.leu.2400750. [DOI] [PubMed] [Google Scholar]
- 32.Zon LI. Developmental biology of hematopoiesis. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]
- 33.Mead PE, Brivanlou IH, Kelley CM, Zon LI. BMP-4-responsive regulation of dorsal-ventral patterning by the homeobox protein Mix.1. Nature. 1996;382:357–360. doi: 10.1038/382357a0. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia M, Wang JCY, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larochelle A, Vormoor J, Hanenberg H, Wang JC, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao XL, Kato I, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 36.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in scidmice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 37.Dick JE. Normal and leukemic human stem cells assayed in SCID mice. Semin Immunol. 1996;8:197–206. doi: 10.1006/smim.1996.0025. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia M, Bonnet D, Kapp U, Wang JC, Murdoch B, Dick JE. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–624. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, Dick JE. High-level multilineage engraftment of human cord blood cells in SCID mice. J Hematother. 1993;2:215–216. doi: 10.1089/scd.1.1993.2.215. [DOI] [PubMed] [Google Scholar]
- 40.Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande K, Spiegle, Miyazono K, Huylebroeck D, Ten P, Dijke Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- 41.Kanatsu M, Nishikawa SI. In vitro analysis of epiblast tissue potency for hematopoietic cell differentiation. Development (Camb) 1996;122:823–830. doi: 10.1242/dev.122.3.823. [DOI] [PubMed] [Google Scholar]
- 42.Williams DA. Ex vivo expansion of hematopoietic stem and progenitor cells—robbing Peter to pay Paul? . Blood. 1993;81:3169–3172. [PubMed] [Google Scholar]