Abstract
Molecular mimicry refers to structural homologies between a self-protein and a microbial protein. A major epitope of myelin basic protein (MBP), p87–99 (VHFFKNIVTPRTP), induces experimental autoimmune encephalomyelitis (EAE). VHFFK contains the major residues for binding of this self-molecule to T cell receptor (TCR) and to the major histocompatibility complex. Peptides from papilloma virus strains containing the motif VHFFK induce EAE. A peptide from human papilloma virus type 40 (HPV 40) containing VHFFR, and one from HPV 32 containing VHFFH, prevented EAE. A sequence from Bacillus subtilis (RKVVTDFFKNIPQRI) also prevented EAE. T cell lines, producing IL-4 and specific for these microbial peptides, suppressed EAE. Thus, microbial peptides, differing from the core motif of the self-antigen, MBPp87–99, function as altered peptide ligands, and behave as TCR antagonists, in the modulation of autoimmune disease.
Keywords: experimental autoimmune encephalomyelitis, mimicry, altered peptide ligand, autoimmunity, multiple sclerosis
When certain neurotropic viruses trigger inflammation in the central nervous system (CNS),1 immune cells in the inflammatory infiltrate attack neighboring myelin antigens in the CNS. This immune response then spreads to various epitopes on various myelin antigens, a process known as epitope spreading (2–4). Certain altered peptide ligands (APLs) actually resemble the immunogenic portion of certain neurotropic viruses, and can be used to subvert epitope spreading. Indeed, we have been able to suppress the spreading response by administering various APLs that mimic the structure of both certain microbes and a component of myelin. These APLs induce IL-4 and either prevent or reverse experimental autoimmune encephalomyelitis (EAE). Administration of such APLs may clear an entire inflammatory infiltrate that contains a diverse collection of T cells and B cells from the brain (2, 3, 5–7).
In the (PLSJL/J)F1 mouse there are numerous pathogenic epitopes of myelin antigens that induce EAE. These include several on myelin basic protein (MBP): pAc1–11, restricted by I-Au; p35–47, restricted by I-Eu; and p87–99, restricted by I-As (8–13). Moreover, the immune response to other epitopes in MBP is actually restricted by hybrid determinants where the α chain is encoded by I-As, and the β chain by I-Au (8). In the (PLSJL/J)F1, the dominant immunogenic and pathogenic epitope of MBP is pAc1–11. MBPp87–99 is restricted by I-As and is a minor determinant (9, 10). Furthermore, after immunization of SJL mice with spinal cord homogenate, other myelin antigens such as proteolipid protein (PLP) are actually more pathogenic than MBP (14, 15).
MBPp87–99 is an immunodominant epitope for T cells and autoantibodies in MS brain lesions (16–19). The main region of MBP recognized by T cells and autoantibodies, found in MS brain, is the core motif, HFFK, from MBPp87–99 in patients who are HLA-DRB1*1501 DQB1*0602 (HLA-DR2). Recently, Smith et al. solved the crystal structure of HLA-DR2 with MBPp84–102, and confirmed that K91 is the major TCR contact site, while F90 is a major anchor to MHC, binding the hydrophobic P4 pocket (20).
Previously, we have compared the structural requirements for autoantibody recognition to those of T cell clones reactive to MBPp87–99. MBP autoantibodies were affinity-purified from CNS lesions of 12 postmortem cases studied. The MBPp87–99 peptide was immunodominant in all cases and it inhibited autoantibody binding to MBP by >95%. Residues contributing to autoantibody binding were located in a 10-amino acid segment p86–95 (VVHFFKNIVT) that also contained the MHC–TCR residues for T cells recognizing MBP in the context of DRB1*1501 and DQB1*0602. In the epitope center, the same residues, VHFFK, were important for T cell binding and MHC recognition. Based on the antibody binding motif, microbial peptides that were bound by purified autoantibodies were identified. Autoantibody binding of microbial peptides required sequence identity at four or five contiguous residues in the epitope center VHFFK. Papilloma viruses (types 7, 13, 40, and 32), EBV, cytomegalovirus, Dhori virus, herpes simplex virus (HSV) type 1, influenza type A, hepatitis A, and adenovirus were efficient at binding autoantibodies to MBP. All these viruses contained the FFK or FFKN motifs. The papilloma virus type 7 (IGGRVHFFKDISPIA) bound both autoantibodies to MBP found in brain, and it stimulated a human MBP-specific T cell clone from an MS patient (19, 21).
In this report we have chosen microbial peptides that bear chemical similarities with MBPp87–99 and demonstrate how these epitopes derived from microbial sequences, can function like an APL, and suppress EAE. In a companion paper, sequences bearing the motif HFFK derived from various microbes including human papilloma virus type 7 (HPV 7) induced EAE after administration in CFA, or after in vitro stimulation of an MBP-specific T cell clone (1).
Materials and Methods
Animals.
6–8-wk-old female (PLSJL/J)F1 mice were purchased from The Jackson Laboratory.
Antigens.
Peptides were synthesized on a peptide synthesizer (model 9050: MilliGen) by standard 9-fluorenylmethoxycarbonyl chemistry. Peptides were purified by HPLC. Structure was confirmed by amino acid analysis and mass spectroscopy. Peptides used for the experiments were: ENPVVHFFKNIVTPR (MBP p85–99); AASQKRPSQRHG (MBPAc1–11); IGGRVHFFKDISPIA (HPV 7); IGGRVHFFKDISPIS (HPV 13); IGGRVHFFRDISPIG (HPV 40); IGSRVHFFHDISPIT (HPV 32); RKVVTDFFKNIPQRI (Bacillus subtilis hyp protein X13); and DMTPADALDDRDLEM (HSV VP16).
Peptide Treatment.
For the peptide treatment a solution of 2 mg/ml of peptide dissolved in PBS, emulsified 1:1 (vol/vol) in IFA was prepared. Mice were injected intradermally with 0.1 ml of the antigen emulsion, twice with a 10-d interval. 10 d after the last injection experimental animals were challenged for EAE.
EAE Induction.
Lyophilized guinea pig spinal cord (gpSCH) was dissolved in PBS to a concentration of 5 mg/ml and emulsified with an equal volume of IFA, supplemented with 4 mg/ml heat-killed Mycobacterium tuberculosis H37Ra (Difco Labs.). Mice were injected subcutaneously with 0.1 ml of the peptide emulsion, and again on the same day and then 48 h later were injected intravenously with 0.1 ml of a solution of 4 μg/ml Bordetella pertussis toxin in PBS. Experimental animals were scored as follows: 0, no clinical disease; 1, tail weakness or paralysis; 2, hind limb weakness; 3, hind limb paralysis; 4, forelimb weakness or paralysis; 5, moribund or dead.
T Cell Lines.
Lymph node cells from experimental animals were taken 20 d after challenge for EAE. Cells (5–10 × 106/ml) were incubated in enriched RPMI (RPMI 1640 supplemented with l-glutamine [2 mM], sodium pyruvate [1 mM], nonessential amino acids [0.1 mM], penicillin [100 U/ml], streptomycin [0.1mg/ml], and 2-ME [5 × 10−5 M]), supplemented with 1% syngeneic mouse sera with 10 μg/ml peptide for 3 d. After incubation, cells were washed and resuspended for 10 d in enriched RPMI completed with 10% FCS and 10% supernatant of spleen cells activated with concanavalin A (Con A sup). After this period of culture the cells were then activated in the presence of syngeneic irradiated spleen cells (107/ml) and 10 μg/ml peptide for 3 d, washed and incubated for 10 d in enriched RPMI complemented with 10% FCS and 10% Con A sup. The cells were continuously grown in the above conditions for 2-wk cycles. The peptide-specific T cells were used for assays 1 wk after antigen stimulation.
T Cell Line Proliferation Assay.
T cells (104) were incubated in 96-well flat-bottomed plates (Corning) with 5 × 105 irradiated syngeneic APC in a total volume of 200 μl of enriched RPMI and 10% FCS, and different concentrations of the peptide. After 24 h 100 μl were removed from each well for cytokine secretion analysis in a sandwich ELISA. The remaining cells were incubated for an additional 24 h, pulsed with [3H]thymidine (0.5 μCi of 5 Ci/mmol), harvested, and counted in a beta counter.
Class II Peptide Binding Assay.
Peptide binding assays were performed as described elsewhere (22). In brief, the B cell lymphoma LS102.9 was used as a source of I-As. The cell line was maintained in vitro by culture in enriched RPMI. Cells were lysed at a concentration of 108 cells/ml in PBS containing 1% NP-40, 1 mM PMSF, 5 mM Na-orthovanadate, and 25 mM iodoacetamide. The lysates were cleared of debris and nuclei by centrifugation at 10,000 g for 20 min.
Mouse class II molecules were purified as previously described (22) using the mAb Y3JP (IAb,s -specific), coupled to Sepharose 4B beads. Purified mouse class II molecules (5–500 nM) were incubated with 1–10 nM 125I-radiolabeled peptides for 48 h in PBS containing 5% DMSO in the presence of a protease inhibitor cocktail. Purified peptides were iodinated using the chloramine-T method.
Peptide inhibitors were typically tested at concentrations ranging from 120 μg/ml to 1.2 ng/ml. The data were then plotted and the dose yielding 50% inhibition (IC50) was measured. Intermediate binding was equivalent to IC50 in the range of 100–1,000 nM. In appropriate stoichiometric conditions, the IC50 of an unlabeled test peptide to the purified MHC is a reasonable approximation of the affinity of interaction (K d). Peptides were tested in two to four completely independent experiments.
Class II peptide complexes were separated from free peptide by gel filtration on TSK2000 columns (TosoHaas 16215), and the fraction of bound peptide calculated as previously described (22). In preliminary experiments, each of the I-A prep was titered in the presence of fixed amounts of radiolabeled peptides to determine the concentration of class II molecules necessary to bind 10–20% of the total radioactivity. All subsequent inhibition and direct binding assays were then performed using these class II concentrations.
TCR Antagonist Assay.
TCR antagonism was tested as previously described (23). In brief, irradiated syngeneic spleen cells were pulsed with a 0.005 μM concentration of MBPp85–99 for 3 h at 37°C. Spleen cells were then washed and used as APCs to the MBPp85–99 (L35) specific T cell line in the presence of different inhibitor concentration. Proliferative responses were measured by [3H]thymidine incorporation. Percentage of inhibition was calculated by the formula described in Table III.
Table III.
Inhibition of Lymph Node Cell Proliferation to MBPp85–99 by Microbial Mimicry Peptides
| Peptide | Sequence | Relative affinity* for I-As | Inhibition of LNC responses‡ | |||
|---|---|---|---|---|---|---|
| % | ||||||
| MBPp85–99 | ENPVVHFFKNIVTPR | 667* | – | |||
| HPV 7 | IGGRVHFFKDISPIA | 208 | 62 | |||
| HPV 40 | IGGRVHFFRDISPIG | 180 | 39 | |||
| Herpes simplex DNA pol | GGRRLFFVKAHVRE | 551 | 39 | |||
| Herpes simplex VP16 | DMTPADALDDRDLEM | ND | 29 |
Peptide binding assays on purified I-As molecules were performed as described in reference 22. Results are expressed as IC50 in nanomolars. E. coli E7 peptide bound at an IC50 of 76 nM.
For inhibition of lymph node cell (LNC) proliferative responses by microbial mimicry peptides, LNCs from (PLSJL/J)F1 mice immunized with the MBPp85–99 peptide were incubated in vitro in the presence of both the MBPp85–99 peptide and a molecular mimicry peptide at a molar ratio of 1:1 (final concentration of each peptide was 0.01 mg/ml). CPM incorporation of the LNCs incubated with MBPp85–99 at 0.01 mg/ml were 5,792 in the absence of inhibitor and CPM of LNCs incubated with medium alone was 1,010. Percentage of inhibition was calculated with the formula: % of inhibition = (1 − SI with inhibitor / SI without inhibitor) × 100.
Results
Microbial Peptides Block EAE Induction with gpSCH in the (PLSJL/J)F1 Mouse.
We asked whether microbial peptides with structural similarities to the self-peptide MBPp85–99 would block EAE. We made slight modifications of a protocol used previously to prevent EAE by APLs (23). Mice were injected intradermally twice at 10-d intervals with 0.1 mg of peptide in IFA. 10 d after the last injection the animals were challenged with gpSCH in order to induce EAE. As seen in Table I, the incidence, mean day of onset, and mean peak severity were significantly lower for papilloma virus 40 (VHFFR), papilloma virus 32 (VHFFH), and Bacillus subtilis open reading frame (ORF) (DFFK) than for the IFA alone control or the MBPp85–99/IFA control (P < 0.001). Mice injected with HPV 7 VHFFK, HPV 13 VHFFK, or the native peptide MBPp85–99 have an increased disease incidence, compared with microbial sequences mutated at the main or secondary TCR contact sites, 91K and 88H respectively, although there was a significant delay of disease onset and mean maximal disease score when compared with IFA control mice (P < 0.01). Nevertheless, only peptides from HPV 40 and Bacillus subtilis ORF were effective in amelioration of all the clinical parameters, clinical incidence, mean day of onset, and mean peak severity of disease, (P < 0.001). Thus, viral peptides with limited homology to a self-MBP peptide induce protection to EAE in (PLSJL/J)F1 mice. As seen in Table I, when the motif found in MBPp85–99 is mutated from VHFFK (found in MBP and in HPV 7) to VHFFR in HPV 40, or VHFFH in HPV 32, these microbial peptides reduce EAE induced with gpSCH from 100% incidence (20 out of 20 with IFA alone and 31 out of 40 with MBPp85–99 or HPV 7), to 20% incidence (8 out of 40 with HPV 40 or 32) (P < 0.01). Thus, a mutation of the major TCR contact site K91 abrogates EAE, a T cell–mediated disease, whereas mutation of the secondary TCR binding site at H88 in Bacillus subtilis ORF totally eliminates disease (P < 0.001). Since EAE is a T cell–mediated autoimmune disease, these studies support the idea that the microbial sequences mutated at 91K or 88H are APLs: Paul M. Allen, the discoverer of APLs, defines them in this way, “We subsequently defined the term ‘altered peptide ligand' to describe analogues of immunogenic peptides in which the TCR contact sites have been manipulated. While these peptides do not stimulate T cell clonal proliferation, they nevertheless have the capacity to activate some TCR-mediated effector functions. Other peptide analogues do not stimulate any detectable function from the T cells and are simply termed peptide analogues” (24). Another refinement of the definition in 1998 by Allen again states: “These peptides bind to [MHC class II] with similar affinities, but have different potencies to induce T cell functions: [two substitutions] do not cause T cell proliferation at any concentration, and [one] causes minimal proliferation only at the highest concentrations; all these peptides, however can act as antagonists” (25). The peptides used in this study are the same: they are APLs that do not cause proliferation of native peptide with VHFFK, but nevertheless antagonize it, and bind with similar affinities to MHC class II (see Table III). They significantly alter the ability of MBPp87–99 TCR to induce EAE. It is important to note that HPV 7, containing the HFFK motif, cross-reacts with MBPp87–99. Thus an HPV 7 peptide can stimulate MBPp87–99 T cell lines and induce relapsing EAE. Likewise, MBPp87–99 can cross-stimulate an HPV 7 T cell line and induce relapsing EAE (1). If the K91 is mutated as it is in HPV 32 or 40, EAE is prevented. This dramatic change in T cell phenotype, induction of autoimmune disease versus maintenance of self-tolerance when mutations are made at TCR contact sites, allow these microbial mutant peptides to be called APLs.
Table I.
Clinical Parameters in (PLSJL/J)F1 Mice Immunized with Microbial Mimicry Peptides and Challenged for EAE Induction with gpSCH
| IFA immunization* | EAE challenge | Percentage of incidence | Mean day of disease onset | Mean peak disease severity | ||||
|---|---|---|---|---|---|---|---|---|
| IFA alone | gpSCH | 100 | 9 ± 1 | 5 | ||||
| MBPp85–99 (ENPVVHFFKNIVTPR) | gpSCH | 95 | 16 ± 2‡ | 3.6 ± 0.9‡ | ||||
| HPV 7 (IGGRVHFFKDISPIA)§ ‖ | gpSCH | 60 | 17 ± 2‡ | 2.3 ± 1.2‡ | ||||
| HPV 13 (IGGRVHFFKDISPIS) | gpSCH | 60 | 16 ± 3‡ | 2.3 ± 0.4‡ | ||||
| HPV 40 (IGGRVHFFRDISPIG) | gpSCH | 20 | 21¶ | 1¶ | ||||
| Bacillus subtilis ORF (RKVVTDFFKNIPQRI) | gpSCH | 0 | 0¶ | 0¶ | ||||
| HPV 32 (IGSRVHFFHDISPIT) | gpSCH | 20 | 13¶ | 3‡ |
Groups of 20 animals were used for data analysis.
P < 0.01 by Student's t test compared with immunization with IFA alone.
Amino acid homologies with the peptide MBPp87–99 are in bold.
Note that the HPV 7 sequence we use is three amino acids shorter at its NH2 terminus than that used by Ufret-Vincenty et al. (1).
P < 0.001 by Student's t test compared with immunization with MBPp87–99.
T Cell Lines Isolated from Mice Immunized with Mimicry Microbial Peptides Produce IL-4 and γ-IFN and Do Not Cross-react with the Native MBPp87–99.
To analyze the immune responses in the protected animals, T cell lines specific for the viral peptides were generated. After the mice recovered from the acute phase of disease, draining lymph node cells were isolated and restimulated in vitro with either MBP or various viral peptides. Table II shows cytokine profiles of T cell lines isolated from the experimental animals. T cell lines stimulated with HPV 13 and Bacillus subtilis ORF produced both IL-4 and γ-IFN, whereas the T cell line stimulated with MBPp85–99 produced IL-4, but not γ-IFN. T cells stimulated with HSV VP16 peptide, used as a control, lacking the HFFK motif (DMTPADALDDRDLEM), failed to proliferate or produce IL-4 or γ-IFN. These experiments demonstrate that IFA is not critical in the protective effect of these viral peptides, as the T cell lines were derived from animals injected with CFA and antigen. It also indicates that within these animals it is possible to select for lines that can be stimulated by sequences from these viral peptides. Once these lines have been selected, there is no cross-reactivity between the viral peptides and MBP. However, in draining lymph nodes from mice injected with CFA MBPp85–99, T cell responses to MBPp85–99 can be inhibited by viral peptides mutated at the main TCR contact site 91K, but retaining the capacity to bind to MHC class II, I-As (see Table III below).
Table II.
Proliferative Responses and Cytokine Profile of T Cell Lines Specific for Microbial Mimicry Peptides
| T cell line specificity | Stimulus* | Proliferation | IL-4‡ | γ-IFN‡ | ||||
|---|---|---|---|---|---|---|---|---|
| CPM | ng/ml | ng/ml | ||||||
| MBPp85–99 | MBPp85–99 | 52,580 | 0.14 | BD | ||||
| MBPp85–99 | HS VP16 | 4,691 | BD | BD | ||||
| MBPp85–99 | none | 3,479 | BD | BD | ||||
| HPV 13 | HPV 13 | 95,834 | 0.16 | 0.295 | ||||
| HPV 13 | MBPp85–99 | 12,955 | BD | BD | ||||
| HPV 13 | none | 11,938 | BD | BD | ||||
| Bacillus subtilis ORF | Bac sub ORF | 8,626 | 0.14 | 0.251 | ||||
| Bacillus subtilis ORF | MBPp85–99 | 2,430 | BD | BD | ||||
| Bacillus subtilis ORF | none | 1,557 | BD | BD |
T cells were isolated from experimental mice after recovery from the acute phase of disease and expanded in vitro by stimulation with the specific peptide. Proliferative responses are shown for each line responding to an antigen concentration of 0.01 mg/ml.
Cytokine concentration was measured from supernatants collected after 48 h of in vitro stimulation assays of T cell lines with a peptide concentration of 0.01 mg/ml. Levels <0.01 ng/ml are reported as below detection limits (BD).
T Cell Lines Isolated from Mice Immunized with Mimicry Microbial Peptides Protect from EAE.
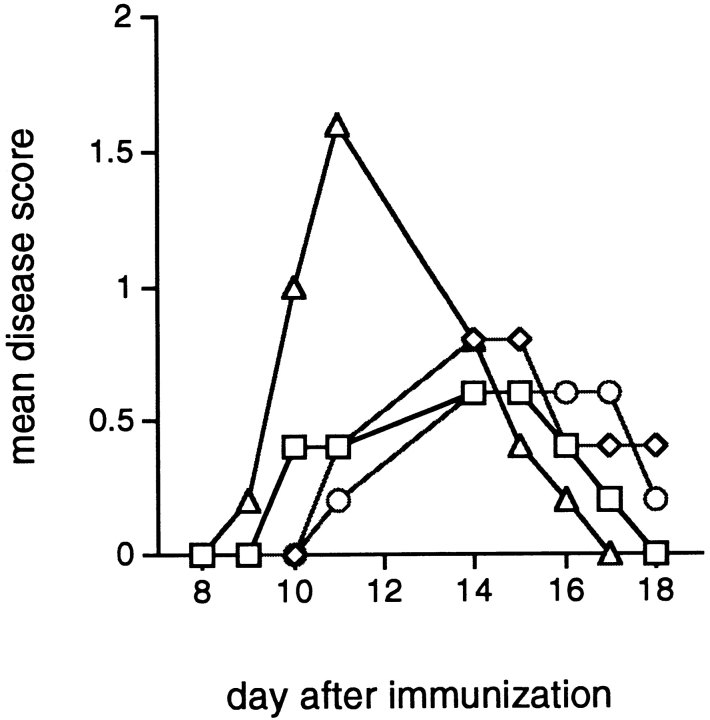
To study the mechanism of disease protection by the mimicry peptides, T cells isolated from the protected mice and expanded in vitro were reinjected to naive animals and those mice were challenged for EAE induction by gpSCH. As seen in Fig. 1, the transfer of activated T cells specific for viral peptides from HPV 13 and from Bacillus subtilis ORF and with sequences similar to MBPp87–99 protects from EAE (P < 0.01 when comparing mean disease score to animals given the T cell line specific for HSV VP16). A Th2 line specific for MBPp87–99 also protects from EAE (P < 0.01) (Fig. 1).
Figure 1.
Prevention of EAE by passive transfer of T cell lines specific for microbial mimicry peptides. Mice were injected intraperitoneally with 5 × 106 T cells specific for either MBPp85–99 (□), HPV 13 peptide (⋄), Bacillus subtilis ORF peptide (○), or HSV VP16 peptide (▵). 10 d after cell transfer, mice were challenged for EAE by immunization with gpSCH. Results are expressed as mean disease score in groups of five animals.
Mimicry Microbial Peptides Bind to MHC Class II I-As Molecules and Inhibit Anti-MBPp85–99 T Cell Proliferative Responses.
We tested the binding of the mimicry microbial peptides to affinity purified I-As molecules. As shown in Table III, all the peptides tested had intermediate binding to the class II molecules (IC50 in the 100–1,000 nM range). The possibility that sequence similarity between microbial peptides used in our experiments and the native MBPp85–99 peptide would influence antigen specific responses against the native MBPp85–99 peptide was tested by in vitro inhibition assay. 10 d after immunization of (PLSJL/J)F1 mice with the MBPp85–99 peptide in CFA, draining lymph node cells were tested for the proliferative response to the immunizing peptide alone or together in the presence of mimicry microbial peptides as inhibitors. As observed in Table III, at 1:1 molar ratio the HPV 7 peptide inhibited proliferative responses 62%, HPV 40 peptide inhibits 39%, and Herpes simplex DNA polymerase peptide inhibits 39%. Therefore, cross-reactivity is observed between the native MBPp85–99 peptide and the mimicry microbial peptides at the level of inhibition of proliferative responses. Inhibition of proliferative responses may have been due to the capacity of the microbial peptides to mimic native MBP, and to bind to the MHC class II I-As molecules, partially inhibiting presentation of the MBPp85-99 to specific T cells.
Microbial Peptides Protect from EAE in an Epitope-specific Manner: Lack of a Bystander Effect.
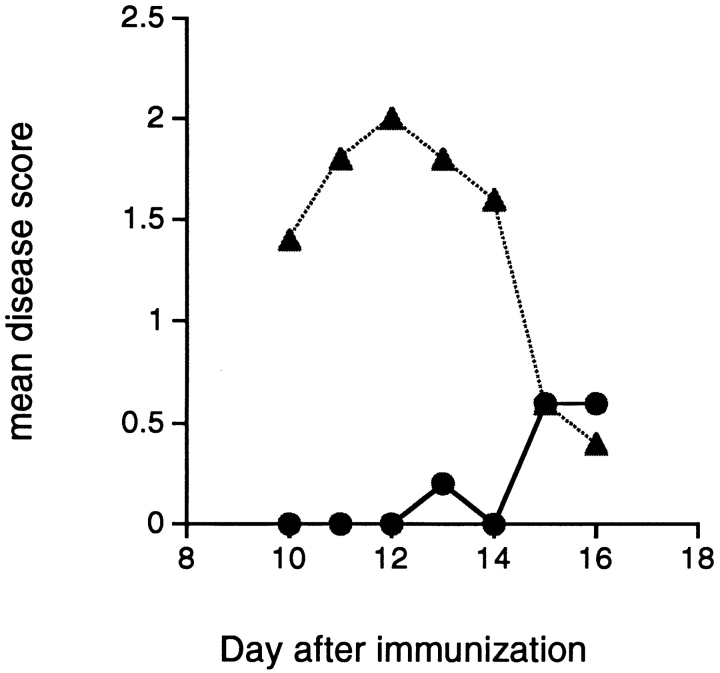
To rule out a bystander effect by the microbial mimicry peptides, we compare the protective effect in EAE induced by either gpSCH or MBPpAc1–11. In Fig. 2, we show that the Bacillus subtilis peptide injected into IFA inhibits induction of EAE induced by gpSCH, but does not inhibit disease induced by MBPpAc1–11. Therefore, the regulatory effect of mimicry peptides requires the presence of the MBPp85–99 epitope in the EAE-inducing antigen.
Figure 2.
A microbial peptide with homology to MBPp85–99 inhibits the induction of EAE by gpSCH but does not prevent disease induction by MBP Ac1–11. Mice were immunized with the a microbial peptide from Bacillus subtilis and challenged for disease with either gpSCH (•) or MBPpAc1–11 (▴) in CFA. Results are expressed as mean disease score in groups of five animals.
HPV 40 Peptide Acts as a TCR Antagonist.
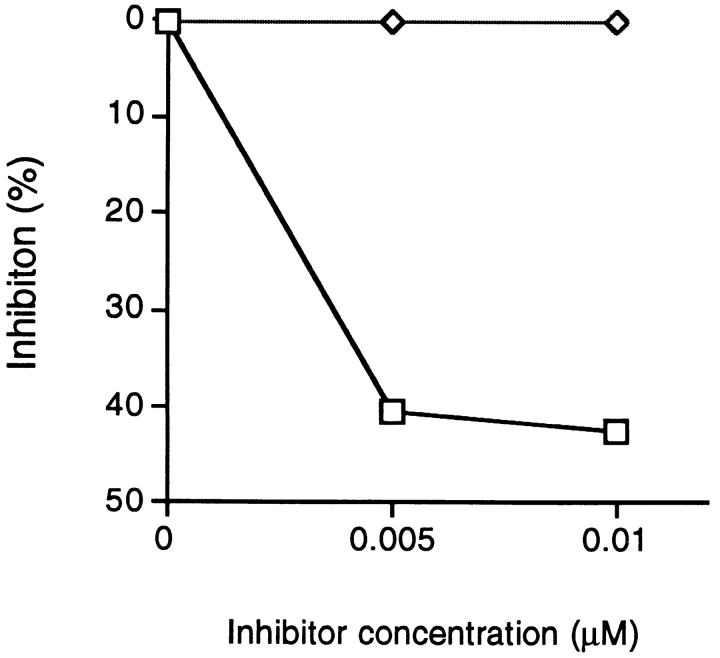
We tested for TCR antagonism using an assay by Karin et al. (23). In this assay we inhibit the proliferative response to wild-type MBPp85–99 by prepulsing irradiated splenic APCs with 0.005 mM of MBPp85–99, then washing and using these APCs to stimulate an MBPp85–99–specific T cell line in the presence of a putative antagonist. The peptide HPV 40 (containing the motif VHFFR) inhibited proliferation by 40%, whereas a control peptide lacking the HFFK motif did not inhibit proliferation at all (Fig. 3).
Figure 3.
TCR antagonism by a peptide from HPV 40. APCs prepulsed with MBPp85–99 (0.005 μM) were used for stimulation of a T cell line (L35) specific for the peptide MBPp85–99, in the presence of a mimicry microbial peptide (□) or a peptide lacking the core motif of MBPp85–99 (⋄). Results are expressed as percentage of inhibition of T cell proliferative responses. Proliferation of the L35 line in the presence of prepulsed cells was 63773.6 CPM. Proliferation of the L35 line in the presence of APCs alone was 423 CPM.
Discussion
“In MS—as in other autoimmune diseases—there has been feverish debate between those who believe that the disease is triggered by an environmental agent and those who champion a genetic basis. But there is actually a reasonable reconciliation of these opposing views: certain genes conferring susceptibility to the disease and certain factors in the environment are both critical for the development of autoimmunity, and this is particularly true for MS” (7).
Molecular mimicry provides a scheme whereby viral sensitization in the blood leads to activation of T cells (26). These enter the brain where they encounter their cognate mimic in myelin. We have detected a number of microbes whose amino acid sequences can activate anti-myelin T cells from MS patients, as well as bind to anti-myelin antibodies eluted from MS brain material (19, 21). Molecular mimicry also allows for reconciliation of the genes versus the environment debate: genomic searches for genes linked to MS susceptibility reveal that the most important gene in determining susceptibility to MS is HLA (28–30). HLA is of course critical for selecting the appropriate mimic and presenting it to the immune system. Moreover, many different viruses mimic various parts of the myelin sheath, so inflammation in the white matter of the brain may ensue from an immune response to a variety of microbes. Thus, the hope of finding the virus that triggers MS may remain elusive forever (7).
Our study shows how molecular mimics may modulate autoimmune disease. Earlier work by Gautam et al. (31) had demonstrated that a polyalanine peptide with only five native MBP residues is able to induce EAE in (PLSJL/J)F1 mice. Further analysis also showed that an 11-amino acid peptide, consisting mostly of alanines with only four native Ac1–11 residues, was able to induce T cell hybridoma proliferation. Taking an approach of introducing either d-amino acids or unnatural amino acids in place of l-amino acids into MBPpAc1–11 analogues, we showed that T cells recognize only a short stretch of six or seven amino acids. More importantly, this stretch contains only four native MBPpAc1–11 residues. We also tested T cell recognition in vivo, using EAE as a measure of activation. We show that a short peptide of six amino acids with a core of only five native Ac1–11 amino acids induces EAE (31, 32).
A herpes virus Saimiri (HVS) peptide, AAQRRPSRPFA, with a limited homology to MBP1–11 peptide, ASQKRPSQRHG, (bold letters show homology) can stimulate a panel of MBP1–11–specific T cell hybridomas, and more importantly cause EAE in mice. We demonstrate that this is due to cross recognition of these two peptides by TCRs. This HSV peptide with homology at just five amino acids with a self-peptide can induce clinical signs and histologic evidence of EAE in mice (33).
Relapsing EAE has been induced with two peptides bearing the HFFK motif containing the primary TCR and MHC contact for I-E in the Lewis rat, I-A in the SJL mouse, and DRB1*1501 in humans (5, 17, 18, 23). Using a passive transfer protocol, T cells specific for an HPV 7 peptide (IGGRVHFFKDISPIASSE) were found to induce relapsing EAE. These T cells could be activated by MBPp87–99 to induce EAE, and MBPp87–99 T cells could also be stimulated by the HPV 7 peptide to induce EAE. Active EAE with this papilloma peptide was also induced. Another viral peptide from EBV (RAHPVYFFKSACPPA) could activate the papilloma virus–specific T cells and induce EAE by passive transfer (1).
These results have practical significance for the success of APL therapy in MS patients. The APLs now in Phase II clinical trials in MS (2) have a K→ A substitution at position 91 and thus, neither bind anti-MBP antibody nor trigger MBP-specific T cells. Administration of soluble native versions of myelin antigens may have dangerous consequences. Genain et al. showed that EAE induced in marmosets by immunization with myelin oligodendroglial glycoprotein (MOG) could be delayed by intraperitoneal treatment with soluble MOG; however, treated animals developed a severe late form of the disease (34). In these animals, MOG-specific T cell proliferative responses were transiently suppressed, cytokine profiles were shifted from a Th1- to a Th2-type pattern, titers of autoantibodies to MOG were enhanced, and autoimmune disease was exacerbated (34). This implies that provoking a vigorous anti-myelin reaction with a native peptide could have dangerous consequences in a clinical setting (35).
APLs work in part by altering cytokine production in T cells that respond to self-antigens (36–40). For example, in the Lewis rat administration of MBPp87–99 (K91→ A), an APL altered at the primary TCR contact residue K91, reversed paralysis in EAE, and reduced production of the proinflammatory cytokine TNF-α (23). Another APL, MBPp87–99 (96P→ A), reversed paralysis in EAE and increased production of IL-4 at the site of disease (5). The effect of this APL was reversed by the in vivo administration of anti–IL-4 antibody (5).
In the studies presented here, T cell lines with specificity for viral sequences that resemble MBPp85–99, but were not identical to MBPp85–99, produced IL-4 and γ-IFN, two cytokines known to suppress EAE (5, 41–45). Despite the fact that systemic administration of γ-IFN is protective in EAE, γ-IFN can induce MHC class II on astrocytes (46), and allow these glial cells to present myelin antigens to encephalitogenic T cells. Although in EAE systemic administration of γ-IFN is protective, in MS administration of γ-IFN provokes exacerbation of disease (47).
Interestingly, a T cell line specific for MBP that produced IL-4 was also able to suppress EAE (Fig. 1). Both IL-4 and γ-IFN are capable of suppressing EAE. Thus, lines specific for microbial sequences like HPV 13 or Bacillus subtilis ORF produce both IL-4 and γ-IFN and suppress EAE, whereas a T cell line specific for MBP producing IL-4 and no γ-IFN also protects. From previously published work we know that antibody to γ-IFN and antibody to IL-4 both exacerbate EAE (5, 48). Thus, the production of antiinflammatory cytokines like IL-4 by T cells responding to microbes, whose sequences resemble but are not identical to the self-epitope MBPp87–99, has potent effects on in vivo disease. These T cell lines can inhibit EAE in (PLSJL/J)F1 animals, induced by gpSCH. This homogenate contains epitopes such as MBPpAc1–11, and MBPp35–47, as well as PLP epitopes, that dominate the pathogenic response (8– 10, 13). The trans-acting effect of such T cells, producing antiinflammatory cytokines, is thus able to shut down a diversity of immune responses (2).
Suppression of autoimmune disease with microbial sequences can also be achieved by naked DNA immunization with minigenes encoding the core motif of MBPp85–99 (48a). Thus, microbial genomes may direct immunization via the information encoded in the DNA itself, and this immunization might include sensitization to altered peptides of self (49, 50).
The notion that microbial sequences can act as APLs and suppress autoimmune disease appears to be novel. Combined with the observation that microbial mimics can also induce EAE (1, 33, 51), the concept of molecular mimicry provides a framework for explaining the modulation of immune responses to self, both in the development of autoimmune disease and in protection from autoimmunity.
A recent report by Zhao et al. demonstrated that a coat protein of HSV type 1 could be recognized by autoreactive T cells that target corneal antigens in a murine model of autoimmune herpes stromal keratitis (52). Mutant HSV type 1 viruses that lacked this epitope did not induce autoimmune disease. Our report reveals that mutant viruses can block autoimmunity, and this appears to be a novel observation.
The interaction of the immune system with microbes may allow the selection of viral subtypes. It is interesting to speculate that attenuation of the immune response by a peptide derived from a papilloma viral subtype, containing an APL-like motif, may be desirable for viral survival. A virus that can subvert immunity might be selected because it could survive and persist, instead of being eradicated in the wake of an autoimmune response. The degeneracy of T cell recognition of MBPp87–99 has been clearly shown with combinatorial peptide libraries (53). At least in the case of T cells specific for MBPp87–99, there may be a delicate physiological interplay between self- and microbial antigens, allowing the modulation of autoimmune disease and the persistence and survival of mutant microbes. Attenuating inflammation in the brain may allow microbes to survive, sequestered within the central nervous system. It is remarkable that certain viral subtypes are mutated exactly at a main TCR contact site, and such mutations may represent an adaptive response of a virus, which then acts as an APL.
Acknowledgments
We thank Ms. Teri Montgomery for the preparation of the manuscript, and Dennis J. Mitchell for excellent technical assistance.
This work was funded by the National Institutes of Health (grant Nos. NS18235 and NS28759) and the Phil N. Allen Trust.
Abbreviations used in this paper
- APL
altered peptide ligand
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- gpSCH
guinea pig spinal cord homogenate
- HPV
human papilloma virus
- HSV
herpes simplex virus
- HVS
herpes virus Saimiri
- MBP
myelin basic protein
- MOG
myelin oligodendroglial glycoprotein
- MS
multiple sclerosis
References
- 1.Ufret-Vincenty R, Quigley L, Tresser N, Pak SH, Gado A, Hausmann S, Wucherpfennig KW, Brocke S. In vivo survival of viral antigen-specific T cells that induce experimental autoimmune encephalomyelitis. J Exp Med. 1998;188:1725–1738. doi: 10.1084/jem.188.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman L, Conlon P. Viral damage and the breakdown of self-tolerance. Nat Med. 1997;3:1085–1087. doi: 10.1038/nm1097-1085. [DOI] [PubMed] [Google Scholar]
- 3.Miller SD, Vanderblugt CL, Begolks WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann P, Forsthuber T, Miller A, Sercarz EE. Spreading of T cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 5.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, et al. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379:343–345. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 6.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 7.Steinman L, Oldstone MBA. More mayhem from molecular mimics. Nat Med. 1997;3:1321–1322. doi: 10.1038/nm1297-1321. [DOI] [PubMed] [Google Scholar]
- 8.Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard J. T cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 9.Sakai K, Sinha A, Mitchell DJ, Zamvil SS, McDevitt HO, Rothbard JB, Steinman L. Involvement of distinct T cell receptors in the autoimmune encephalitogenic response to nested epitopes of myelin basic protein. Proc Natl Acad Sci USA. 1988;85:8608–8612. doi: 10.1073/pnas.85.22.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai K, Zamvil SS, Mitchell DJ, Lim M, Rothbard JB, Steinman L. Characterization of a major encephalitogenic T cell epitope in SJL/J mice with synthetic oligopeptides of myelin basic protein. J Neuroimmunol. 1988;19:21–32. doi: 10.1016/0165-5728(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 11.Zamvil SS, Mitchell D, Power M, Sakai K, Rothbard J, Steinman L. Multiple discrete epitopes of the autoantigen myelin basic protein. J Exp Med. 1988;168:1181–1186. doi: 10.1084/jem.168.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acha-Orbea H, Mitchell DJ, Timmerman L, Wraith DC, Waldor MK, Tausch GS, Zamvil SS, McDevitt HO, Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988;54:263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- 13.Wraith DC, Smilek DE, Mitchell DJ, Steinman L, McDevitt HO. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide mediated immunotherapy. Cell. 1989;59:247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- 14.Yu M, Johnson JM, Touchy VK. A predictable sequential determinant spreading cascade invariably accompanies progression of EAE: a basis for peptide-specific therapy after onset of clinical disease. J Exp Med. 1996;183:1771–1778. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholson LB, Murtaza A, Hafler DA, Sette A, Kuchroo VJ. A TCR antagonist peptide induces T cells that mediate bystander suppression and prevent autoimmune encephalomyelitis induced with multiple myelin antigens. Proc Natl Acad Sci USA. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oksenberg JR, Panzara MA, Begovich AV, Mitchell D, Erlich HA, Murray RS, Shimonkevitz R, Sherritt M, Rothbard J, Bernard CCA, Steinman L. Selection for T cell receptor Vβ-Dβ-Jβ gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993;362:68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- 17.Wucherpfennig KW, Sette A, Southwood S, Oseroff C, Matsui M, Strominger JL, Hafler DA. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994;179:279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt AB, Kropshofer H, Kalbacher M, Kalbus H, Rammensee HG, Coligan J, Martin R. Ligand motifs of HLA BRB5*0101 and DRB1*1501 molecules delineated from self-peptides. J Immunol. 1994;151:1665–1673. [PubMed] [Google Scholar]
- 19.Wucherpfennig KW, Catz I, Hausmann S, Strominger JL, Steinman L, Warren KG. Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2 restricted T cell clones from multiple sclerosis patients: identity of key contact residues in the B cell and T cell epitopes. J Clin Invest. 1997;100:1114–1122. doi: 10.1172/JCI119622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sette A, Buus S, Colon SM, Miles C, Grey HM. Structural analysis of peptides capable of binding to more than one Ia antigen. J Immunol. 1989;142:35–40. [PubMed] [Google Scholar]
- 23.Karin N, Mitchell D, Ling N, Brocke S, Steinman L. Reversal of experimental autoimmune encephalomyelitis by a soluble variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon γ and tumor necrosis factor α production. J Exp Med. 1994;180:2227–2237. doi: 10.1084/jem.180.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanism and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Kersh EN, Shaw A, Allen PM. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 26.Oldstone MBA. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- 27.Oldstone MBA. Viruses and autoimmune diseases. Scand J Immunol. 1997;46:320–325. doi: 10.1046/j.1365-3083.1997.d01-145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpuj MV, Steinman L, Oksenberg JR. Multiple sclerosis: a polygenic disease involving epistatic interactions, germline rearrangements and environmental effects. Neurogenetics. 1997;1:21–28. doi: 10.1007/s100480050003. [DOI] [PubMed] [Google Scholar]
- 29.Multiple Sclerosis Genetics Group. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatibility complex. Nat Genet. 1996;13:469–471. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- 30.Sawcer S, Jones HB, Feakes R, Gray J, Smaldon N, Chataway J, Robertson N, Clayton D, Goodfellow PN, Compston A. A genome screen in MS reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet. 1996;13:464–468. doi: 10.1038/ng0896-464. [DOI] [PubMed] [Google Scholar]
- 31.Gautam AM, Pearson C, Smilek D, Steinman L, McDevitt HO. A polyalanine peptide containing only five native myelin basic protein residues induces autoimmune encephalomyelitis. J Exp Med. 1992;176:605–609. doi: 10.1084/jem.176.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautam AM, Lock C, Smilek DE, Pearson CI, Steinman L, McDevitt HO. Minimum structural requirements for peptide presentation by major histocompatibility complex class II molecules: implications in induction of autoimmunity. Proc Natl Acad Sci USA. 1994;91:767–771. doi: 10.1073/pnas.91.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautam AM, Liblau R, Chelvanayagam G, Steinman L, Boston T. A viral peptide with limited homology to a self peptide can induce clinical signs of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:60–64. [PubMed] [Google Scholar]
- 34.Genain CP, Abel K, Belmar N, Vilinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- 35.Garren H, Steinman L, Lock C. The specificity of the antibody response in multiple sclerosis. Ann Neurol. 1998;43:4–6. doi: 10.1002/ana.410430105. [DOI] [PubMed] [Google Scholar]
- 36.Evavold BD, Allen P. Separation of IL–4 production from Th cell proliferation by an altered TCR ligand. Science. 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 37.Adorini L, Muller S, Cardinaux F, Lehmann PV, Falcioni F, Nagy ZA. In vivo competition between self peptides and foreign antigens in T cell activation. Nature. 1988;334:623–625. doi: 10.1038/334623a0. [DOI] [PubMed] [Google Scholar]
- 38.Windhagen A, Scholz C, Hollsberg P, Fukuara H, Sette A, Hafler D. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity. 1995;2:373–380. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 39.Vergelli M, Hemmer B, Utz U, Vogt A, Kalbus M, Tranquill L, Conlon P, Ling N, Steinman L, McFarland H, Martin R. Differential activation of human autoreactive T cell clones by altered peptide ligands derived from myelin basic protein peptide (87–99) Eur J Immunol. 1996;26:2624–2634. doi: 10.1002/eji.1830261113. [DOI] [PubMed] [Google Scholar]
- 40.Gaur A, Boehme SA, Chalmers D, Crowe PD, Pahuja A, Ling N, Brocke S, Steinman L, Conlon P. Amelioration of relapsing experimental autoimmune encephalomyelitis with altered myelin basic protein peptides involves different cellular mechanisms. J Neuroimmunol. 1997;74:149–158. doi: 10.1016/s0165-5728(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 41.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billiau A, Hereman H, Vanderkerckhove F, Dijkmans R, Sobis H, Muelepas E, Carton H. Enhancement of EAE in mice by antibodies to IFN-γ. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- 43.Krakowski M, Owens T. Interferon-γ confers resistance to EAE. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 44.O'Garra A, Steinman L, Gijbels K. CD4+ T cell subsets in autoimmunity. Curr Opin Immunol. 1997;9:872–883. doi: 10.1016/s0952-7915(97)80192-6. [DOI] [PubMed] [Google Scholar]
- 45.Ferber I, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of EAE. J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 46.Fierz W, Endler B, Reske K, Wekerle H, Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression in astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985;134:3785–3793. [PubMed] [Google Scholar]
- 47.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbation associated with activation of the immune system. Neurology. 1987;37:1097–1101. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 48.Gijbels K, Brocke S, Abrams J, Steinman L. Administration of neutralizing antibodies to IL-6 reduces EAE and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1:795–805. [PMC free article] [PubMed] [Google Scholar]
- 48a.Ruiz PJ, Garren H, Ruiz IU, Hirschberg DL, Nguyen LV, Karpuj MV, Cooper MJ, Mitchell DJ, Fathman CG, Steinman L. Suppressive immunization with DNA encoding a self-peptide prevents autoimmune disease: modulation of T cell costimulation. J Immunol. 1999;162:3336–3341. [PubMed] [Google Scholar]
- 49.Cohen IR, Steinman L. Exploring the potential of DNA vaccination. Hosp Pract (Off Ed) 1997;32:169–178. doi: 10.1080/21548331.1997.11443488. [DOI] [PubMed] [Google Scholar]
- 50.Waisman A, Ruiz PJ, Hirschberg DL, Gelman A, Oksenberg JR, Brocke S, Mor F, Cohen IR, Steinman L. Suppressive vaccination with DNA encoding a variable region gene of the T cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat Med. 1996;2:899–906. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 51.Fujinami RS, Oldstone MBA. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z, Granule F, Yeah L, Schooner P, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1988;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 53.Hemmer B, Fleckenstein BT, Vergelli M, Jung G, McFarland H, Martin R, Wiesmuller K. Identification of high potency microbial and self-ligands for a human autoreactive class II–restricted T cell clone. J Exp Med. 1997;185:1651–1659. doi: 10.1084/jem.185.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]