Abstract
To investigate the potential involvement of T helper (Th)2-type responses in murine models of intestinal inflammation, we used trinitrobenzene sulfonic acid (TNBS)–hapten to induce inflammatory bowel disease in situations where Th1-type responses with interferon (IFN)-γ synthesis are either diminished or do not occur. Intracolonic administration of TNBS to either normal (IFN-γ+/+) or Th1-deficient IFN-γ knockout (IFN-γ−/−) BALB/c mice resulted in significant colitis. In IFN-γ−/− mice, crypt inflammation was more severe than in IFN-γ+/+ mice and was accompanied by hypertrophy of colonic patches with a lymphoepithelium containing M cells and distinct B and T cell zones resembling Peyer's patches. Hapten-specific, colonic patch T cells from both mouse groups exhibited a Th2 phenotype with interleukin (IL)-4 and IL-5 production. TNBS colitis in normal mice treated with anti–IL-4 antibodies or in IL-4−/− mice was less severe than in either IFN-γ+/+ or IFN-γ−/− mice. Our findings now show that the Th2-type responses in TNBS colitis are associated with colonic patch enlargement and inflammation of the mucosal layer and may represent a model for ulcerative colitis.
Keywords: inflammatory bowel diseases, mouse T cells, cytokines, hapten-induced colitis
The normal mucosal immune system in the gastrointestinal tract maintains a delicate balance between immunity to microbial pathogens and tolerance to food antigens and indigenous microflora. Despite its importance in protecting against disease and maintaining homeostasis, mucosal immunity is only partially understood. What is clear is that the mucosal immune system can be divided into inductive sites and effector regions. The former are termed gut-associated lymphoreticular tissues (GALT)1 and include Peyer's patches, whereas the latter is a lamina propria characterized by more diffuse collections of lymphocytes and plasma cells. Active induction of selected immune responses in the GALT has been shown to lead to either upregulation of antipathogenic responses or to actual suppression of systemic immunity to ubiquitous oral antigens.
Inflammatory bowel diseases (IBD) represent chronic, relapsing, and tissue-destructive diseases. Although the etiology of IBD remains unknown, there is circumstantial evidence to link IBD to the mucosal immune system's failure to attenuate immunity to luminal antigens (1, 2). A recent study has shown a primary role for T cells in IBD by demonstrating that anti-CD4 monoclonal antibodies are effective in treating the disease (3). Various experimental murine models of IBD also support a central role for T cells in chronic intestinal inflammation (4–8). These models were characterized by an imbalance of regulatory cytokines, most notably by an excessive production of IFN-γ. A central role for IFN-γ has been reported in murine colitis following transfer of CD45RBhigh T cells to scid mice (4, 9, 10), by adoptive transfer of T cell–depleted bone marrow cells from normal mice into T cell–deficient CD3ε-transgenic mice (11), and in IL-10−/− mice, which spontaneously develop a severe focal inflammation in both the small and large intestines (12).
Another important mouse model has been introduced to study specific T cell subsets in the intestinal inflammation resulting from 2,4,6-trinitrobenzene sulfonic acid (TNBS)- induced colitis, a system first established in rats (13). The colonic administration of TNBS in 50% ethanol has been shown to induce a chronic colitis (14, 15) as a result of covalent binding of TNP residues to autologous host proteins with subsequent stimulation of delayed-type hypersensitivity to the TNP-modified self antigens (16). Previous studies using SJL/J mice have emphasized that Th1-type responses with production of IL-2 and IFN-γ are associated with this induced colitis, and treatment with anti–IL-12 antibody markedly decreased the severity of TNBS-induced colitis (14, 17).
Thus, most mouse IBD models are associated with activated T cells producing cytokines characteristic of a Th1 phenotype, a finding in agreement with clinical observations of Crohn's disease (18–20). However, as the production of Th1-type cytokines is not as pronounced in ulcerative colitis as in Crohn's disease (21, 22), we hypothesized that a Th2-type response is also operative in the ulcerative colitis type of chronic intestinal inflammation. In this study, we examined the development of TNBS-induced inflammation in cytokine-deficient mice to determine whether the colitis that develops could be associated with a Th2-type cytokine array. Our results provide the first evidence that Th2-type responses are predominant in TNBS colitis in BALB/c mice and that the resultant disease is characterized by hypertrophy of colonic patches. Furthermore, we show that mice undergoing Th2-type responses develop a disease that more closely resembles ulcerative colitis than Crohn's disease.
Materials and Methods
Mice.
Normal (IFN-γ+/+), IFN-γ gene–disrupted (IFN-γ−/−), and IL-4 gene–disrupted (IL-4−/−) mice, all on the BALB/c background, were purchased from The Jackson Laboratory. Mice were kept in microisolator cages in animal facilities at The University of Alabama at Birmingham Immunobiology Vaccine Center. Mice were provided sterile food and water ad libitum and were free of microbial pathogens as determined by antibody screening and routine histologic analysis of organs and tissues. All mice used in this study were between 10 and 16 wk of age.
Hapten-induced Colitis.
Mice were given a solution of TNBS (Research Organics) dissolved in a mixture of PBS, pH 7.2, and then mixed with an equal volume of ethanol for a final concentration of 2% TNBS in 50% ethanol. Enemas were performed on mice anesthetized with ketamine/xylazine with a glass microsyringe equipped with a gastric intubation needle. One series of experiments was performed to compare the sensitivity of IFN-γ+/+ or IFN-γ−/− mice to TNBS colitis. In brief, groups of mice were given TNBS at a dose of 25, 36, or 50 μg/g of body weight on days 0 and 7. The severity of disease was assessed by weight loss, fur ruffling, rectal prolapse, and death. A dose of 36 μg TNBS/g of body weight was chosen and given on days 0 and 7, and tissues and cells were assessed on day 10.
Treatment with Monoclonal Anticytokine Antibodies.
One set of experiments was performed with mAbs to IFN-γ or IL-4 to determine the roles of these cytokines in the development of TNBS colitis. In brief, mice were given 1 mg of either rat anti–IFN-γ (XMG 1.2) or anti–IL-4 (11B11) mAbs by the intraperitoneal route on the same days as the TNBS enema (days 0 and 7). Control groups received an intraperitoneal dose of normal rat IgG (Jackson ImmunoResearch Labs, Inc.) along with a TNBS enema.
Histological Analysis.
The colon was removed from its mesentery to the pelvic brim by blunt dissection. The pelvis was severed, and the rectum was carefully removed from the sacral lymph nodes (SLN) and adjacent tissue. The distal half of the colon was opened longitudinally and fixed in 5% glacial acetic acid in ethanol (vol/vol). After embedding in paraffin, 4-μm-thick serial sections were prepared and stained with hematoxylin and eosin for histologic grading. Thickness of lymphoid follicles was determined on sections that contained follicles extending from the mucosal layer to the serosa using a micrometer. Histologic grading was done blindly according to the criteria listed in Table I. A maximum score of 8 indicated severe colitis with acute ulcers and an overall diffuse pattern of chronic changes.
Table I.
Histological Scoring System Used in TNBS-induced Colitis
| Histological changes | Scores | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ||||
| Crypt distortion | None | Focal | Numerous or diffuse | |||
| Crypt with loss of goblet cells | None | A few crypts | Many crypts | |||
| Acute inflammation (ulcers with infiltration of polymorphonuclear cells) | None | Superficial | Beyond the layer of Muscularis mucosae | |||
| Chronic inflammation (infiltration of mononuclear cells and fibrosis in the mucosal layer) | No increase | Focal | Multifocal | |||
Immunohistochemistry.
Tissues were freshly frozen in Tissue-Tec OCT compound (Miles-Yeda, Inc.). 7-μm cryostat sections were fixed in ice-cold acetone for 10 min, dried, and rehydrated in Tris-buffered saline. This step was followed by blocking with Tris-buffered saline containing a 1:50 dilution of Fc-blocking 2.4G2 mAb (PharMingen) and 10% heat-aggregated rabbit serum (Sigma Chemical Co.). Sections were stained for 1 h in the same buffer with PE–anti-CD4 mAb (1:50 dilution; PharMingen) and biotinylated anti-B220 mAb (1:50 dilution; PharMingen). These sections were washed with buffer and then stained with streptavidin–FITC (Southern Biotechnology Associates) for 30 min. Reactivity with peanut agglutinin (PNA) was demonstrated using a 1:50 dilution of biotinylated PNA (Vector Labs, Inc.) and streptavidin–FITC. The sections were mounted and viewed using 100× optics and a dual red/green filter. Images from each staining were analyzed for red and green fluorescence using identical settings in Photoshop 4.0 (Adobe Systems, Inc.).
Electron Microscopy.
For transmission electron microscopy, the colon was prefixed with cold 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 h and washed extensively with 0.1 M phosphate buffer. The tissues were then post-fixed with osmium tetraoxide, dehydrated, and embedded in Epon. Sections were cut and examined with an electron microscope.
Preparation of Lymphoid Cells from Tissues.
Peyer's, cecal, and colonic patches were excised from the intestinal wall. The colonic lymphoid follicles in naive mice were identified with a magnifying lens, and ∼3–5 follicles/colon were seen. Patches and follicles were washed once with RPMI 1640 (Cellgro Mediatech) and dissociated with collagenase (type IV; Sigma Chemical Co.) at a concentration of 0.5 mg/ml in RPMI 1640 with 100 U/ml penicillin, 100 μg/ml streptomycin, and 40 μg/ml gentamicin for 20 min at 37°C to obtain single-cell preparations (23). The cell dissociation was repeated two additional times in fresh collagenase solution each time. The single-cell suspensions were then pooled and washed with RPMI 1640 twice more. Mononuclear cells were further purified using a discontinuous Percoll gradient to avoid contamination with epithelial cells. The SLN were teased with forceps, and the resulting cell suspension was washed with RPMI 1640 two additional times. Colonic lamina propria lymphocytes were prepared as previously described with modifications (24). In brief, after excision of all visible lymphoid follicles, colonic tissue was treated with 1 mM EDTA in PBS for 20 min to remove the epithelium. The tissue was then digested with collagenase (type IV; Sigma Chemical Co.) for 20 min, and this step was repeated two more times. The isolated cells were further purified and separated from epithelial cells by centrifugation through a Percoll gradient as described elsewhere (24). The isolated lamina propria lymphocytes were >99% viable.
Flow Cytometry and Cell Sorting.
In these studies, isolated lymphoid cells were stained for various membrane receptors with FITC-, PE-, or biotin-labeled mAbs. Lymphoid cells were first preincubated with an Fc-blocking mAb (clone 2.4G2; PharMingen) at a concentration of 12.5 μg/ml for 5 min on ice. The cells were then incubated with FITC-, PE-, or biotin-labeled antibodies for 30 min in ice. Biotinylated antibodies were detected with PE– or FITC–streptavidin. The following conjugated or unconjugated anti–mouse antibodies were used: anti-CD3 (clone 145-2C11), anti-CD8 (clone 53.6.7), anti-B220 (clone RA3-6B2), anti–TCR-β (clone H57-597), anti–TCR-γ/δ (clone GL3), anti-IgD (11–26c.2a), anti-IgM (clone II/41), anti-IgA (clone R5-140), and anti–I-Ad (clone AMS-32.1) (PharMingen). Goat anti–IgG (Fab′) fragment was purchased from Southern Biotechnology Associates. Two-color analysis was performed with a FACStarPLUS® (Becton Dickinson). In some experiments, cells were stained with FITC-labeled anti-CD4 mAb (clone GK1.5; University of Alabama at Birmingham Core Facility) and subjected to sorting with a FACStarPLUS® to obtain purified CD4+ T cells (>99% CD4+ T cells).
Culture Conditions and Proliferation Assays for T Cells.
For stimulation of antigen-specific T cells, cells were coupled to TNBS. In brief, cells isolated from patches, lymph nodes, or lamina propria were treated with 0.3 mg/ml TNBS in RPMI 1640 for 15 min at room temperature. Cells were then extensively washed and cultured in RPMI 1640 supplemented with 10% FCS, sodium pyruvate, l-glutamine, Hepes, 50 μM 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamicin, and 1 μg/ml amphotericin B (complete medium) at 37°C in an atmosphere of 5% CO2 in air. For the proliferation assay, 2 × 105 cells were placed in each well of 96-well plates, and 0.5 μCi/well of tritiated [3H]thymidine was added 18 h before harvesting. Peyer's, cecal, and colonic patch cells were incubated for 4 d, and SLN cells were incubated for 3 d. The amount of [3H]thymidine incorporation was determined by scintillation counting. Cells cultured without TNBS treatment were used as controls. In some experiments, TNBS-treated cells were cultured in complete medium for 24 h at a concentration of 3 × 106 cells/ml. Culture supernatants were subjected to cytokine-specific ELISA. For mRNA analysis, nonadherent cells were harvested after 24 h of incubation, and CD4+ T cells were purified by flow cytometry. Extracted total RNA was subjected to RT-PCR to assess cytokine-specific mRNA. The CD4+ T cells purified from lamina propria lymphocytes isolated from the colon were subjected to RT-PCR.
Cytokine-specific ELISA.
The details of this assay have been described previously (24–26). In brief, Immunoplates (Nunc MaxiSorp; Nunc, Inc.) were coated with antibodies to individual cytokines and incubated overnight at 4°C. After blocking with 3% BSA in PBS at 37°C for 2 h, diluted samples were added to wells and incubated overnight at 4°C. The wells were then washed and incubated with detecting antibodies, and bound antibody was detected by peroxidase-labeled antibiotin mAb (Vector Labs). 3,3′,5,5′-tetramethylbenzidine was used as a substrate for peroxidase. The following anti-cytokine antibodies for coating or biotinylated antibodies for detection, respectively, were used in this ELISA: anti–IFN-γ, R4-6A2 and XMG 1.2 mAbs; anti–IL-2, JES6-1A12 and JES6-5H4 mAbs; anti–IL-4, BVD4-1D11 and BVD6-24G2 mAbs; anti–IL-5, TRFK-5 and TRFK-4 mAbs; anti–IL-6, MP5-20F3 and MP5-32C11 mAbs; and anti–IL-10, JES5-2A5 and JES5-16E3 mAbs. The ELISA assays were capable of detecting 0.02 ng/ml IFN-γ, 0.05 U/ml IL-2, 1.0 pg/ml IL-4, 0.1 U/ml IL-5, 0.1 ng/ml IL-6, and 0.04 ng/ml IL-10.
Cytokine-specific RT-PCR.
Total RNA fractions were prepared from antigen-stimulated CD4+ T cells by the acid guadinium–thiocyanate, phenol–chloroform extraction method (27). Cytokine-specific RT-PCR was performed as previously described in detail with minor modifications (28). In brief, a standard protocol was used for the RT reaction, and the resultant DNA was amplified by repeating PCR for 35 cycles at 95°C for 1 min and at 60°C for 1 min.
Statistics.
Statistical significance was determined by the Mann– Whitney U test using the Statview-J 4.11 statistical program (Abacus Concepts, Inc.) for Macintosh computers. To analyze survival distributions, the Mantel–Cox test was performed, and the significance level chosen was P = 0.05.
Results
Wasting Disease in IFN-γ+/+ and IFN-γ–deficient Mice.
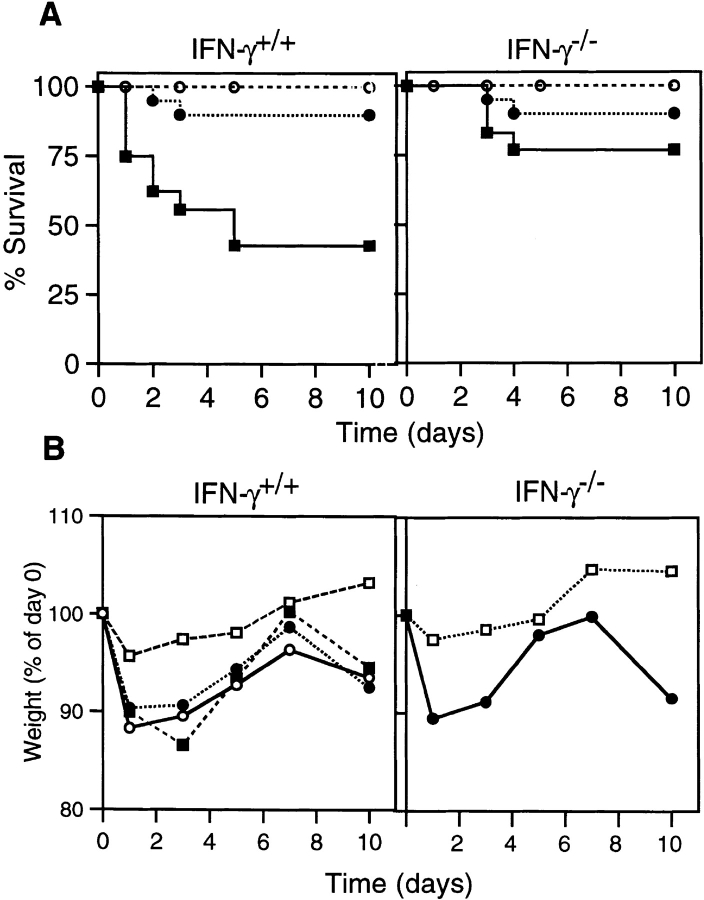
Previous studies have provided compelling evidence that the Th1-type cytokine IFN-γ plays a major role in experimental IBD in mice. To examine the possible significance of Th2-type responses, we first queried if colitis would develop in mice deficient in IFN-γ production. In these studies, we found that weight loss, colitis, and even death were dependent upon the dose of TNBS given, though the differences in colitis-inducing versus lethal doses were small in normal IFN-γ+/+ mice. Interestingly, IFN-γ−/− mice were more resistant to TNBS–ethanol enemas, with an approximate LD50 dose for IFN-γ+/+ mice only causing the death of 25% of IFN-γ−/− mice (Fig. 1 A). At the intermediate dose of 36 μg TNBS/g weight, >90% of mice in both groups survived. In both groups, autopsy showed large necrotizing ulcer rings and obstructed colons. With the low dose of TNBS (25 μg/g of weight), all mice survived without symptoms of colitis.
Figure 1.
The course of TNBS colitis in normal and IFN-γ–deficient mice. (A) Survival rate of mice given TNBS enema. IFN-γ+/+ (left) or IFN-γ−/− (right) mice were given 50 (▪), 36 (•), or 25 μg TNBS/g weight (○) intracolonically on days 0 and 7. Each group contained 16–18 mice. The survival rate after administration of 50 μg/g weight was significantly higher in IFN-γ−/− than in IFN-γ+/+ mice. (B) Wasting disease in mice given TNBS enema. Left, weight loss of IFN-γ+/+ mice after administration of ethanol only (□), 36 μg/g weight TNBS–ethanol (○), TNBS–ethanol together with intraperitoneal administration of rat anti– IFN-γ antibody XMG1.2 (▪), or rat IgG as a control (•) on days 0 and 7. Right, weight loss of IFN-γ−/− mice given TNBS–ethanol (•) or ethanol only (□). The data shown represent average values, and each group contained 5–10 mice. The weights of mice treated with ethanol only were significantly higher than in other groups on days 1, 3, 5, and 10 (left) and on days 1, 3, 7, and 10 (right).
We used the intermediate dose of TNBS to compare wasting disease in IFN-γ−/− and IFN-γ+/+ mice (Fig. 1 B). Administration of ethanol only had little effect on body weight and produced no symptoms of colitis in mice of either group. On the other hand, the intermediate dose of TNBS (36 μg/g) together with ethanol induced significant weight loss and diarrhea in both mouse groups (Fig. 1 B). Of interest was the finding that treatment of IFN-γ+/+ mice with anti–IFN-γ mAb did not prevent development of wasting disease (Fig. 1 B). These findings show that although IFN-γ−/− mice were more resistant than IFN-γ+/+ mice to TNBS–ethanol enemas, they did develop significant TNBS-induced wasting disease in the absence of IFN-γ.
Histologic Characteristics of TNBS-induced Colitis in IFN-γ−/− Mice.
The pathological features of TNBS colitis were essentially the same in IFN-γ−/− and IFN-γ+/+ mice given TNBS enemas of 36 μg/g. The entire colonic wall became thick from edema. The major colitis lesion was observed in the distal half of the colon, and focal ulcers were detected in ∼70% of colonic tissues from either IFN-γ−/− or IFN-γ+/+ mice. The ulcers often penetrated the colon and adhered to adjacent tissues. Distortion of crypts, loss of goblet cells, and infiltration of mononuclear cells were observed in all mice, and most IFN-γ−/− mice showed these changes in a more extended area of the colon than did IFN-γ+/+ mice. Some parts of the mucosal layer lost crypts and were replaced with lymphocytes, macrophages, and fibrotic tissue (Fig. 2, B, C, E, and F). Crypt abscesses were less frequent.
Figure 2.
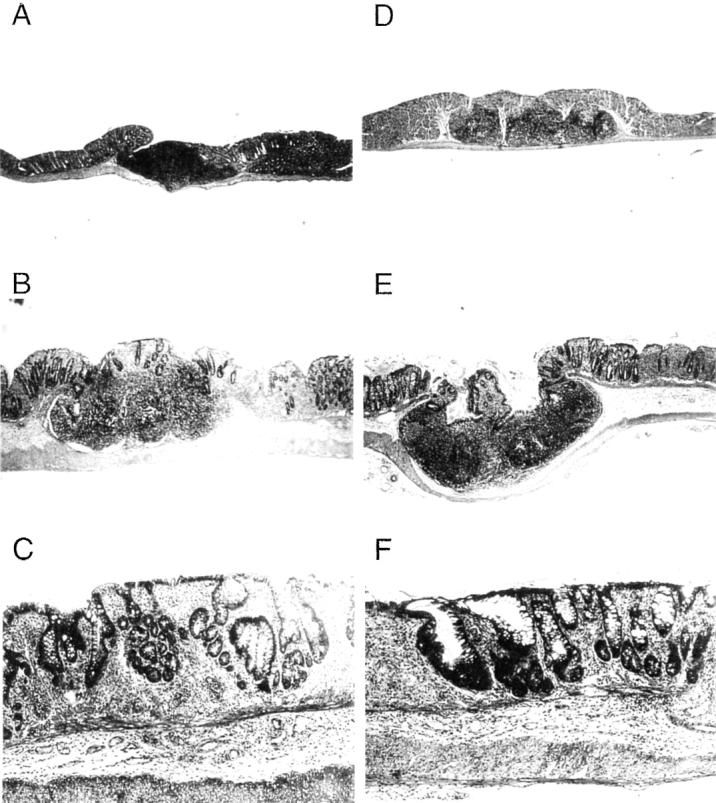
Histologic features of TNBS-induced colitis. (A–C) IFN-γ+/+ mice; (D–F) IFN-γ−/− mice. A and D show normal colon with colonic patches, and B, C, E, and F illustrate the colon after a TNBS enema. Original magnification was 10 in A, B, D, and E and 40 in C and F.
Another major finding in this model was an enlargement of lymphoid follicles in the colon. The inflamed colon developed white patches that resembled Peyer's patches in the small intestine and protruded from the colonic wall into the mesenteric side. When examined microscopically, these white patches, which were distinct from other parts of the colonic wall, were found to be lymphoid follicles (Fig. 2, B and E). Before TNBS enema, the colonic lymphoid follicles were difficult to detect macroscopically but were microscopically identified in tissue sections of both IFN-γ−/− and IFN-γ+/+ mice (Fig. 2, A and D). When TNBS colitis was induced, ∼7–8 white follicles per mouse were visible and thicker than colonic follicles observed in mice before TNBS enema (Table II). The cell yield from colonic patches was dramatically increased after induction of colitis, and the follicles in IFN-γ−/− mice contained higher numbers of mononuclear cells than did the follicles in IFN-γ+/+ mice (Table II). This finding indicates that the enlargement of these follicles in the inflamed colon was actually enhanced by the absence of IFN-γ.
Table II.
Comparison of Lymphoid Follicles in the Colons of IFN-γ+/+ and IFN-γ−/− Mice with TNBS-induced Colitis
| TNBS enema | Mouse strain | |||||
|---|---|---|---|---|---|---|
| IFN-γ+/+ | IFN-γ−/− | |||||
| Thickness of lymphoid follicles (mm)a | − | 0.39 ± 0.06* | 0.37 ± 0.05‡ | |||
| + | 0.55 ± 0.17* | 0.57 ± 0.15‡ | ||||
| Cell yield from lymphoid folliclesb (106 cells/mouse) | − | 1.7 ± 0.62 | 0.7 ± 0.20 | |||
| + | 4.55 ± 1.13§ | 6.37 ± 1.90§ | ||||
a 10 patches were measured for each group. Values shown are the average ± 1 SD.
P < 0.02,
P < 0.05. b Values shown are the average ± 1 SD from three to seven separate experiments using 5–7 mice/experiment.
P < 0.02.
Characterization of Colonic Follicles in TNBS Colitis.
We next investigated whether the follicles that appeared after TNBS enema were secondary lymphoid aggregates or enlarged GALT, e.g., Peyer's patch–like structures. As IFN-γ−/− mice developed larger follicles than control mice, we considered it possible that there was a difference in lymphocytes between IFN-γ−/− and IFN-γ+/+ mice that could account for the severity of the disease. Immunohistochemical analysis showed that these follicles had distinct T cell areas and B cell zones with germinal centers (Fig. 3). Flow cytometry analysis of mononuclear cells from these follicles indicated that T cell subsets were similar to those of Peyer's patches in the small intestine and to cecal patches. In IFN-γ−/− mice, CD4+ T cells were significantly less frequent in Peyer's or cecal patches. Increases in macrophages or other inflammatory cell types were not observed in colonic patches. When compared with naive mice, both IFN-γ+/+ and IFN-γ−/− mice tended to show decreased percentages of CD4+ T cells (Table III). On the other hand, the percentage of B cell subsets was slightly elevated in both groups of mice after induction of colitis (Table IV). Colonic follicles in IFN-γ−/− mice exhibited higher numbers of B cells when compared with either cecal or Peyer's patches. IFN-γ−/− mice had more surface IgA–positive B cells than IFN-γ+/+ mice. The higher percentages of B cells noted in IFN-γ−/− when compared with IFN-γ+/+ mice were also observed before induction of colitis. Collectively, the colonic follicles of mice with TNBS colitis showed a dramatic expansion of both T and B cells that was accompanied by a relative increase in all B cell subsets.
Figure 3.
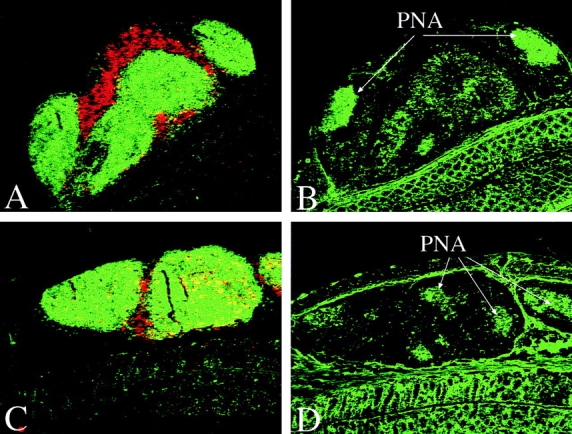
Immunohistochemistry of colonic lymphoid follicles of mice with TNBS colitis. The distribution of T (red) and B (green) cells in a colonic patch from an IFN-γ+/+ (A) or IFN-γ−/− (C) mouse. Serial sections were stained with PNA in B (IFN-γ+/+) and D (IFN-γ−/−). Original magnification was 100.
Table III.
T Cell Composition of Colonic Lymphoid Follicles, Cecal Patches, and Peyer's Patches from IFN-γ+/+ and IFN-γ−/− Mice with TNBS Colitis
| CD3+ T cells | Colonic lymphoid follicles | Cecal patches | Peyer's patches | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ+/+ | IFN-γ−/− | IFN-γ+/+ | IFN-γ−/− | IFN-γ+/+ | IFN-γ−/− | |||||||
| CD4−CD8+ | 6.7 ± 2.7 (4.5) | 5.9 ± 0.8 (2.0) | 5.1 ± 0.2 | 9.2 ± 3.5 | 6.4 ± 0.8 | 6.7 ± 0.3 | ||||||
| CD4+CD8− | 19.7 ± 3.8 (28.9) | 18.8 ± 2.4* (24.3) | 26.4 ± 2.3 | 31.9 ± 4.9* | 29.7 ± 3.9 | 26.2 ± 1.0* | ||||||
| CD4+CD8+ | 0.2 ± 0.0 (0.56) | 0.8 ± 1.0 (0.83) | 0.1 ± 0.0 | 1.2 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.3 | ||||||
| CD4−CD8− | 1.3 ± 1.7 (2.8) | 0.7 ± 0.1 (1.1) | 2.6 ± 1.3 | 1.8 ± 0.9 | 1.2 ± 0.5 | 1.0 ± 0.6 | ||||||
| α/β-TCR+ | 29.6 ± 3.3 (26.0) | 28.8 ± 3.0 (23.2) | 34.2 ± 3.0 | 42.6 ± 7.5 | 32.9 ± 04.5 | 36.1 ± 1.4 | ||||||
| γ/δ-TCR+ | 0.5 ± 0.1 (1.2) | 0.8 ± 0.1 (1.7) | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.7 ± 0.2 | 0.8 ± 0.2 | ||||||
Values shown are the average ± 1 SD of the percentage of mononuclear cells in three independently prepared cell fractions. Values shown in parentheses are for colonic lymphoid follicles from naive mice and were obtained from pooled cell fractions from 10 mice.
P < 0.05.
Table IV.
B Cell Composition of Colonic Lymphoid Follicles, Cecal Patches, and Peyer's Patches from IFN-γ+/+ and IFN-γ−/− Mice with TNBS Colitis
| B220+ B cells | Colonic lymphoid follicles | Cecal patches | Peyer's patches | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ+/+ | IFN-γ−/− | IFN-γ+/+ | IFN-γ−/− | IFN-γ+/+ | IFN-γ−/− | |||||||
| Ig+ | 50.4 ± 3.9 (32.9) | 55.7 ± 1.9* (50.7) | 47.0 ± 7.8 | 44.2 ± 0.7 | 40.2 ± 5.9 | 33.7 ± 2.5* | ||||||
| I-Ad+ | 45.5 ± 0.3 (23.4) | 55.6 ± 8.7 (47.1) | 40.4 ± 8.9 | 40.3 ± 3.8 | 45.1 ± 7.9 | 38.9 ± 7.4 | ||||||
| IgM+ | 40.1 ± 5.1 (30.2) | 55.7 ± 2.5 (47.0) | 33.5 ± 9.9 | 31.0 ± 3.6 | 35.2 ± 0.9 | 27.9 ± 1.6 | ||||||
| IgA+ | 5.4 ± 3.2* (6.5) | 11.4 ± 4.5* (10.1) | 6.5 ± 0.1 | 9.6 ± 3.3 | 8.7 ± 2.1* | 13.9 ± 1.1* | ||||||
| IgG+ | 2.3 ± 0.2 (3.8) | 2.2 ± 0.2 (4.2) | 2.4 ± 0.2 | 1.1 ± 0.5 | 1.6 ± 0.2 | 1.9 ± 0.7 | ||||||
| IgD+ | 30.9 ± 1.8 (18.3) | 30.9 ± 8.3 (30.5) | 24.4 ± 1.4 | 17.1 ± 11.7 | 21.1 ± 7.5 | 21.8 ± 3.4 | ||||||
Values shown are the average ± 1 SD of percentage of mononuclear cells in three independently prepared cell fractions. Values shown in parentheses are for colonic lymphoid follicles from naive mice and were obtained from pooled cell fractions from 10 mice.
P < 0.05.
M cells were identified in the epithelial cells covering the follicles by electron microscopy. M cells exhibited irregular microvilli with attached bacteria as well as interlaced macrophages and lymphocytes that extended into the mitochondria- and vesicle-rich cytoplasm (Fig. 4). M cells were found in follicles of both IFN-γ−/− and IFN-γ+/+ mice. From these studies, we conclude that the lymphoid follicles induced by TNBS colitis are colonic patches that retain an overall structure similar to that of Peyer's patches of the small intestine. The number of patches found in mice with colitis was comparable to numbers reported in naive adult BALB/c mice, as determined by an enumeration method consisting of fixation and illumination of the colon (29), leading us to conclude that these patches were not induced de novo but were instead enlargements of existing patches which became visible after TNBS enema.
Figure 4.
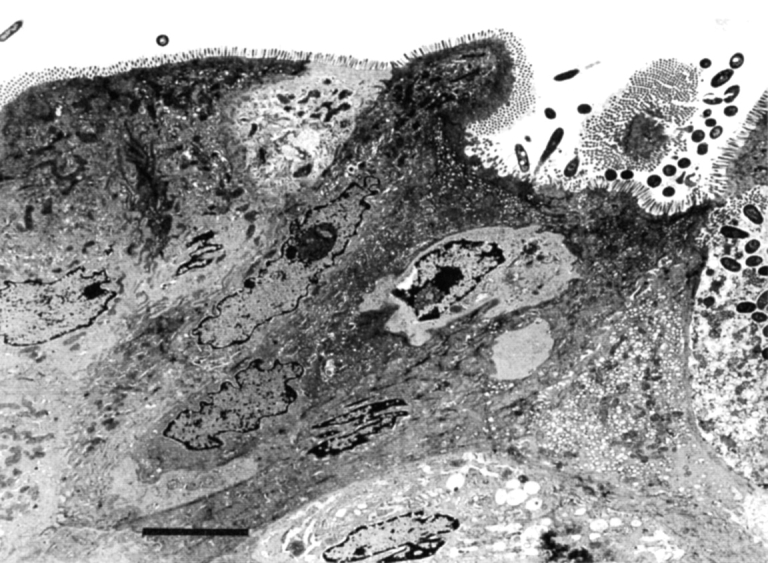
M cells detected in a colonic lymphoid follicle of an IFN-γ−/− mouse after TNBS enema. Irregular microvilli with attached bacteria and an underlying macrophage are shown. Bar, 5 μm.
Antigen-specific Proliferative Responses and Cytokine Profiles of Colonic Patch Cells from Mice with TNBS Colitis.
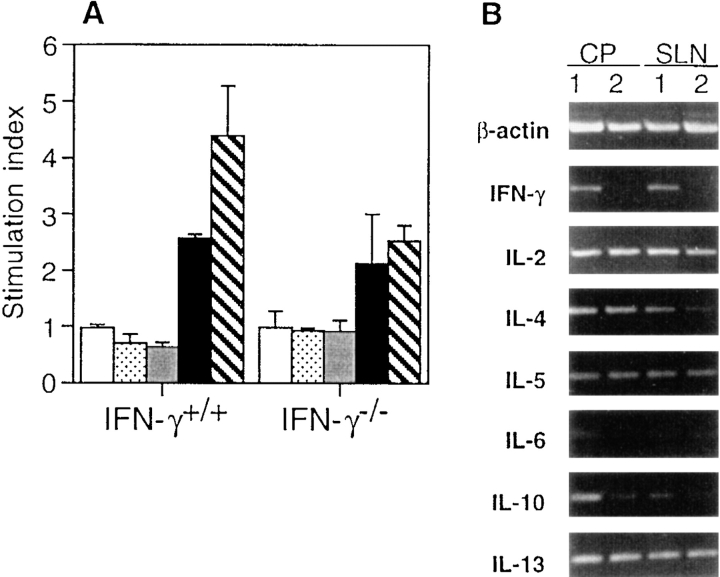
Antigen (TNBS)-specific responses of colonic patch cells were examined. Mononuclear cells isolated from colonic patches and SLN of mice with TNBS colitis responded to stimulation with TNBS, whereas cells from Peyer's or cecal patches or colonic lamina propria did not undergo TNBS-specific responses (Fig. 5 A). Cells from SLN of IFN-γ−/− mice had lower proliferative responses than did those of IFN-γ+/+ mice, perhaps due to the lack of response of cells normally activated by the presence of IFN-γ. These results show that TNBS-responding cells are localized in the colonic patches of both groups of mice.
Figure 5.
TNBS-specific proliferative responses in IFN-γ+/+ or IFN-γ−/− mice with TNBS colitis. (A) Proliferative responses of GALT and SLN cells. Cells were prepared from small intestinal Peyer's patches (white bar), cecal patches (dotted bar), colonic lamina propria lymphocytes (gray bar), colonic patches (black bar), and SLN (hatched bar). The stimulation index was determined as cpm of wells with antigen (TNBS)/cpm of wells without TNBS. The level of [3H]thymidine incorporation for control wells without TNBS was <600 cpm. Values are shown as the average ± 1 SD and are representative of three separate experiments using pooled cells from 3–5 mice in each group. (B) Expression of cytokine-specific mRNA in TNBS-stimulated CD4+ T cells. TNBS-stimulated total cell suspensions, which were prepared from SLN or colonic patches (CP) from IFN-γ+/+ (lane 1) or IFN-γ−/− (lane 2) mice with TNBS colitis, were cultured for 24 h. CD4+ T cells were purified by sorting and subjected to cytokine-specific RT-PCR.
Cytokine production accompanied by TNBS-stimulated proliferative responses was also measured (Table V). Cells from colonic patches and SLN of IFN-γ+/+ mice produced both IL-4 and IL-5 in addition to IFN-γ and IL-2, and the former also produced IL-6. Colonic patch and SLN cells from IFN-γ−/− mice produced IL-4, IL-5, and IL-10. Secretion of IL-5 by cells from IFN-γ−/− mice was higher than that by cells from IFN-γ+/+ mice, and this difference reached statistical significance in cells from SLN. Although it has been shown that Th2-type cytokine responses can be downregulated by IFN-γ (30), as much or more IL-4 was produced in IFN-γ+/+ mice as in IFN-γ−/− mice. Cytokine levels were also assessed in culture supernatants of lamina propria lymphocytes; however, low levels were seen with or without TNBS stimulation. These results indicate that Th2-type cytokines were produced by colonic patch T cells in the inflamed colon and SLN obtained from both IFN-γ+/+ and IFN-γ−/− mice. To confirm this, purified CD4+ T cells were subjected to analysis of cytokine-specific mRNA by RT-PCR (Fig. 5 B). The expression of mRNA from cytokines in TNBS-specific CD4+ T cells was similar to the results obtained for secreted proteins. In addition, mRNA for IL-13, another Th2-type cytokine, was also detected by RT-PCR.
Table V.
Cytokine Production by TNBS-stimulated Cells
| Cells | Mice | TNBS enema | Stimulation with TNBS | Th1-type cytokines | Th2-type cytokines | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | |||||||||||||
| ng/ml | U/ml | pg/ml | U/ml | ng/ml | ng/ml | |||||||||||||
| Colonic patches | IFN-γ+/+ | + | + | 0.08 ± 0.04 | 0.63 ± 0.11 | 255 ± 49 | 0.95 ± 0.20 | 6.26 ± 0.21* | 0.05 ± 0.00 | |||||||||
| + | − | <0.05 | 0.10 ± 0.01 | 24 ± 16 | 0.17 ± 0.14 | <0.1 | <0.04 | |||||||||||
| − | − | <0.05 | 0.10 | <5 | 0.10 | <0.1 | <0.04 | |||||||||||
| IFN-γ−/− | + | + | <0.05 | 1.02 ± 0.25 | 114 ± 24 | 2.68 ± 1.00 | 0.66 ± 0.18* | 0.32 ± 0.25 | ||||||||||
| + | − | <0.05 | 0.42 ± 0.25 | 5 ± 4 | 0.47 ± 0.34 | 0.55 ± 0.08 | <0.04 | |||||||||||
| − | − | <0.05 | <0.05 | 5 | 0.65 | <0.1 | 0.1 | |||||||||||
| IFN-γ+/+ | + | + | 0.30 ± 0.11 | 1.62 ± 0.19 | 215 ± 41 | 1.15 ± 0.36* | 0.25 ± 0.03 | 0.20 ± 0.13 | ||||||||||
| + | − | 0.06 ± 0.04 | 0.58 ± 0.25 | <5 | <0.1 | 0.01 ± 0.01 | 0.17 ± 0.00 | |||||||||||
| SLN | − | − | 0.07 | <0.05 | <5 | <0.1 | <0.1 | <0.04 | ||||||||||
| + | + | <0.05 | 1.43 ± 0.11 | 174 ± 14 | 3.38 ± 0.42* | 0.19 ± 0.10 | 0.32 ± 0.25 | |||||||||||
| IFN-γ−/− | + | − | <0.05 | 0.31 ± 0.25 | 2 ± 2 | 0.46 ± 0.23 | 0.11 ± 0.10 | <0.04 | ||||||||||
| − | − | <0.05 | <0.05 | <5 | <0.1 | <0.1 | <0.04 | |||||||||||
Values shown are the average ± 1 SD of three independently prepared cell fractions. Values of mice without TNBS enema were obtained from a pooled cell fraction taken from 10 mice. TNBS specific cytokine responses were not observed for IL-6 in IFN-γ−/− mice and for IL-10 in SLN of IFN-γ+/+ mice. In other studies, lamina propria lymphocytes from IFN-γ−/− and IFN-γ+/+ mice were assessed for Th1 and Th2 cytokines and in all instances, neither Th1- nor Th2-type cytokines were detected.
P < 0.02.
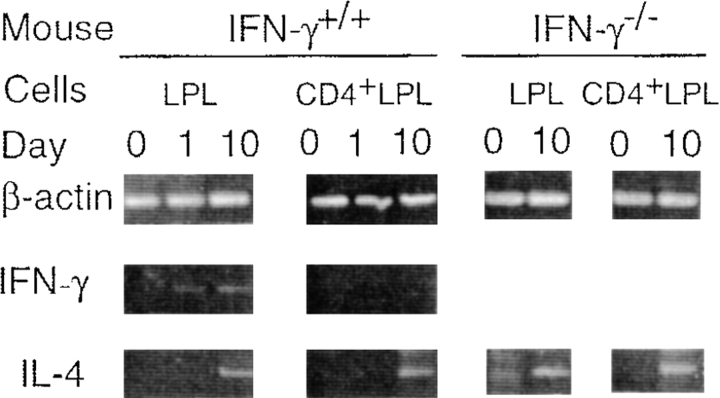
In additional studies, we examined the time course of mucosal cytokine responses in IFN-γ+/+ and IFN-γ−/− mice. Lamina propria CD4+ T cells were isolated on days 0, 1, and 10 and subjected to cytokine-specific RT-PCR. On day 1, ulcers were present in the colon; however, the colonic patches were poorly visible, and the size of SLN was much smaller than that observed on day 10. In IFN-γ+/+ mice, lamina propria CD4+ T cells obtained on days 0, 1, or 10 did not express IFN-γ mRNA, although the total lamina propria cell population contained IFN-γ on days 1 and 10 (Fig. 6). Furthermore, freshly isolated CD4+ T cells taken from colonic patches or SLN on day 0, 1, or 10 also failed to express mRNA for IFN-γ. mRNA specific for IL-4 was detected in CD4+ T cells from lamina propria on day 10 in both groups of mice (Fig. 6). When colonic lamina propria CD4+ T cells isolated from IFN-γ+/+ or IFN-γ−/− mice were stimulated with anti-CD3 mAbs, mRNA for both IL-4 and IL-5 was detected by RT-PCR (data not shown). Thus, it was shown that both IFN-γ+/+ and IFN-γ−/− mice with TNBS colitis exhibit Th2-type cytokine responses in colonic patches, SLN, and lamina propria.
Figure 6.
Expression of cytokine-specific mRNA from freshly isolated lamina propria lymphocytes. Total colonic lamina propria lymphocytes or CD4+ T cells purified by flow cytometry were prepared on days 0 (before TNBS enema), 1, and 10 and subjected to cytokine-specific RT-PCR. 50 ng of total RNA was used for each reaction. Representative results from three individual experiments are shown.
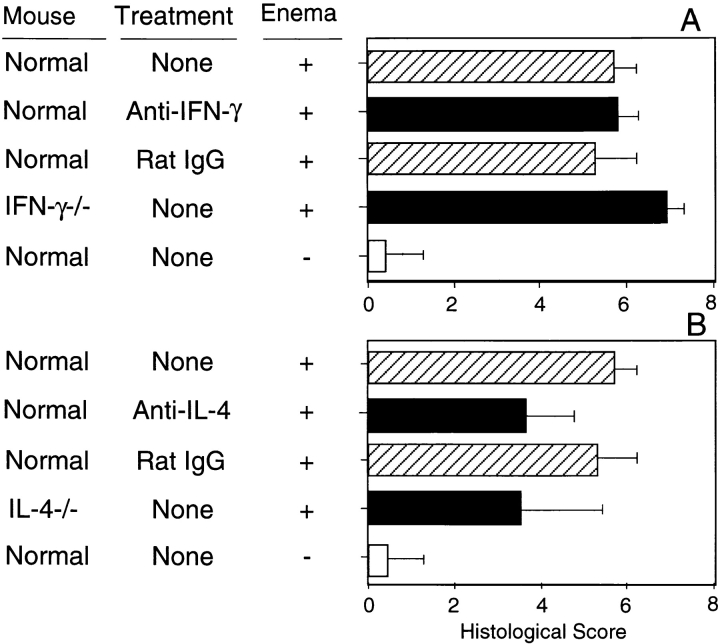
TNBS Colitis in IL-4–deficient Mice.
Our results to this point have shown that Th2-type, rather than Th1-type cytokines, are important in the lymphoid tissues of the inflamed colonic site. We next studied TNBS colitis in the absence of IL-4. Anti–IL-4 mAb treatment failed to prevent the initial weight loss after TNBS enema in normal IL-4+/+ mice. IL-4−/− mice given TNBS also suffered from severe wasting disease and died within 3 d at the same rate (10%) as untreated mice. However, the colitis in anti–IL-4 mAb-treated or in IL-4−/− surviving mice was different from that in IFN-γ+/+ or IFN-γ−/− mice (Fig. 7). IFN-γ−/− mice showed higher histologic scores than control mice. On the other hand, IL-4−/− mice as well as normal mice treated with anti–IL-4 mAb generally exhibited mild lesions, especially in terms of deformation of crypts and chronic changes that resulted in significantly lower histological scores.
Figure 7.
Histological scores for Th1 and Th2 cytokine-deficient mice with TNBS colitis. (A) IFN-γ–deficient mice; (B) IL-4–deficient mice. Each group included 4–10 mice. Values are shown as the average ± 1 SD. The score of IFN-γ−/− mice was significantly higher than that of other groups in A (P < 0.02). IL-4−/− mice and mice treated with anti–IL-4 mAb showed significantly lower values than did normal BALB/c and IFN-γ−/− mice (P < 0.05). −, rectal administration of ethanol only.
Taken together, our present data indicate that Th2 cells and the cytokine IL-4 play important roles in the induction of crypt inflammation and hypertrophy of colonic patches in the chronic phase of TNBS colitis induced in BALB/c mice.
Discussion
Two major new findings emerged from this study. Our studies have provided the first evidence that TNBS colitis develops in the absence of IFN-γ in BALB/c mice. In addition, the TNBS colitis lesion is an enhanced expansion of colonic patches where Th2-type cytokine responses are prominent. The enlargement of colonic patches in TNBS colitis was more evident in Th1-deficient IFN-γ−/− mice than in IFN-γ+/+ mice. These findings indicate that Th2-type responses derived from colonic patches play a significant role in TNBS colitis without a requirement for IFN-γ.
Hypertrophy of patches in the inflamed colon appears to be due to localized recurrent antigenic stimulation from luminal TNP-haptenated antigens. Other GALT, e.g., cecal and Peyer's patches, did not respond to TNBS, suggesting that colonic patch cells were responding to locally derived luminal antigens. Although the abundant presence of colonic patches in humans has been well documented (31, 32), there are no studies reporting a direct relationship between IBD and colonic lymphoid tissues. However, we would certainly expect that antigen uptake and processing by colonic patches would be important during the process of inflammation. In this regard, it has been reported that the abnormal immune responses to intestinal flora that follow a loss of tolerance may be one of the mechanisms for IBD in humans (33, 34) and in mouse models (35, 36). Because colonic patches are more exposed to bacterial antigens than are the Peyer's patches of the small intestine, the immunoregulatory functions of colonic patches as mucosal inductive sites are important in the pathogenesis of IBD.
Recent clinical studies have shown different cytokine profiles in Crohn's disease and ulcerative colitis. In Crohn's disease, the production of IFN-γ and the number of IFN-γ–producing cells were increased (18–20), whereas in ulcerative colitis, the increase was less pronounced. Lamina propria CD4+ T cells from Crohn's disease patients showed increased production of IFN-γ after stimulation via the CD28/CD2 accessory pathway, whereas cells from ulcerative colitis patients showed enhanced production of IL-5 but not IFN-γ (21). Furthermore, upregulation of IL-12 was observed with Crohn's disease but not with ulcerative colitis (22). Thus, it is generally recognized that Th1-type T cells are mainly involved in Crohn's disease, whereas Th2-type responses may have a more significant role in ulcerative colitis. To this end, our findings support the notion that Th2-type cells are involved in the development of an intestinal inflammation characterized by diffuse damage to the mucosal layer that bears resemblance to ulcerative colitis.
A high dose of TNBS enema induced deep ulcers in the acute phase of colitis that led to rapid weight loss and early death in IFN-γ+/+ mice. On the other hand, IFN-γ−/− mice were more resistant to this initial lethal phase of the disease. These results suggest that IFN-γ is important in the acute phase of the disease. This notion was supported by the presence of IFN-γ in total lamina propria lymphocytes from the early phase of disease in IFN-γ+/+ mice (Fig. 6). However, lack of mRNA for IFN-γ in CD4+ T cells indicates that the production of IFN-γ was likely induced in other cell types that comprise innate immunity, rather than by TNBS-specific Th1-type T cells in this experimental system. Lesions observed in the later phase of colitis induced by the intermediate dose of TNBS included distortion of crypts, loss of goblet cells, and infiltration by mononuclear cells. These lesions were frequently found to be accompanied by a total loss of crypts with fibrosis and the presence of a small number of granulomas. These morphological changes in crypts parallel those in ulcerative colitis.
Although both IFN-γ+/+ and IFN-γ−/− mice showed these crypt changes with Th2-type responses, the histological score in IFN-γ−/− mice was somewhat higher than in IFN-γ+/+ mice (Fig. 7 A). Furthermore, IFN-γ−/− mice developed larger colonic patches with higher numbers of B cells than did IFN-γ+/+ mice, which was due in part to increased Th2-type responses. The lesions that resulted in all of these mouse groups included enlargement of colonic patches. These findings indicate that Th2-type responses play an important role in chronic inflammation associated with TNBS colitis. In this regard, we were unable to detect mRNA for IFN-γ in CD4+ T cells isolated from the colonic lamina propria throughout the course of disease; however, we did detect mRNA for IL-4 on day 10. TNBS colitis induced in SJL/J mice (14) or other models of colitis (4, 10, 12) where Th1-type responses are predominant are characterized by a dense cell infiltration with active destruction of all layers throughout the entire colon. In contrast, the inflammation that was induced in IFN-γ+/+ or IFN-γ−/− BALB/c mice on day 10 was characterized by atrophic changes of the mucosal layer in the distal colon, which occurred in the absence of extensive lymphocyte infiltration or destruction of tissue. Thus, histological features in our model likely explain the lack of antigen-specific proliferative responses and cytokine production by lamina propria lymphocytes. We postulate that this is a feature of Th2-type inflammation in response to antigen that occurs in the inductive site itself, e.g., the colonic patches where constant stimulation of Th2-type cells disturbs the normal tissue repair processes in lamina propria and regeneration of epithelial cells, leading to fibrosis and the deformity seen in crypts. Further support for this concept was offered by our finding that IL-4−/− and normal BALB/c mice treated with anti–IL-4 mAb developed an initial wasting disease and severe ulcers but exhibited a milder lesion than did control mice in the chronic phase of the disease.
Our study demonstrated that TNBS colitis in IFN-γ+/+ mice was similar to Th2 cytokine-mediated colitis observed in IFN-γ−/− mice. This is not surprising, as BALB/c mice are known to favor Th2-type development of T cells, whereas other mouse strains, such as B10.D2, favor Th1-type development (37, 38). In this regard, the colonic patch cells from IFN-γ+/+ mice tended to produce larger amounts of IL-4 and IL-6 when compared with identical cell fractions taken from IFN-γ−/− mice. The low production of IL-6 in cell cultures from IFN-γ−/− mice is most likely due to insufficient activation of macrophages in cultures lacking IFN-γ. We also consider the decreased IL-4 responses in IFN-γ−/− mice to be due to insufficient cell activation, as determined by proliferation assays. These findings suggest that upregulation of IL-4 production associated with TNBS colitis in BALB/c mice may be independent of a cytokine regulatory network involving IFN-γ. Evidence for a Th2-type response with colitis has been reported in TCR-α–deficient mice, which develop spontaneous colitis. In these mice, cytokine production by mesenteric lymph node cells showed a marked increase in IL-4 synthesis (5). Furthermore, studies have also shown that local CD4+ TCR-α−/βdim+ T cells were responsible for production of IL-4 (8). Recent studies of hapten-induced colitis using oxazolone have also shown that Th2-type cells can mediate intestinal inflammation (39). Thus, it is possible that Th2-type responses are also involved in other mouse models of IBD.
Previous studies by others have emphasized the major importance of IFN-γ production by Th1-type T cells in TNBS colitis in a different mouse strain (SJL/J; 14). In contrast, our study has now shown that TNBS colitis is associated with Th2-type responses and with a pattern of inflammation that resembles ulcerative colitis. Thus, the immune responses to intracolonic TNBS can induce either Th1- or Th2-type responses, which are associated with distinct types of colitis. The apparent differences in experimental conditions between our studies and those of others are in the mouse strain and the dose of TNBS (14). However, luminal components such as food antigens or intestinal microflora may also be involved. These congenital and acquired factors could also contribute to the differentiation of phenotypes of T cell responses and colonic inflammation. It is also clear that there are clinical cases of IBD that are difficult to diagnose as either Crohn's disease or ulcerative colitis. Furthermore, the phenotype of disease can shift from one to the other during disease progression. It is possible that environmental factors influence induction of Th1- or Th2-prone responses that reflect distinct pathological features. Further investigation of the phenotypic shift in TNBS colitis using normal and cytokine-deficient mice will contribute to a better understanding of the complex pathogenesis of IBD and may reveal the underlying immunologic differences that give rise to Crohn's disease and ulcerative colitis.
Acknowledgments
This work was supported by U.S. Public Health grants DK 44240, AI 18958, AI 43197, DE 09837, DE 12242, and AI 35932 and grants from the Japanese Ministry of Education, Science, Sports and Culture, the Japanese Ministry of Health and Welfare, and OPSR (Japan).
Abbreviations used in this paper
- GALT
gut-associated lymphoreticular tissues
- IBD
inflammatory bowel diseases
- PNA
peanut agglutinin
- SLN
sacral lymph nodes
- TNBS
2,4,6-trinitrobenzene sulfonic acid
References
- 1.Braegger CP, MacDonald TT. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy. 1994;72:135–141. [PubMed] [Google Scholar]
- 2.Brandtzaeg P, Haraldsen G, Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin Immunopathol. 1997;18:555–589. doi: 10.1007/BF00824058. [DOI] [PubMed] [Google Scholar]
- 3.Emmrich J, Seyfarth M, Fleig WE, Emmrich F. Treatment of inflammatory bowel disease with anti-CD4 monoclonal antibody. Lancet. 1991;338:570–571. doi: 10.1016/0140-6736(91)91133-f. [DOI] [PubMed] [Google Scholar]
- 4.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor α mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizoguchi A, Mizoguchi E, Tonegawa S, Bhan AK. Alteration of a polyclonal to an oligoclonal immune response to cecal aerobic bacterial antigens in TCRα mutant mice with inflammatory bowel disease. Int Immunol. 1996;8:1387–1394. doi: 10.1093/intimm/8.9.1387. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi I, Kiyono H, Hamada S. CD4+T-cell population mediates development of inflammatory bowel disease in T-cell receptor α chain-deficient mice. Gastroenterology. 1997;112:1876–1886. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]
- 9.Powrie F, Leach MW, Mause S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 scidmice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 10.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45RBhi CD4+T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 11.Simpson SJ, Hollander GA, Mizoguchi E, Allen D, Bhan AK, Wang B, Terhorst C. Expression of pro-inflammatory cytokines by TCRαβ+ and TCRγδ+T cells in an experimental model of colitis. Eur J Immunol. 1997;27:17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- 12.Kühn R, Löhler D, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 13.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 14.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: mucosal immune responses and protection by oral tolerance. J Immunol. 1996;157:2174–2185. [PubMed] [Google Scholar]
- 16.Turk, J.L. 1975. Delayed Hypersensitivity. North Holland Publishing Company, Amsterdam. 319 pp.
- 17.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 18.Niessner M, Vold BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breese E, Braegger CP, Corrigan CJ, Walker-Smith JA, MacDonald TT. Interleukin-2 and interferon-γ-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78:127–131. [PMC free article] [PubMed] [Google Scholar]
- 20.Autschbach F, Schurmann G, Qiao L, Merz H, Wallich R, Meuer SC. Cytokine messenger RNA expression and proliferation status of intestinal mononuclear cells in noninflamed gut and Crohn's disease. Virchows Arch. 1995;426:51–60. doi: 10.1007/BF00194698. [DOI] [PubMed] [Google Scholar]
- 21.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 22.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza R, Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 23.Fujihashi K, McGhee JR, Kweon M-N, Cooper MD, Tonegawa S, Takahashi I, Hiroi T, Mestecky J, Kiyono H. γ/δ T cell–deficient mice have impaired mucosal immunoglobulin A responses. J Exp Med. 1996;183:1929–1935. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taguchi T, McGhee JR, Coffman RL, Beagley KW, Eldridge JH, Takatsu K, Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+T cells that secrete IFN-γ and IL-5. J Immunol. 1990;145:68–77. [PubMed] [Google Scholar]
- 25.VanCott JL, Staats HF, Pascual DW, Roberts M, Chatfield SN, Yamamoto M, Coste M, Carter PB, Kiyono H, McGhee JR. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. . J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 26.Fujihashi K, Yamamoto M, McGhee JR, Beagley KW, Kiyono H. Function of αβ TCR+ intestinal intraepithelial lymphocytes: Th1- and Th2-type cytokine production by CD4+CD8− and CD4+CD8+T cells for helper activity. Int Immunol. 1993;5:1473–1481. doi: 10.1093/intimm/5.11.1473. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Hiroi T, Fujihashi K, McGhee JR, Kiyono H. Polarized Th2 cytokine expression by both mucosal γδ and αβ T cells. Eur J Immunol. 1995;25:2743–2751. doi: 10.1002/eji.1830251005. [DOI] [PubMed] [Google Scholar]
- 29.Owen RL, Piazza AJ, Ermak TH. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991;190:10–18. doi: 10.1002/aja.1001900103. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 31.Jacob E, Baker SJ, Swaminathan SP. ‘M' cells in the follicle-associated epithelium of the human colon. Histopathology. 1987;11:941–952. doi: 10.1111/j.1365-2559.1987.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Leary AD, Sweeney EC. Lymphoglandular complexes of the colon: structure and distribution. Histopathology. 1986;10:267–283. doi: 10.1111/j.1365-2559.1986.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 33.Duchmann R, Marker-Hermann E, Meyer zum Buschenfelde KH. Bacteria-specific T-cell clones are selective in their reactivity toward enterobacteria or H. pyloriand increased in inflammatory bowel disease. Scand J Immunol. 1996;44:71–79. doi: 10.1046/j.1365-3083.1996.d01-273.x. [DOI] [PubMed] [Google Scholar]
- 34.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 36.Brandwein SL, McCabe RP, Cong Y, Waites KB, Ridwan BU, Dean PA, Ohkusa T, Birkenmeier EH, Sundberg JP, Elson CO. Spontaneously colitic C3H/HeJBir mice demonstrate selective antibody reactivity to antigens of the enteric bacterial flora. J Immunol. 1997;159:44–52. [PubMed] [Google Scholar]
- 37.Gorham JD, Güler ML, Steen RG, Mackey AJ, Daly MJ, Frederick K, Dietrich WF, Murphy KM. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci USA. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Güler ML, Gorham JD, Hsieh C-S, Mackey AL, Steen RG, Dietrich WF, Murphy KM. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 39.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]