Abstract
Using a snake toxin as a proteic antigen (Ag), two murine toxin–specific monoclonal antibodies (mAbs), splenocytes, and two murine Ag–specific T cell hybridomas, we showed that soluble protein A (SpA) from Staphylococcus aureus and protein G from Streptococcus subspecies, two Ig binding proteins (IBPs), not only abolish the capacity of the mAbs to decrease Ag presentation but also increase Ag presentation 20–100-fold. Five lines of evidence suggest that this phenomenon results from binding of an IBP–Ab–Ag complex to B cells possessing IBP receptors. First, we showed that SpA is likely to boost presentation of a free mAb, suggesting that the IBP-boosted presentation of an Ag in an immune complex results from the binding of IBP to the mAb. Second, FACS® analyses showed that an Ag–Ab complex is preferentially targeted by SpA to a subpopulation of splenocytes mainly composed of B cells. Third, SpA-dependent boosted presentation of an Ag–Ab complex is further enhanced when splenocytes are enriched in cells containing SpA receptors. Fourth, the boosting effect largely diminishes when splenocytes are depleted of cells containing SpA receptors. Fifth, the boosting effect occurs only when IBP simultaneously contains a Fab and an Fc binding site. Altogether, our data suggest that soluble IBPs can bridge immune complexes to APCs containing IBP receptors, raising the possibility that during an infection process by bacteria secreting these IBPs, Ag-specific T cells may activate IBP receptor–containing B cells by a mechanism of intermolecular help, thus leading to a nonspecific immune response.
Keywords: Ag presentation, protein A, B cell superantigen
Previous studies have shown that an Ag-specific Ab can modulate presentation of an Ag to T cells (1–5). At least two mechanisms can be associated with these observations. First, some of the Fcγ receptors expressed on APCs can mediate the internalization of the immune complex and thus may enhance Ag presentation (6–8). Second, Ag-specific Abs can affect the processing pattern of an Ag (9, 10), causing an enhanced or suppressed presentation of various T cell determinants (11, 12). This raises the question of whether components that interact with Abs, such as soluble microbial Ig binding proteins (IBPs),1 can also influence presentation of Ag–Ab complexes.
Microbial IBPs are produced by protozoa, viruses, and both gram-positive and gram-negative bacteria (13) and play important physiological roles (14). In particular, protein A (SpA) from Staphylococcus aureus can modify opsonization, phagocytosis, complement consumption (15, 16), Ab-dependent cell-mediated cytotoxicity (17), and mitogenesis (18). Molecular properties of SpA are well documented. It is a monomeric protein that can bind to an Ig molecule, free or bound to its Ag (19–21). SpA can bind to the Fcγ part of the Ab (22) through any of its five Fcγ binding domains, called EDABC (23, 24). It can also bind to the Fab region of an Ab via an alternative recognition site (25–30) located in the VH domain (31). This site is present in a large proportion of IgM, IgA, and IgG F(ab′)2 that are restricted to the VHIII family in humans (32–34) and to the S107 and J606 families in mice (35, 36). Thus, SpA can interact with a large proportion of B cells and is therefore considered a B cell superantigen (37, 38). As SpA is naturally and efficiently secreted by S. aureus (39–41), we investigated its possible influence on presentation of an Ag–Ab complex.
In this study, we examined, in a murine model, the influence of SpA on T cell presentation of Ag–Ab complexes, using a snake toxin as a proteic Ag, two toxin-specific mAbs, splenocytes, and two Ag-specific T cell hybridomas. We show that SpA not only abolishes the capacity of mAbs to diminish Ag presentation but also increases Ag presentation by 20–100-fold. In addition, we show that (i) SpA targets Ag–Ab complex to a subpopulation of splenocytes containing SpA receptors and mainly composed of B lymphocytes; (ii) APCs that possess SpA receptors are responsible for the boosting effect; (iii) the boosting effect occurs only when the IBP simultaneously possesses a Fab and an Fc binding site; (iv) in the absence of its Ag, an Ab also undergoes a SpA-specific boosted presentation. Altogether, our observations suggest that SpA boosts Ag presentation by bridging an immune complex to SpA receptors present at the surface of appropriate APCs. We also show that protein G from Streptococcus subspecies (ssp.) group C, another bacterial IBP, can also boost presentation of Ag–Ab complexes. Our observations raise the possibility that during an infection process by bacteria secreting these IBPs, Ag-specific T cells may activate IBP receptor–containing B cells by a mechanism of intermolecular help, thus leading to a nonspecific immune response. Several reports suggest that these observations may also be extended to humans.
Materials and Methods
Proteins and Reagents
Protein A from S. aureus, protein G from group C Streptococcus ssp., the IgG binding fragment BB, and protein G′ were purchased from Sigma Chemical Co. ZZ was expressed and purified as previously described (49). Toxin α was purified from Naja nigricollis venom (Pasteur Institute, Paris, France). Purity of the toxin was assessed by both reverse phase HPLC and isoelectric focusing. Biorex 70 and Bio-Gel P2 were obtained from Bio-Rad Labs. N-succinimidyl-3(2-pyridyldithio) proprionate (SPDP) was purchased from Pharmacia, and N-succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC) was purchased from Pierce Chemical Co. N-hydroxysuccinimidyl-fluorescein-5(6)-carboxamidocaproate (NHS–FCC) was obtained from Sigma Chemical Co. The HPLC C18 column (25 cm × 10 mm) was from Vydac. All solvents were obtained from Merck and used without further purification. mAb B220, Mac-2, anti-CD4, and anti-CD8 were from the American Type Culture Collection. Streptavidin-PE (SAPE) was purchased from Caltag Labs.
Chemical Modification of Proteins
Chemical Modification with NHS–FCC and NHS–biotin.
SpA was dialyzed overnight at 4°C against PBS before its modification with NHS–FCC. 52 nmol of SpA was subsequently incubated for 1 h at room temperature with 10× excess NHS–FCC. The reaction mixture was filtered through a Sephadex G15 column (26 × 1.5 cm) equilibrated in 50 mM phosphate buffer, pH 7.2. The protein that eluted in the void volume was used without further purification. 73.5 nmol of toxin α was modified with 735 nmol of NHS–FCC according to the same protocol. The procedure used for the biotinylation of protein G was similar to that described for NHS–FCC.
Covalent Coupling of Toxin α to mAb MST2
For the covalent coupling of toxin α to MST2, we used a modified procedure of the protocol previously described for coupling of toxin α to peroxidase (42).
Monothiolation of Toxin α.
200 μl of SPDP (17 μM) in absolute ethanol was added to 3.38 μmol of toxin α in 1.8 ml of 0.1 M phosphate buffer, pH 7.5, containing 0.1 M NaCl. The mixture was stirred for 30 min at room temperature. The reaction mixture was filtered through a Bio-Gel P2 column (26 × 1.5 cm) equilibrated in 10% acetic acid. The protein which eluted in the void volume was freeze dried. The modified protein was dissolved in 1 ml of 0.05 M ammonium acetate, pH 7.1, and applied onto a Bio-Rex 70 column (70 × 2.5 cm) equilibrated in the same buffer. The toxin was eluted with a linear gradient of 0.05– 0.4 M ammonium acetate, pH 7.1. Fractions containing monomodified derivatives were roughly identified by analogy with the elution profile previously obtained with monoacetylated derivatives. The modified amino groups were unambiguously identified by determination of the affinities of each derivative for two toxin-specific mAbs. Each derivative was rechromatographed on a C18 10-mm Vydac column equilibrated in water containing 0.1% TFA. The derivatives were eluted by using acetonitrile as secondary solvent. Elution was over a linear gradient of 0–50% acetonitrile. Each derivative that was freeze dried had incorporated a 2-pyridyl-dithiopropionate moiety on a single lysine.
Incorporation of Maleimido Groups into mAb MST2.
5.5 μl of dimethylformamide containing 83 nmol of SMCC was added to 8.3 nmol of MST2 dissolved in 312 μl of 0.1 M phosphate buffer, pH 7, containing 0.1 M NaCl. The mixture was left for 1 h with stirring at room temperature. The reaction mixture was then filtered through a Sephadex G15 column (20 × 0.5 cm) equilibrated in 0.1 M phosphate buffer, pH 6, containing 0.1 M NaCl.
Coupling Procedure.
The coupling reaction was performed in three steps. (i) The extra disulfide bond at Lys 27 was reduced. 8.3 nmol of the derivative was dissolved in 200 μl of 0.1 M acetate buffer, pH 4.5, containing 0.1 M NaCl and 25 mM dithiothreitol. The mixture was left under stirring at room temperature for 20 min, and the solution was filtered through a Sephadex G15 column (20 × 0.5 cm) equilibrated in 0.1 M phosphate buffer, pH 6.1, containing 0.1 M NaCl. The monothiolated toxin eluted in the void volume. (ii) (27-Nε mono thio-propionyl-lysine)– toxin α (8.3 nmol) was reacted with maleimido MST2 (8.3 nmol) overnight at 4°C. Uncoupled toxin α was removed by affinity chromatography on a Sepharose–protein G column. The conjugate was then concentrated using a Centricon® 10 and stored at −20°C with 0.1% BSA.
ELISA
Microtiter ELISA plates were coated overnight with either 0.3% BSA or polyclonal human (h) IgMs (1 μg/well) in 0.05 M phosphate buffer, pH 7.4. Plates were then saturated with 0.3% BSA in 0.1 M phosphate buffer, pH 7.4. Dilutions of biotinylated toxin α, Mα2-3, ZZ, and BB were performed in 0.1 M phosphate buffer, pH 7.4, containing 0.1% BSA. For the assessment of the binding of Mα2-3 to IgM-coated plates, the mAb (3 nM) was incubated overnight at 4°C in BSA-coated plates in the presence or absence of ZZ or BB (8 nM for each SpA derivative). The incubated solutions were then transferred into IgM-coated plates and left at room temperature for 4 h. The wells were then washed five times with 0.01 M phosphate buffer, pH 7.4, containing Tween 20, and a F(ab′)2 goat anti–mouse IgG peroxidase conjugate (Immunotech) was added and incubated for 30 min. After extensive washings, 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) was added, coloration was developed for 30 min, and the resulting absorbance was measured at 414 nm. For the assessment of the binding of biotinylated toxin α to IgM-coated plates, Mα2-3 and biotinylated toxin α were incubated overnight at 4°C in BSA-coated plates in the presence or absence of ZZ or BB. The incubated solutions were then transferred into IgM-coated plates and left at room temperature for 4 h. The wells were washed, and a streptavidin peroxidase conjugate (Immunotech) was added and incubated for 30 min. ABTS was used as a substrate as described above.
T Cell–stimulating Assay
All experiments were performed using DCCM1 (Biological Industries) as a synthetic culture medium, in the absence of FCS. Fixed or serial dilutions of the different Ags were preincubated in microculture wells for one night at 4°C. 5 × 105 splenocytes per well were then added, along with 5 × 104 of either T1B2 or T1C9 hybridoma. Cells were cultured for 24 h at 37°C in a humidified 7% CO2 atmosphere. For the assessment of the presenting capacity of SpA-specific cells, various amounts of splenocytes, splenocytes enriched with SpA-specific cells, or splenocytes depleted of SpA-specific cells were added to the wells in the presence of 5 × 104 T1B2.
The presence of IL-2 in culture supernatants from T cell hybridoma and bulk culture was evaluated by determining the proliferation of an IL-2–dependent cytotoxic T cell line (CTLL) using methyl [3H]TdR (5 Ci/mmol; CEA). The data are expressed in cpm.
Flow Cytometric Analysis
Binding to Splenocytes.
Biotinylated toxin α (0.7 μM) was incubated for one night at 4°C in the presence or absence of SpA and Mα1 (0.6 and 0.1 μM, respectively). Splenocytes (5 × 105 cells) were then added and incubated for 30 min at 4°C in PBS/ 0.5% BSA. Cells were washed three times and incubated for 30 min at 4°C with SAPE (6 μg/ml). After three washes, 5,000 viable cells were analyzed on a FACS® (Becton Dickinson). The binding of SpAF to splenocytes was assessed by incubating 5 × 105 cells for 30 min at 4°C with 50 μl SpAF (0.6 μM final) in the presence or absence of 10× excess unlabeled SpA. After three washes, 5,000 viable cells were analyzed on a FACS®. For the assessment of the binding of the two IBPs to splenocytes, biotinylated protein G (0.2 μM final) was incubated for 30 min at 4°C with 5 × 105 cells in the presence or absence of SpAF. Splenocytes were subsequently washed three times and incubated for 30 min at 4°C with SAPE (6 μg/ml). After three washes, 5,000 viable cells were analyzed on a FACS®.
Cell Sorting of Splenocytes.
Cells were incubated for 30 min at 4°C in the presence of SpAF. After three washings, splenocytes were filtered through nylon and sorted at 2,000 cells/s under sterile conditions on an EPICS V flow cytometer (Coulter) using a 76-μM nozzle and the 488-nm line of an argon laser set at 300 mW. PBS was used as a sheath fluid. The sorting criteria were normal forward-angle light scatter and bright fluorescein fluorescence (515–560 nm) taken on a log scale; ∼2% of cells were positive.
Binding to Cell-sorted Splenocytes.
Biotinylated toxin α (0.7 μM) and SpA (0.6 μM) were incubated for one night at 4°C in the presence or absence of Mα1 (0.1 μM). 105 splenocytes, SpA unbound splenocytes, or SpA-bound cell–enriched splenocytes were then added and incubated for 30 min at 4°C in PBS/0.5% BSA. Cells were washed three times, and binding to cells was analyzed as described above. To determine the type of cells bound by SpAF, SpA-bound cell–enriched splenocytes were incubated for 30 min at 4°C with mAb–B220, Mac-2, anti-CD4, and anti-CD8, respectively (0.1 μM final for each). Cells were washed three times, and binding to cells was analyzed as described above.
Results
SpA Enhances Presentation of Ag–Ab Complex.
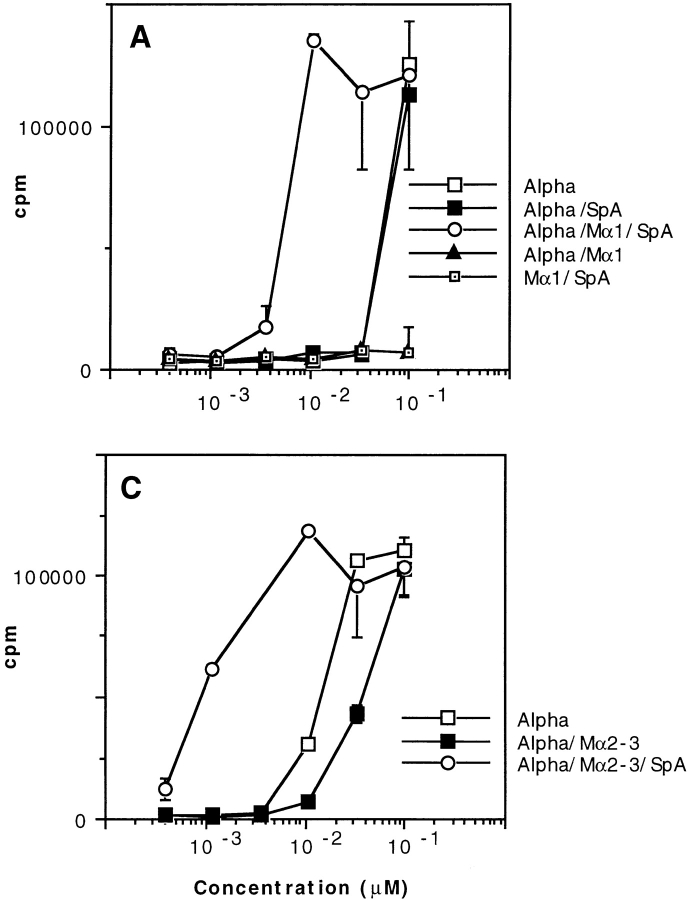
We investigated the influence of SpA from S. aureus on presentation of an Ag–Ab complex to Ag-specific T cells. We used a snake toxin, toxin α (43), as an Ag, two toxin α–specific T cell hybridomas, called T1C9 and T1B2 (44), and two IgG2a toxin α–specific mAbs, called Mα1 (45) and Mα2-3 (46), whose epitopes are topographically unrelated to each other. Serial dilutions of toxin α were preincubated overnight at 4°C in the presence of fixed quantities of a toxin-specific mAb (Mα1 or Mα2-3) and SpA before addition of the appropriate T cells and splenocytes from BALB/c mice. Secretion of IL-2 was determined 24 h later. As shown in Fig. 1, the capacity of toxin α to stimulate T1B2 and T1C9 in the presence of splenocytes decreased when the Ag was incubated with either Mα1 (Fig. 1, A and B) or Mα2-3 (Fig. 1 C). However, the suppressing effect caused by the two toxin-specific mAbs not only vanished in the presence of soluble SpA, but the efficacy of Ag presentation dramatically increased. Fig. 1 shows that 20–100-fold lower amounts of toxin α sufficed to cause secretion of the same amounts of IL-2 when the Ag was incubated with both toxin-specific mAbs and SpA. Therefore, the presence of an Ig-binding protein can specifically enhance presentation of an Ag complexed to an Ag-specific Ab.
Figure 1.
T cell presentation of toxin α in the presence of soluble SpA. Toxin α (Alpha) was serially diluted and incubated overnight at 4°C in the presence or absence of fixed concentrations of SpA and either mAb Mα1 (0.2 μM final) or Mα2-3 (0.1 μM final). 5 × 105 splenocytes from BALB/c mice were then added to each well in the presence of 5 × 104 of either T1B2 (A) or T1C9 (B and C). Cells were cultured for 24 h at 37°C, and IL-2 secretion was subsequently determined by CTLL assay. The experiments shown in A were repeated in the presence of variable concentrations of SpA and a fixed concentration of Mα1 (2 nM final; D). In this experiment, a control was performed using an unrelated IgG2a and SpA (0.1 μM final for each).
In these experiments, we used relatively high proportions of SpA and mAbs (0.2 μM). Nevertheless, when their concentrations decreased to 4 nM and 2 nM, respectively, being thus probably closer to physiological conditions, a potent boosting effect remained observable (Fig. 1 D). It is notable that, in these experiments, the initial suppressing effect of the mAb apparently disappeared (Fig. 1 D). This is no surprise, as the ratio of concentrations of mAb (2 nM) to antigen (0.1 μM) was low and, as a result, the presentation associated with the free toxin dominated and overshadowed the inhibiting effect of the mAb. The same phenomenon was observed in all experiments in which the toxin concentration was higher than that of the antibody (see below).
This is not the first time that an Ag presentation has been shown to be affected by mAbs. In particular, presentation of tetanus toxin was shown to increase or decrease in the presence of some specific mAbs (11, 12). In our study, we observed only a decrease in presentation by both toxin-specific mAbs, although they bound to topographically unrelated epitopes (45, 46). This result might be associated with the small size (6.8 kD) of the antigen, whose mAbs cover a large proportion of the toxin surface and are thus likely to interfere with presentation of the toxin T cell epitopes, located between residues 24–41 and 32–49 (44).
It was previously shown that when SpA is expressed at the surface of Staphylococcus or coupled to Sepharose, it can exert a mitogenic stimulating effect (18, 47). To rule out the possibility that the SpA-specific boosting phenomenon described above was related to such a nonspecific property, various controls were performed. First, in the absence of any mAbs, Mα1, or Mα2-3, the toxin α stimulation efficacy was unaffected by SpA (Fig. 1, A and B). Second, in the absence of toxin α, no IL-2 stimulation was triggered by soluble SpA and Mα1 (Fig. 1, A and B). Third, SpA did not modify Ag presentation when the Ab was an unrelated IgG2a (Fig. 1 D). Fourth, no stimulation occurred when splenocytes and T1B2 were incubated separately with SpA, toxin α, and Mα1 (data not shown). Therefore, besides the necessary appropriate immune cells, three additional components were absolutely required for the boosting effect to be observable: the Ag, an Ag-specific Ab, and soluble SpA.
The SpA-boosted Presentation of Ag Remains MHC Restricted.
The presentation of free toxin α to T1B2 and T1C9 is restricted to I-Ad and I-Ed, respectively (44). We investigated whether or not this MHC restriction was still associated with the SpA-boosted presentation of the toxin using splenocytes from D2GD mice, which express I-Ad and I-Eb molecules. We observed that the capacity of toxin α to stimulate T1B2 was boosted when the toxin was added concomitantly with both the mAb and SpA (Fig. 2 A), whereas under the same experimental conditions, T1C9 was not stimulated (Fig. 2 B). Therefore, the SpA-dependent boosting presentation of an Ag complexed to an Ag-specific Ab remains MHC restricted and is not related to the MHC-independent genetic background of BALB/c mice.
Figure 2.
T cell presentation of toxin α in the presence of toxin-specific antibody and SpA is MHC restricted. Toxin α was serially diluted and incubated overnight at 4°C in the presence or absence of fixed concentrations of SpA and Mα1 (0.1 μM final for each protein). 5 × 105 of splenocytes from D2GD mice were then added to each well in the presence of 5 × 104 of either T1B2 (A) or T1C9 (B). T cell stimulation was assessed as in the Fig. 1 legend.
Ag–Ab Complexes Are Targeted to APC Possessing SpA Receptors.
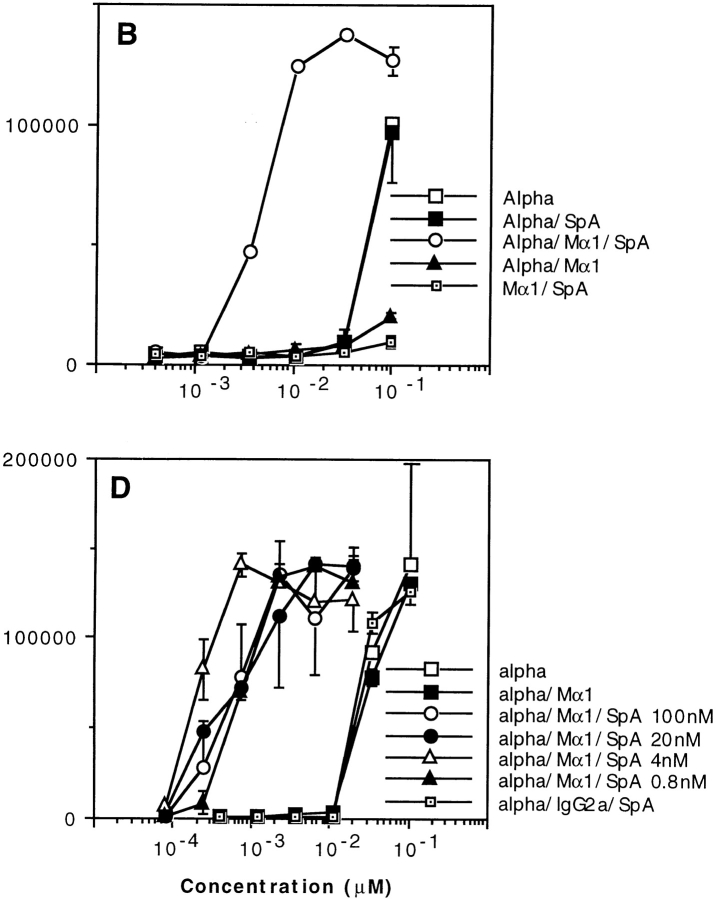
What are the mechanisms that may account for an SpA-dependent boosting presentation of an Ag complexed to an Ag-specific Ab? As already mentioned, SpA possesses multiple Ig binding sites (23, 24) and therefore could bind simultaneously to several immune complexes, thus generating an artificial concentration effect. In other words, incubation of stoichiometric concentrations of Ab and SpA could favor formation of heterogeneous multimolecular complexes, and those possessing several toxin molecules could predominantly generate the boosting effect. To examine such a possibility, we incubated overnight a constant concentration of SpA with varying dilutions of toxin α and either a fixed concentration of Mα1 or varying concentrations of Mα1 so that the ratios of SpA/Mα1 were equal to 1.5, 4.5, 13.5, 40.5, 121.5, or 364.5 for the first to the sixth dilution, respectively. Thus, in each toxin dilution, at most one molecule of the immune complex should have bound to one SpA molecule. As shown in Fig. 3, T cell presentation evolved similarly with toxin dilution, irrespective of whether the concentrations of Mα1 were fixed or variable. Therefore, the observed boosted T cell stimulation seems to be unrelated to the number of Ab–Ag molecules associated to an SpA molecule.
Figure 3.
T cell presentation of toxin α in the presence of fixed or decreasing concentrations of Mα1. Toxin α was serially diluted and incubated overnight at 4°C in the presence or absence of fixed concentrations of SpA (0.3 μM final) and either serial dilutions of Mα1 or a fixed amount of mAb (0.2 μM final). 5 × 105 splenocytes from BALB/c mice were then added to each well in the presence of 5 × 104 T1B2. T cell stimulation was then assessed as previously described.
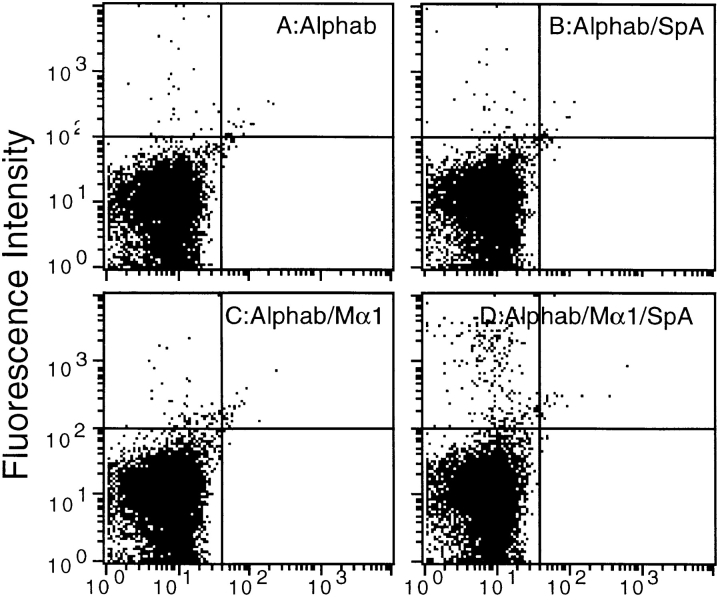
Another explanation that may account for the SpA- enhanced presentation of an Ag is that SpA targets Ag–Ab complexes to APCs that possess SpA receptors on their surfaces. To investigate this possibility, we performed a series of FACS® analyses. At first, we examined the direct binding of SpA to splenocytes using SpA labeled with FCC (SpAF). As shown in Fig. 4, E–G, labeled SpA binds specifically to a subpopulation of splenocyte cells. We then investigated the binding of the Ag on splenocytes using SpA, a toxin-specific mAb (Mα1), and the biotinylated toxin α, which was detected by SAPE. When the biotinylated toxin α was added alone, <0.5% of splenocytes were labeled (Fig. 4 A). The same result was obtained when SpA (Fig. 4 B) or Mα1 (Fig. 4 C) was added separately. In sharp contrast, when the biotinylated toxin was concomitantly incubated with Mα1 and SpA, the proportion of labeled cells increased to nearly 3% (Fig. 4 D). These findings support the view that splenocytes possess SpA-specific cells and that an Ag–Ab complex binds to this subpopulation.
Figure 4.
Binding of the immune complex and SpA on splenocytes. Biotinylated toxin α (Alphab) was incubated overnight at 4°C (A), in the presence of a fixed concentration of SpA (B), Mα1 (C), or both (D). Splenocytes (5 × 105 cells) were added and incubated for 30 min at 4°C. Binding of biotinylated toxin α to cells was assessed using SAPE. FACS® analyses E–G show splenocytes (5 × 105 cells) incubated for 30 min at 4°C without SpAF (E), in the presence of SpAF alone (F), or SpAF diluted with a 10× excess unlabeled SpA (G).
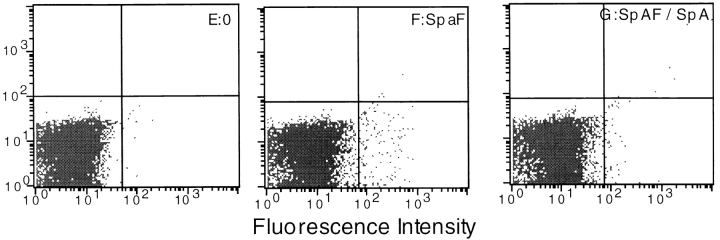
To further validate this conclusion, we sorted SpAF- labeled splenocytes (2% of the whole population) and investigated the binding of the biotinylated Ag to this population enriched in SpA-specific cells. 32% of the positively sorted splenocytes were effectively labeled with SpAF (Fig. 5 E, bottom right). In the absence of both SpA and Mα1, quite a small proportion of these cells were labeled with the biotinylated toxin (Fig. 5 E, top right). In contrast, when both Mα1 and SpA were present, nearly 60% of the SpAF-labeled cells were labeled with the biotinylated Ag (Fig. 5 F, top right). However, a proportion of SpAF-labeled splenocytes were not labeled with the biotinylated toxin, even when both the mAb and SpA were present in the medium (Fig. 5 F, bottom right). No definite explanation presently accounts for this observation. Control experiments were carried out with the population of negatively sorted cells, which contained only 0.4% of SpAF-labeled cells (Fig. 5 C, bottom right). Only 0.6% of the depleted splenocytes were labeled with the biotinylated Ag in the presence of the Ab and SpA (Fig. 5 D, top left and top right), and this value did not increase to more than 2.5% when the splenocytes were unsorted (Fig. 5 B, top left). Altogether, these experiments provide direct evidence that in the presence of SpA, an Ag–Ab complex is preferentially targeted to a particular subpopulation of splenocytes that possess SpA receptors.
Figure 5.
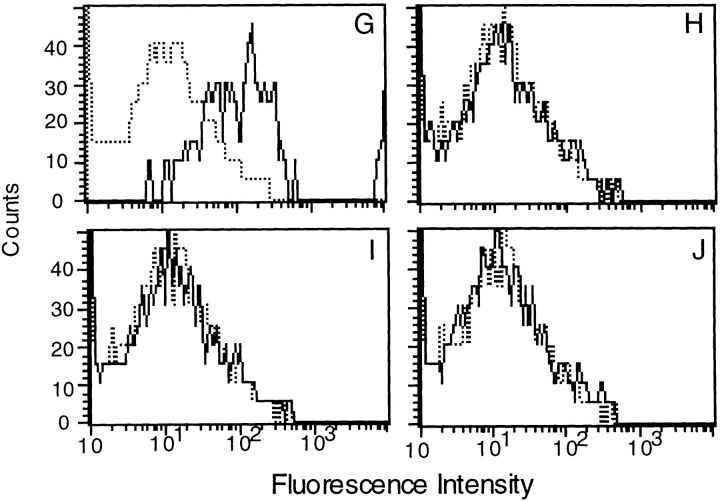
Binding of toxin α on cell-sorted splenocytes. Biotinylated toxin α and SpA were incubated overnight at 4°C with (B, D, and F) or without (A, C, and E) Mα1. 105 unsorted splenocytes (A and B), splenocytes depleted of cells labeled with SpA–FCC (C and D), or splenocytes enriched in cells labeled with SpA–FCC (E and F) were added and incubated for 30 min at 4°C. Binding of biotinylated toxin α to cells was assessed using SAPE. Analyses G–J show characterization of splenocytes enriched in cells labeled with SpAF, using biotinylated mAbs B220, Mac-2, GK1.2, and H35 (G, H, I, and J, respectively).
To identify the cell types that predominantly populated splenocytes enriched in sorted SpAF-labeled cells, we used four biotinylated mAbs (mAb B220, mAb Mac 2, mAb GK1.2, and mAb H35) and SAPE. As shown in Fig. 5 G, a shift in fluorescence intensity was observed with the B cell marker, whereas no shift was observed using macrophage, CD4, and CD8 cell surface markers (Fig. 5, H–J). Therefore, SpAF-bound cells are mainly composed of B cells.
The Boosted Presentation Requires APCs Possessing SpA Receptor.
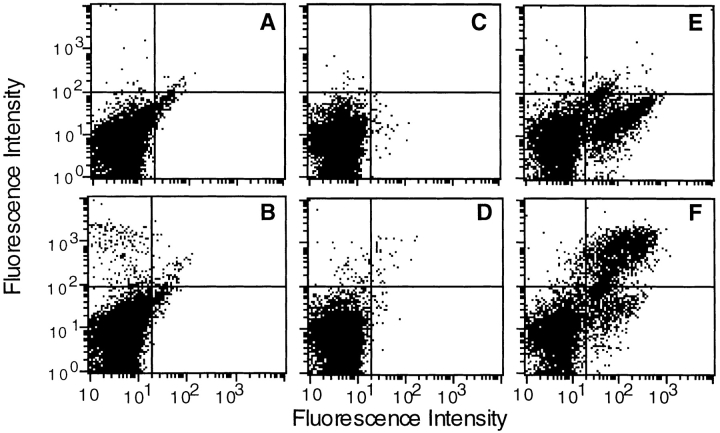
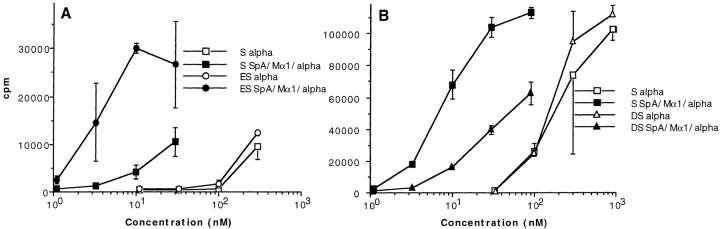
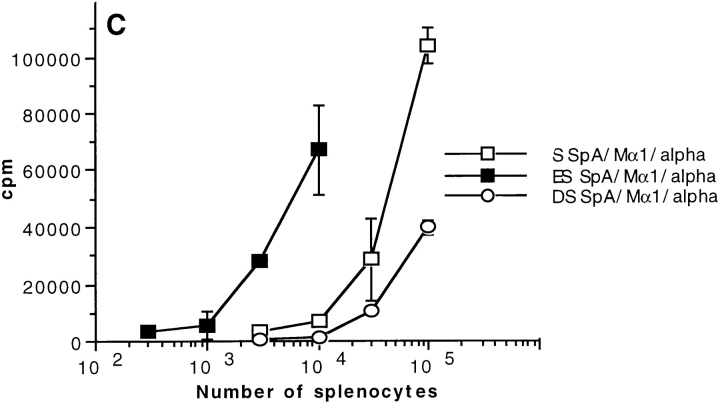
We investigated the ability of both unsorted splenocytes (S) and splenocytes enriched with (ES) or depleted of (DS) SpAF-bound cells to stimulate T1B2 in the presence of toxin α alone or in combination with both SpA and Mα1. As shown in Fig. 6 A, 104 splenocytes enriched with SpA receptor–possessing cells were ∼10-fold more efficient than 104 unsorted splenocytes at boosting toxin α presentation in the presence of Mα1 and SpA. In contrast, 105 unsorted splenocytes were ∼10-fold more efficient than 105 splenocytes depleted of SpA receptor–containing cells (Fig. 6 B). The observed differences in efficiency are not related to a modification of the processing efficiency of cells treated with SpAF during the sorting experiments, as control experiments showed that splenocytes enriched with SpA receptor–containing cells were equally as efficient as unsorted splenocytes at presenting toxin α alone (Fig. 6). Further demonstration that positively sorted cells are more efficient than unsorted cells, which in turn are more efficient than the negatively sorted splenocytes, is also shown in Fig. 6 C, where stimulation of T1B2 by the Ab–Ag complex in the presence of SpA is shown as a function of the number of cells. Therefore, the subpopulation of splenocytes possessing SpA receptors is mainly responsible for the capacity of SpA to boost the T cell stimulation of Ag–Ab complex.
Figure 6.
T cell presentation of toxin α in the presence of cell-sorted splenocytes. Toxin α was serially diluted and incubated overnight at 4°C in the presence or absence of fixed concentrations of SpA and Mα1 (0.1 μM final concentration for both). Then, the different mixtures were incubated in the presence of T1B2 (5 × 104 cells) and (A) 104 APCs consisting of either splenocytes enriched in SpA specific cells (ES) or unsorted splenocytes (S); or (B) 105 APCs consisting of either splenocytes depleted of SpA-specific cells (DS) or unsorted splenocytes (S). C shows the stimulation of T1B2 in the presence of variable numbers of unsorted, positively sorted, and negatively sorted splenocytes, and a fixed concentration of toxin α (30 nM), Mα1 (0.1 μM), and SpA (0.1 μM).
SpA Receptors May Be Surface Immunoglobulins.
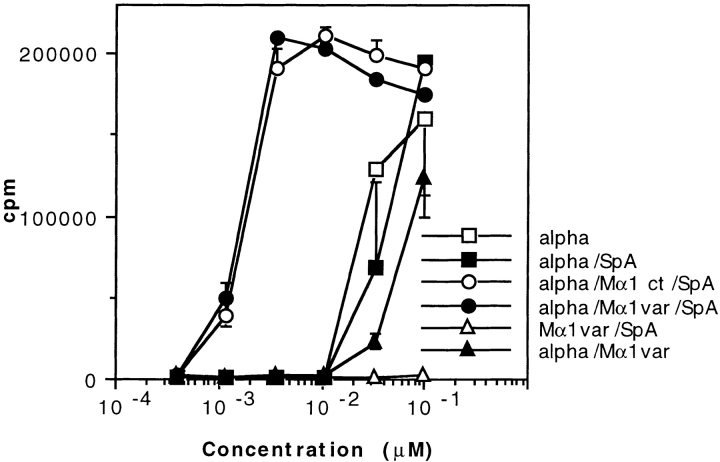
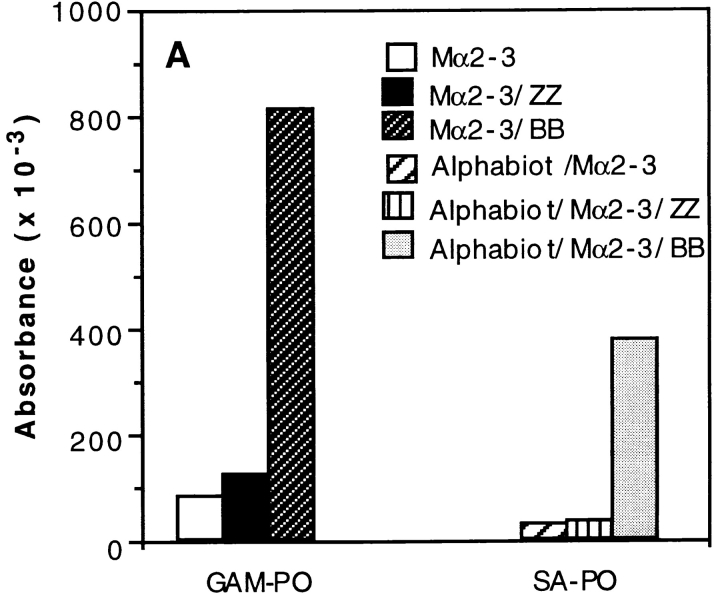
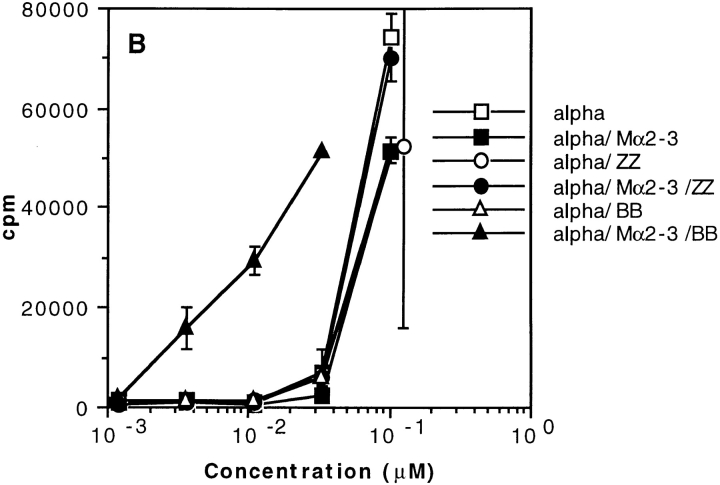
The above data indicated that the Ag–Ab complexes are targeted to SpA receptors present at the surface of APCs containing predominantly B cells. In other words, the boosting effect can be interpreted as resulting from the formation of complexes that consist of a SpA receptor bound to SpA, which in turn is bound to Ab–Ag complex. To investigate the possibility that the SpA receptors may be surface Igs, we proceeded as follows. First, we selected two SpA derivatives which differ in their recognition specificity toward Igs. One of them is composed of two B domains (BB), and, like SpA, recognizes the Fab region of IgMs and IgGs as well as the Fcγ part of IgG (48). The other derivative is ZZ (Z is a mutant of domain B [49]) and binds to the Fcγ part of IgG but very weakly to the Fab region (50). Second, we examined the binding of either a mouse mAb or biotinylated toxin α to hIgM-coated plates. As shown in Fig. 7 A (left), the mouse IgG2a mAb Mα2-3 can bind to coated hIgMs in the presence of BB but not in the presence of ZZ. Furthermore, the presence of Mα2-3 and BB is a prerequisite for the binding of biotinylated toxin α to coated hIgMs (Fig. 7 A, right). Therefore, formation of a ternary complex, IgM–SpA derivative–IgG, or a quaternary complex, IgM–SpA derivative–IgG–Ag, is possible, provided that the derivative can bind to both IgG and IgM. In addition, the VH domain of Mα2-3 is coded by the VH germline gene J558 (51), a characteristic that precludes the Fab of this IgG to bind SpA and BB (35, 36). As a result, this IgG can be recognized only through its Fcγ. Therefore, the complex observed in the ELISA consists of an IgM bound by its Fab to BB, which in turn binds to the Fcγ region of Mα2-3.
Figure 7.
Effect of two SpA derivatives on the binding to coated hIgMs and on the T cell presentation of toxin α. (A) Mα2-3 was incubated overnight in the presence or absence of fixed concentrations of toxin α biotinylated at the NH2 terminus (Alphabiot) and either ZZ or BB in BSA-coated microwell plates. The solutions were transferred in hIgM-coated plates. Binding of Mα2-3 to the wells was determined using a goat anti–mouse IgG peroxidase conjugate (GAM–PO), whereas binding of biotinylated toxin α was determined using a streptavidin peroxidase conjugate (SA–PO). (B) Toxin α (alpha) was serially diluted and incubated overnight at 4°C in the presence or absence of mAb Mα2-3 (25 nM final) and either ZZ or BB (0.1 μM final for each derivative). 5 × 105 splenocytes from BALB/c mice were then added to each well in the presence of 5 × 104 T1B2. T cell stimulation was assessed as previously described.
Third, we investigated the effect of the two SpA derivatives on the labeling of splenocytes from BALB/c mice by a mouse IgG–FITC. In these unpublished experiments, we observed that 10.8% of the splenocytes were bound by the mouse IgG2a–FITC, a proportion which increased to 23.8% in the presence of BB and decreased to 6.4% in the presence of ZZ. Fourth, we investigated the capacity of BB and ZZ to boost the presentation of the Mα2-3–toxin complex using T1C9 and splenocytes. As shown in Fig. 7 B, a boosting effect is observed when the Ab–Ag complex is incubated in the presence of BB but not in the presence of ZZ. Thus, the boosting effect requires that the SpA derivative possesses both a binding site to the Fcγ region of Mα2-3 and a binding site to the Fab of Igs, strongly suggesting that the BB–Mα2-3–Ag complex is targeted to the Fab region of surface Igs of APCs.
The Presentation of an Immune Complex Is Also Boosted by Protein G.
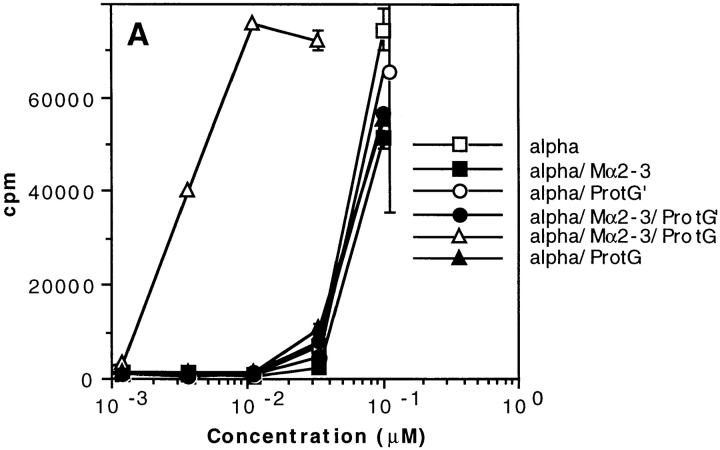
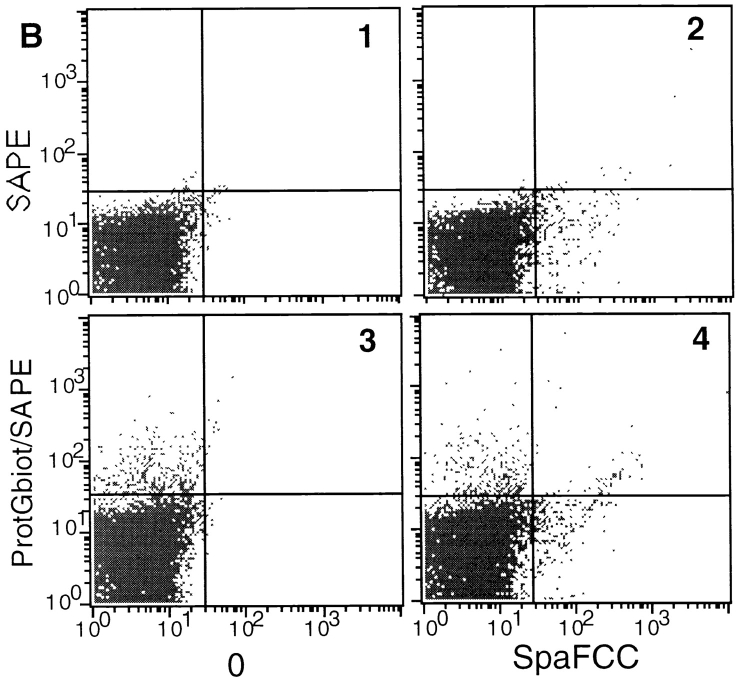
We investigated whether a bacterial IBP other than SpA could also trigger a boosting effect. Several of the above experiments were therefore repeated using protein G from Streptococcus ssp. group C (52), which contains both a Fab binding site and an Fcγ binding site (53, 54). As shown in Fig. 8 A, protein G boosted presentation of toxin α to T1B2 only when the toxin, Mα2-3, and protein G were concomitantly added to appropriate immune cells. In contrast, the boosting effect was not observed with protein G′, a protein G derivative that binds exclusively to the Fcγ of Igs (55). Therefore, these data suggest that the boosted presentation of an Ab–Ag complex results from a targeting by protein G to the Fab region of surface Igs of APCs. This raises the question of whether SpA and protein G recognize the same APC subpopulation. To approach this question, we performed FACS® analyses using both biotinylated protein G and SpAF. As shown in Fig. 8, protein G (B3, top left) and SpAF (B2, bottom right) bind to approximately the same proportion of the whole splenocyte population. Furthermore, the data presented in Fig. 8 (B4) suggest that SpA and protein G may recognize different splenocyte subpopulations. However, as we observed a slight increase in the upper right area of B4, we cannot preclude that a proportion of cells recognized by SpA and protein G are the same.
Figure 8.
T cell presentation of toxin α in the presence of protein G from Streptococcus ssp. (A) Toxin α (alpha) was serially diluted and incubated overnight at 4°C in the absence or presence of fixed concentrations of mAb Mα2-3 (25 nM final) and protein G or protein G′ (0.1 μM final concentration for each derivative). 5 × 105 splenocytes from BALB/c mice were then added to each well in the presence of 5 × 104 T1B2. Cells were cultured for 24 h at 37°C, and IL-2 secretion was subsequently determined by CTLL assay. (B) Splenocytes (5 × 105 cells) were incubated in the absence of IBP (B1) or presence of biotinylated protein G (protGbiot; B2), SpA–FCC (B3), or both (B4) for 30 min at 4°C. Cells were all incubated with SAPE and analyzed by flow cytometry.
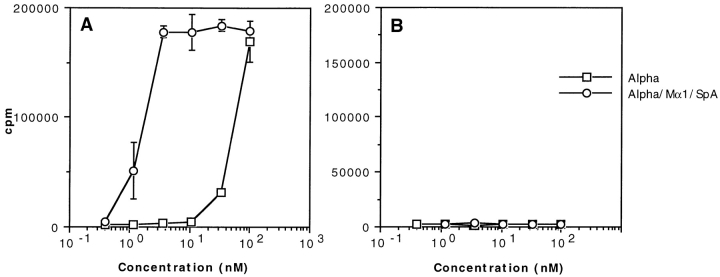
SpA May Also Boost T Cell Presentation of a Free Ab.
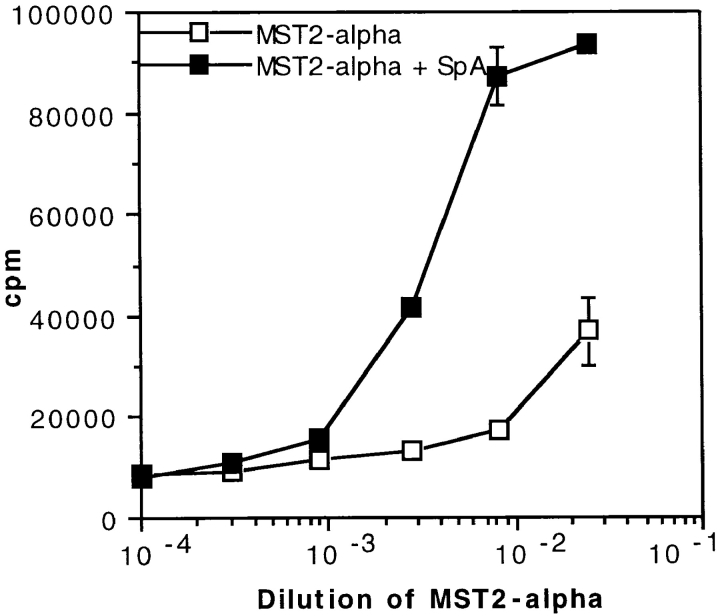
The view that emerges from the above data is that presentation of an Ag is boosted in the presence of an Ag-specific Ab as a result of the targeting of the complex by an IBP to APCs that contain IBP receptors. According to this scenario, both the mAb and SpA should also undergo a boosted presentation. This was demonstrated earlier to be the case for SpA (56). To provide evidence that it is also the case for the mAb, one would need mAb-specific T cells; however, they were unavailable in our laboratory. To overcome this difficulty, we used the toxin α–specific T1B2 to tentatively monitor the presentation of an mAb that was selected for its inability to recognize toxin α (57). This mAb, an IgG1 called MST2, was covalently linked to toxin α, which became, therefore, a label to probe the presentation of MST2. As shown in Fig. 9, the MST2–toxin α couple was efficiently presented to T1B2, and its presentation was clearly boosted by SpA. As a control, no such boosting effect was observed when the free toxin α was incubated with both the uncoupled MST2 and SpA (not shown). Therefore, only the toxin that was covalently linked to the Ab underwent a boosted presentation. This effect most probably reflects a boosting effect of the Ab itself. We conclude, therefore, that the boosting presentation of an Ab–Ag complex, as previously perceived via the stimulation of the Ag, reflects an increased presentation of both the Ag and Ag-specific Ab, a finding in agreement with the proposed scenario that an immune complex may be targeted by SpA to APC containing SpA receptors.
Figure 9.
Boosted presentation of MST2, an mAb which does not recognize toxin α as an antigen. In the absence of MST2-specific T cell hybridoma, we took advantage of the availability of T1B2 and coupled MST2 covalently to toxin α, which, therefore, was a labeling probe to monitor the presentation of the mAb. The covalent MST2–toxin α complex (called MST2-alpha) was serially diluted and incubated overnight at 4°C in the presence or absence of 0.2 μM SpA. 5 × 105 splenocytes from BALB/c mice were added to each well in the presence of 5 × 104 T1B2. Cells were cultured for 24 h at 37°C, and IL-2 secretion was subsequently determined by CTLL assay.
Discussion
Using a snake toxin as an Ag and two toxin-specific mAbs (Mα1 and Mα2-3), we showed that two soluble bacterial IBPs, SpA and protein G, dramatically increase the Ag-specific T cell stimulation of Ag–Ab complex. These findings are in sharp contrast to the observation that, in the absence of IBP, either of the Ag-specific mAbs inhibits presentation of the Ag.
A number of observations cast some light on the origin of this IBP-dependent boosting phenomenon. Thus, for the boosted presentation of the Ag to be seen, the Ag, an Ag-specific Ab, and an IBP, SpA or protein G, are absolutely required. As SpA is likely to boost presentation of a free Ab, we propose that the SpA-dependent boosted presentation of an immune complex, as perceived through presentation of its Ag, results from the binding of SpA to the Ab of the immune complex. Three lines of evidence indicate that the Ag-boosted presentation may result from binding of a ternary complex SpA–Ab–Ag to B cells containing SpA receptors. First, FACS® analyses demonstrated that an Ag–Ab complex is preferentially targeted by SpA to a subpopulation of splenocytes possessing SpA-specific receptors and mainly composed of B cells. Second, the boosted presentation further increased when splenocytes were enriched in cells containing SpA-specific receptors. Third, the boosting effect largely decreased when APCs were depleted of cells containing SpA-specific receptors. Altogether, these observations suggest that the two soluble IBPs can bridge an immune complex to IBP receptors present at the surface of a particular subpopulation of APCs, thus enhancing endocytosis and presentation of the Ag.
For SpA to bridge an immune complex to SpA receptors, appropriate binding sites must exist on the Ag–Ab complex. It is known that SpA can interact with the Fcγ region of mouse IgGs without impairing the binding of the Ags to their paratopes (19). In addition, SpA can bind to an alternative site located within the Fab domain of some mouse Igs (25, 29) that is coded by VH families S107 or J606 (35, 36). The VH domain of Mα2-3, one of the two toxin-specific mAbs that triggered the boosting effect, is coded by the VH germline gene J558 (51), whose gene product is, therefore, not recognized by SpA (35, 36). Thus, the Fcγ region of Mα2-3 and perhaps of Mα1, whose amino acid sequence is still unknown, are likely to be responsible for the interaction of the immune complex with SpA.
For SpA to bridge the Ab–Ag complexes to APCs, these cells must also possess appropriate SpA receptors. Four lines of evidence suggest that these receptors are surface Igs and most probably IgMs. First, we observed that the quaternary complex IgM–SpA (or BB)–Mα2-3–Ag can occur in vitro. Second, binding of a soluble IgG to splenocytes is increased only in the presence of an IBP derivative that possesses the capacity of binding concomitantly to Fab and Fcγ moieties. Third, for the boosting effect to be observed, an IBP with the same characteristics is also strictly required. Fourth, SpA preferentially targets Ab–Ag complex to a subpopulation of B cells. Because the surface IgMs offer an alternative binding site on the Fab domain, we suggest that they constitute the most plausible SpA receptors. Other possible SpA receptors are the Igs bound in vivo to FcRs present at the surfaces of macrophages (58). Although we detected no macrophages in the subpopulation of splenocytes enriched in cells containing SpA receptor, we cannot rule out their participation in the boosting effect. Therefore, our findings are compatible with the view that SpA may link the Fcγ part of the Ab moiety of an immune complex to cell surface, SpA-specific receptor structures such as IgMs present at the surface of a subpopulation of murine splenocytes.
Protein G from Streptococcus ssp. also efficiently boosts the presentation of Ag in immune complexes. Like SpA, protein G binds to the Fc region of IgGs in the Cγ2–Cγ3 interface (53, 54), their respective epitopes partially overlapping (59). Unlike SpA, however, protein G binds to Fab regions of IgG via the CH1 domain (60, 61) and not to IgMs (52). Therefore, if soluble protein G acts, like SpA, as an enhancer of immune complex presentation, its bridging capacity is likely to be different. Presumably, protein G predominantly focuses immune complexes to APCs carrying IgGs.
Several lines of evidence, mostly taken from the literature, indicate that SpA may react similarly with both human and mouse Igs. First, hIgs bind at two independent sites on SpA (14, 26–28, 30). Second, mouse Igs S107 and J606 are related to the human VH genes of family 3 (62), and several works have shown that hIgG F(ab′)2 and hIgM reacting with SpA derive from the VHIII family but do not have markers for other families (32–34). More precisely, 16/26 potentially functional germline VHIII genes encode SpA reactivity (63, 64). Third, SpA–Sepharose or SpA-containing S. aureus can stimulate B cells expressing VH gene segments encoded by a set of genes belonging to the VHIII family (47). Fourth, as reported above, the quaternary complex hIgM–SpA–mAb–toxin is sufficiently stable to be detected in ELISA assays. Plausibly, therefore, soluble SpA could bridge the Fc region of soluble hIgs to cell surface hIgs coded by one or more of the 16 germline VHIII genes encoding SpA reactivity. Remarkably, up to 54% of human B cells are capable of binding SpA (64), whereas VH families J606 and S107 are expressed in only 10–20% of adult B lymphocytes of BALB/c mice (65–68) and are likely to bind SpA. Therefore, it is not impossible that the binding of SpA with hIgs could cause a boosting effect in humans similar to the one that we described here for mice.
During an humoral immune response, an Ag is captured by dendritic cells, macrophages, and Ag-specific B cells, processed, and presented to Ag-specific T helper cells. This cascade of events leads to Ab secretion. The secreted Ag-specific Abs bind to the Ag, forming an heterogeneous population of immune complexes. It has been proposed that these complexes can cause a negative feedback on the specific antibody response, as a result of the diversion of Ags from their specific B cells, toward cells possessing Ig receptors, i.e., FcγR-expressing cells and RF-producing B cells (69). In this scenario, the Ag-specific helper T cells may activate B cells producing RF by intermolecular help. The data presented in this paper suggest another mechanism that might affect the immune response during an infection process by IBP-secreting bacteria. As a result of the presence of a soluble IBP, SpA for example, the Ab–Ag complexes may be targeted to B cell IgMs that can bind SpA. The SpA-binding B cells may thus present T cell epitopes from the targeted immune complexes and stimulate the corresponding T cells and may be activated by an intermolecular help mechanism. Although their specific Ags may not necessarily be present in the medium, the targeted B cells may nevertheless secrete their natural antibodies. The specificities of the produced antibodies cannot be identified a priori. However, they are likely to be large because the VH-reactive B cells are not bound by SpA via the traditional antigen binding site. It is tempting to speculate that this predicted diverting mechanism by an IBP may help the bacteria to evade the immune response.
Acknowledgments
We are indebted to the Service de Cytométrie, Institut des Sciences Végétales, Centre Nationale de la Recherche Scientifique-Unité de Recherche Propre 040, 91198 Gif-sur-Yvette, France, where we performed our cell sorting experiments. We gratefully acknowledge Dr. Pascal Kessler for providing us with monothiolated toxin α and Dr. Gilles Mourier for providing us with the NH2-terminally biotinylated toxin α.
Abbreviations used in this paper
- CTLL
cytotoxic T cell line
- FCC
fluorescein-5(6)-carboxamidocaproic
- hIg
human immunoglobulin
- IBP
immunoglobulin binding protein
- SAPE
streptavidin-PE
- SpA
staphylococcal protein A
- ssp.
subspecies
Footnotes
J. Galon was a recipient of fellowships from Ministère de la Recherche and of Association de Recherche contre le Cancer.
References
- 1.Celis E, Chang TW. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science. 1984;224:297–299. doi: 10.1126/science.6231724. [DOI] [PubMed] [Google Scholar]
- 2.Celis E, Zurawski VR, Jr, Chang TW. Regulation of T-cell function by antibodies: enhancement of the response of human T-cell clones to hepatitis B surface antigen by antigen-specific monoclonal antibodies. Proc Natl Acad Sci USA. 1984;81:6846–6850. doi: 10.1073/pnas.81.21.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corradin G, Engers HD. Inhibition of antigen-induced T-cell clone proliferation by antigen-specific antibodies. Nature. 1984;308:547–548. doi: 10.1038/308547a0. [DOI] [PubMed] [Google Scholar]
- 4.Corradin G, Juillerat MA, Engers HD. Differential effects of two anti-apo-cytochrome c-specific monoclonal antibodies on the function of apo-cytochrome c-specific murine T cell clones. J Immunol. 1984;133:2915–2919. [PubMed] [Google Scholar]
- 5.Schalke BC, Klinkert WE, Wekerle H, Dwyer DS. Enhanced activation of a T cell line specific for acetylcholine receptor (AChR) by using anti-AChR monoclonal antibodies plus receptors. J Immunol. 1985;134:3643–3648. [PubMed] [Google Scholar]
- 6.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet J-G, Webster P, Sautès C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 7.Amigorena S, Salamero J, Davoust J, Fridman WH, Bonnerot C. Tyrosine containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature. 1992;358:337–341. doi: 10.1038/358337a0. [DOI] [PubMed] [Google Scholar]
- 8.Amigorena S, Lankar D, Briken V, Gapin L, Viguier M, Bonnerot C. Type II and III receptors for immunoglobulin G (IgG) control the presentation of different T cell epitopes from single IgG-complexed antigens. J Exp Med. 1998;187:505–515. doi: 10.1084/jem.187.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manca F, Fenoglio D, Kunkl A, Cambiaggi C, Sasso M, Celada F. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J Immunol. 1988;140:2893–2898. [PubMed] [Google Scholar]
- 10.Davidson HW, Watts C. Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol. 1989;109:85–92. doi: 10.1083/jcb.109.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med. 1993;178:1459–1463. doi: 10.1084/jem.178.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–1963. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widders, P.R. 1990. Fc receptors and the pathogenesis of bacterial infections in animals. In Bacterial Immunoglobulin-binding Proteins, Vol. 1. M.D.P. Boyle, editor. Academic Press, Inc., Orlando, FL. 375–396.
- 14.Langone JJ. Protein A of Staphylococcus aureusand related immunoglobulin receptors produced by streptococci and pneumococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- 15.Ghetie V, Mota G, Dobre-Ghetie MA, Laky M, Olinescu A, Dima S, Moraru I, Sjoquist J. Modulation of IgG effector functions by a monovalent fragment of staphylococcal protein A. Mol Immunol. 1986;23:377–384. doi: 10.1016/0161-5890(86)90135-5. [DOI] [PubMed] [Google Scholar]
- 16.Kozlowski LM, Soulika AM, Silverman GJ, Lambris JD, Levinson AI. Complement activation by a B cell superantigen. J Immunol. 1996;157:1200–1206. [PubMed] [Google Scholar]
- 17.Rosenblatt J, Zeltzer PM, Portaro J, Seeger RC. Inhibition of antibody-dependent cellular cytotoxicity by protein A from Staphylococcus aureus. . J Immunol. 1977;118:981–985. [PubMed] [Google Scholar]
- 18.Romagnani S, Giudizi MG, Del Prete G, Maggi E, Biagiotti R, Almerigogna F, Ricci M. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureusCowan I on human B cells. J Immunol. 1982;129:596–602. [PubMed] [Google Scholar]
- 19.Langone JJ. [125I] protein A: a tracer for general use in immunoassay. J Immunol Methods. 1978;24:269–285. doi: 10.1016/0022-1759(78)90131-x. [DOI] [PubMed] [Google Scholar]
- 20.Endresen C. The binding to protein A of immunoglobulin G and of Fab and Fc fragments. Acta Pathol Microbiol Scand Sect C Immunol. 1979;87:185–189. [PubMed] [Google Scholar]
- 21.Zikan J. Interactions of pig Fab gamma fragments with protein A from Staphylococcus aureus. . Folia Microbiol (Praha) 1980;25:246–253. doi: 10.1007/BF02877346. [DOI] [PubMed] [Google Scholar]
- 22.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureusat 2.9- and 2.8-Å resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 23.Uhlén M, Guss B, Nilsson B, Gatenbeck S, Philipson L, Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984;259:1695–1702. [PubMed] [Google Scholar]
- 24.Moks T, Abrahmsen L, Nilsson B, Hellman U, Sjöquist J, Uhlén M. Staphylococcal protein A consists of five IgG-binding domains. Eur J Biochem. 1986;156:637–643. doi: 10.1111/j.1432-1033.1986.tb09625.x. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie MR, Gutman GA, Warner NL. The binding of murine IgM to staphylococcal A protein. Scand J Immunol. 1978;7:367–370. doi: 10.1111/j.1365-3083.1978.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 26.Inganäs M, Johansson SGO, Bennich HH. Interaction of human polyclonal IgE and IgG from different species with protein A from Staphylococcus aureus: demonstration of protein-A-reactive sites located in the Fab′2fragment of human IgG. Scand J Immunol. 1980;12:23–31. doi: 10.1111/j.1365-3083.1980.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 27.Inganäs M. Comparison of mechanisms of interaction between protein A from Staphylococcus aureus and human monoclonal IgG, IgA and IgM in relation to the classical Fc gamma and the alternative F(ab′)2epsilon protein A interactions. Scand J Immunol. 1981;13:343–352. doi: 10.1111/j.1365-3083.1981.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 28.Biguzzi S. Fc gamma-like determinants on immunoglobulin variable regions: identification by staphylococcal protein A. Scand J Immunol. 1982;15:605–618. doi: 10.1111/j.1365-3083.1982.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 29.Young WW, Jr, Tamura Y, Wolock DM, Fox JW. Staphylococcal protein A binding to the Fab fragments of mouse monoclonal antibodies. J Immunol. 1984;133:3163–3166. [PubMed] [Google Scholar]
- 30.Vidal MA, Conde FP. Alternative mechanism of protein A-immunoglobulin interaction: the VH-associated reactivity of a monoclonal human IgM. J Immunol. 1985;135:1232–1238. [PubMed] [Google Scholar]
- 31.Reichmann L, Davies J. Backbone assignment, secondary structure and protein A binding of an isolated, human antibody VH domain. J Biomol NMR. 1995;6:141–152. doi: 10.1007/BF00211778. [DOI] [PubMed] [Google Scholar]
- 32.Sasso EH, Silverman GJ, Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989;142:2778–2783. [PubMed] [Google Scholar]
- 33.Sasso EH, Silverman GJ, Mannik M. Human IgA and IgG F(ab′)2 that bind to staphylococcal protein A belong to the VHIII subgroup. J Immunol. 1991;147:1877–1883. [PubMed] [Google Scholar]
- 34.Sasano M, Burton DR, Silverman GJ. Molecular selection of human antibodies with an unconventional bacterial B cell antigen. J Immunol. 1993;151:5822–5839. [PubMed] [Google Scholar]
- 35.Seppälä I, Kaartinen M, Ibrahim S, Mäkelä O. Mouse Ig coded by VHfamilies S107 or J606 bind to protein A. J Immunol. 1990;145:2989–2993. [PubMed] [Google Scholar]
- 36.Ibrahim S, Kaartinen M, Seppälä I, Matoso-Ferreira A, Makela O. The alternative binding site for protein A in the Fab fragment of immunoglobulins. Scand J Immunol. 1993;37:257–264. doi: 10.1111/j.1365-3083.1993.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 37.Silverman GJ. Human antibody responses to bacterial antigens: studies of a model conventional antigen and a proposed model B cell superantigen. Int Rev Immunol. 1992;9:57–78. doi: 10.3109/08830189209061783. [DOI] [PubMed] [Google Scholar]
- 38.Silverman GJ. B-cell superantigens. Immunol Today. 1997;18:379–386. doi: 10.1016/s0167-5699(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 39.Forsgren A. Protein A from Staphylococcus aureus. VIII. Production of protein A by bacterial and l-forms of S. aureus. . Acta Pathol Microbiol Scand. 1969;75:481–490. [PubMed] [Google Scholar]
- 40.Forsgren A. Significance of protein A production by Staphylococci. . Infect Immun. 1970;5:672–673. doi: 10.1128/iai.2.5.672-673.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Movitz J. Formation of extracellular protein A by Staphylococcus aureus. . Eur J Biochem. 1976;68:291–299. doi: 10.1111/j.1432-1033.1976.tb10788.x. [DOI] [PubMed] [Google Scholar]
- 42.Léonetti M, Pillet L, Maillère B, Lamthanh H, Frachon P, Couderc J, Ménez A. Immunization with a peptide having both T cell and conformationally restricted B cell epitopes elicits neutralizing antisera against a snake neurotoxin. J Immunol. 1990;145:4214–4221. [PubMed] [Google Scholar]
- 43.Ménez, A., L. Pillet, M. Léonetti, F. Bontems, and B. Maillère. 1992. Snake toxins as antigens. In Structure of Antigens, Vol. 1. M.H.V. Van Regenmortel, editor. CRC Press, Boca Raton, FL. 293–320.
- 44.Maillère B, Cotton J, Mourier G, Léonetti M, Leroy S, Ménez A. Role of thiols in the presentation of a snake toxin to murine T cells. J Immunol. 1993;150:5270–5280. [PubMed] [Google Scholar]
- 45.Boulain JC, Ménez A. Neurotoxin-specific immunoglobulins accelerate dissociation of the neurotoxin-acetylcholine receptor complex. Science. 1982;217:732–733. doi: 10.1126/science.7100919. [DOI] [PubMed] [Google Scholar]
- 46.Trémeau O, Boulain JC, Couderc J, Fromageot P, Ménez A. A monoclonal antibody which recognized the functional site of snake neurotoxins and which neutralizes all short chain variants. FEBS Lett. 1986;208:236–240. doi: 10.1016/0014-5793(86)81024-9. [DOI] [PubMed] [Google Scholar]
- 47.Kristiansen SV, Pascual V, Lipsky PE. Staphylococcal protein A induces biased production of Ig by VH3-expressing B lymphocytes. J Immunol. 1994;153:2974–2982. [PubMed] [Google Scholar]
- 48.Jansson B, Uhlen M, Nygren PA. All individual domains of staphylococcal protein A show Fab binding. FEMS Immunol Med Microbiol. 1998;20:69–78. doi: 10.1111/j.1574-695X.1998.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson B, Moks T, Jansson B, Abrahmsén L, Elmblad A, Holmgren E, Henrichson C, Jones TA, Uhlén M. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;2:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 50.Ljundberg UK, Jansson B, Niss U, Nilsson R, Sandberg BE, Nilsson B. The interaction between different domains of staphylococcal protein A and human polyclonal IgG, IgA, IgM, and F(ab′)2: separation of affinity from specificity. Mol Immunol. 1993;30:1279–1285. doi: 10.1016/0161-5890(93)90044-c. [DOI] [PubMed] [Google Scholar]
- 51.Ducancel F, Mérienne K, Fromen-Romano C, Trémeau O, Pillet L, Drevet P, Zinn-Justin S, Boulain J-C, Ménez A. Mimicry between receptors and antibodies. Identification of snake toxin determinants recognized by the acetylcholine receptor and an acetylcholine receptor-mimicking monoclonal antibody. J Biol Chem. 1996;271:31345–31353. doi: 10.1074/jbc.271.49.31345. [DOI] [PubMed] [Google Scholar]
- 52.Björck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984;2:969–974. [PubMed] [Google Scholar]
- 53.Erntell M, Myhre EB, Sjöbring U, Björck L. Streptococcal protein G has affinity for both Fab- and Fc-fragments of human IgG. Mol Immunol. 1988;25:121–126. doi: 10.1016/0161-5890(88)90059-4. [DOI] [PubMed] [Google Scholar]
- 54.Woof, J.M., and D.R. Burton. 1990. The nature of the interaction of bacterial Fc receptors and IgG. In Bacterial Immunoglobulin-binding Proteins, Vol. 1. M.D.P. Boyle, editor. Academic Press, Inc., Orlando, FL. 305–316.
- 55.Goward CR, Murphy JP, Atkinson T, Barstow DA. Expression and purification of a truncated recombinant streptococcal protein G. Biochem J. 1990;267:171–177. doi: 10.1042/bj2670171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Léonetti M, Thai R, Cotton J, Leroy S, Drevet P, Ducancel F, Boulain J-C, Ménez A. Increasing immunogenicity of antigens fused to Ig binding proteins by cell-surface targeting. J Immunol. 1998;160:3820–3827. [PubMed] [Google Scholar]
- 57.Charpentier I, Pillet L, Karlsson E, Couderc J, Ménez A. Recognition of the acetylcholine receptor binding site of a long-chain neurotoxin by toxin-specific monoclonal antibodies. J Mol Recognit. 1990;3:74–81. doi: 10.1002/jmr.300030204. [DOI] [PubMed] [Google Scholar]
- 58.Cohen BE, Rosenthal AS, Paul WE. Antigen-macrophage interaction. II. Relative roles of cytophilic antibody and other membrane sites. J Immunol. 1973;111:820–828. [PubMed] [Google Scholar]
- 59.Tashiro M, Montelione GT. Structures of bacterial immunoglobulin-binding domains and their complexes with immunoglobulins. Curr Opin Struct Biol. 1995;5:471–481. doi: 10.1016/0959-440x(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 60.Derrick JP, Wigley DB. Crystal structure of a streptococcal protein G domain bound to an Fab fragment. Nature. 1992;359:752–754. doi: 10.1038/359752a0. [DOI] [PubMed] [Google Scholar]
- 61.Derrick JP, Wigley DB. The third IgG-binding domain from streptococcal protein G. An analysis by X-ray crystallography of the structure alone and in a complex with Fab. J Mol Biol. 1994;243:906–918. doi: 10.1006/jmbi.1994.1691. [DOI] [PubMed] [Google Scholar]
- 62.Rathbun, G., J. Berman, G. Yancopoulos, and F.W. Alt. 1989. Organization and expression of the mammalian heavy-chain variable-region locus. In Immunoglobulin Genes. T. Honjo, F.W. Alt, and T.H. Rabbits, editors. Academic Press, Inc., Orlando, FL. 63–90.
- 63.Hillson JL, Karr NS, Oppliger IR, Mannik M, Sasso EH. The structural basis of germline-encoded VHIII immunoglobulin binding to staphylococcal protein A. J Exp Med. 1993;178:331–336. doi: 10.1084/jem.178.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hakoda M, Kamatani N, Hayashimoto-Kurumada S, Silverman GJ, Yamanaka H, Terai C, Kashiwazaki S. Differential binding avidities of human IgM for staphylococcal protein A derive from specific germ-line VH3 gene usage. J Immunol. 1996;157:2976–2981. [PubMed] [Google Scholar]
- 65.Silverman GJ, Sasano M, Wormsley SB. Age-associated changes in binding of human B lymphocytes to a VHIII-restricted unconventional bacterial antigen. J Immunol. 1993;151:5840–5855. [PubMed] [Google Scholar]
- 66.Wu GE, Paige CJ. VHgene family utilization in colonies derived from B and pre-B cells detected by the RNA colony blot assay. EMBO (Eur Mol Biol Organ) J. 1986;5:3475–3481. doi: 10.1002/j.1460-2075.1986.tb04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong HD, Teale JM. Comparison of the fetal and adult functional B cell repertoires by analysis of VHgene family expression. J Exp Med. 1988;168:589–603. doi: 10.1084/jem.168.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yancopoulos GD, Malynn BA, Alt FW. Developmentally regulated and strain-specific expression of murine VHgene families. J Exp Med. 1988;168:417–435. doi: 10.1084/jem.168.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roosnek E, Lanzavecchia A. Efficient and selective presentation of antigen–antibody complexes by rheumatoid factor B cells. J Exp Med. 1991;173:487–489. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]