Abstract
TRAIL (tumor necrosis factor [TNF]-related apoptosis-inducing ligand) is a molecule that displays potent antitumor activity against selected targets. The results presented here demonstrate that human monocytes rapidly express TRAIL, but not Fas ligand or TNF, after activation with interferon (IFN)-γ or -α and acquire the ability to kill tumor cells. Monocyte-mediated tumor cell apoptosis was TRAIL specific, as it could be inhibited with soluble TRAIL receptor. Moreover, IFN stimulation caused a concomitant loss of TRAIL receptor 2 expression, which coincides with monocyte acquisition of resistance to TRAIL-mediated apoptosis. These results define a novel mechanism of monocyte-induced cell cytotoxicity that requires TRAIL, and suggest that TRAIL is a key effector molecule in antitumor activity in vivo.
Keywords: TRAIL, apoptosis, tumor, monocyte, human
Mononuclear phagocytes (Mφ)1 circulate in the peripheral blood before differentiation into non-lymphoid and lymphoid tissue–associated macrophages (1). Mφ mediate several host defense mechanisms through activation of MHC class I– and class II–restricted T lymphocytes (2, 3), release of inflammatory mediators (4), and killing of virus-infected cells (5). In addition, Mφ effectively kill tumor cells through both antibody-dependent and antibody-independent mechanisms (6, 7). A critical prerequisite for these Mφ functions is cellular activation. Two potent mediators of Mφ activation, IFN-γ and IFN-α, dramatically enhance the cytolytic potential of human Mφ (8, 9). The mechanisms responsible for this IFN-induced tumoricidal activity remain poorly defined. Nitric oxide (NO) is known to be produced by Mφ and is a potent mediator of tumor cell death (10, 11). However, several reports have demonstrated that IFN-γ and IFN-α stimulation of human Mφ does not increase the level of NO release, suggesting that NO does not play a significant role in the cytotoxic activity of IFN-stimulated human Mφ (12–14). Although TNF possesses cytolytic activity and is produced by Mφ after certain types of activation (e.g., LPS), several studies have shown that IFN does not induce TNF production in human Mφ (15– 17). These data indicate that other cell death–inducing molecules may be involved with the Mφ-mediated tumoricidal activity after IFN stimulation.
Two TNF family members capable of inducing cell death are Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL). FasL participates in many physiological events, including autoimmunity, activation-induced cell death (AICD) of lymphocytes, immune privilege, and tumor evasion from the immune system (18–21). Dysregulation of physiological Fas/FasL interactions results in immune disease states characterized by enhanced levels of Fas-mediated apoptosis and a variety of forms of hepatitis (22–24) or diseases with decreased levels of lymphocyte death (e.g., human autoimmune lymphoproliferative syndrome [18]). Human Mφ contain high levels of intracellular FasL that can be released after cellular activation (25). Recent evidence indicates that FasL-expressing Mφ are essential in the elimination of activated effector T cells in homeostasis and in disease, suggesting that they may also play an important physiological role in a variety of other immunological settings (23, 26–29).
In contrast to FasL, the role of TRAIL in immune regulation remains enigmatic (30). Recent studies have identified four distinct cell surface TRAIL receptors, with two (DR4 and DR5/TRAIL-R2; hereafter referred to as TRAIL-R1 and -R2, respectively) that contain a cytoplasmic death domain and signal for apoptotic cell death upon receptor cross-linking, and two (TRID/DcR1/TRAIL-R3 and TRAIL-R4/DcR2; hereafter referred to as TRAIL-R3 and -R4, respectively) that lack a death domain, making them unable to signal for cell death (31–37). Recombinant, soluble forms of TRAIL are potent mediators of tumor cell apoptosis, while demonstrating minimal cytotoxicity toward normal tissues in vitro and in vivo (38–40). The cell death induced by TRAIL displays many of the same characteristics observed with other apoptotic molecules (i.e., FasL and TNF), such as caspase activation, DNA fragmentation into oligonucleosomal “ladders,” annexin V binding, and the morphological membrane blebbing and apoptotic body release (30, 38, 41). This tumoricidal activity occurs on ∼2/3 of the >30 hematopoietic and nonhematopoietic tumor cell lines tested (30, 36, 38, 42). Such studies not only support the potential use for TRAIL as an antitumor therapeutic agent (30, 38, 40), but suggest that TRAIL may be an innate effector molecule involved in the elimination of a broad range of spontaneously arising tumor cells. The results presented here identify a novel mechanism by which Mφ induce tumor cell apoptosis via TRAIL.
Materials and Methods
Reagents and mAbs.
Reagents and sources were as follows: IL-1, IL-2, IL-3, IL-4, IL-6, IL-7, IL-15, GM-CSF (100 ng/ml; Immunex); IFN-α, IFN-γ, IL-10, IL-12 (100 ng/ml; Genzyme Corp.); LPS (5 ng/ml; DIFCO); MOPC-21, nonspecific IgG1 isotype control; MOPC-173, nonspecific IgG2a isotype control; M181, IgG1 anti-TRAIL (Immunex); mAb11, IgG1 anti-TNF; NOK-1, IgG1 anti-FasL (PharMingen). The mAbs against the four TRAIL receptors (M271, IgG2a anti–TRAIL-R1; M413, IgG1 anti–TRAIL-R2; M430, IgG1 anti–TRAIL-R3; and M444, IgG1 anti–TRAIL-R4) were produced at Immunex by immunizing BALB/c mice (The Jackson Laboratory) with a purified fusion protein consisting of the extracellular domain of human TRAIL-R1, -R2, -R3, or -R4 coupled to the constant region of human IgG1 (huTRAIL-R:Fc) in Titermax (CytRx Corporation). Mice were boosted three times, and spleen cells were fused with the murine myeloma NS1 in the presence of 50% polyethylene glycol in PBS followed by culture in DMEM/HAT and DMEM/HT selective media. Supernatants from positive wells were tested for the ability to bind the appropriate TRAIL receptor in an ELISA (cell-based ELISA using CV1 cells transfected with TRAIL receptor cDNA) and reactivity to huTRAIL-R:Fc in Western blots. Hybridomas that produced antibodies that bound to huTRAIL-R:Fc, but not human IgG1, were cloned by three rounds of limiting dilution. All mAbs were purified by protein A affinity chromatography. The matrix metalloproteinase inhibitors, TAPI (TNF protease inhibitor [43]) and KB8301, were obtained from Immunex and PharMingen, respectively. The soluble fusion proteins TRAIL-R2:Fc, Fas:Fc, and TNFR:Fc were produced at Immunex. The leucine zipper (LZ)-TRAIL expression plasmid (34) and the production and purification of LZ-huTRAIL (40) have been described previously.
Cell Lines.
The ovarian carcinoma cell line (OVCAR3) was obtained from Dr. Richard F. Camalier, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). The human melanoma cell lines (WM 793 and 164) were obtained from Dr. M. Herlyn, Wistar Institute (Philadelphia, PA). The human prostate carcinoma cell line (PC-3) was obtained from Dr. Michael Cohen, University of Iowa (Iowa City, IA). The human colon carcinoma cell line (Colo205) and the human lung adenocarcinoma cell line (H2126) were provided by Dr. Brian Gliniak and Tim Lofton, respectively (Immunex). L929 cells and normal human foreskin fibroblasts were obtained from American Type Culture Collection. All tumor cell lines were cultured as directed. The normal human lung fibroblasts were purchased from Clonetics Corporation and cultured as directed.
Isolation of Human Mφ.
Peripheral blood Mφ were enriched using countercurrent elutriation. Cells from leukopheresis packs obtained from healthy volunteers were loaded onto a JE-5 elutriator (Beckman), and 50-ml fractions were collected while increasing the flow rate from 65 to 85 ml/min at 2,000 rpm. Mφ-enriched fractions generated from a flow rate >75 ml/min were >90% CD14+ as assessed by flow cytometric analysis using TUK-4, an IgG2a anti-CD14 (Caltag Laboratories, Inc.).
Flow Cytometry.
Untreated or cytokine-stimulated Mφ were incubated with the following unlabeled primary mAbs for 1 h at 4°C: MOPC-21, MOPC-173, M181, M271, M413, M430, M444, mAb11, and NOK-1. After three washes, primary antibody binding was detected with a PE-conjugated, Fc-specific, mouse anti–human F(ab′)2 (Jackson ImmunoResearch Laboratories). Staining for TNF was done in the presence of the matrix metalloproteinase inhibitor, TAPI (50 μM). Staining for FasL was done in the presence of the matrix metalloproteinase inhibitor, KB8301 (10 μM). Cells were analyzed immediately after staining or fixed in 1% paraformaldehyde until analysis on a FACSCalibur™ (Becton Dickinson).
TRAIL-mediated Killing of Human Tumor Cells.
Mφ were cultured for 12 h in medium alone, GM-CSF, IFN-γ, or IFN-α, washed, and resuspended in complete medium. Tumor cells were labeled with 100 μCi of 51Cr for 1 h at 37°C, washed three times, and resuspended in complete medium. To determine TRAIL- induced death, 51Cr-labeled tumor cells (104/well) were incubated with varying numbers of Mφ effector cells for 8 h. As a positive control, soluble LZ-TRAIL was added to the target cells at the indicated concentrations. In some cultures, TRAIL-R2:Fc, Fas:Fc, or TNFR:Fc (20 μg/ml) was added to the Mφ effector cells 15 min before adding tumor cell targets. All cytotoxicity assays were performed in round-bottomed 96-well plates, and the percent specific lysis was calculated as: 100 × (experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm). Spontaneous and total release were determined in the presence of either medium alone or 1% NP-40, respectively. The presence of TRAIL-R2:Fc, Fas:Fc, or TNFR:Fc during the assay had no effect on the level of spontaneous release of 51Cr by the target cells. For analysis of tumor cell apoptosis, tumor cell targets were incubated with unstimulated or cytokine-stimulated Mφ as described above. Apoptotic cell death of the tumor cells was measured by flow cytometry using FITC-conjugated annexin V and propidium iodide (Apoptosis detection kit; R&D Systems) as per the manufacturer's protocol. Light scatter characteristics were used to identify the tumor cells.
Inhibition of NO Synthesis.
The specific inhibitor of NO synthase, N G-monomethyl-l-arginine (L-NMMA; AerBio, Ltd.), was used to block Mφ NO production (44). Mφ were stimulated with the appropriate cytokine and then incubated with the tumor cell targets as above. Mφ were cultured in the presence of 300 μM L-NMMA at all steps throughout the assay. The presence of L-NMMA had no effect on the level of spontaneous release of 51Cr by the target cells during the assay.
TNF-mediated Killing of L929 Cells.
Mφ were cultured for 2 or 12 h in medium alone or LPS (5 ng/ml), washed, and resuspended in complete medium. L929 cells were labeled with 100 μCi of 51Cr for 1 h at 37°C, washed three times, and resuspended in complete medium. To determine TNF-induced death, 51Cr-labeled L929 cells (104/well) were incubated with varying numbers of Mφ effector cells for 8 h. As a positive control, soluble TNF was added to the target cells at the indicated concentrations. In some cultures, TNFR:Fc or TRAIL-R2:Fc (20 μg/ml) was added to the Mφ effector cells 15 min before adding tumor cell targets. L929 cytotoxicity assays were performed in an identical manner as for the human tumor cell lines. The presence of TNFR:Fc or TRAIL-R2:Fc during the assay had no effect on the level of spontaneous release of 51Cr by the L929 target cells.
TRAIL-mediated Killing of Mφ.
Mφ were cultured for 12 h in medium alone or with cytokine (IL-1, IL-2, IL-3, IL-4, IL-7, IL-10, IL-12, IL-15, GM-CSF, or IFN-γ; all cytokines were used at 100 ng/ml), and then labeled with 100 μCi of 51Cr for 1 h at 37°C, washed three times, and resuspended in complete medium. To determine sensitivity to TRAIL-induced death, 51Cr-labeled Mφ (106/well) were incubated with LZ-TRAIL for 8 h. Assays were performed as described above.
Reverse Transcription PCR.
Total RNA was isolated from Mφ with TRIzol reagent (Life Technologies) as per the manufacturer's instructions. RNA samples (1 μg each) were tested for DNA contamination by 30 cycles of PCR with human β-actin primers. After it was shown that there was no DNA contamination, cDNA synthesis was performed using an RNA PCR kit (Perkin-Elmer) with the supplied oligo d(T)16 primer. Reverse transcription was performed using a thermal program of 25°C for 10 min, 42°C for 30 min, and 95°C for 5 min. PCR reactions were performed using the following primers: human β-actin (forward: 5′-GAAACTACCTTCAACTCCATC-3′; reverse: 5′-CGAGGCCAGGATGGAGCCGCC-3′); human TRAIL-R1 (forward: 5′-CTGAGCAA-CGCAGACTCGCTGTCCAC-3′; reverse: 5′-TCCAAGGACA-CGGCAGAGCCTGTGCCAT-3′); human TRAIL-R2 (forward: 5′-GCCTCATGGACAATGAGATAAAGGTGGCT-3′; reverse: 5′-CCAAATCTCAAAGTACGCACAAACGG-3′); human TRAIL-R3 (forward: 5′-GAAGAATTTGGTGCCAATGCCACTG-3′; reverse: 5′-CTCTTGGACTTGGCTGGGAGATGTG-3′); human TRAIL-R4 (forward: 5′-CTTTTCCGGCGGCGTTCATGTCCTTC-3′; reverse: 5′-GTTTCTTCCAGGCTGCTTCCCTTTGTAG-3′); and human TRAIL (forward: 5′-CAACTCCGTCAGCTCGTTAGAAAG-3′; reverse: 5′-TTAGACCAACAACTATTTCTAGCACT-3′), giving products of 219, 506, 502, 612, 453, and 443 bp, respectively. β-actin PCR cycle conditions were 95°C for 45 s, 55°C for 1 min, and 72°C for 45 s for 30 cycles. TRAIL-R1, -R2, and -R3 cycle conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min for 30 cycles. TRAIL-R4 cycle conditions were 95°C for 4 min 15 s, followed by 30 cycles of 95°C for 45 s, 60°C for 45 s, and 72°C for 45 s. TRAIL cycle conditions were 95°C for 45 s, 55°C for 45 s, and 72°C for 45 s for 30 cycles. Samples were resolved on a 2% agarose gel and visualized with ethidium bromide.
Results
Human Mφ Stimulated with IFN Upregulate TRAIL.
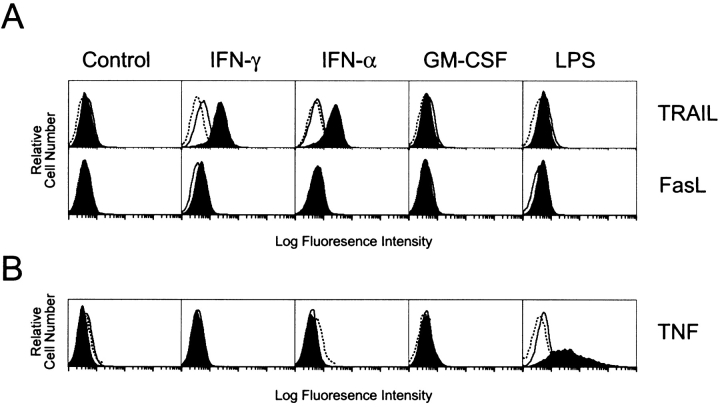
To compare the difference in TRAIL, FasL, and TNF surface expression, peripheral blood human Mφ were isolated and cultured with several molecules known to induce Mφ activation and differentiation (IFN-γ, IFN-α, GM-CSF, and LPS), and then examined by flow cytometry. Significant TRAIL expression was detected on the Mφ cultured for 12 h in the presence of either IFN-γ or IFN-α, but not with either GM-CSF or LPS (Fig. 1 A). In contrast, FasL expression on Mφ was undetectable after either 2- or 12-h stimulation with any of the cytokines or LPS in the presence of the metalloproteinase inhibitor, KB8301 (Fig. 1 A). Analysis of the surface levels of TNF demonstrated no measurable increase after stimulation with IFN-γ or IFN-α; however, stimulation with LPS for 2 h led to increased surface levels of TNF that disappeared by 12 h (Fig. 1 B). To inhibit the cleavage of membrane TNF, Mφ were cultured in the presence of the metalloproteinase inhibitor, TAPI (TNF protease inhibitor [43]). These data demonstrate that TRAIL, but not FasL and TNF, is induced after IFN stimulation, and that the expression of TRAIL and TNF on Mφ is regulated by distinct activation stimuli and with different kinetics.
Figure 1.
TRAIL, FasL, and TNF expression on human Mφ. (A) Mφ were incubated for 2 or 12 h in the absence or presence of IFN-γ, IFN-α, GM-CSF, or LPS and then analyzed for TRAIL or FasL surface expression. Filled histograms represent staining at 12 h by either M181 (anti-TRAIL mAb) or NOK-1 (anti-FasL mAb). Open histograms represent staining at 2 h with the same mAb, whereas dotted histograms represent staining with isotype control mAb. (B) Mφ were incubated as in A and then analyzed for TNF surface expression. Filled histograms represent staining at 2 h by mAb11 (anti-TNF mAb). Open histograms represent staining at 12 h with the same mAb, whereas dotted histograms represent staining with isotype control mAb. Histograms represent 104 gated Mφ in all conditions, and viability was >95% as assessed by propidium iodide exclusion. These observations were reproduced using Mφ from at least five different donors.
IFN-stimulated Mφ Kill Tumor Cells via a TRAIL-dependent Mechanism.
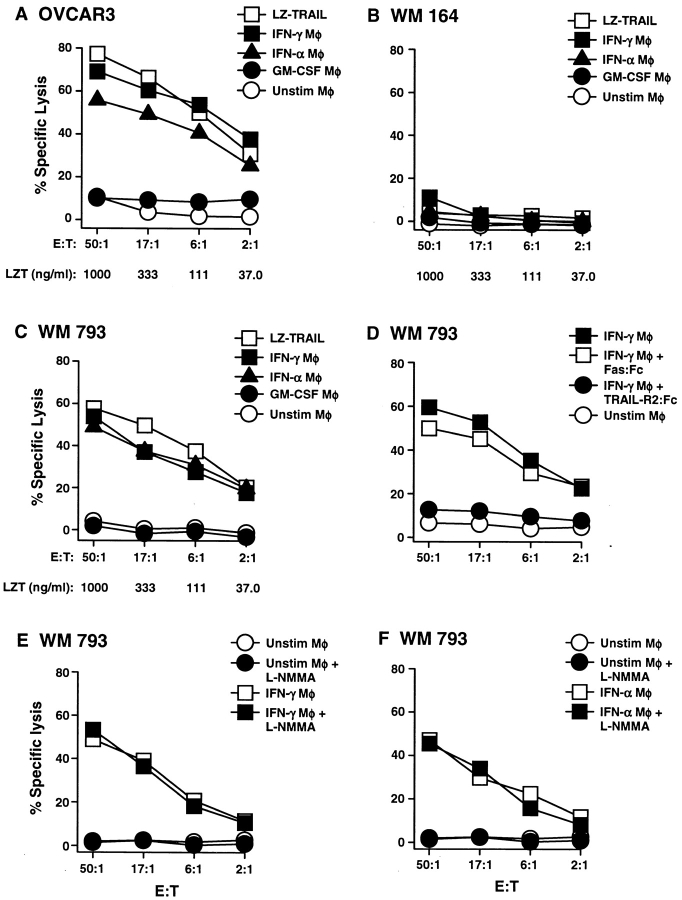
The results from Fig. 1 demonstrate that Mφ stimulated with IFN upregulate the expression of TRAIL on the cell surface. Thus, to examine the functional activity of TRAIL in this setting, Mφ were stimulated with either GM-CSF, IFN-γ, or IFN-α for 12 h and then cultured in the presence of OVCAR3, a TRAIL-sensitive human ovarian carcinoma cell line. Although the unstimulated or GM-CSF–treated Mφ demonstrated minimal tumoricidal activity toward OVCAR3, the Mφ stimulated with IFN-γ or IFN-α were potent killers of these TRAIL-sensitive tumor cells over a broad range of E/T ratios (Fig. 2 A). A titration of both IFN-γ and IFN-α concentrations revealed that as little as 10 pg/ml led to enhanced Mφ cytotoxicity against the tumor cells; however, GM-CSF did not induce any antitumor activity at any concentration tested (100 ng/ml to 10 pg/ml; data not shown). Moreover, the IFN-stimulated Mφ were as effective in killing the tumor target cells as recombinant, soluble TRAIL (LZ-TRAIL [34]). The tumoricidal activity of IFN-stimulated Mφ was also examined on a TRAIL-resistant human melanoma cell line, WM 164, and a TRAIL-sensitive human melanoma cell line, WM 793 (38). The TRAIL-resistant melanoma (WM 164) was also resistant to the Mφ-mediated cytotoxicity, whereas the TRAIL-sensitive melanoma (WM 793) was quite sensitive to the cytotoxic activity of either IFN-γ– or IFN-α–stimulated Mφ (Fig. 2, B and C). The tumoricidal activity of both IFN-γ– or IFN-α–stimulated Mφ was seen from multiple donors and with other TRAIL-sensitive tumor cells (Table I). Two normal human fibroblast cell types were also tested for sensitivity to the cytokine-stimulated Mφ and were found to be resistant in all conditions from multiple donors.
Figure 2.
TRAIL-mediated tumoricidal activity by human Mφ occurs after stimulation with IFN. (A–D) Mφ were incubated for 12 h in the absence or presence of either GM-CSF, IFN-γ, or IFN-α and then cultured for 8 h with 51Cr-labeled (A) OVCAR3, (B) WM 164, or (C) WM 793 target cells at the indicated E/T ratios. As a positive control, soluble LZ-TRAIL (LZT) was added to target cells at the indicated concentrations. (D) Inclusion of the fusion protein TRAIL-R2:Fc (20 μg/ml) to 12-h IFN-γ–stimulated Mφ inhibited killing of WM 793 target cells, whereas addition of Fas:Fc (20 μg/ml) did not. (E and F) Addition of the NO inhibitor L-NMMA (300 μM) did not alter the antitumor activity of (E) IFN-γ– or (F) IFN-α–stimulated Mφ against WM 793 target cells. Data points represent the mean of triplicate wells, and experiments were repeated at least three times with similar results. For clarity, SD bars were omitted from the graphs, but were <10% of the value of all points.
Table I.
Tumoricidal Activity of Cytokine-stimulated Mφ
| Tumor cell target | Donors tested | Mφ* | LZ-TRAIL‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unstimulated | GM-CSF | IFN-γ | IFN-α | |||||||||
| H2126 (lung adenocarcinoma) | 3 | 1.3 ± 0.9 | 1.3 ± 1.3 | 47.5 ± 4.0 | 36.1 ± 11.9 | 59.0 ± 5.9 | ||||||
| PC-3 (prostate carcinoma) | 4 | 3.3 ± 2.0 | 2.0 ± 1.7 | 57.2 ± 7.6 | 41.5 ± 5.2 | 67.5 ± 4.2 | ||||||
| Colo205 (colon carcinoma) | 3 | 2.3 ± 2.1 | 1.8 ± 4.2 | 46.1 ± 3.0 | 38.4 ± 7.1 | 58.3 ± 3.1 | ||||||
| OVCAR3 (ovarian carcinoma) | 5 | 5.8 ± 4.3 | 6.3 ± 3.8 | 59.4 ± 13.2 | 47.5 ± 7.5 | 65.4 ± 11.0 | ||||||
| MDA 231 (breast adenocarcinoma) | 3 | 2.0 ± 0.7 | 2.4 ± 0.3 | 39.6 ± 6.8 | 31.2 ± 8.8 | 57.7 ± 10.7 | ||||||
| WM 164 (melanoma) | 3 | 0.9 ± 0.4 | 2.4 ± 0.8 | 9.3 ± 2.7 | 4.4 ± 2.1 | 3.2 ± 0.9 | ||||||
| WM 793 (melanoma) | 5 | 3.6 ± 4.2 | 4.8 ± 4.2 | 45.6 ± 14.6 | 43.7 ± 4.5 | 48.6 ± 6.8 | ||||||
| Normal lung fibroblasts | 2 | 4.1 ± 0.1 | 4.1 ± 2.3 | 3.2 ± 1.3 | 0.9 ± 1.2 | 1.0 ± 1.0 | ||||||
| Normal foreskin fibroblasts | 2 | 3.9 ± 1.2 | 3.2 ± 2.0 | 2.9 ± 1.0 | 2.6 ± 1.4 | 2.1 ± 1.1 | ||||||
Mean percent specific lysis (+SD) at 50:1 Mφ to target cell ratio.
Mean percent specific lysis (+SD) with 1 μg/ml LZ-TRAIL. Means were calculated from experiments performed with Mφ from the indicated number of donors.
Although FasL was not detected on the surface of the activated Mφ (Fig. 1 A), activated Mφ have been shown to release soluble FasL from intracellular stores (25). Therefore, to confirm that the observed tumoricidal activity was specific to TRAIL and not FasL, IFN-γ–stimulated Mφ were pretreated with either TRAIL-R2:Fc (35) or Fas:Fc before adding the tumor cell targets. The TRAIL-R2:Fc reduced target cell death to control (unstimulated Mφ effector) levels, whereas Fas:Fc did not alter the ability of the IFN-γ–treated Mφ to mediate tumor lysis (Fig. 2 D). Finally, to determine whether Mφ NO production contributed to the measured cytotoxic activity, Mφ were stimulated as above but in the absence or presence of the NO synthase inhibitor, L-NMMA (44). The cytotoxic activity of the IFN-γ– and IFN-α–stimulated Mφ was not decreased in the presence of L-NMMA compared with Mφ stimulated in the absence of the inhibitor (Fig. 2, E and F). Similar results were observed with other tumor cell targets (data not shown). Furthermore, analysis of NO production by the Mφ after 12 h stimulation, as measured by the accumulation of nitrite, revealed no increase in nitrite levels in the culture supernatants with any of the different stimuli compared with unstimulated Mφ (data not shown). Collectively, these results confirm that the TRAIL expressed on Mφ mediates the killing of tumor cells, demonstrating a novel mechanism of Mφ-mediated apoptosis.
TRAIL-expressing Mφ Induce Apoptotic Cell Death of Sensitive Tumor Cells.
Although the release of 51Cr from the tumor cell targets as measured in Fig. 2 indicates the amount of cell death, it does not discriminate between apoptotic and necrotic cell death. Previous reports have demonstrated that TRAIL-induced cell death occurs through an apoptotic mechanism (30, 34, 38, 41). To confirm that the tumor cell death induced by the IFN-stimulated Mφ was mediated through an apoptotic mechanism, the binding of FITC-conjugated annexin V to the tumor cells was analyzed. Annexin V preferentially binds to phosphatidylserine, a phospholipid component of the inner leaflet of the plasma membrane that is rapidly externalized during apoptosis (45, 46). Upon staining the OVCAR3 tumor cells after 6 h incubation with unstimulated or cytokine-stimulated Mφ (E/T ratio 2:1) or soluble LZ-TRAIL, only those tumor cells incubated with IFN-stimulated Mφ or LZ-TRAIL were positive for FITC-annexin V binding (Fig. 3), indicating that these cells were dying from the induction of apoptosis. Morphological changes (membrane blebbing and release of apoptotic bodies) were also observed using light microscopy (data not shown).
Figure 3.
Phosphatidylserine externalization on OVCAR3 tumor cells during apoptosis induced by IFN-stimulated, TRAIL- expressing Mφ. OVCAR3 tumor cells were cultured for 6 h in medium alone or in the presence of LZ-TRAIL (1 μg/ml), unstimulated, or cytokine (GM-CSF, IFN-γ, IFN-α [100 ng/ml for 12 h])–stimulated Mφ (E/T ratio 2:1). Cells were then stained with FITC–annexin V and analyzed by flow cytometry. The percent of FITC–annexin V positive tumor cells is indicated for each condition. Histograms represent 104 gated tumor cells. Similar results were seen with Mφ from three other donors.
The Principal Mechanism of Mφ Tumoricidal Activity Differs after IFN and LPS Stimulation.
Having demonstrated that IFN-stimulated Mφ express functional TRAIL, the differences between TRAIL and TNF-mediated tumoricidal activity by Mφ were examined. The results presented in Fig. 1 show that TNF was only expressed on Mφ after incubation with LPS. Thus, to demonstrate the biologic activity of the cell surface TNF detected after LPS stimulation, 2- and 12-h LPS-stimulated Mφ were evaluated for the ability to kill the TNF-sensitive target cell, L929 (17). In direct correlation with the flow cytometric results, the 2-h LPS-stimulated Mφ, but not the 12-h LPS-stimulated Mφ, killed L929 target cells to levels comparable to soluble TNF (Fig. 4 A). When tested for sensitivity to LZ-TRAIL, the L929 cells were found to be resistant (data not shown). This killing was TNF-specific as demonstrated by the significant inhibition of the lysis of L929 cells upon the addition of TNFR:Fc, but not TRAIL-R2:Fc (Fig. 4 B). To further demonstrate that the cytotoxic activity of IFN-γ–stimulated Mφ was mediated by TRAIL and not TNF, tumor cell lysis was measured in the presence of TRAIL-R2:Fc or TNFR:Fc. Only TRAIL-R2:Fc, and not TNFR: Fc, inhibited the IFN-γ–stimulated Mφ from killing the tumor cell targets (Fig. 4 C). Thus, human Mφ have multiple mechanisms for killing a variety of target cells depending on the activation mechanism.
Figure 4.
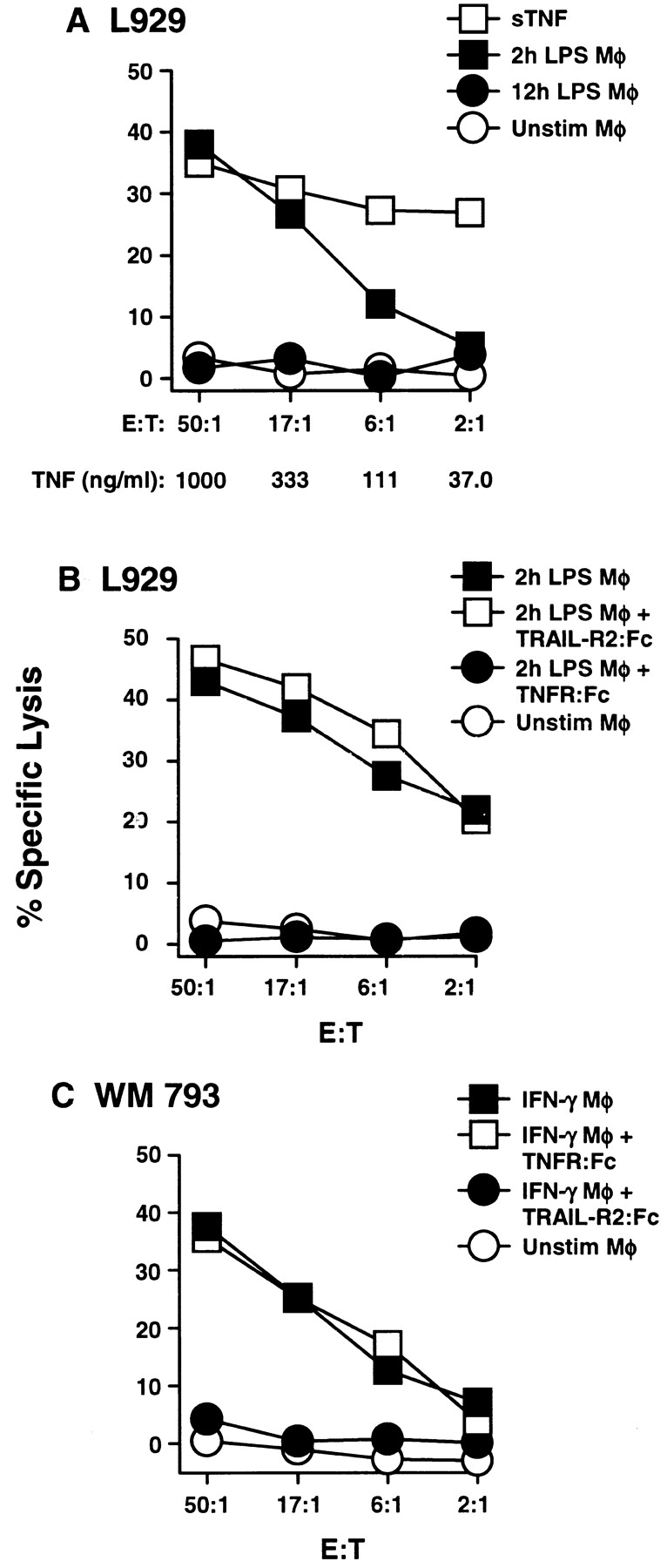
TNF-mediated apoptosis by human Mφ occurs after stimulation with LPS but not IFN-γ. (A) Mφ were incubated in the absence or presence of LPS for 2 or 12 h and then cultured for 8 h with 51Cr-labeled L929 target cells at the indicated E/T ratios. As a positive control, soluble (s)TNF was added to targets cells at the indicated concentrations. (B) Inclusion of TNFR: Fc (20 μg/ml) to 2-h LPS-stimulated Mφ inhibited the killing of L929 target cells, whereas addition of TRAIL-R2:Fc (20 μg/ml) did not. (C) Killing of WM 793 tumor cells by Mφ stimulated with IFN-γ for 12 h can be inhibited by TRAIL-R2:Fc (20 μg/ml), but not TNFR:Fc (20 μg/ml). Data points represent the mean of triplicate wells, and the experiments were repeated at least three times with similar results. For clarity, SD bars were omitted from the graphs, but were <10% of the value of all points.
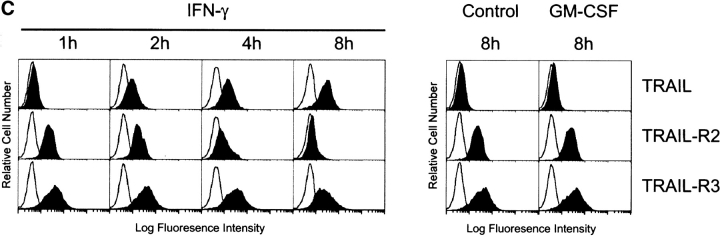
TRAIL Receptor Expression on Human Mφ Is Altered by IFN.
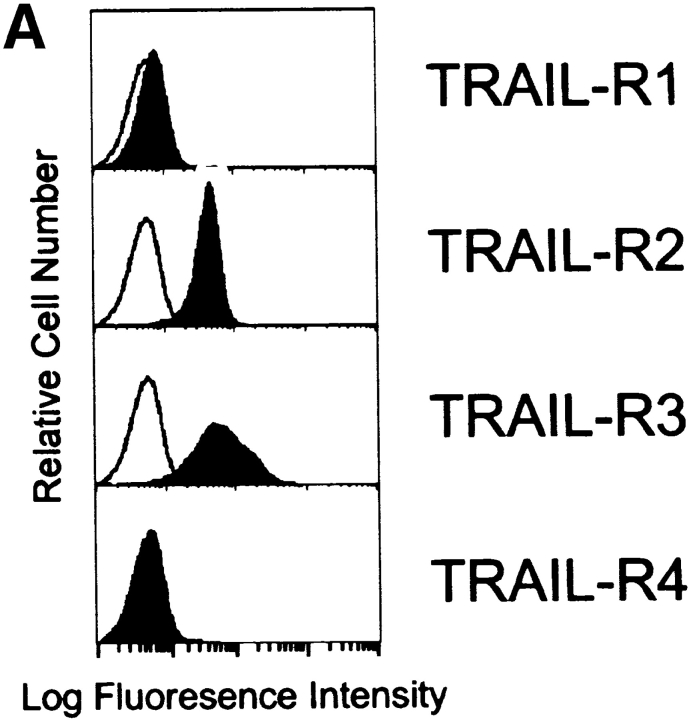
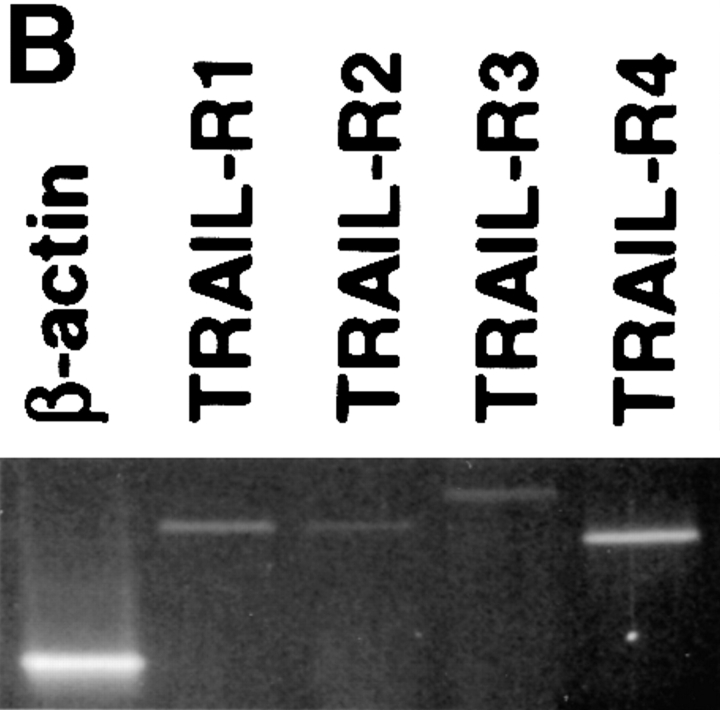
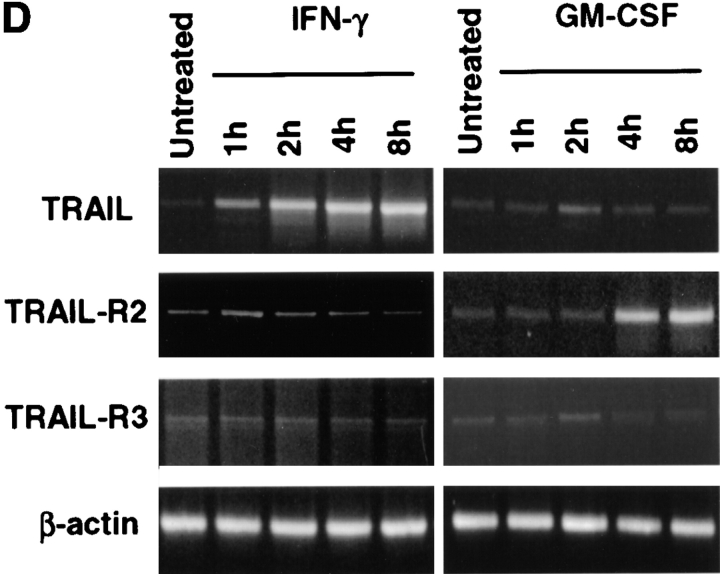
Because TRAIL can interact with two death-inducing and two non-death-inducing receptors, the distribution of the four known TRAIL receptors on the Mφ surface using receptor-specific mAbs was investigated. Unstimulated Mφ expressed both TRAIL-R2 and -R3, whereas the levels of TRAIL-R1 and -R4 were at or below detection (Fig. 5 A). However, mRNA for each of the four TRAIL receptors could be detected by reverse transcription (RT)- PCR analysis (Fig. 5 B). The kinetics of TRAIL, TRAIL-R2, and TRAIL-R3 expression after IFN-γ stimulation were then measured at both the protein level by flow cytometry and the mRNA level by RT-PCR. Increased TRAIL expression could be detected by 2 h on the cell surface after addition of IFN-γ (Fig. 5 C), whereas RT-PCR analysis demonstrated that TRAIL mRNA levels increased by 1 h (Fig. 5 D). No consistent change in TRAIL protein or mRNA levels was detected after GM-CSF treatment compared with untreated Mφ (Fig. 5, C and D).
Figure 5.
Surface analysis of IFN-γ–stimulated Mφ reveals simultaneous increase in TRAIL expression with downregulation of TRAIL-R2 expression. (A) Flow cytometric analysis of TRAIL-R1, -R2, -R3, and -R4 expression on unstimulated Mφ. Filled histograms represent staining by M271 (anti–TRAIL-R1 mAb), M413 (anti–TRAIL-R2 mAb), M430 (anti–TRAIL-R3 mAb), or M444 (anti–TRAIL-R4 mAb), and open histograms represent staining with isotype control mAb. (B) RT-PCR analysis of TRAIL receptor mRNA expression in normal Mφ. (C) TRAIL, TRAIL-R2, and TRAIL-R3 expression after various times of IFN-γ stimulation. Filled histograms represent staining by M413 (anti–TRAIL-R2 mAb), M430 (anti–TRAIL-R3 mAb), or M181 (anti-TRAIL mAb), and open histograms represent staining with isotype control mAb. For comparison, Mφ cultured in the absence or presence of GM-CSF for 8 h were also stained for TRAIL, TRAIL-R2, and TRAIL-R3. (D) RT-PCR analysis of TRAIL, TRAIL-R2, and TRAIL-R3 mRNA levels after Mφ culture in the absence or presence of IFN-γ and GM-CSF. β-actin was used as a control over the same time course. Similar results were observed with Mφ from two other donors.
Examination of the surface levels of TRAIL-R2 and -R3 during this same 8-h period revealed that IFN-γ–stimulated Mφ downregulated TRAIL-R2 expression, whereas TRAIL-R3 was only slightly downmodulated (Fig. 5 C). In contrast, incubation with GM-CSF for 8 h resulted in a slight increase in TRAIL-R2 expression. Analysis of mRNA from IFN-γ–stimulated Mφ revealed that TRAIL-R2 mRNA levels remained relatively constant over the 8-h period, whereas the TRAIL-R2 mRNA levels increased in Mφ stimulated with GM-CSF over time (Fig. 5 D). No changes in TRAIL-R3 mRNA were observed with IFN-γ or GM-CSF incubation during this period of time (Fig. 5 D), nor were any changes in TRAIL-R1 or -R4 mRNA or protein detected (data not shown). Thus, TRAIL expression can be detected on Mφ within 2 h after IFN-γ stimulation, paired with a concomitant loss in cell surface expression of the cognate death-inducing TRAIL-R2. Similar results examining TRAIL and TRAIL receptor expression on IFN-α–stimulated Mφ were also detected (data not shown).
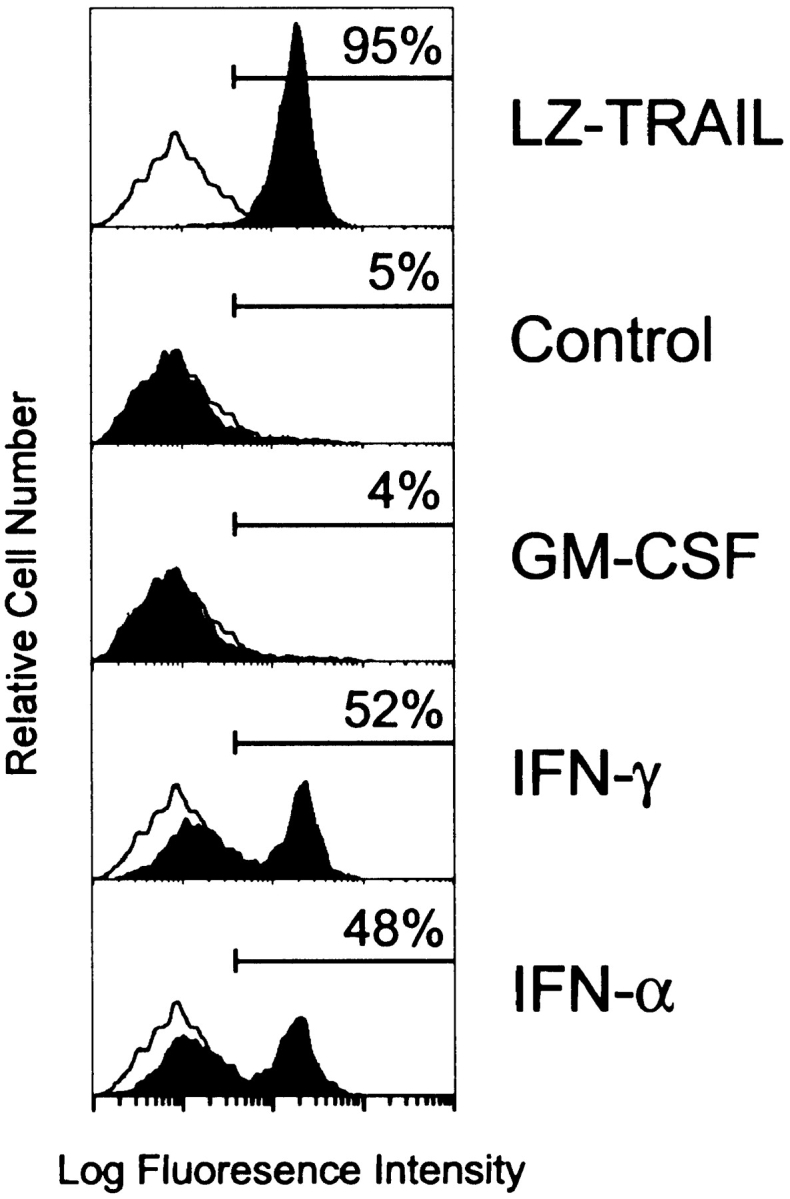
IFN-stimulated Mφ Are Resistant to TRAIL-induced Death.
The loss of TRAIL-R2 expression suggested that peripheral blood Mφ stimulated with IFN-γ would be resistant to TRAIL-mediated death. Thus, Mφ were cultured in the absence or presence of GM-CSF or IFN-γ and then examined for sensitivity to LZ-TRAIL. Mφ treated with GM-CSF displayed increased sensitivity to TRAIL-induced death compared with untreated Mφ; in contrast, IFN-γ treatment significantly decreased TRAIL-induced Mφ death (Fig. 6 A). Similar results were obtained with IFN-α–stimulated Mφ (data not shown). No significant changes in Mφ sensitivity to TRAIL were seen with the other cytokines tested (IL-1, IL-2, IL-3, IL-4, IL-6, IL-7, IL-10, IL-12, and IL-15; data not shown) compared with untreated Mφ. These results suggest that Mφ stimulated with IFN-γ minimize TRAIL-mediated suicide or fratricide with the downregulation of TRAIL-R2 surface levels. However, it remains possible that additional mechanisms may contribute to the protection of the Mφ from TRAIL-induced death.
Figure 6.
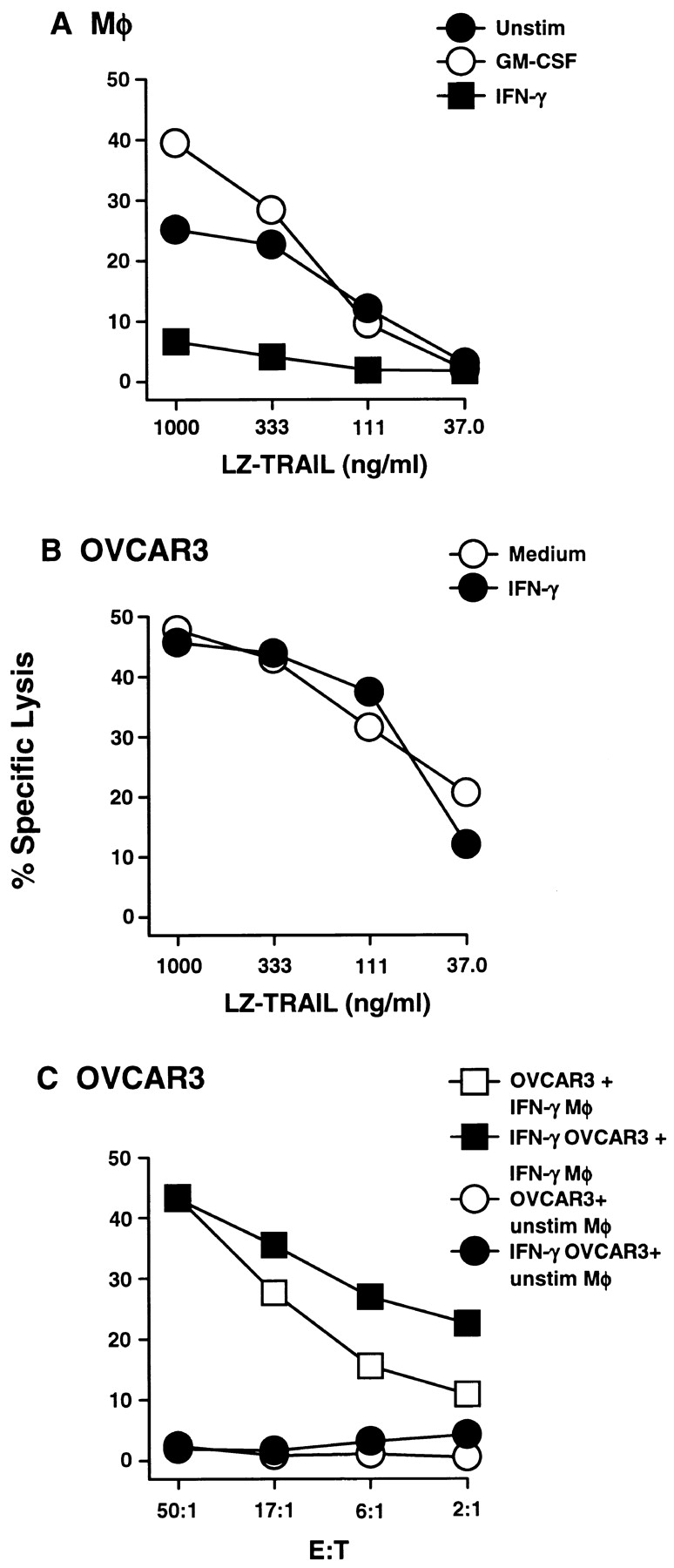
IFN-γ stimulation renders Mφ resistant to TRAIL-induced death, but not tumor cell targets. (A) Peripheral blood Mφ were incubated for 12 h in the absence or presence of GM-CSF and IFN-γ, then tested for sensitivity to LZ-TRAIL. (B) OVCAR3 tumor cells were incubated for 12 h in the absence or presence of IFN-γ, then tested for sensitivity to LZ-TRAIL. (C) OVCAR3 tumor cells were cultured for 12 h in the absence or presence of IFN-γ, then incubated with unstimulated or IFN-γ–stimulated Mφ. Percent specific lysis was measured by 51Cr release after 8 h, and each data point represents the mean of triplicate wells. For clarity, SD bars were omitted from the graphs, but were <10% of the value of all points. These experiments were repeated at least three times with similar results using Mφ from at least three different donors.
The rendering of Mφ resistant to TRAIL-mediated apoptosis after stimulation with IFN-γ suggested that the tumor cell targets used in Fig. 2 could be affected in a similar fashion upon culture with IFN-γ. OVCAR3 tumor cells were incubated in the absence or presence of IFN-γ for 12 h, and then tested for sensitivity to LZ-TRAIL or IFN-γ–stimulated Mφ. In contrast to the Mφ, the sensitivity of OVCAR3 tumor cells was not altered after incubation with IFN-γ (Fig. 6, B and C). Similar results were seen with other tumor cells (WM 793 and PC-3; data not shown). These results imply that not all cell types respond to IFN-γ by gaining resistance to TRAIL-induced death as observed with the Mφ.
Discussion
Mφ not only influence the activities of other immune and nonimmune cells in the body, but also function as effector cells under a variety of conditions (1). Activated Mφ display potent tumoricidal activity against several different tumor cell types (7, 47–49). The results presented here demonstrate that one of the mechanisms by which Mφ kill tumor cells is through expression of TRAIL. Mφ stimulation with either IFN-γ or IFN-α resulted in the rapid expression of TRAIL on the cell surface, but not FasL or TNF. Because TRAIL mediates apoptosis in a high percentage (approximately two thirds) of hematopoietic and nonhematopoietic cell types (30, 36, 38, 42), Mφ have the potential to mediate apoptosis of a broad range of tumor cell types via TRAIL. Expression of TRAIL appeared to be specific to the IFNs, as Mφ stimulation with either IL-1, IL-2, IL-3, IL-4, IL-6, IL-7, IL-10, IL-12, IL-15, or GM-CSF resulted in no detectable TRAIL expression (data not shown). Interestingly, a concomitant loss of TRAIL-R2 (and to a lesser extent TRAIL-R3) expression was detected upon IFN stimulation, rendering the Mφ resistant to TRAIL-mediated death. To our knowledge, this is the first demonstration of inducible TRAIL expression on a particular human peripheral blood cell population, as well as modulation of the death-inducing TRAIL-R2 by a proinflammatory cytokine on the same cell population.
Although the expression of TRAIL on the IFN-stimulated Mφ is critical for the tumoricidal activity in our assay system, the sensitivity of the tumor cell to TRAIL-induced apoptosis is also an essential component of this phenomenon, as demonstrated by the fact that the tumor cell lines and normal cells that were resistant to TRAIL-mediated apoptosis were also resistant to TRAIL-expressing Mφ. The identification of two TRAIL receptors with death- inducing ability and two without led to the initial hypothesis that the expression of TRAIL-R3 and/or -R4 conferred resistance to TRAIL-induced death (32, 33, 37). However, it is important to note that this hypothesis was formulated from reports examining the distribution of TRAIL receptor mRNA in several normal tissues and tumor cell lines and from experiments where TRAIL-R3 or -R4 was overexpressed in transfected cells. Most of the tumors used in this study express TRAIL-R3 and/or -R4 (38, 42). When the OVCAR3, WM793, and PC3 tumor cells were cultured with IFN-γ before incubation with LZ-TRAIL or IFN-stimulated Mφ, the TRAIL receptor levels remained unchanged, and no significant change in the level of TRAIL sensitivity was observed (data not shown). Thus, the differences in sensitivity of the tumor cells to the TRAIL expressed on the Mφ or the recombinant TRAIL added in solution are probably regulated by a variety of molecular mechanisms, both inside the cell and at the surface.
While our results focused on the tumoricidal activity of TRAIL-expressing Mφ, previous reports have shown these cells can also produce cytotoxic inorganic oxidants, such as NO (10, 11). A role for NO in tumor cell killing has been documented for both human and mouse activated macrophages (4, 44, 50), where the toxicity of NO is mediated via mitochondrial damage, inhibition of DNA synthesis, and disruption of the tricarboxylic acid cycle, ultimately resulting in apoptosis (51, 52). Although murine macrophages release high levels of NO after either LPS or IFN-γ stimulation, studies with human peripheral blood Mφ have reported contradictory findings (10). In some reports, Mφ stimulated with either LPS or IFN-γ (or in combination) failed to release significant levels of NO (12–14, 44, 53), whereas others have reported that IFN-α stimulation results in a slight increase in NO production (54). In our studies, addition of the NO inhibitor L-NMMA to the cytotoxicity assays did not decrease the ability of the IFN-γ– and IFN-α–stimulated Mφ to kill the tumor cell targets. Moreover, analysis of the culture supernatants for nitrites revealed no increase after 12 h stimulation with GM-CSF, IFN-γ, or IFN-α (data not shown). These observations, coupled with the fact that TRAIL-R2:Fc completely inhibited the tumoricidal activity of the Mφ to background (unstimulated Mφ) levels, imply that TRAIL is the primary mediator of the tumoricidal activity after IFN stimulation. Coexpression of TRAIL and TNF, and perhaps other unidentified death-inducing molecules, would theoretically increase both the cytolytic potential of the Mφ and the range of different tumor targets susceptible to Mφ-mediated death.
The importance of IFN in the management of spontaneously arising tumors was recently demonstrated in vivo using mice that lack sensitivity to IFN-γ (55). Compared with wild-type mice, the IFN-γ–insensitive mice develop tumors more rapidly and with greater frequency when challenged with a chemical carcinogen. Part of this “IFN effect” is via the interaction of the IFN with the tumor cells by enhancing the tumor cell tumorigenicity through heightened MHC class I expression. IFN-γ may also enhance an innate antitumor mechanism through the induction of TRAIL on cells of the Mφ lineage (55). Although our data suggest that Mφ would confer this antitumor activity, further studies are required to determine if other cell types, such as NK cells, neutrophils, and dendritic cells, are also able to express TRAIL after IFN stimulation (56).
Finally, in addition to a role in tumoricidal activity, these data suggest that Mφ TRAIL-expression may contribute to other physiologic and pathologic situations, such as the AICD of T cells during HIV infection. Recently, Katsikis et al. (57, 58) have demonstrated that activation-induced peripheral blood T cell apoptosis in HIV-infected individuals was Fas independent, and a potential role for TRAIL in this phenomenon was identified. It was observed that a blocking mAb to TRAIL could inhibit the AICD of T cells in a mixed population of HIV+ PBMCs (58). However, it was unclear which PBMC subset was expressing TRAIL and responsible for death of the T cells. Thus, the results presented here may provide an explanation for these experimental observations, as well as a basis for examining other activities mediated by activated Mφ.
Acknowledgments
We thank Drs. David Cosman, Ray Goodwin, David Lynch, Craig Smith, Michael Widmer, and Doug Williams for careful reading of the manuscript, Gary Carlton for figure preparation, and Anne Aumell for editorial assistance.
Abbreviations used in this paper
- AICD
activation-induced cell death
- L-NMMA
N G-monomethyl-l-arginine
- LZ
leucine zipper
- Mφ
mononuclear phagocyte(s)
- NO
nitric oxide
- RT
reverse transcription
- TRAIL
TNF-related apoptosis-inducing ligand
References
- 1.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 2.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 3.Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol. 1992;149:3477–3481. [PubMed] [Google Scholar]
- 4.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 5.Jewett A, Giori JV, Bonavida B. Antibody- dependent cellular cytotoxicity against HIV-coated target cells by peripheral blood monocytes from HIV seropositive asymptomatic patients. J Immunol. 1990;145:4065–4071. [PubMed] [Google Scholar]
- 6.Steplewski Z, Lubeck MD, Koprowski H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science. 1983;221:865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- 7.te Velde AA, Figdor CG. Monocyte mediated cytotoxic activity against melanoma. Melanoma Res. 1992;1:303–309. doi: 10.1097/00008390-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 9.Webb DS, Gerrard TL. IFN-alpha and IFN-gamma can affect both monocytes and tumor cells to modulate monocyte-mediated cytotoxicity. J Immunol. 1990;144:3643–3648. [PubMed] [Google Scholar]
- 10.Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 12.Zembala M, Siedlar M, Marcinkiewicz J, Pryjma J. Human monocytes are stimulated for nitric oxide release in vitroby some tumor cells but not by cytokines and lipopolysaccharide. Eur J Immunol. 1994;24:435–439. doi: 10.1002/eji.1830240225. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 14.Konur A, Krause SW, Rehli M, Kreutz M, Andreesen R. Human monocytes induce a carcinoma cell line to secrete high amounts of nitric oxide. J Immunol. 1996;157:2109–2115. [PubMed] [Google Scholar]
- 15.Feinman R, Henriksen-DeStefano D, Tsujimoto M, Vilcek J. Tumor necrosis factor is an important mediator of tumor cell killing by human monocytes. J Immunol. 1987;138:635–640. [PubMed] [Google Scholar]
- 16.Philip R, Epstein LB. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, γ-interferon and interleukin-1. Nature. 1986;323:86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- 17.Solomon KA, Covington MB, DeCicco CP, Newton RC. The fate of pro-TNF-α following inhibition of metalloprotease-dependent processing to soluble TNF-α in human monocytes. J Immunol. 1997;159:4524–4531. [PubMed] [Google Scholar]
- 18.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 19.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 21.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 22.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus–infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badley AD, Dockrell D, Simpson M, Schut R, Lynch DH, Leibson P, Paya CV. Macrophage- dependent apoptosis of CD4+T lymphocytes from HIV- infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (Apo-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC. Human monocytic cells contain high levels of intracellular Fas ligand. J Immunol. 1997;159:1594–1598. [PubMed] [Google Scholar]
- 26.Oyaizu N, Adachi Y, Hashimoto F, McCloskey TW, Hosaka N, Kayagaki N, Yagita H, Pahwa S. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+T cells apoptosis. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 27.Wu MX, Daley JF, Rasmussen RA, Schlossman SF. Monocytes are required to prime peripheral T cells to undergo apoptosis. Proc Natl Acad Sci USA. 1995;92:1525–1529. doi: 10.1073/pnas.92.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cottrez F, Manca F, Dalgleish AG, Arenzana-Seisdedos F, Capron A, Groux H. Priming of human CD4+antigen-specific T cells to undergo apoptosis by HIV-infected monocytes: a two step process involving the gp120 molecule. J Clin Invest. 1997;99:257–266. doi: 10.1172/JCI119154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dockrell DH, Badley AD, Villacian JS, Heppelmann CJ, Algeciras A, Ziesmer S, Yagita H, Lynch DH, Roche PC, Leibson PJ, Paya CV. The expression of Fas ligand by macrophages and its upregulation by human immunodeficiency virus infection. J Clin Invest. 1998;101:2394–2405. doi: 10.1172/JCI1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang C-P, Nicholl JK, Sutherland GR, Davis T, Smith, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 31.Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 32.Pan G, Ni J, Wei Y-F, Yu G-I, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 33.Sheridan JP, Marsters SA, Pitti PM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 34.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO (Eur Mol Biol Organ) J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang C-P, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 37.Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 38.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL- induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 39.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walczak H, Miller RE, Gliniak B, Ariail K, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of TRAIL in vivo. . Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 41.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 42.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 43.Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K, et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- 44.Martin JHJ, Edwards SW. Changes in mechanisms of monocyte/macrophage-mediated cytotoxicity during culture. J Immunol. 1993;150:3478–3486. [PubMed] [Google Scholar]
- 45.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 46.Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie RCAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams DO. Molecular interactions in macrophage activation. Immunol Today. 1989;10:33–35. doi: 10.1016/0167-5699(89)90298-3. [DOI] [PubMed] [Google Scholar]
- 48.Nissen-Meyer J, Espevik T. Effect of antisera against recombinant tumor necrosis factor and the monocyte-derived cytotoxin(s) on monocyte-mediated killing of various tumor cells. Cell Immunol. 1987;109:384–396. doi: 10.1016/0008-8749(87)90321-2. [DOI] [PubMed] [Google Scholar]
- 49.Nissen-Meyer J, Hofsli E, Espevik T, Austgulen R. Involvement of tumor necrosis factor in cytotoxicity mediated by human monocytes. Nat Immun Cell Growth Regul. 1988;7:266–279. [PubMed] [Google Scholar]
- 50.Keller R, Keist R. Abilities of activated macrophages to manifest tumoricidal activity and to generate reactive nitrogen intermediates: a comparative study in vitro and ex vivo. . Biochem Biophys Res Commun. 1989;164:968–973. doi: 10.1016/0006-291x(89)91764-6. [DOI] [PubMed] [Google Scholar]
- 51.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 52.Pellat C, Heney Y, Drapier JC. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between l-arginine-derived nitric oxide and non-heme iron products. Biochem Biophys Res Commun. 1990;166:119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- 53.Harwix S, Andreesen R, Ferber E, Schwamberger G. Human macrophages secrete a tumoricidal activity distinct from tumour necrosis factor-α and reactive nitrogen intermediates. Res Immunol. 1992;143:89–94. doi: 10.1016/0923-2494(92)80084-x. [DOI] [PubMed] [Google Scholar]
- 54.Sharara AI, Perkins DJ, Misukonis MA, Chan SU, Dominitz JA, Weinberg JB. Interferon (IFN)-α activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression: possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-α in vivo. J Exp Med. 1997;186:1495–1502. doi: 10.1084/jem.186.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell–mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;88:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katsikis PD, Garcia-Ojeda ME, Wunderlich ES, Smith CA, Yagita H, Okumura K, Kayagaki N, Alderson M, Herzenberg LA, Herzenberg LA. Activation- induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int Immunol. 1996;8:1311–1317. doi: 10.1093/intimm/8.8.1311. [DOI] [PubMed] [Google Scholar]
- 58.Katsikis PD, Garcia-Ojeda ME, Torres-Roca JF, Tijoe IM, Smith CA, Herzenberg LA, Herzenberg LA. Interleukin-1β converting enzyme–like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]