Abstract
Resuscitation from hemorrhage induces profound pathophysiologic alterations and activates inflammatory cascades able to initiate neutrophil accumulation in a variety of tissues. This process is accompanied by acute organ damage (e.g., lungs and liver). We have previously demonstrated that significant leukocyte–endothelium interactions occur very early in other forms of ischemia/reperfusion (i.e., splanchnic ischemia/reperfusion and traumatic shock) which are largely mediated by increased expression of the adhesion molecule, P-selectin, on the vascular endothelium. Here we postulated that increased endothelial expression of P-selectin in the microvasculature would play an essential role in initiating the inflammatory signaling of hemorrhagic shock. Using intravital microscopy, we found that hemorrhagic shock significantly increased the number of rolling and adherent leukocytes in the mouse splanchnic microcirculation. In contrast, mice genetically deficient in P-selectin, or wild-type mice given either an anti–P-selectin monoclonal antibody or a recombinant soluble P-selectin glycoprotein ligand (PSGL)-1 immunoglobulin, exhibited markedly attenuated leukocyte–endothelium interaction after hemorrhagic shock. Thus, activation of P-selectin protein on the microvascular endothelium is essential for the initial upregulation of the inflammatory response occurring in hemorrhagic shock. Moreover, endogenous levels of PSGL-1 mRNA were significantly increased in the lung, liver, and small intestine of wild-type mice subjected to hemorrhagic shock. Since PSGL-1 promotes adhesive interactions largely through P-selectin expressed on the vascular endothelium, this result further supports the crucial role played by P-selectin in the recruitment of leukocytes during hemorrhagic shock.
Keywords: mRNA, leukocyte, endothelium, immunohistochemistry, intravital microscopy
Hemorrhagic shock initiates an inflammatory response characterized by the upregulation of cytokine expression (1) and emigration of neutrophils into a variety of tissues (2). The accumulation of neutrophils in splanchnic and thoracic organs is likely to contribute to end-organ damage and resultant dysfunction after this form of shock. The mechanism by which hemorrhage triggers this inflammatory response remains poorly understood. Heightened adrenergic activity (3), increased production of reactive free radicals (4), and systemic release of proinflammatory agents from the gut (5, 6) have been hypothesized to contribute to acute organ injury after hemorrhage. Nevertheless, none of these studies has clarified the specific pathway that promotes leukocyte recruitment into visceral organs during hemorrhagic shock.
Before transmigration into inflamed tissue, leukocytes must initially interact with the vascular endothelium (7), as demonstrated during ischemia/reperfusion (8) and in several experimental models of circulatory shock (9). Moreover, we have reported that under shock conditions very early endothelial dysfunction (i.e., 2.5–5 min) is associated with increased endothelial dysfunction (10, 11) and expression of the cell adhesion molecule, P-selectin (12). P-selectin is able to initiate the cascade of events that increases cell adherence and leukocyte infiltration into injured tissues by first promoting leukocyte rolling along the vascular endothelium (13, 14). Therefore, we hypothesized that increased surface expression of P-selectin in the microvascular endothelium would exert an essential role in recruitment of leukocytes in the case of hemorrhagic shock. Hemorrhage was therefore carried out in control wild-type mice, in wild-type mice treated with anti–P-selectin mAb or with a recombinant soluble form of P-selectin glycoprotein ligand (PSGL)-1,1 and in mice genetically deficient in P-selectin. Furthermore, mRNA lung, liver, and small intestine levels for the counter-ligand of P-selectin, PSGL-1, were investigated in mice subjected to hemorrhagic shock. We report here that P-selectin deficiency or blockade prevents the increase in leukocyte–endothelium interaction observed in the mouse microcirculation after reinfusion of hemorrhaged mice. These data strongly indicate that increased P-selectin surface expression on the surface of the microvascular endothelium plays an essential role in the initiation of the inflammatory response observed in hemorrhage and reinfusion, and that this mechanism can be abrogated in a variety of ways.
Materials and Methods
Hemorrhagic Shock Protocol.
This study was performed in accordance with the National Institute of Health guidelines for the use of experimental animals, and all animal protocols have been approved by the Institutional Animal Care and Use Committee (IACUC) of Thomas Jefferson University.
Wild-type (C57BL/6) mice and P-selectin–deficient (C57BL/ 6J-Sel) mice were obtained from The Jackson Laboratory. Male and female mice (8–14 wk old and 20–30 g body wt) were anesthetized with sodium pentobarbital (120 mg/kg) injected intraperitoneally. A tracheotomy was performed to maintain a patent airway throughout the experiment. The left carotid artery was cannulated for continuous blood pressure monitoring, and the right jugular vein was cannulated for blood withdrawal and fluid or antibody administration. Mice were subjected to hemorrhage by withdrawal of blood to allow mean arterial blood pressure (MABP)1 to be maintained at 40 mmHg for 45 min. The mean bleedout volume was 0.72 ± 0.12 ml and 0.81 ± 0.09 ml for the wild-type and P-selectin–deficient mouse, respectively. Blood was collected in a heparinized (5 U) syringe and kept at 37°C until reinfusion. Mice were then resuscitated by infusion of the shed blood and intravenous injection of 0.5 ml 0.9% NaCl alone or with either an anti–P-selectin mAb or rs.PSGL.Ig. Mice were killed by exsanguination 45 min after resuscitation, after completion of intravital microscopy studies. Control wild-type mice underwent cannulation and anesthesia for the same period of time as hemorrhaged mice, but were not bled (n = 6). Wild-type and P-selectin–deficient mice were randomly assigned to one of four experimental hemorrhage groups: (i) wild-type mice receiving saline (n = 7); (ii) P-selectin–deficient mice receiving saline (n = 6); (iii) wild-type mice receiving 1 mg/kg anti–P-selectin mAb (RB40.34; PharMingen) (n = 6); and (iv) wild-type mice receiving 1 mg/kg high affinity mutant rs.PSGL.Ig (rsPSGL.47mutFc; Genetics Institute, Inc.) (n = 6).
The total number of circulating white blood cells in all experimental groups of mice was determined by hemocytometric count of smears of blood, which was obtained through the jugular vein cannula.
Intravital Microscopy of Mouse Peri-intestinal Venules.
All intravital microscopy experiments were conducted in anesthetized mice, which were surgically prepared as reported in the above hemorrhagic shock protocol section. Intravital microscopy was performed on mouse peri-intestinal venules, after exteriorization of a loop of ileal tissue via a midline laparotomy. The ileum was placed in a temperature-controlled fluid-filled plexiglas chamber and transilluminated for brightfield observation of the peri-intestinal microcirculation according to a previously described procedure (15). The ileum and mesentery were superfused throughout the experiment with a buffered Tyrode solution (pH 7.4, 37 ± 1°C). Preparations were allowed to stabilize for 15 min. Observations of rolling and adherent leukocytes were made with a Microphot microscope and a 40× salt water–immersion lens (Nikon Corp.). Images were projected by a high-resolution color video camera (DC-330; DAGE-MTI, Inc.) onto a color Sony high resolution video monitor (Multiscan 200-sf), and the image was recorded with a videocassette recorder. All images were then analyzed using computerized imaging software (Phase 3 Image System; Media Cybernetics) on a Pentium based IBM-compatible computer (Micron Millenia Mxe; Micron Electronics Inc.). Red blood cell velocity was determined on-line using an optical Doppler velocimeter (16) obtained from the Microcirculation Research Institute, College Station, TX. This method gives an average red blood cell velocity, which is digitally displayed on a meter, and allows for the calculation of shear rates (17). The number of rolling and adhered leukocytes was determined off-line by playback analysis of the videotape.
Histologic Assessment of Neutrophil Infiltration and Immunohistochemistry of P-selectin.
The number of neutrophils (PMNs) infiltrating into lung, liver, and intestine was determined in both wild-type and P-selectin–deficient mice. At the end of the 45 min reinfusion period, mice were killed and the pulmonary circulation was flushed by injecting PBS into the right ventricle. Lungs were injected with formalin through the trachea, then removed and placed into 4% formalin. Similarly, the splanchnic organs were perfused through the superior mesenteric artery and fixed in vivo by infusion of formalin (15). Lungs, liver, and intestine were then cut into blocks and dehydrated in graded acetone washes at 4°C. Tissue blocks were embedded in plastic (Immunobed; Polysciences, Inc.), and 4-μm-thick sections were cut and transferred to Vectabond-coated slides (Vector Laboratories, Inc.). The slides were soaked in ethanol for 10 min to remove the plastic embedding material and to allow staining of the tissue. After the 10-min ethanol wash, the tissue sections were stained with hematoxylin and eosin and examined blindly. The number of PMNs was counted and tallied. Five fields from each of two slides were counted from each organ, and three mice were studied per group. In addition, grading of the histopathologic changes in the lung (i.e., neutrophil infiltration, interstitial edema, and intraparenchymal hemorrhage) was performed on a 0 to 3 scale (with 0 being normal and 3 being the most severe abnormality).
Immunohistochemical localization of P-selectin was determined in ileal samples after intravital microscopy was completed, according to previously described methods (15). mAb PB1.3 only recognizes surface expressed P-selectin (18). Quantification of P-selectin was accomplished using the avidin-biotin immunoperoxidase technique (Vectastain ABC Reagent; Vector Labs.) as previously described by Weyrich et al. (18). 50 venules were analyzed per tissue section, 20 sections were examined per group, and the percentage of positive staining venules was tallied.
Quantification of P-selectin and PSGL-1 mRNA Expression by Ribonuclease Protection Assays.
Immediately after intravital microscopy was performed, mouse lungs, liver, and small intestine were removed for total RNA extraction. Total RNA was extracted from these tissues using the acid guanidium-phenol-chloroform extraction method described by Chomczynski and Sacchi (19). The concentration of RNA suspended in ribonuclease-free water was determined spectrophotometrically.
The plasmid containing the full-length mouse P-selectin cDNA was provided by Professor Dietmar Vestweber (Institut fur Zellbiologie, ZMBE, Westfalische Wilhelms Universitat Munster, Munster, Germany). The plasmid for synthesis of mouse tissue P-selectin probe for ribonuclease protection assays was created by recloning of a PstI–AvrII fragment of mouse P-selectin (from 1878 to 2270; sequence data available from EMBL/GenBank/ DDBJ under accession number M87861) into a PstI–SpeI site of pBluescript II SK+ vector (Stratagene). This plasmid was digested with XhoI and used as a template for in vitro transcription of a 474-base radiolabeled antisense probe containing a 393-base protected fragment using T3 RNA-polymerase (Boehringer Mannheim) in the presence of [32P]UTP (Amersham Corp.).
Mouse PSGL-1 cDNA was synthesized by reverse transcriptase PCR using mouse lung total RNA and oligo(dT), and was amplified using forward primer (5′-CCTGGGAATTCACCTGCCCC-3′) and reverse primer (5′-GAGAGTGGAGCTAGCAAAGG-3′). These oligonucleotides correspond to amino acid sequences 267–283 and 394–388 of mouse PSGL-1, respectively (sequence data available from EMBL/GenBank/DDBJ under accession number X91144). A 384-bp PCR fragment was cloned using PCR 2.1-TOPO Cloning Kit (a gift from Invitrogen Corp.). The Sst I–XbaI fragment of this plasmid was recloned in pTRIPLEscript vector (pTRIamp 18; Ambion). This plasmid was digested with XbaI to make, with T7 polymerase, a 570-base radiolabeled antisense probe that contained a 384-base protected fragment. All constructs used in this investigation were verified by sequencing the insert in the plasmid. These sequences were found to be 100% identical to the published sequences. The expression of PSGL-1 mRNA was analyzed by reverse transcriptase PCR analysis. Amplified PCR products were analyzed by electrophoresis on a 1.5% agarose gel. The intensity of each P-selectin and PSGL-1 mRNA band was normalized for GAPDH (data not shown).
Mouse β-actin antisense RNA probes were used to evaluate total RNA, which in turn were used for P-selectin and PSGL-1 mRNA expression analysis. The mouse β-actin plasmid for the synthesis of the antisense RNA probe was received from Ambion. For the ribonuclease protection assay, 2 μg of total RNA for detection of β-actin mRNA, and 60 μg for detection of PSGL-1 mRNA in the lung, liver, and small intestine have been used accordingly to previously described procedures (20). The intensity of each P-selectin and PSGL-1 mRNA band was normalized for β-actin mRNA levels.
Statistical Analyses.
All values for data listed in the text and figures are presented as means ± SEM of n independent experiments. Data were compared by analysis of variance using post-hoc analysis with Fisher's correct t test. P ≤ 0.05 was considered significant in all cases.
Results
Hemodynamic Changes Induced by Hemorrhagic Shock.
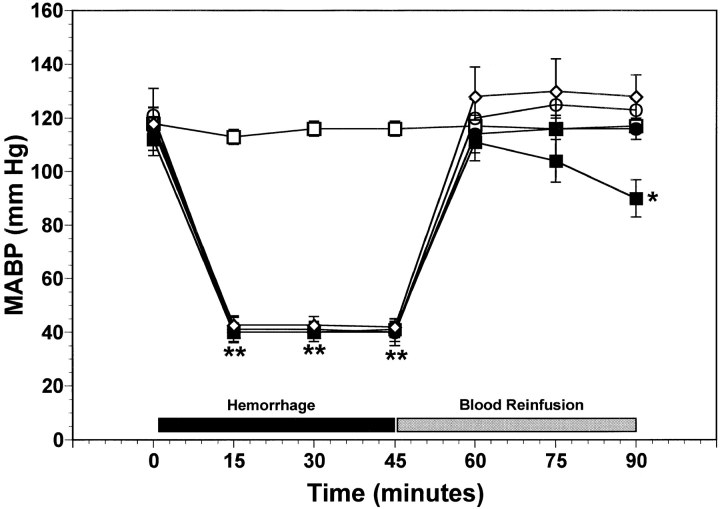
Fig. 1 illustrates the time course of systemic MABP in the five experimental groups of mice. All groups of mice exhibited initial MABP values in the range of 110–120 mmHg (Fig. 1). In control wild-type mice, MABP did not significantly change over the entire 90-min observation period (Fig. 1). In hemorrhaged mice, MABP was maintained at 40 mmHg for 45 min. After reinfusion of the shed blood to hemorrhaged mice, MABP increased to values not significantly different from control wild-type mice at that time (Fig. 1). In the wild-type hemorrhaged group receiving only saline, MABP progressively decreased to 90 ± 5 mmHg at the end of the experiment. In contrast, hemorrhaged P-selectin–deficient mice as well as hemorrhaged wild-type mice receiving either the anti–P-selectin mAb or the rs.PSGL.Ig maintained a significantly higher MABP at the end of the 45-min observation period in the range of 115–125 mmHg (P < 0.05, Fig. 1). This higher MABP was not due to decreased bleedout volumes, since the volume of shed blood was not significantly different among all groups of mice. These final blood pressures were also not statistically different from the initial MABP in these same groups of mice. Thus, either gene deficiency or functional inactivation of P-selectin expressed on the vascular endothelium limits the systemic hemodynamic consequences of hemorrhagic shock.
Figure 1.
Time course of MABP over the course of hemorrhage and reinfusion for the five experimental groups of mice. Wild-type and P-selectin–deficient (P-selectin−/−) mice were subjected to hemorrhagic shock. Functional blockade of P-selectin in wild-type mice was achieved by systemic administration of either anti–P-selectin mAb (1 mg/kg) or rs.PSGL.Ig (1 mg/kg). Each point represents mean values ± SEM; numbers indicate surviving rats at each interval. *P < 0.05 and **P < 0.01 versus wild-type control mice. □, control wild-type (n = 6); ▪, hemorrhage wild-type (n = 7); •, hemorrhage P-selectin−/− (n = 6); ○, hemorrhage wild-type + anti–P-selectin mAb (n = 5); ⋄, hemorrhage wild-type + rs.PSGL.Ig (n = 5).
Venular shear rates for the five experimental groups of mice are reported in Table I. No significant differences were observed in initial shear rates among the five groups of mice. After hemorrhage, shear rates in peri-intestinal venules abruptly decreased to less than half of the observed initial control values. Therefore, the present hemorrhagic shock model is characterized by a marked hypoperfusion of the splanchnic microvasculature during the oligemic phase. However, upon reinfusion of shed blood, venular shear rates returned to normal values (Table I). This strongly suggests that blood flow was reestablished to control levels during the postoligemic phase. Since shear rates were normal after reinfusion, the adhesive interactions observed between leukocytes and the microvascular endothelium during resuscitation from hemorrhage could not be attributed to alterations in physical hydrodynamic forces brought about by perturbations in local hemodynamics.
Table I.
Diameters and Shear Rates for Mouse Peri-intestinal Venules
| Group | n | Venular diameter | Venular shear rate (s−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (0 min) | Hemorrhage (45 min) | Resuscitation (90 min) | ||||||||
| Control wild-type | 6 | 35 ± 3.3 | 620 ± 19 | 612 ± 33 | 563 ± 42 | |||||
| Hemorrhage P-selectin gene deleted | 7 | 33 ± 2.7 | 653 ± 20 | 320 ± 41 | 625 ± 40 | |||||
| Hemorrhage wild-type | 6 | 31 ± 6.3 | 616 ± 39 | 303 ± 28 | 618 ± 46 | |||||
| Hemorrhage wild-type + anti–P-selectin mAb | 5 | 36 ± 6.5 | 614 ± 41 | 310 ± 51 | 609 ± 37 | |||||
| Hemorrhage wild-type + rs.PSGL.Ig | 5 | 32 ± 8.1 | 626 ± 54 | 287 ± 47 | 617 ± 24 | |||||
All values are means ± SEM. n = numbers of mice studied.
P-selectin Is Required for the Upregulation of Leukocyte– Endothelium Interaction in Hemorrhagic Shock.
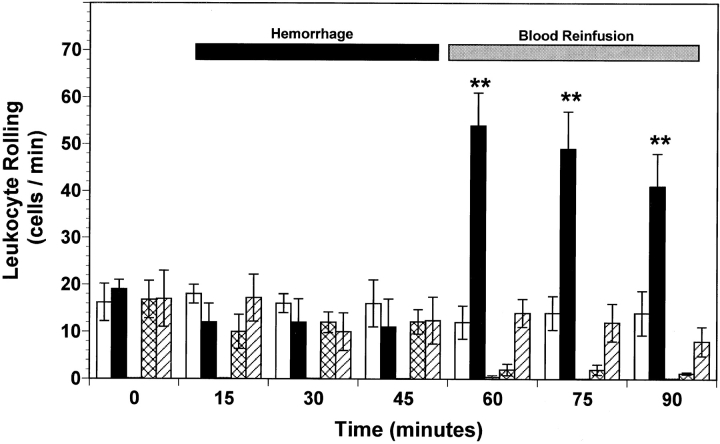
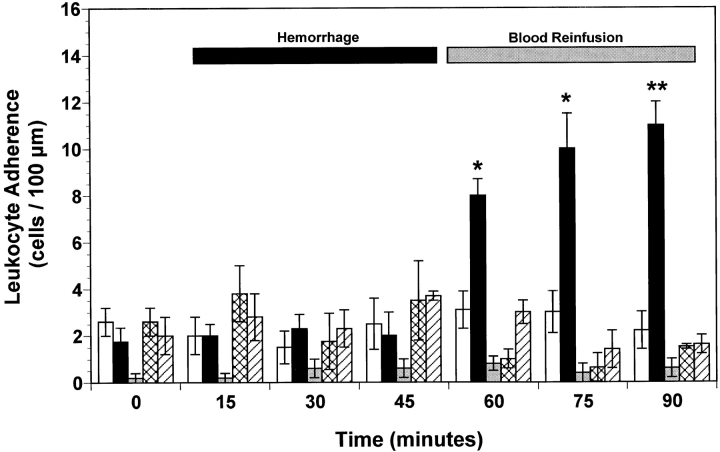
A low baseline number of rolling (i.e., 10–20 cells/min; Fig. 2) and adherent (i.e., 2–3 cells/100 μm; Fig. 3) leukocytes was observed in the mesenteric microvasculature for all experimental groups of wild-type mice. Furthermore, neither rolling (Fig. 2) nor adherence of (Fig. 3) leukocytes increased in peri-intestinal venules of P-selectin–deficient mice at any time. Baseline leukocyte rolling (Fig. 2) and adherence (Fig. 3) were not significantly changed during the first 45 min of the hemorrhage period in all wild-type and P-selectin–deficient mice. However, the number of rolling and adherent leukocytes in untreated hemorrhaged wild-type mice exhibited a threefold (P < 0.01) increase after reinfusion compared with normal control values (Figs. 2 and 3). In contrast, no significant increase in the number of rolling or adhered leukocytes was observed in the peri-intestinal venules of P-selectin–deficient mice (Figs. 2 and 3). Similarly, intravenous infusion of either 1 mg/kg of an anti–P-selectin mAb or 1 mg/kg of rs.PSGL.Ig significantly attenuated both leukocyte rolling (Fig. 3) and leukocyte adherence (Fig. 3) induced by hemorrhage and reinfusion of shed blood. In addition, no significant change in the total number of circulating leukocytes was observed in the five experimental groups of mice, so that the changes in rolling and adherence could not be attributed to leukopenia. The average number of circulating leukocytes in wild-type mice and P-selectin–deficient mice was 5.9 ± 0.6 and 7.2 ± 0.4 103 cells/mm3 (mean ± SEM), respectively. These values are not significantly different from each other, nor was leukopenia observed at the end of the experimental protocol or after systemic administration of either anti– P-selectin mAb or rs.PSGL.Ig. Therefore, functional expression of P-selectin protein on the mouse splanchnic microvascular endothelium exerts a crucial role in triggering inflammatory events after hemorrhage and fluid resuscitation.
Figure 2.
Leukocyte rolling observed in peri-intestinal venules of wild-type mice, P-selectin–deficient (P-selectin−/−) mice, and wild-type mice given either anti–P-selectin mAb or rs.PSGL.Ig, and subjected to hemorrhagic shock. Bar heights represent means and brackets indicate ± SEM. *P < 0.05 and **P < 0.01 from control wild-type mice. White bars, control wild-type (n = 6); black bars, hemorrhage wild-type (n = 7); gray bars, hemorrhage P-selectin−/− (n = 6); cross-hatched bars, hemorrhage wild-type + anti–P-selectin mAb (n = 5); hatched bars, hemorrhage wild-type + rs.PSGL.Ig (n = 5).
Figure 3.
Leukocyte adherence observed in peri-intestinal venules of wild-type mice, P-selectin–deficient (P-selectin−/−) mice, and wild-type mice given either anti–P-selectin mAb or rs.PSGL.Ig, and subjected to hemorrhagic shock. Bar heights represent means and brackets indicate ± SEM. *P < 0.05 and **P < 0.01 from control wild-type mice. White bars, control wild-type (n = 6); black bars, hemorrhage wild-type (n = 7); gray bars, hemorrhage P-selectin−/− (n = 6); cross-hatched bars, hemorrhage wild-type + anti–P-selectin mAb (n = 5); hatched bars, hemorrhage wild-type + rs.PSGL.Ig (n = 5).
Determination of P-selectin Surface Expression In Situ by Immunohistochemical Localization.
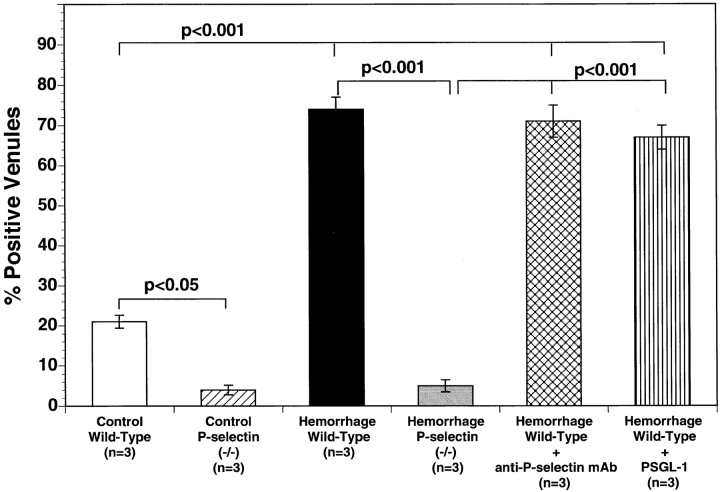
Immunolocalization of P-selectin was studied in the venular endothelium of the mouse ileum immediately after completion of intravital microscopic measurements. The percentage of venules staining positively for P-selectin in ileal sections from control wild-type mice was consistently low (21 ± 4% positive venules; Fig. 4). Moreover, virtually no surface P-selectin expression was detected by immunohistochemistry at any time in P-selectin gene–deficient mice (Fig. 4). However, hemorrhage plus reperfusion resulted in a significant increase in P-selectin expression in wild-type mice (Fig. 4). Intravenous infusion of either anti–P-selectin mAb or rs.PSGL.Ig did not attenuate the number of venules staining positively for P-selectin after hemorrhage and reinfusion (74 ± 4% and 70 ± 6 positive venules respectively; NS versus hemorrhage wild-type mice). This clearly indicates that inhibition of leukocyte–endothelium interaction induced by anti–P-selectin mAb or rs.PSGL.Ig is due to functional neutralization of P-selectin on the endothelial cell surface rather than to significant attenuation of P-selectin expression on the microvascular endothelium.
Figure 4.
Immunohistochemistry of mouse ileal microvessels: percentage of vessels staining positive for P-selectin in the five experimental groups of mice. Bar heights represent mean values, and brackets indicate SEM. 20 sections were studied in each of three mice, and 50 vessels were studied in each section.
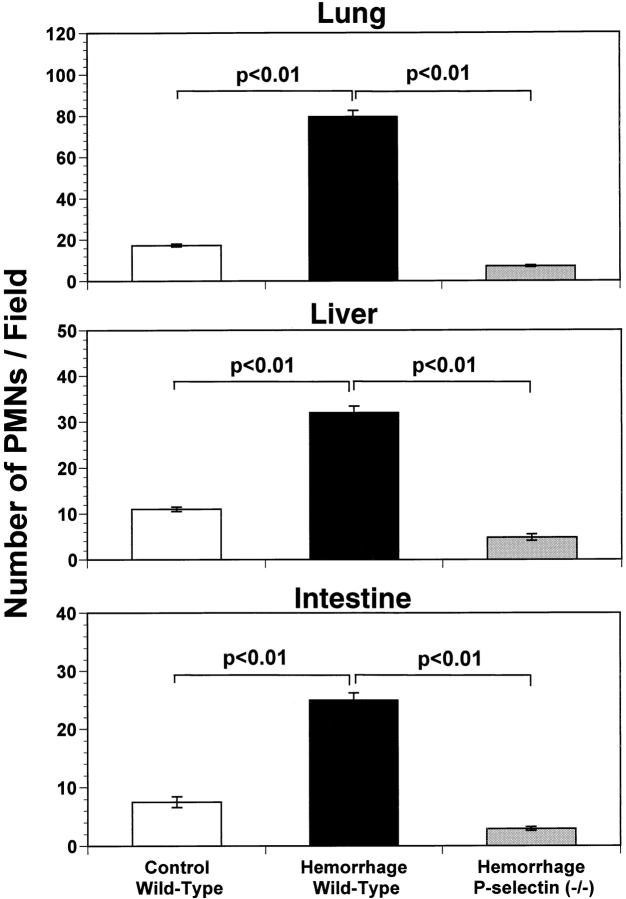
Inhibition of Neutrophil Infiltration into Lung, Liver, and Intestine Is Associated with Decreased Organ Injury in P-selectin– deficient Mice.
As an additional verification of organ injury, we performed histological analysis of lung, liver, and intestine in control and hemorrhaged mice. After hemorrhage and reinfusion, P-selectin–deficient mice had fewer infiltrated neutrophils in lung, liver, and intestine (Fig. 5) than did wild-type mice. Moreover, P-selectin–deficient mice subjected to hemorrhage and reinfusion developed less interstitial lung edema and intra-alveolar hemorrhage compared with hemorrhaged wild-type mice, as assessed by histological analysis (histopathological score 0.45 ± 0.08 and 2.7 ± 0.15, respectively; P < 0.01). This decreased inflammation and injury in lungs and splanchnic organs of P-selectin–deficient mice strongly demonstrates the crucial role exerted by selectin-mediated leukocyte recruitment during the early pathophysiologic events of hemorrhagic shock.
Figure 5.
Quantification of histological staining of neutrophils in lung (top), liver (center) and small intestine (bottom) of wild-type and P-selectin–deficient (P-selectin−/−) mice subjected to hemorrhagic shock. Bar heights represent mean values, and brackets indicate ± SEM. Five fields were counted on two slides of each organ from three mice in each group. Hemorrhage followed by reinfusion significantly enhanced accumulation of neutrophils in wild-type mice but not in P-selectin–deficient mice (P < 0.01).
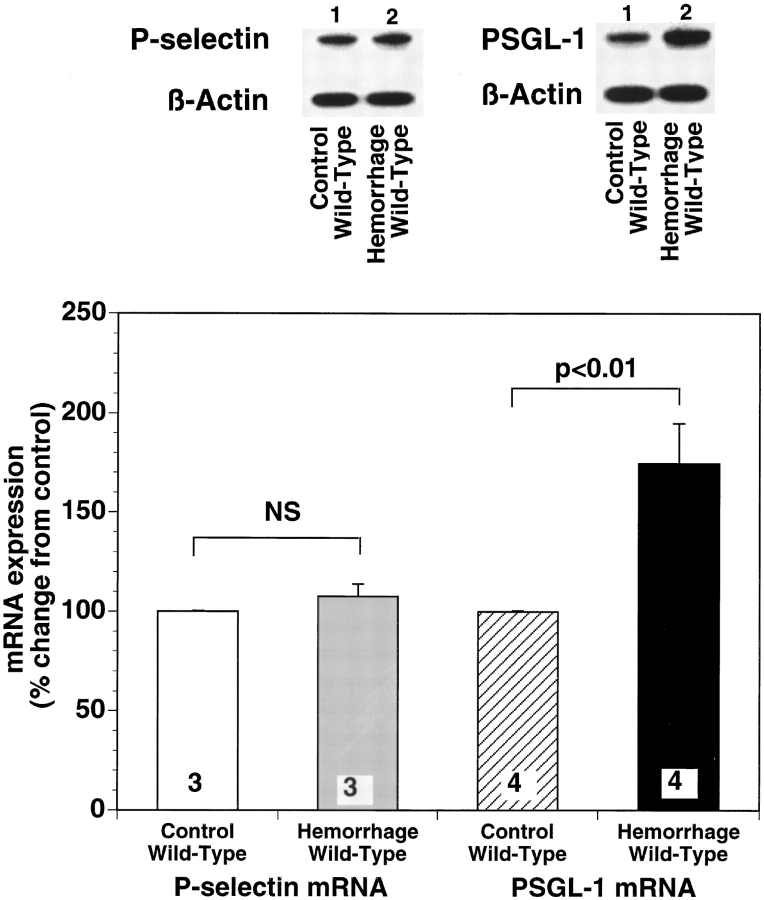
Hemorrhagic Shock Increases PSGL-1 mRNA Expression in Several Organs in the Wild-type Mouse.
Levels of mRNA codifying for endogenous PSGL-1 were assessed in hemorrhaged wild-type mice, using a ribonuclease protection assay. As shown in Fig. 6, the intensity of each PSGL-1 mRNA band was normalized to that of β-actin. After the 45-min resuscitation period, PSGL-1 transcripts were significantly increased in the lungs of mice subjected to hemorrhagic shock (Fig. 6). Similar results were also observed in the liver and small intestine of hemorrhaged wild-type mice. After hemorrhage and reinfusion PSGL-1 transcripts in both liver and intestine increased 34 ± 8.4% and 32 ± 2%, respectively (P < 0.001 versus control mouse tissue). In contrast, no significant changes were observed for the P-selectin mRNA transcript in wild-type mice after hemorrhage and reperfusion. This suggests that in the early phase of hemorrhagic shock, increased de novo synthesis of the counter-ligands for P-selectin in marginated blood cells entrapped in vital organs, more than de novo synthesis of P-selectin protein in the vascular endothelium, is responsible for further exacerbation of selectin-mediated leukocyte– endothelium interaction.
Figure 6.
Representative autoradiograph of a polyacrylamide gel used in typical ribonuclease protection assay comparing PSGL-1 and P-selectin mRNA expression in lungs of sham-operated control and hemorrhaged wild-type mice. Compared with lanes containing PSGL-1, mRNA levels from sham-operated wild-type mice (lane 1) there is a marked increase in PSGL-1 mRNA in lung isolated from wild-type mice subjected to hemorrhagic shock (lane 2). No significant difference was observed in P-selectin mRNA levels between the two groups of mice. Densitometric quantification of the effect of hemorrhage on lung PSGL-1 mRNA expression is summarized in the right panel of the figure. Bar heights represent means and brackets indicate ± SEM. Numbers at the bottom of each bar represent the number of mice studied.
Discussion
This study was undertaken to determine the role of P-selectin in the early inflammatory response occurring after resuscitation from hemorrhagic shock. Using either mice genetically deficient in P-selectin protein or functionally blocking P-selectin in wild-type mice, by either an anti–P-selectin mAb or rs.PSGL.Ig, we first demonstrate that P-selectin plays an essential role in the pathological recruitment of leukocytes observed in hemorrhagic shock. We also found that upon resuscitation from hemorrhage, an increased expression of mRNA codifying for endogenous PSGL-1 occurs in the lung as well as in the liver and intestine of hemorrhaged wild-type mice. These data provide compelling evidence that P-selectin plays a key role in the activation of the inflammatory cascades occurring in hemorrhagic shock, thus designating P-selectin as a possible strategic target in the therapy of hemorrhagic shock. Our findings are also consistent with an earlier report that an mAb against P-selectin markedly reduced the volume of fluid necessary for resuscitation of hemorrhaged rabbits (21), although no analysis of leukocyte–endothelium interactions was attempted.
A multistep series of adhesive and signaling events regulates inflammatory responses to infection or injury (22, 23). To initiate these responses, circulating leukocytes must first roll along the endothelium, and then adhere to the vascular wall under shear forces. Selectins mediate the first adhesive step, which is characterized by tethering and rolling of leukocytes on endothelial cells, platelets, or other leukocytes (24). In particular, P-selectin, expressed on activated platelets and endothelial cells, binds to ligands on most leukocytes (25). The regulated expression of the selectins and their high affinity ligand (i.e., PSGL-1), helps modulate the inflammatory response. However, inappropriate expression of these molecules contributes to leukocyte-mediated tissue damage in a variety of acute inflammatory disorders (26, 27). In this regard, several investigators have demonstrated that inhibition of the rolling phase of leukocytes plays a key role in attenuating the acute inflammatory response (28, 29). Consistent with such findings, we now demonstrate that soon after resuscitation from hemorrhage, leukocyte– endothelium interactions are significantly upregulated in the microcirculation, an event that is associated with increased expression of P-selectin on the microvascular endothelium. Moreover, as confirmed in P-selectin–deficient mice, in the absence of P-selectin protein virtually no leukocyte–endothelium interaction occurs after hemorrhage and reinfusion of shed blood. This finding agrees with previous observations showing severe attenuation of leukocyte rolling and extravasation in P-selectin–deficient mice (14). In addition, we observed de novo synthesis of PSGL-1 in the lungs and splanchnic organs of hemorrhaged wild-type mice, as confirmed by quantification of PSGL-1 mRNA. This increase in PSGL-1 mRNA levels may contribute to widespread increases in cell-to-cell interaction during acute inflammatory conditions such as hemorrhagic shock.
The inhibitory effect on leukocyte–endothelium interaction exerted by blockade of P-selectin may contribute to normalization of the pathophysiologic events in hemorrhagic shock. One possible explanation is that inhibition of the initial P-selectin–mediated tethering of leukocytes to endothelium may diminish the localized production of proinflammatory cytokines, which subsequently induce expression of endothelial cell adhesion molecules. In this regard, other investigators have demonstrated that after hemorrhagic shock increased infiltration of blood cells into vital organs increases intraparenchymal cytokine expression, starting 2 h after reinfusion and reaching a peak value after 3 d (30, 31). Moreover, inhibition of leukocyte extravasation exerts a key role during inflammation because activated neutrophils, which have adhered to the endothelium, release cytotoxic mediators including proteases, eicosanoids, cytokines, and oxygen-derived free radicals (1, 32), each of which can promote tissue injury and exacerbate endothelial dysfunction in hemorrhagic shock.
One may speculate on the mechanism triggering the upregulation of P-selectin to the vascular endothelial cell surface during hemorrhagic shock. Hemorrhagic shock represents a severe form of whole body ischemia/reperfusion. Several investigators have demonstrated that a common pathophysiologic event occurring during ischemia/reperfusion is the early occurrence of acute endothelial dysfunction characterized by impaired endothelial release of nitric oxide (8, 9, 11, 28, 33). In this connection, acute endothelial dysfunction associated with severe organ injury has been reported in myocardial ischemia/reperfusion (11), splanchnic ischemia/reperfusion (9), traumatic shock (10), and hemorrhagic shock (33). Moreover, a functional relationship between loss of endothelium-derived nitric oxide and the upregulation of P-selectin on the venular endothelium has been reported previously (12). Therefore, hemorrhage-induced loss of endothelium-derived nitric oxide release is probably responsible for increased expression of cell adhesion molecules in the microvascular endothelium. This conclusion is supported by the observation that endothelial cell dysfunction occurs very early after hemorrhage and persists despite fluid resuscitation (33), and that an mAb against P-selectin attenuated fluid leakage in hemorrhagic shock (21).
This study provides the first clear in vivo evidence of a purely P-selectin–dependent leukocyte–endothelium interaction in hemorrhagic shock. This work also suggests that neutralization of P-selectin in the early phase of hemorrhagic shock may limit infiltration of leukocytes into inflamed organs.
Acknowledgments
We thank Ms. Irina Opentanova for her expert technical assistance in the molecular biological measurements used in this study.
This work was supported in part by Research Grant No. GM-45434 from the National Institutes of General Medical Science of the National Institutes of Health.
Abbreviations used in this paper
- MABP
mean arterial blood pressure
- PMNs
polymorphonuclear leukocytes
- PSGL-1
P-selectin glycoprotein ligand 1
References
- 1.Barroso-Aranda, J., B.W. Zweifach, J.C. Mathison, and G.W. Schmid-Schönbein. 1995. Neutrophil activation, tumor necrosis factor, and survival after endotoxic and hemorrhagic shock. J. Cardiovasc. Pharmacol. 25(Suppl. 2):S23–S29. [DOI] [PubMed]
- 2.Harbrecht BG, Wu B, Watkins SC, Billiar TR, Peitzman AB. Inhibition of nitric oxide synthesis during severe shock but not after resuscitation increases hepatic injury and neutrophil accumulation in hemorrhaged rats. Shock. 1997;8:415–421. [PubMed] [Google Scholar]
- 3.LeTulzo Y, Shenkar R, Kaneko D, Moine P, Fantuzzi G, Dinarello CA, Abraham E. Hemorrhage increases cytokine expression in lung mononuclear cells in mice: involvement of catecholamines in nuclear factor-κB regulation and cytokine expression. J Clin Invest. 1997;99:1516–1524. doi: 10.1172/JCI119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 5.Deitch EA, Bridges W, Berg R, Specian RD, Granger DN. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Peitzman AB, Udekwu AO, Ochoa J, Smith S. Bacterial translocation in trauma patients. J Trauma. 1991;31:1083–1086. [PubMed] [Google Scholar]
- 7.McEver RP. Leukocyte-endothelial cell interactions. Curr Opin Cell Biol. 1992;4:840–849. doi: 10.1016/0955-0674(92)90109-p. [DOI] [PubMed] [Google Scholar]
- 8.Lefer AM, Ma XL, Weyrich A, Lefer DJ. Endothelial dysfunction and neutrophil adherence as critical events in the development of reperfusion injury. Agents Actions. 1993;41:127–135. [PubMed] [Google Scholar]
- 9.Lefer AM, Lefer DJ. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- 10.Scalia R, Pearlman S, Campbell B, Lefer AM. Time course of endothelial dysfunction and neutrophil adherence and infiltration during murine traumatic shock. Shock. 1996;6:177–182. [PubMed] [Google Scholar]
- 11.Tsao PS, Aoki N, Lefer DJ, Johnson G, Lefer AM. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990;82:1402–1412. doi: 10.1161/01.cir.82.4.1402. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier TW, Davenpeck KL, Lefer AM. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am J Physiol. 1994;267:G562–G568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- 13.Lorant DE, Topham MK, Whatley RE, McEver RP, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory roles of P-selectin. J Clin Invest. 1993;92:559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in Pselectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 15.Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Lipoxin A4stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc Natl Acad Sci USA. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borders JL, Granger HJ. An optical doppler intravital velocimeter. Microvasc Res. 1984;27:117–127. doi: 10.1016/0026-2862(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 17.Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol. 1989;257:G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- 18.Weyrich AS, Buerke M, Albertine KH, Lefer AM. Time course of coronary vascular endothelial adhesion molecule expression during reperfusion of the ischemic feline myocardium. J Leukocyte Biol. 1995;57:45–55. doi: 10.1002/jlb.57.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- 21.Winn RK, Paulson JC, Harlan JM. A monoclonal antibody to P-selectin ameliorates injury associated with hemorrhagic shock in rabbits. Am J Physiol. 1994;267:H2391–H2397. doi: 10.1152/ajpheart.1994.267.6.H2391. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman GA, McIntyre TM, Prescott SM. Adhesion and signaling in vascular cell–cell interactions. J Clin Invest. 1996;98:1699–1702. doi: 10.1172/JCI118967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 24.McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 25.Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- 26.Lefer AM, Weyrich AS, Buerke M. Role of selectins, a new family of adhesion molecules, in ischaemia-reperfusion injury. Cardiovasc Res. 1994;28:289–294. doi: 10.1093/cvr/28.3.289. [DOI] [PubMed] [Google Scholar]
- 27.Lowe JB, Ward PA. Therapeutic inhibition of carbohydrate-protein interactions in vivo. J Clin Invest. 1997;100:S47–S51. [PubMed] [Google Scholar]
- 28.Weyrich AS, Ma XY, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993;91:2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubes P, Jutila M, Payne D. Therapeutic potential of inhibiting leukocyte rolling in ischemia/reperfusion. J Clin Invest. 1995;95:2510–2519. doi: 10.1172/JCI117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenkar R, Coulson WF, Abraham E. Hemorrhage and resuscitation induce alterations in cytokine expression and the development of acute lung injury. Am J Respir Cell Mol Biol. 1994;10:290–297. doi: 10.1165/ajrcmb.10.3.8117448. [DOI] [PubMed] [Google Scholar]
- 31.Abraham E, Bursten S, Shenkar R, Allbee J, Tuder R, Woodson P, Guidot DM, Rice G, Singer JW, Repine J. Phosphatidic acid signaling mediates lung cytokine expression and lung inflammatory injury after hemorrhage in mice. J Exp Med. 1995;181:569–575. doi: 10.1084/jem.181.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki M, Inauen W, Kvietys PR, Grisham MB, Meininger C, Schelling ME, Granger HJ, Granger DN. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol. 1989;257:H1740–H1745. doi: 10.1152/ajpheart.1989.257.5.H1740. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs very early following trauma-hemorrhage and persists despite fluid resuscitation. Am J Physiol. 1993;265:H973–H979. doi: 10.1152/ajpheart.1993.265.3.H973. [DOI] [PubMed] [Google Scholar]