Abstract
The effectiveness of interleukin 10 (IL-10) in the treatment of autoimmune-mediated central nervous system inflammation is controversial. Studies of the model system, experimental autoimmune encephalomyelitis (EAE), using various routes, regimens, and delivery methods of IL-10 suggest that these variables may affect its immunoregulatory function. To study the influence of these factors on IL-10 regulation of EAE pathogenesis, we have analyzed transgenic mice expressing human IL-10 (hIL-10) transgene under the control of a class II major histocompatibility complex (MHC) promoter. The hIL-10 transgenic mice are highly resistant to EAE induced by active immunization, and this resistance appears to be mediated by suppression of autoreactive T cell function. Myelin-reactive T helper 1 cells are induced but nonpathogenic in the IL-10 transgenic mice. Antibody depletion confirmed that EAE resistance is dependent on the presence of the transgenic IL-10. Mice expressing the hIL-10 transgene but not the endogenous murine IL-10 gene demonstrated that transgenic IL-10 from MHC class II–expressing cells is sufficient to block induction of EAE. This study demonstrates that IL-10 can prevent EAE completely if present at appropriate levels and times during disease induction.
Keywords: major histocompatibility complex class II promoter–regulated interleukin 10, myelin-reactive T cells, immune regulation, autoimmunity
IL-10 is an immune regulatory factor that has potential therapeutic value for organ-specific autoimmune diseases (1). Administration of rIL-10 reduces disease in experimental models of diabetes, rheumatoid arthritis, and inflammatory bowel disease (2–6); however, its utility in autoimmune encephalomyelitis is unclear. Studies using various routes, regimens, and delivery methods of rIL-10 have yielded conflicting results. In an adoptive transfer model of experimental autoimmune encephalomyelitis (EAE), intravenous injection of rIL-10 exacerbated rather than suppressed disease (7). In contrast, subcutaneous rIL-10 treatment partially inhibited disease in rat and mouse models of EAE induced by active immunization (8, 9). In these active immunization models, daily rIL-10 treatment regimens from the day of immunization to just before the expected day of disease onset were necessary for significant EAE inhibition. These results suggest that systemic rIL-10 treatment may inhibit EAE either by suppressing in vivo development or migration of encephalitogenic Th1 cells or by inhibiting the inflammatory effector function of these Th1 cells. Recent studies of targeted delivery of IL-10 to the central nervous system (CNS) have also yielded conflicting results. Intracranial injection of soluble rIL-10 or plasmids containing a retroviral promoter–directed IL-10 cDNA at day 12 after active immunization did not suppress EAE (10). Adoptive transfer of retroviral transduced myelin basic protein (MBP)–specific T cell hybridoma also did not inhibit EAE (11). In contrast, antigen-inducible IL-10 produced by proteolipid protein (PLP)-specific T memory cells suppressed EAE when adoptively transferred to PLP peptide–immunized mice 1 d before expected disease onset (12). These results suggest that the timing of IL-10 production and possibly the localization of IL-10 may critically affect its immunoregulatory function.
To study the influence of these factors on IL-10 regulation of EAE pathogenesis, we have analyzed transgenic mice expressing human IL-10 (hIL-10) under the control of a class II MHC promoter. hIL-10 is fully active in mice (1), and the species difference allows for the specific detection and depletion of transgenic and endogenous IL-10. In the present study, we have demonstrated that hIL-10 transgenic (hIL-10Tg) mice are resistant to EAE induced by active immunization and that this resistance is a consequence of a reduced autoreactive Th1 response.
Materials and Methods
Mice.
SJL/J and CSJLF1/J mice were obtained from The Jackson Laboratory. BALB/cAnN mice were obtained from Taconic Farms, Inc. hIL-10Tg mice were constructed using a hIL-10 cDNA sequence regulated by a class II MHC Eα promoter sequence (13). For this study, male hemizygous BALB/c IL-10Tg mice were backcrossed with female BALB/cAnN mice to generate hIL-10Tg and nonTg littermates. SJL × BALB/c F1 transgene–positive and nonTg littermates were generated by crossing male hemizygous hIL-10Tg BALB/cAnN mice with female SJL/J mice. To generate mice expressing the hIL-10 transgene but lacking endogenous murine IL-10, the hIL-10 transgene was backcrossed onto the BALB/cAnN IL-10KO background. Male BALB/cAnN hemizygous hIL-10Tg IL-10KO mice were bred with female BALB/cAnN IL-10KO mice to produce transgene-positive and nonTg littermate mice on the IL-10KO background.
Induction of EAE.
Mouse spinal cord homogenate (MSCH) was prepared from 8–12-wk-old BALB/cAnN mice as previously described (14). Bovine MBP was obtained from Sigma Chemical Co. For active induction of EAE, mice were immunized intradermally with 2.5 mg of MSCH and 200 μg of Mycobacterium tuberculosis (strain H37RA; Difco) at days 0 and 7 as described (14). Mice were examined and scored for clinical signs of EAE, and routine histopathological analyses of hematoxylin and eosin– or Luxol fast blue–stained paraffin sections were performed in a masked fashion as described (14).
Antibodies.
All mAb were purified by column chromatography from either ascites fluid or tissue culture supernatants and contained <4 EU endotoxin/mg protein. 1B1.2 is a blocking mAb reactive with mouse IL-10 receptor used in in vitro cultures (15). The following mAbs were used in vivo: JES3-9D7 (rat IgG1), a hIL-10–specific mAb that does not cross-react with mouse IL-10 (16); and GL113 (rat IgG1), an isotype control mAb reactive with β-galactosidase. For in vivo mAb treatment, mice were injected i.p. with 1 mg mAb/dose in 100 μl of PBS. Each animal received three injections, once per week.
Cell Purification.
CD4+ draining lymph node (DLN) cells were purified by positive selection using MACS® L3T4 microbeads and MiniMACS® columns (Miltenyi Biotec). The microbead-labeled cell suspensions were processed through the magnetic column twice, and the purity was routinely >95% CD4+ cells. To prepare T cell–depleted APC, spleen cells from naive CSJLF1/J mice were depleted of CD4 (GK1.5, 20 μg/ml)- and CD8 (2–43, 20 μg/ml)-staining cells by negative selection using anti–rat Ig-coated Dynabeads (Dynal).
Cell Culture and Cytokine Detection.
Mice were immunized with MSCH following the same procedure as for active induction of EAE. 10 d after immunization, DLN cells (4 × 106/ml) and purified CD4+ DLN cells (106/ml) were stimulated with 50 μg/ml of MBP as described (14). Anti–IL-10 receptor mAb (1B1.2, 10 μg/ml) was added to cultures where indicated. Culture supernatants were harvested after 60 h and levels of IFN-γ and IL-4 were determined using a sandwich ELISA technique as described (14).
Results
Transgenic IL-10 Prevents Induction of EAE.
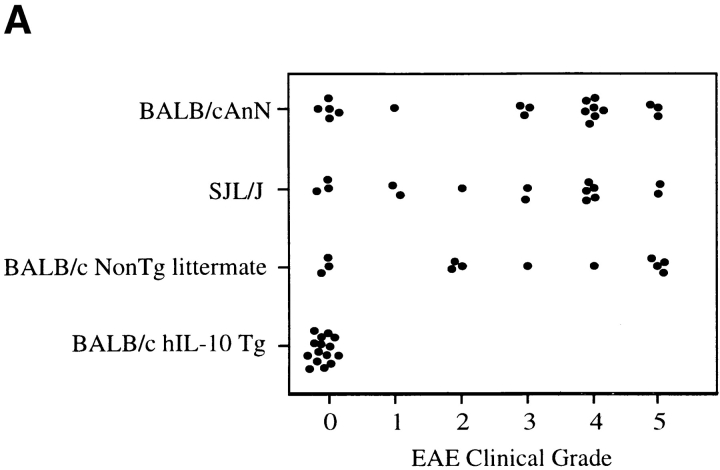
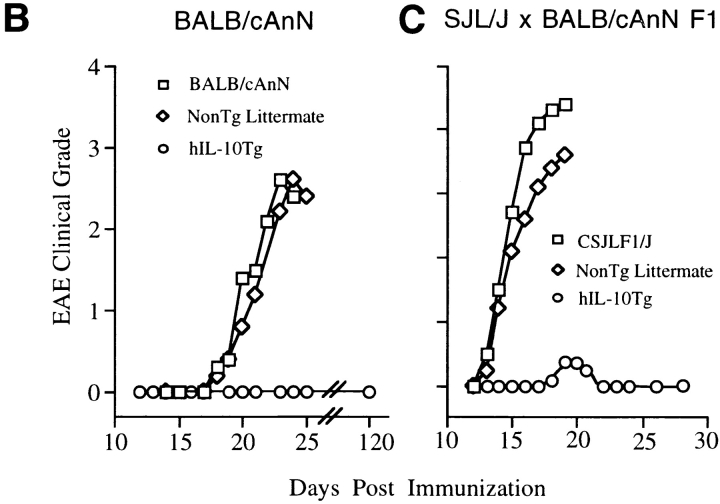
For the initial study, hIL-10Tg mice and nonTg littermate controls on the BALB/cAnN genetic background were compared for EAE susceptibility. Although BALB/c mice are generally considered to be EAE resistant, it has been reported that BALB/cAnN and BALB/cByJ substrains are susceptible to actively induced EAE (17). Consistent with these results, EAE was reproducibly induced in these strains of mice by intradermal immunization on days 0 and 7 with MSCH in CFA (84%, n = 86; average EAE grade = 3.2; day of disease onset = 17–30 d after immunization). With this immunization, hIL-10Tg mice were completely EAE resistant (0/23 mice), whereas control littermate mice were highly EAE susceptible and exhibited the same EAE severity as wild-type BALB/cAnN and SJL/J mice (Fig. 1, A and B). No inflammatory infiltrates were found in the spinal cords of EAE-resistant Tg mice, whereas intense inflammatory infiltrates and demyelination were found in the white matter of EAE-susceptible nonTg littermate mice (Fig. 2).
Figure 1.
hIL-10Tg mice are EAE resistant. (A) Circles represent peak severity of clinical disease for individual mice on the indicated genetic backgrounds. Data compiled from three separate experiments. (B) Average severity score for hIL-10Tg mice on the BALB/cAnN background. One representative experiment of five is shown. (C) Average severity score for hIL-10Tg mice on SJL/J × BALB/cAnN F1 background. One representative experiment of four is shown.
Figure 2.
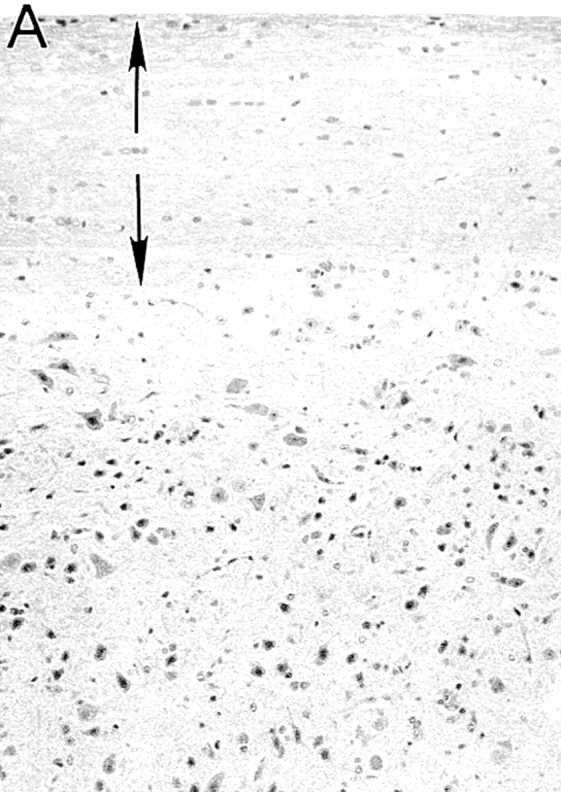
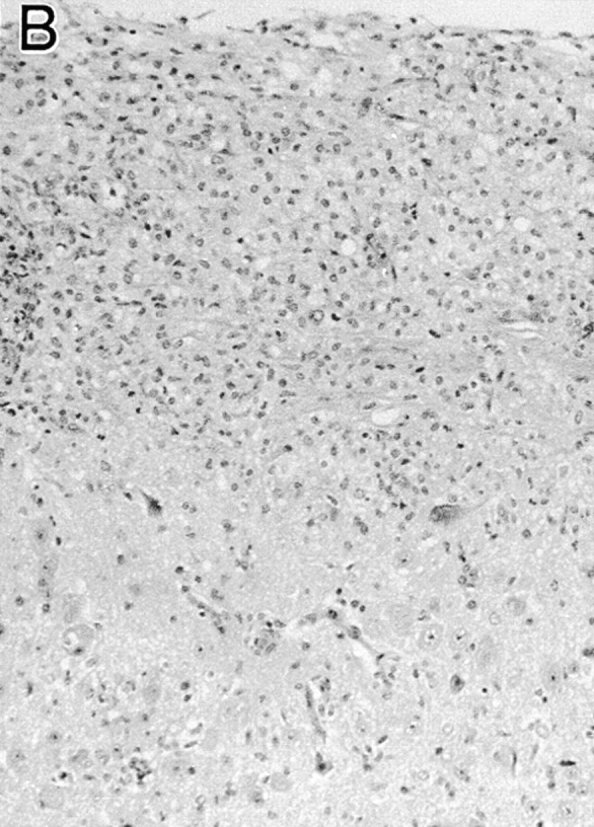
Histology of spinal cords of hIL-10Tg mice and nonTg littermates immunized to induce EAE. (A) hIL-10Tg mice were EAE resistant and spinal cords showed no inflammatory infiltrates or plaques of demyelination in the white matter (normal white matter outlined by arrows). (B) NonTg littermates were highly EAE susceptible and showed inflammatory infiltrates and extensive demyelination in the white matter. Luxol fast blue stain with hematoxylin counterstain. ×125.
To confirm these findings in a more conventional model of EAE, hemizygous male BALB/c hIL-10Tg mice were crossed with SJL/J mice to generate transgene-positive and nonTg littermates. These F1 mice were compared to CSJLF1/J, which have a highly predictable disease onset at 14 d after immunization with MSCH and a relatively strong in vitro recall response to MBP. Transgene-positive SJL/J × BALB/cAnN F1 mice had a low EAE incidence (4/55 mice) and disease severity compared to nonTg littermate control mice (43/46 mice), which were highly susceptible to EAE. The nonTg littermates exhibited an EAE severity and disease course similar to wild-type CSJLF1/J mice (Fig. 1 C). No difference in EAE severity or day of disease onset was observed when comparing CSJLF1/J with SJL/J mice (data not shown).
Transgenic IL-10 Must Be Present during the Induction of Disease to Prevent EAE.
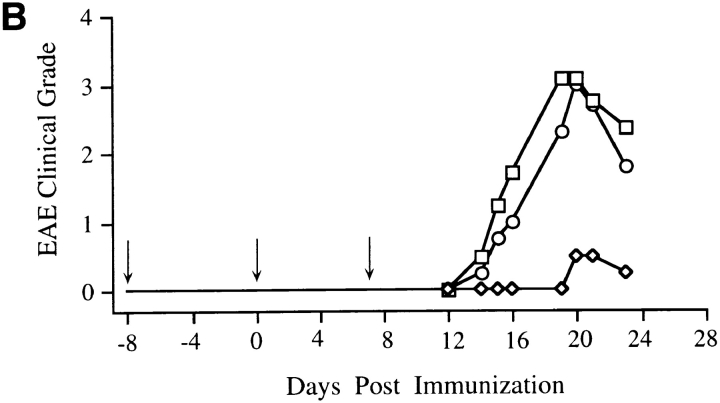
To show that disease resistance was due to the direct effects of the transgenic IL-10, immunized transgenic SJL/J × BALB/cAnN F1 mice were injected with either JES3-9D7 mAb (hIL-10–specific mAb [16] that does not cross-react with mouse IL-10) or GL113 mAb (an isotype-matched mAb control). Tg mice receiving the first anti–hIL-10 mAb injection on the day of immunization were susceptible to EAE, whereas the isotype control mAb–injected mice were resistant to EAE (Fig. 3 A). This result suggests that EAE resistance of hIL-10Tg mice was the consequence of hIL-10 present during immunization, rather than of developmental effects of the transgene. A consistent delay in the day of disease onset was found in the anti–hIL-10 mAb–treated mice compared to control littermates (Fig. 3 A). To determine the cause of this delay, anti–hIL-10 mAb treatment was initiated 8 d before MSCH immunization. With this treatment regimen, the EAE clinical grade and day of disease onset for hIL-10Tg and control littermate mice were indistinguishable (Fig. 3 B). Similar results were obtained when hIL-10Tg mice on the BALB/cAnN genetic background were treated with the anti–hIL-10 mAb (data not shown).
Figure 3.
Induction of EAE in hIL-10Tg mice treated with anti– hIL-10 mAb. Days of mAb treatment are indicated by arrows. (A) Tg mice were injected with anti–hIL-10 mAb (1 mg/dose) or isotype-matched mAb starting at the day of MSCH immunization. One representative experiment out of five is shown. (B) Mice were injected with the mAb starting 8 d before MSCH immunization. One representative experiment out of three is shown.
MBP-specific Th1 Cells Were Generated Normally in IL-10Tg Mice.
Three possible mechanisms for the regulatory effect of IL-10Tg in EAE are as follows: inhibition of the initial development of autoreactive Th1 cells, active inhibition of Th1 cells by IL-10, or immune deviation toward a Th2-type response. To test these, the cytokine secretion profiles of DLN cells from MSCH-immunized SJL × BALB/c F1 hIL-10Tg and control littermate mice were analyzed. 10 d after immunization, DLN cells were prepared for culture in the presence of MBP or MBP plus anti–IL-10 receptor mAb. After 3 d, the culture supernatants were tested for secreted IFN-γ and IL-4. Cells from nonTg littermate mice stimulated with MBP secreted higher levels of IFN-γ than cells from Tg mice, a result consistent with the association of EAE pathogenesis with IFN-γ production (Fig. 4). However, when stimulated with MBP in the presence of anti–IL-10 receptor mAb, cells from both hIL-10Tg mice and littermate controls produced similar levels of IFN-γ. To eliminate APC differences between hIL-10Tg and control littermate mice, CD4+ T cells were purified and cultured with irradiated, T-depleted splenocytes from CSJLF1/J donor mice. In the absence of APC-derived hIL-10Tg, T cells from both hIL-10Tg and control littermate mice produced equivalent levels of IFN-γ (Fig. 4 B). No MBP-specific IL-4 production was detected in cultures of DLN cells or CD4+ T cells from hIL-10Tg or control littermate mice, suggesting that EAE resistance in the Tg mice was not associated with a Th2-type response (data not shown). These results support the interpretation that myelin-reactive Th1 cells were induced in the hIL-10Tg mice and suggest that their ability to exert an effector function was actively suppressed by IL-10 in vivo.
Figure 4.
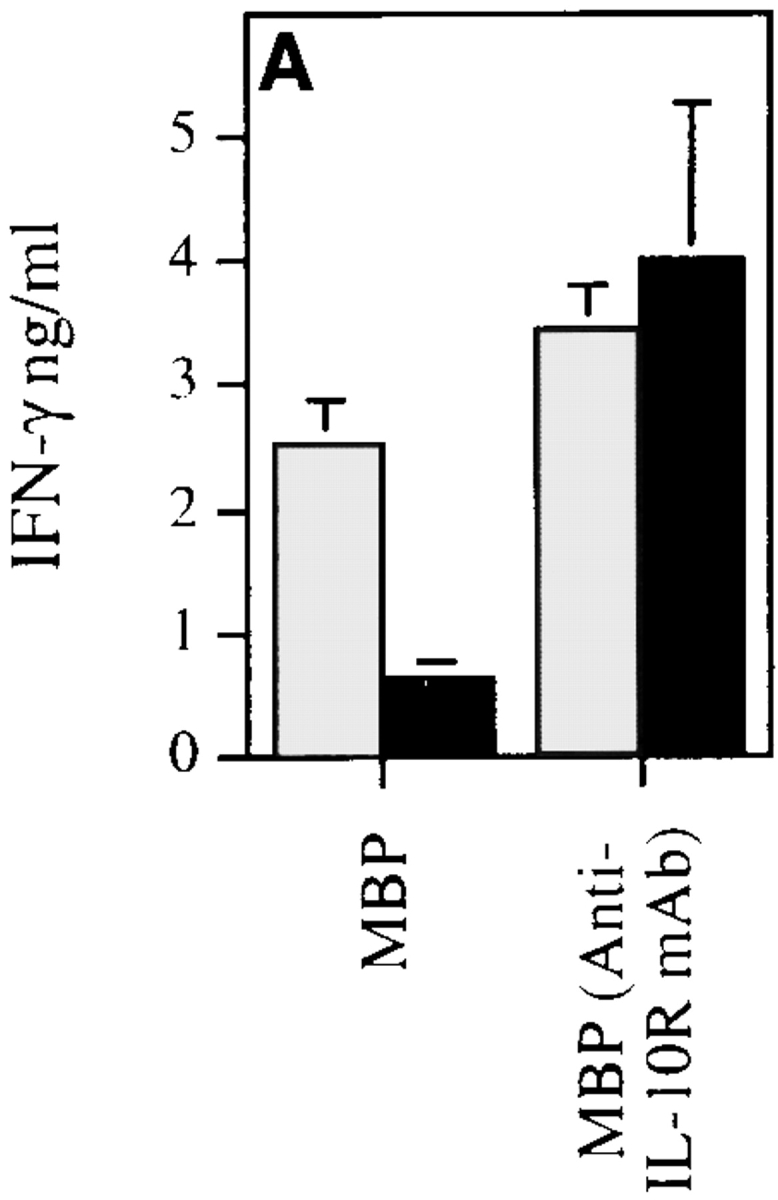
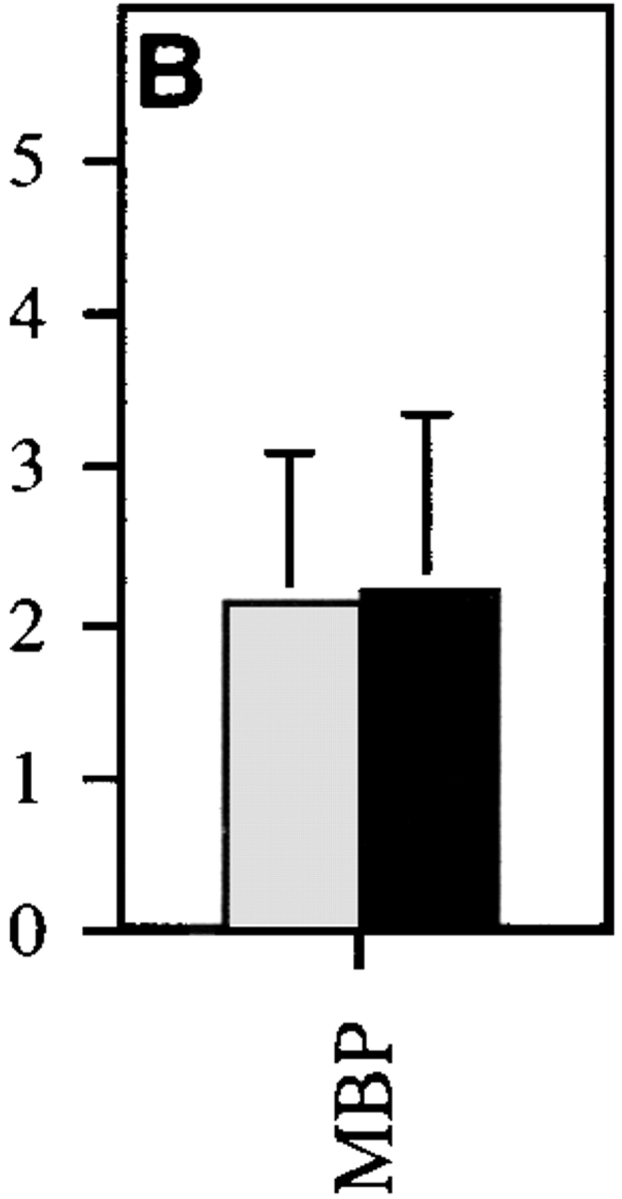
MBP-specific Th1 cells are induced in hIL-10Tg mice. hIL-10Tg mice (black bar) and control littermate mice (gray bar) were immunized twice at 1-wk intervals with MSCH emulsified in CFA. (A) 3 d after the last immunization, DLN cells from hIL-10Tg or control littermate mice were stimulated with MBP or MBP plus anti–IL-10 receptor mAb. One representative experiment out of three is shown. (B) CD4+ T cells were purified and stimulated in vitro with MBP plus irradiated T cell–depleted splenocytes from control CSJLF1/J mice for 60 h. The amount of IFN-γ in the supernatants was measured by ELISA. One representative experiment out of two is shown. The results are the mean of three or four individual mice/group ± SEM.
hIL-10Tg in the Absence of Endogenous Mouse IL-10 Can Prevent EAE.
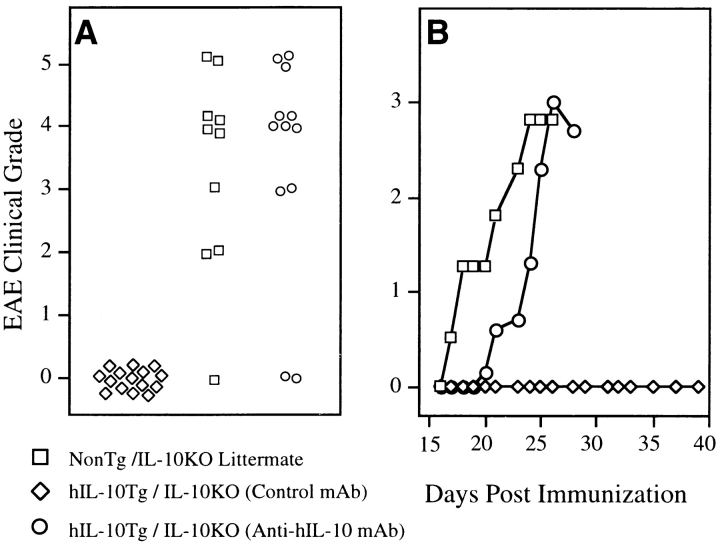
To assess whether T cell–derived endogenous mouse IL-10 was required for EAE resistance, the IL-10Tg mice were backcrossed onto an IL-10 null (IL-10KO) background to generate mice that expressed hIL-10Tg but lacked endogenous mouse IL-10. Because murine T cells do not express MHC class II molecules, no T cell–derived IL-10 is present in these mice. The hIL-10Tg IL-10KO mice and nonTg IL-10KO littermates were tested for susceptibility to EAE. Transgene-positive IL-10KO mice, which only have MHC class II–positive cell–derived IL-10, were completely resistant to EAE, whereas nonTg IL-10KO littermates were highly susceptible to EAE (Fig. 5). No difference in EAE susceptibility was observed when nonTg IL-10KO littermates were compared with BALB/cAnN IL-10KO mice (data not shown). Treatment with anti– hIL-10 mAb reversed this protection in the Tg IL-10KO mice, which became as susceptible to EAE as nonTg IL-10KO littermates (Fig. 5). To determine the extent of the protection provided by the IL-10Tg in the absence of endogenous murine IL-10, the CNS of the three groups of mice from Fig. 5 were examined. Intense inflammatory infiltrates and extensive demyelination as determined by the loss of Luxol fast blue staining were found in the white matter of nonTg IL-10KO littermates, consistent with the clinical disease (Fig. 6 A). In contrast, the CNS of the EAE-resistant mice expressing the IL-10 transgene but lacking endogenous murine IL-10 were completely free of inflammatory infiltrates and demyelination (Fig. 6 B). Examination of the CNS of hIL-10 transgene–positive IL-10KO mice treated with anti–hIL-10 mAb showed extensive inflammation and demyelination similar to the pathological changes found in the nonTg littermates (data not shown). These data suggest that APC production of IL-10 alone, despite the complete absence of T cell–produced IL-10, is sufficient to protect mice from EAE.
Figure 5.
hIL-10Tg mice on the IL-10KO genetic background are EAE resistant. (A) Peak severity scores of individual mice treated with isotype control mAb or anti–hIL-10 mAb (1 mg/dose) on days 0, 7, and 14. Results shown are compiled from two separate experiments. (B) Average severity score over time for the three groups of mice shown in A.
Figure 6.


Histology of spinal cords of hIL-10Tg mice on the IL-10KO background shown in Fig. 5. (A) NonTg IL-10KO mice were EAE susceptible and showed inflammatory infiltrates and plaques of demyelination in spinal cords (arrowheads). (B) hIL-10Tg IL-10KO mice were EAE resistant and their spinal cords showed no inflammatory infiltrates or demyelination. Luxol fast blue stain with hematoxylin counterstain. ×125.
Discussion
In this study, we have demonstrated that Tg mice expressing hIL-10 under the control of the MHC class II promoter are completely resistant to induction of EAE and that this resistance is independent of T cell–derived IL-10. The use of human IL-10, which has similar specific activity to murine IL-10 (1, 2), allowed for the specific measurement and inhibition of Tg and endogenous IL-10. Treatment of MSCH-immunized mice with neutralizing anti–hIL-10 at the time of immunization rendered transgene-positive mice fully susceptible to EAE, although the disease onset was delayed by several days (Figs. 3 and 5). Treatment of hIL-10Tg mice with anti–hIL-10 mAb 8 d before MSCH immunization resulted in kinetics of disease onset identical to that of nonTg littermates, suggesting that the elevated IL-10 level before immunization can also influence the response to antigen, consistent with our previous study showing that IL-10 has significant effects on the function of APC prior to antigen-induced activation (18). Furthermore, APC-derived IL-10Tg is sufficient for EAE prevention, as shown by the resistance of transgene-positive BALB/c mice homozygous for a mutated mouse IL-10 gene (Fig. 5). Therefore, IL-10 need not be provided by T cells to prevent EAE.
Several observations suggest that myelin-reactive Th1 cells are induced in hIL-10Tg mice despite the absence of disease. Similar levels of IFN-γ were produced by purified, MBP-specific CD4+ cells from EAE-resistant hIL-10Tg mice and EAE-susceptible nonTg littermates when stimulated in vitro with wild-type APC and MBP (Fig. 4). No MBP-induced IL-4 was produced by T cells from Tg or nonTg mice, suggesting that EAE resistance was not associated with a Th2 response. These results are consistent with a report showing that, although systemic treatment with rIL-10 decreased the severity of EAE in SJL mice, PLP-specific Th1 cells were induced and no PLP-specific Th2 cells were found (9). These data are also in agreement with the original finding that IL-10 does not substantially inhibit the development of Th1 cells but does inhibit their effector function (1, 19).
EAE in BALB/c and SJL × BALB/c F1 mice is characterized by an intense inflammatory infiltrate found predominantly in the white matter of the CNS and occasionally extending into the gray matter region, causing neuronal destruction and death (not shown). The absence of an inflammatory infiltrate in the CNS of EAE-resistant Tg mice (Figs. 2 and 6) suggests that either autoreactive T cells did not enter the CNS or the few T cells entering the CNS were not able to recruit additional inflammatory cells. In vitro, IL-10 has been shown to inhibit antigen presentation and production of IL-1, IL-6, TNF-α, CD80, and CD86 by LPS- and/or IFN-γ–activated microglia (20, 21). Therefore, it is possible that IL-10Tg in the CNS may inhibit microglia antigen presentation and activation of myelin-reactive Th1 cells.
Several parameters, including the amount of IL-10 and the mode of gene regulation in specific local regions, may account for the effectiveness of IL-10Tg in inhibition of EAE. hIL-10 was detectable in the serum of the hIL-10Tg mice at levels of 400–700 pg/ml (13). In vitro, hIL-10 and mouse IL-10 were detected in the lymph node cell cultures at similar levels of 2–6 ng/ml (data not shown). Thus, IL-10Tg was not present systemically at high levels even though it significantly protected mice from EAE.
Two separate studies have shown that IL-10 regulated by lymphocyte-specific promoters can inhibit EAE. Adoptive transfer of PLP-specific memory T cells expressing IL-2 promoter–regulated IL-10 partially reduced or reversed disease in PLP-immunized recipient mice (12). FVB × SJL F1 mice expressing Tg murine IL-10 under the lymphocyte-specific CD2 promoter are resistant to EAE induced by PLP immunization (22). These results and the findings in the present study suggest that, in addition to lymphocyte-derived IL-10, MHC class II–positive cell-derived IL-10Tg, which may be upregulated early in an inflammatory response, can dramatically protect mice from EAE. Furthermore, our preliminary results show that adoptive transfer of MBP-specific encephalitogenic Th1 cells can not induce EAE in hIL-10Tg mice, suggesting a role for local inhibition of Th1 cells by MHC class II promoter-regulated IL-10Tg within the CNS (data not shown). These studies suggest that IL-10, applied with appropriate localization, in appropriate amounts, and at the appropriate time, can completely protect animals from EAE, despite their generation of potentially pathogenic Th1-like cells. This model should permit a more detailed understanding of the conditions necessary for IL-10 inhibition of autoimmune-mediated CNS inflammation, as this insight will provide information about how to use IL-10 in therapeutic situations.
Acknowledgments
We would like to thank Drs. Jonathon Sedgwick, Amy Beebe, and Anne O'Garra for critical review of the manuscript and useful discussion.
Footnotes
DNAX is supported by the Schering Plough Corporation.
References
- 1.Moore KW, O'Garra A, de Waal R, Malefyt, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 2.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71:169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 3.Balasa B, Sarvetnick N. The paradoxical effects of interleukin 10 in the immunoregulation of autoimmune diabetes. J Autoimmun. 1996;9:283–286. doi: 10.1006/jaut.1996.0036. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Otsuka T, Hotokebuchi T, Miyahara H, Nakashima H, Kuga S, Nemoto Y, Niiro H, Niho Y. Effect of IL-10 on collagen-induced arthritis in mice. Inflamm Res. 1996;45:283–288. doi: 10.1007/BF02280992. [DOI] [PubMed] [Google Scholar]
- 5.Persson S, Mikulowska A, Narula S, O'Garra A, Holmdahl R. Interleukin-10 suppresses the development of collagen type II-induced arthritis and ameliorates sustained arthritis in rats. Scand J Immunol. 1996;44:607–614. doi: 10.1046/j.1365-3083.1996.d01-355.x. [DOI] [PubMed] [Google Scholar]
- 6.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 7.Cannella B, Gao YL, Brosnan C, Raine CS. IL-10 fails to abrogate experimental autoimmune encephalomyelitis. J Neurosci Res. 1996;45:735–746. doi: 10.1002/(SICI)1097-4547(19960915)45:6<735::AID-JNR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Rott O, Fleischer B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- 9.Nagelkerken L, Blauw B, Tielemans M. IL-4 abrogates the inhibitory effect of IL-10 on the development of experimental allergic encephalomyelitis in SJL mice. Int Immunol. 1997;9:1243–1251. doi: 10.1093/intimm/9.9.1243. [DOI] [PubMed] [Google Scholar]
- 10.Croxford JL, Triantaphyllopoulos K, Podhajcer OL, Feldmann M, Baker D, Chernajovsky Y. Cytokine gene therapy in experimental allergic encephalomyelitis by injection of plasmid DNA-cationic liposome complex into the central nervous system. J Immunol. 1998;160:5181–5187. [PubMed] [Google Scholar]
- 11.Shaw MK, Lorens JB, Dhawan A, DalCanto R, Tse HY, Tran AB, Bonpane C, Eswaran SL, Brocke S, Sarvetnick N, et al. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathisen PM, Yu M, Johnson JM, Drazba JA, Tuohy VK. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J Exp Med. 1997;186:159–164. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groux H, Cottrez F, Rouleau M, Mauze S, Antonenko S, Hurst S, McNeil T, Bigler M, Roncarolo M-G, Coffman RL. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 14.Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J Immunol. 1995;155:4052–4059. [PubMed] [Google Scholar]
- 15.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 17.Teuscher C, Blankenhorn EP, Hickey WF. Differential susceptibility to actively induced experimental allergic encephalomyelitis and experimental allergic orchitis among BALB/c substrains. Cell Immunol. 1987;110:294–304. doi: 10.1016/0008-8749(87)90124-9. [DOI] [PubMed] [Google Scholar]
- 18.Cua DJ, Coffman RL, Stohlman SA. Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J Immunol. 1996;157:2830–2836. [PubMed] [Google Scholar]
- 19.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 20.Frei K, Lins H, Schwerdel C, Fontana A. Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol. 1994;152:2720–2728. [PubMed] [Google Scholar]
- 21.Menendez Iglesias, B., J. Cerase, C. Ceracchini, G. Levi, and F. Aloisi. Analysis of B7-1 and B7-2 costimulatory ligands in cultured mouse microglia: upregulation by interferon-gamma and lipopolysaccharide and downregulation by interleukin-10, prostaglandin E2 and cyclic AMP-elevating agents. J Neuroimmunol. 1997;72:83–93. doi: 10.1016/s0165-5728(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]