Abstract
A panel of cDNAs encoding allergenic proteins was isolated from an Aspergillus fumigatus cDNA library displayed on the surface of filamentous phage. Solid phase–immobilized serum immunoglobulin E (IgE) from A. fumigatus–allergic individuals was used to enrich phage displaying IgE-binding molecules. One of the cDNAs encoded a 11.1-kD protein that was identified as acidic ribosomal phosphoprotein type 2 (P2 protein). The allergen, formally termed rAsp f 8, shares >62% sequence identity and >84% sequence homology to corresponding eukaryotic P2 proteins, including human P2 protein. The sequences encoding human and fungal P2 protein were subcloned, expressed in Escherichia coli as His6-tagged fusion proteins, and purified by Ni2+–chelate affinity chromatography. Both recombinant P2 proteins were recognized by IgE antibodies from allergic individuals sensitized to the A. fumigatus P2 protein and elicited strong type 1–specific skin reactions in these individuals. Moreover, human and fungal P2 proteins induced proliferative responses in peripheral blood mononuclear cells of A. fumigatus– allergic subjects sensitized to the fungal P2 protein. These data provide strong evidence for in vitro and in vivo humoral and cell-mediated autoreactivity to human P2 protein in patients suffering from chronic A. fumigatus allergy.
Keywords: phage display, cDNA libraries, IgE, allergens, autoimmunity
A spergillus fumigatus, a ubiquitous mold (1), is considered an opportunistic pathogen responsible for a vast variety of pulmonary complications in humans and animals. The spectrum of diseases associated with the fungus ranges from mild forms, like saprophytic colonization of the lung and allergy, to life-threatening diseases, such as invasive systemic aspergillosis or allergic bronchopulmonary aspergillosis (ABPA) (2). These different clinical presentations indicate that not only the virulence of the fungus itself, but also other underlying conditions, including impaired immune status, play a role in the development of opportunistic mycoses (3). Cloning and sequencing of allergen-encoding cDNAs from A. fumigatus (4–6) have permitted characterization of the biochemical function of some allergens by sequence comparison. The 18-kD protein Asp f 1, a member of the ribotoxin family (4, 7), represents a major allergen of the fungus (7, 8) and was found in the urine of patients with invasive aspergillosis (9). Serologic studies with rAsp f 1 and other A. fumigatus allergens (10) clearly demonstrated the existence of disease-specific allergens able to elicit IgE responses exclusively in patients suffering from ABPA (10– 12). Although the pathophysiologic mechanisms leading to Aspergillus-related pulmonary complications remain largely unknown, the availability of recombinant allergens from the fungus substantially contributed to improved diagnosis of the diseases (11–14). At the molecular level, the cloned allergens can be subdivided into two categories: secreted and cytoplasmatic proteins (10). Interestingly, at least one of the ABPA-specific allergens, manganese-dependent superoxide dismutase (MnSOD), a phylogenetically highly conserved protein (15), also shows cross-reactivity with human MnSOD (16). Phylogenetically conserved proteins are often involved in fungal allergy (17) and also have the potential to be involved in humoral and cell-mediated autoimmune reactions (15, 18). We have cloned a large panel of cDNAs encoding allergenic A. fumigatus proteins using phage surface display technology (5, 6, 19). Here, we describe the sequence and properties of one of these allergens identified as an A. fumigatus acidic ribosomal phosphoprotein type 2 (P2 protein) by sequence homology. The acidic ribosomal phosphoproteins P0 (38 kD), P1 (13 kD), and P2 (13 kD), contained in the 60 S ribosomal subunit, are highly conserved among eukaryotes and are required for the functional activity of the ribosome (20). These proteins have been reported as antigens capable of inducing IgG antibody responses in systemic lupus erythematosus, the prototypic systemic autoimmune disease (21, 22). Additionally, the P2 proteins from Alternaria alternata and Cladosporium herbarum have been reported to be minor allergens of these molds (17). The A. fumigatus P2 protein is recognized by IgE antibodies of individuals sensitized to the mold and shows significant humoral cross-reactivity to human P2 protein. Both human and A. fumigatus P2 proteins induce strong type 1 skin reactions and proliferative responses in PBMCs of individuals sensitized to the fungal P2 protein.
Materials and Methods
Construction and Screening of an A. fumigatus cDNA Library Displayed on Phage Surface.
Phage displaying IgE-binding proteins were enriched from an A. fumigatus cDNA library constructed in phagemid pJuFo (19) and displayed on the surface of filamentous phage M13 as described (5, 6). Serum IgE from A. fumigatus–sensitized individuals was captured in microtiter plates coated with anti– human mAb TN 142 and used as ligand for selective enrichment of allergen-displaying phage (6). Sera used for screening were selected according to case history, skin test reactivity to commercial A. fumigatus extracts, and specific IgE to A. fumigatus determined by radioallergosorbent test (RAST) (8). The screening procedure yielded a wide variety of phage able to bind specifically to human serum IgE and thus displaying allergenic molecules (5, 6).
Identification of a Clone Encoding A. fumigatus P2 Protein.
Inserts carried by phage displaying IgE-binding proteins differing in length were sequenced as described (23) on an ABI prism 373A sequencer using the d-rhodamine terminator cycle sequencing kit (Perkin-Elmer) according to the manufacturer's instructions. Both DNA strands were sequenced using vector-derived primers. Homology searches and sequence comparisons were performed with BLAST and the Genetics Computer Group program FASTA (24). One clone revealed strong homology with nucleotide sequences encoding acidic ribosomal phosphoprotein type 2 (see Fig. 1).
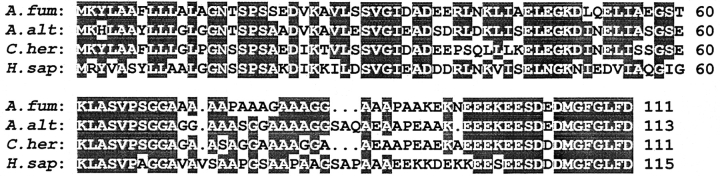
Figure 1.
Alignment of the deduced amino acid sequences A. fumigatus (A. fum), A. alternata (A. alt), C. herbarum (C. her), and human (H. sap) P2 protein sequences. Identical amino acid residues in at least three of the sequences are noted by shading, and gaps are indicated by dots. Sequence identity between A. fumigatus, human, C. herbarum, and A. alternata P2 proteins are 62.16, 72.07, and 71.17%, respectively. Numbers after the sequences indicate the residue numbers, without gaps, starting at the NH2-terminal methionine residues.
Cloning of the Human P2 Protein; Production and Characterization of Recombinant Proteins.
The cDNA encoding human P2 protein was amplified by PCR from a commercial human lung cDNA library (Stratagene) using the following primers: 5′-primer, 5′-GCGGATCCATGCGCTACGTCGCCTCCTACC-3′; 3′-primer, 5′-GCTCTAGATTAATCAAAAAGGCCAAATCCC-3′. The complete cDNA coding for the putative A. fumigatus P2 protein was amplified from the original clone by PCR using the following primers: 5′-primer, 5′-GCGGATCCATGAAGTACCTCGCAGCTTTCC-3′; 3′-primer, 5′-CCCGGACTTTAAGTCGAAGAGACCGAAGCCC-3′. PCR cycling conditions were 30 cycles of 95°C for 60 s, 57°C for 60 s, and 72°C for 60 s, followed by a terminal extension cycle at 72°C for 10 min. The amplification products were purified using a commercial kit (QIAquick; QIAGEN, Inc.), digested with BamHI and XbaI or BamHI and HindIII, respectively, and ligated to appropriately restricted pHis6–DHFR (dihydrofolate reductase) vector (4). Ligation mixtures were transformed into Escherichia coli strain M 15; transformants were grown in liquid to verify the nucleotide sequence (23) and used to produce hexahistidine-tagged recombinant proteins (4, 6). After a single-step purification over Ni2+–chelate affinity columns (6), molecular size and purity of the recombinant proteins were analyzed by polyacrylamide gradient gels (4.5–20%) and 1-mg samples lyophilized for long term storage (14).
ELISA and IgE Immunoblots.
The specific binding properties of serum IgE from A. fumigatus–sensitized individuals to recombinant fungal and human P2 protein were analyzed by an allergen-specific ELISA (8). Absorbency was measured at 405 nm with a Molecular Devices reader and optical densities converted into arbitrary ELISA units (EU/ml) calibrated against an in-house serum pool arbitrarily defined as 100 EU/ml (8, 11, 12). Values below 1 EU/ml were set as 1 EU/ml for graphic display and nonparametric statistical analysis (Mann–Whitney U test). For IgE immunoblots, proteins were separated on SDS–polyacrylamide gradient gels (4–20%), transferred to nitrocellulose, incubated with patient sera diluted 1:10, and processed as described (16, 25).
Proliferative Responses of PBMCs.
PBMCs were isolated from heparinized peripheral venous blood by Ficoll density gradient centrifugation, washed three times, and resuspended in RPMI 1640 supplemented with 1 mM sodium pyruvate, 2 mM l-glutamine, 50 μM 2-ME, 1% MEM nonessential amino acids and vitamins, 100 μg/ml streptomycin, 100 U/ml penicillin (all from Life Technologies), and 10% heat-inactivated FCS (Sera-Lab). Samples of 5 × 105 cells/100 μl were stimulated with different concentrations of recombinant A. fumigatus or human P2 protein or with A. fumigatus extract in triplicate for 7 d. Proliferation was measured as incorporation of tritiated thymidine (DuPont-NEN) during the final 16 h of culture. A stimulation index >3 was considered positive.
Skin Tests with Recombinant P2 Protein.
Recombinant proteins were dissolved in 0.9% saline at concentrations ranging from 10−5 to 1 μg/ml. Intradermal skin tests were performed on the patient's back by injecting 100 μl test solution containing 1 ng recombinant protein. If no positive reaction could be observed after 15 min, testing was continued by injecting a 10-fold higher amount of protein. The test was stopped and considered positive when the wheal surface reached at least half of the histamine wheal size (8, 16) or after injection of 1 μg protein. 0.01% histamine dihydrochloride and 0.9% saline were used as positive and negative controls, respectively (8). An ethical approval for skin testing human subjects with recombinant proteins was obtained from the local ethics committee (Davos, Switzerland). A full explanation of the procedure was given to all participants, and their written consent was obtained before testing.
Results and Discussion
Isolation of cDNA Clones and Sequence Analysis.
The cloning technology based on phage surface display of expression products from cDNA libraries (5, 6, 19) is particularly suitable for selective isolation of cDNAs encoding IgE-binding proteins from complex allergenic systems (26). Starting from a phage surface–displayed cDNA library generated from mRNA of A. fumigatus (6), we selectively enriched phage able to bind human serum IgE from individuals sensitized to the fungus. cDNAs isolated from single phagemids after four rounds of affinity selection, carrying inserts of different lengths, were sequenced and shown to code for different allergenic proteins (10). Among these, a clone containing an open reading frame spanning 333 bp (sequence data available from EMBL under accession no. AJ224333) revealed strong homology with sequences encoding eukaryotic type 2 acidic ribosomal phosphoproteins. The deduced amino acid sequence of this cDNA clone was homologous to P2 proteins, showing a high sequence identity to the human (62%), C. herbarum (71%), and A. alternata (72%) P2 proteins (Fig. 1). Acidic ribosomal phosphorylated (P) proteins have been isolated and characterized from a variety of eukaryotic cells and share significant sequence identity and similarity (20).
Production and Characterization of Recombinant A. fumigatus and Human P2 Proteins.
Both complete cDNAs encoding the putative A. fumigatus P2 protein and the human P2 protein were amplified by PCR, subcloned into the high level expression plasmid pHis6–DHFR, verified by sequencing, and used to produce hexahistidine-tagged P2 proteins (see Materials and Methods). The constructs yielded, after single-step purification by Ni2+–chelate affinity chromatography, 33 and 28 mg/liter E. coli culture of virtually pure A. fumigatus and human P2 protein, respectively. Purity was analyzed by reducing, denaturing SDS-PAGE, and Coomassie blue staining. In each preparation, only one band with estimated molecular mass in agreement with the calculated values of ∼12.6 kD for the hexahistidine-tagged A. fumigatus and human P2 protein was visible (data not shown).
Allergenic Properties of the P2 Proteins.
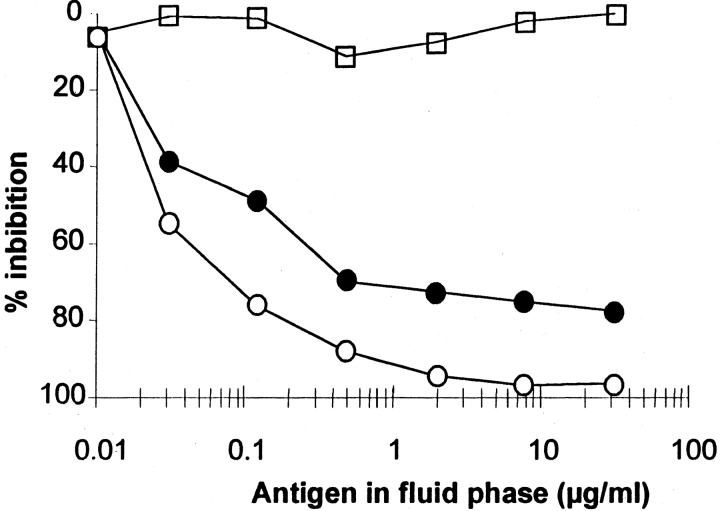
As expected from the selection procedure devoted to isolate allergens from a phage surface display library, the A. fumigatus P2 protein was able to bind IgE present in the serum used for screening. However, both A. fumigatus and human P2 proteins were identified as allergens by ELISA with sera from individuals allergic to A. fumigatus (Fig. 2) and by IgE immunoblots (data not shown). Inhibition experiments showed that increasing amounts of human recombinant P2 protein added to the fluid phase were able to inhibit the binding of serum IgE from patients sensitized to A. fumigatus P2 protein (Fig. 3), demonstrating that the proteins share common IgE-binding epitopes. 14/92 patients studied that were suffering from ABPA and 6/75 patients that were allergic to A. fumigatus and suffering from severe atopic dermatitis studied showed relevant levels of serum IgE antibodies to the A. fumigatus P2 protein, resulting in an incidence of sensitization in the range of 15 and 8%, respectively. Sensitization to P2 protein was not observed in A. fumigatus–sensitized individuals with mild forms of atopic dermatitis or in patients without ABPA.
Figure 2.
Competitive inhibition of IgE binding to solid phase–coated A. fumigatus recombinant P2 protein. Serum from A. fumigatus–sensitized patients was preincubated with increasing amounts of recombinant A. fumigatus (○), human P2 protein (•), or Asp f 1 as negative control (□). Preincubated serum samples were transferred to wells coated with A. fumigatus P2 protein, and bound IgE was analyzed by antigen-specific ELISA (8, 16).
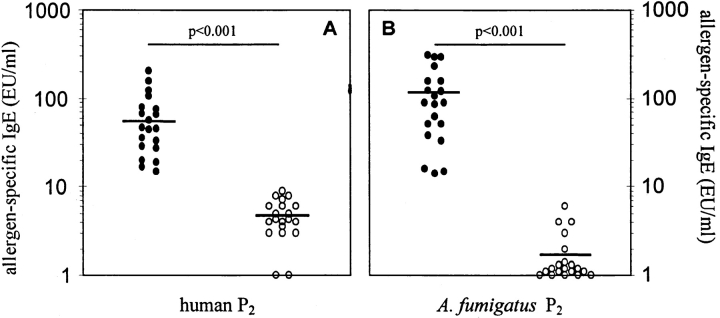
Figure 3.
20 sera from individuals sensitized to (▪) and 20 sera of individuals lacking IgE to the A. fumigatus P2 protein (○) were analyzed for their content of serum IgE able to recognize human P2 protein (A). (B) IgE binding of the same sera to A. fumigatus P2 protein is shown. Units are expressed as arbitrary ELISA units (EU/ml) calculated from the absorbency of an in-house reference serum pool, which was set as 100 EU/ml (8). Bars, mean values.
Proliferative Responses of PBMC from Individuals Sensitized to A. fumigatus P2 Protein.
T cell help is required for the production of allergen-specific IgE (27). Therefore, we measured the proliferative responses of mononuclear cells from six individuals sensitized to the A. fumigatus P2 protein to fungal extract, recombinant A. fumigatus, and human P2 protein. The mean proliferative responses to optimal concentrations of extract, fungal, and human P2 protein were 39,500; 20,092; and 11,790 cpm, respectively (mean SI 11.9, 6.5, and 5.7). The mean proliferative responses of 15,608; 1,601; and 1,273 cpm (mean SI 12.6, 0.8, and 1) to fungal extract, A. fumigatus, and human P2 protein, respectively, obtained for four individuals sensitized to A. fumigatus lacking IgE antibodies against the fungal P2 protein indicate that the recombinant antigens did not induce nonspecific effects. Three control individuals did not respond to any of the antigen preparations (mean SI < 1). Comparison of the values by the rank sum test indicates highly significant differences (P < 0.01). The proliferative responses induced by human P2 protein in individuals sensitized to the A. fumigatus P2 protein indicate a pathogenesis related to autoreactive T cells. Intense local inflammatory responses to A. fumigatus occurring in the lungs of patients suffering from ABPA (11, 12) and in the skin of patients suffering from severe atopic dermatitis might result in release of autoantigens as a consequence of tissue damage due to the inflammatory process (28). Exposure to autoantigens containing cross-reactive determinants (molecular mimicry) can recruit the memory T cell repertoire at the site of inflammation where lymphokine expression is upregulated. These lymphokines can induce expression of MHC II on naive T cells and upregulate accessory molecules that function as costimulatory signals for T cell activation, creating a microenvironment in which all requirements for priming a T cell response are present (29). Molecular mimicry at the T cell level could be a possible pathogenic mechanism to explain autoaggression remaining confined to the local area of inflammation (29).
Allergenicity of the Recombinant P2 Proteins In Vivo.
The ability of a protein to bind IgE in ELISA and Western blots provides strong evidence for the allergenicity of the protein. However, the final demonstration that a protein preparation acts as an allergen and therefore possesses biological activity in vivo is its ability to elicit a type I skin reaction in sensitized individuals. We have investigated whether the IgE-mediated cross-reactivity against A. fumigatus and human P2 protein shown in vitro is sufficient to provoke allergic reactions in vivo through skin tests (8, 16). Four individuals with high IgE levels against A. fumigatus P2 protein, four individuals allergic to the fungus lacking IgE responses to the P2 protein, and two nonallergic control individuals were investigated for their ability to respond to intradermal challenge with recombinant A. fumigatus and human P2 proteins. As expected, none of the individuals without detectable IgE antibodies to A. fumigatus P2 protein reacted against the recombinant protein preparations. A positive skin reaction to A. fumigatus P2 protein was detected only in individuals who had IgE levels >10 EU/ml to the fungal protein. The amounts of recombinant protein needed to elicit a classical type I reaction ranged from 1 to 10 ng, depending on the subject. All individuals reacting to A. fumigatus P2 protein also showed strong skin reaction to challenges with comparable amounts of human P2 protein (Fig. 4). These results show that human P2 protein can cross-link IgE on mast cells in vivo (30) and suggest humoral autoimmune response in some patients suffering from mold allergy. IgE reactivity to fungal and human P2 protein was, however, only detectable in individuals sensitized to A. fumigatus suffering from ABPA or severe atopic dermatitis. This was also the case for the IgE autoreactivity to human MnSOD described earlier (16). Therefore, these two human proteins may serve as a tool to study the role of IgE autoreactivity in tissue damage and release of autoantigens at the site of inflammation.
Figure 4.
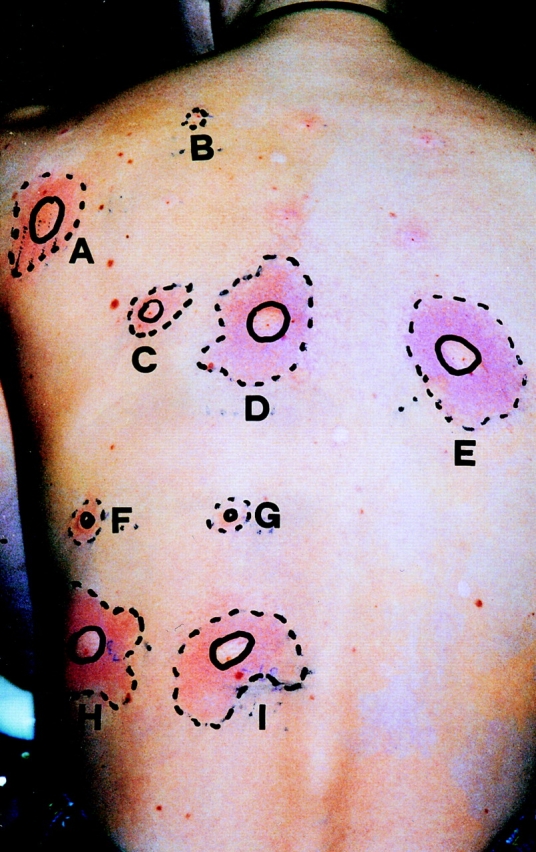
Skin test reactivity to recombinant A. fumigatus and human proteins in a patient sensitized to MnSOD and P2 protein. For intradermal skin tests, 100 μl of the protein solutions (10−1 μg/ml) was injected with a syringe. 0.01% histamine dihydrochloride (A) and 0.9% saline (B) were used as positive and negative controls, respectively. The reactions show that 10 ng fungal (D) and human (E) P2 protein or fungal (H) and human (I) MnSOD are able to elicit a wheal that is comparable to the size of the skin reaction induced by the positive histamine control. C shows the reaction to a challenge with 1 ng human P2 protein. The patient lacks IgE to rAsp f 3 (F) and rAsp f 11 (G), two additional A. fumigatus allergens (10). The absence of reactions to skin challenges with these allergens demonstrates the specificity of the test.
Acknowledgments
We are grateful to Dr. G. Menz (Hochgebirgsklinik, Davos-Wolfgang, Switzerland) for fruitful discussions and careful reading of the manuscript. We thank Dr. D. Stüber (Hoffmann LaRoche, Basel, Switzerland) for providing expression vectors and Dr. C. Heusser (Novartis, Basel, Switzerland) for the TN-142 mAb.
This study was supported in part by Swiss National Science Foundation grants 31-39429.93 and 31-50515.97.
References
- 1.Mullis J, Harvey R, Seaton A. Sources and incidence of airborne Aspergillus fumigatus. . Clin Allergy. 1976;6:209–217. doi: 10.1111/j.1365-2222.1976.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 2.Bardana EJ. The clinical spectrum of aspergillosis. Part 2: classification and description of saprophytic allergic and invasive variants of human diseases. Crit Rev Clin Lab Sci. 1980;13:85–159. doi: 10.3109/10408368009106445. [DOI] [PubMed] [Google Scholar]
- 3.Romani L, Howard DH. Mechanisms of resistance to fungal infections. Curr Opin Immunol. 1995;7:517–523. doi: 10.1016/0952-7915(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 4.Moser M, Crameri R, Menz G, Schneider T, Dudler T, Virchow C, Gmachl M, Blaser K, Suter M. Cloning and expression of recombinant Aspergillus fumigatus allergen I/a (rAsp fI/a) with IgE binding and type I skin test activity. J Immunol. 1992;149:454–460. [PubMed] [Google Scholar]
- 5.Crameri R, Jaussi R, Menz G, Blaser K. Display of expression products of cDNA libraries on phage surfaces. A versatile screening system for selective isolation of genes by specific gene-product/ligand interaction. Eur J Biochem. 1994;226:53–58. doi: 10.1111/j.1432-1033.1994.tb20025.x. [DOI] [PubMed] [Google Scholar]
- 6.Crameri R, Blaser K. Cloning Aspergillus fumigatusallergens by the pJuFo filamentous phage display system. Int Arch Allergy Immunol. 1996;110:41–45. doi: 10.1159/000237308. [DOI] [PubMed] [Google Scholar]
- 7.Arruda LK, Platts-Mills TA, Fox JW, Chapman MD. Aspergillus fumigatusallergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins. J Exp Med. 1990;172:1529–1532. doi: 10.1084/jem.172.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser M, Crameri R, Brust E, Suter M, Menz G. Diagnostic value of recombinant Aspergillus fumigatusallergen I/a for skin testing and serology. J Allergy Clin Immunol. 1994;93:1–11. doi: 10.1016/0091-6749(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 9.Lamy G, Moutaouakil M, Latgé JP, Davies J. Secretion of a potential virulence factor, a fungal ribonucleotoxin, during human aspergillosis infections. Mol Microbiol. 1991;5:1811–1815. doi: 10.1111/j.1365-2958.1991.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 10.Crameri R. Recombinant Aspergillus fumigatusallergens: from the nucleotide sequences to clinical applications. Int Arch Allergy Immunol. 1998;115:99–114. doi: 10.1159/000023889. [DOI] [PubMed] [Google Scholar]
- 11.Crameri R, Hemmann S, Ismail C, Menz G, Blaser K. Disease-specific recombinant allergens for the diagnosis of allergic bronchopulmonary aspergillosis. Int Immunol. 1998;10:1211–1216. doi: 10.1093/intimm/10.8.1211. [DOI] [PubMed] [Google Scholar]
- 12.Hemmann S, Nikolaizik WH, Schöni MH, Blaser K, Crameri R. Differential IgE recognition of recombinant Aspergillus fumigatus allergens by cystic fibrosis patients with allergic bronchopulmonary aspergillosis or Aspergillusallergy. Eur J Immunol. 1998;28:1155–1160. doi: 10.1002/(SICI)1521-4141(199804)28:04<1155::AID-IMMU1155>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Barnerjee B, Kurup VP, Phadnis S, Greenberger PA, Fink JN. Molecular cloning and expression of a recombinant Aspergillus fumigatusprotein Asp f 2 with significant immunoglobulin E reactivity in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1996;127:253–262. doi: 10.1016/s0022-2143(96)90093-1. [DOI] [PubMed] [Google Scholar]
- 14.Crameri R, Lidholm J, Grönlund H, Stüber D, Blaser K, Menz G. Automated specific IgE assay with recombinant allergens: evaluation of the recombinant Aspergillus fumigatusallergen I in the Pharmacia CAP system. Clin Exp Allergy. 1996;26:1411–1419. [PubMed] [Google Scholar]
- 15.Mayer C, Hemmann S, Faith A, Blaser K, Crameri R. Cloning, production, characterization and IgE cross- reactivity of different manganese superoxide dismutases in individuals sensitized to Aspergillus fumigatus. . Int Arch Allergy Immunol. 1997;113:213–215. doi: 10.1159/000237550. [DOI] [PubMed] [Google Scholar]
- 16.Crameri R, Faith A, Hemmann S, Jaussi R, Ismail C, Menz G, Blaser K. Humoral and cell-mediated autoimmunity in allergy to Aspergillus fumigatus. . J Exp Med. 1996;184:265–270. doi: 10.1084/jem.184.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achatz G, Oberkofler H, Lechnauer E, Simon B, Unger A, Kandler D, Ebner C, Prillinger H, Kraft D, Breitenbach M. Molecular cloning of major and minor allergens of Alternaria alternata and Cladosporium herbarum. . Mol Immunol. 1995;32:213–227. doi: 10.1016/0161-5890(94)00108-d. [DOI] [PubMed] [Google Scholar]
- 18.Valenta R, Duchêne M, Pettenburger K, Sillaber C, Valent P, Bettelheim P, Breitenbach M, Rumpold H, Kraft D, Scheiner O. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991;253:557–560. doi: 10.1126/science.1857985. [DOI] [PubMed] [Google Scholar]
- 19.Crameri, R. 1997. pJuFo: a phage surface display system for cloning genes based on protein-ligand interaction. In Gene Cloning and Analysis: Current Innovations. B. Schaefer, editor. Horizon Scientific Press, Wymondham, UK. 29–45.
- 20.Rich BE, Steitz JA. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987;7:4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkon KB, Parnassa AP, Forster CL. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985;162:459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkon K, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1986;83:7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmann S, Blaser K, Crameri R. Allergens of Aspergillus fumigatus and Candida boidiniishare IgE-binding epitopes. Am J Respir Crit Care Med. 1997;156:1956–1960. doi: 10.1164/ajrccm.156.6.9702087. [DOI] [PubMed] [Google Scholar]
- 26.Crameri R, Hemmann S, Blaser K. pJuFo: a phagemid for display of cDNA libraries on phage surface suitable for selective isolation of clones expressing allergens. Adv Exp Med. 1996;409:103–110. doi: 10.1007/978-1-4615-5855-2_14. [DOI] [PubMed] [Google Scholar]
- 27.Zubler RH, Werner-Favre C, Wen L, Sekita KI, Straub C. Theoretical and practical aspects of B-cell activation: murine and human systems. Immunol Rev. 1987;99:281–299. doi: 10.1111/j.1600-065x.1987.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 29.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigen determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 30.Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]