Abstract
Mycobacterium tuberculosis and other pathogenic mycobacteria export abundant quantities of proteins into their extracellular milieu when growing either axenically or within phagosomes of host cells. One major extracellular protein, the enzyme glutamine synthetase, is of particular interest because of its link to pathogenicity. Pathogenic mycobacteria, but not nonpathogenic mycobacteria, export large amounts of this protein. Interestingly, export of the enzyme is associated with the presence of a poly-l-glutamate/glutamine structure in the mycobacterial cell wall. In this study, we investigated the influence of glutamine synthetase inhibitors on the growth of pathogenic and nonpathogenic mycobacteria and on the poly-l-glutamate/glutamine cell wall structure.
The inhibitor l-methionine-S-sulfoximine rapidly inactivated purified M. tuberculosis glutamine synthetase, which was 100-fold more sensitive to this inhibitor than a representative mammalian glutamine synthetase. Added to cultures of pathogenic mycobacteria, l-methionine- S-sulfoximine rapidly inhibited extracellular glutamine synthetase in a concentration-dependent manner but had only a minimal effect on cellular glutamine synthetase, a finding consistent with failure of the drug to cross the mycobacterial cell wall. Remarkably, the inhibitor selectively blocked the growth of pathogenic mycobacteria, all of which release glutamine synthetase extracellularly, but had no effect on nonpathogenic mycobacteria or nonmycobacterial microorganisms, none of which release glutamine synthetase extracellularly. The inhibitor was also bacteriostatic for M. tuberculosis in human mononuclear phagocytes (THP-1 cells), the pathogen's primary host cells. Paralleling and perhaps underlying its bacteriostatic effect, the inhibitor markedly reduced the amount of poly-l-glutamate/glutamine cell wall structure in M. tuberculosis.
Although it is possible that glutamine synthetase inhibitors interact with additional extracellular proteins or structures, our findings support the concept that extracellular proteins of M. tuberculosis and other pathogenic mycobacteria are worthy targets for new antibiotics. Such proteins constitute readily accessible targets of these relatively impermeable organisms, which are rapidly developing resistance to conventional antibiotics.
Keywords: tuberculosis, enzyme inhibitors, poly-l-glutamate/glutamine, drug design
The seemingly inevitable emergence of strains of pathogenic microorganisms resistant to current antibiotics imposes a continuing need for novel strategies for antibiotic development. This need is particularly urgent in the case of tuberculosis, the world's leading cause of death from a single infectious agent. Strains of Mycobacterium tuberculosis, the primary causative agent of tuberculosis, have emerged worldwide that are resistant to the major antibiotics used to treat this disease (1).
Antibiotics typically target key cell wall or intracellular molecules of microorganisms that are involved in cell wall, protein, or DNA synthesis, or in an essential metabolic pathway. On the basis of studies in this report, we shall propose an additional site for antibiotic targeting: extracellular enzymes released by a bacterium.
M. tuberculosis, along with other pathogenic mycobacteria, is unusual among bacterial species in that it secretes or otherwise releases a large number of proteins in considerable quantities into its extracellular milieu. Such extracellular proteins are released by M. tuberculosis organisms when growing either in broth medium or intraphagosomally in human mononuclear phagocytes, the bacterium's primary host cells (2, 3). Approximately 100 proteins are released into broth medium by M. tuberculosis, 11 of which are released in great abundance (4). One of the abundantly released proteins is the enzyme glutamine synthetase (EC 6.3.1.2), which is surprising because this enzyme is typically located in the bacterial cytoplasm (5). Even more surprising, only pathogenic mycobacteria such as M. tuberculosis and Mycobacterium bovis release large amounts of glutamine synthetase extracellularly, whereas nonpathogenic mycobacteria, such as Mycobacterium smegmatis and Mycobacterium phlei, and nonmycobacterial microorganisms, such as Legionella pneumophila and Escherichia coli, do not (5).
Interestingly, the release of glutamine synthetase by pathogenic mycobacteria is correlated with the presence of a poly-l-glutamate/glutamine component in the cell walls of these organisms; nonpathogenic mycobacteria lack this component (6, 7). This suggests the possibility that extracellular glutamine synthetase is involved in the synthesis of poly-l-glutamate/glutamine and that the enzyme's extracellular presence is significant to virulence.
In this report, we shall demonstrate that an irreversible inhibitor of M. tuberculosis extracellular glutamine synthetase blocks bacterial multiplication both in broth medium and in human mononuclear phagocytes and that growth inhibition is correlated with a marked reduction in the amount of the virulence-associated cell wall component poly-l-glutamate/glutamine. Remarkably, the enzyme inhibitor has no effect against nonpathogenic mycobacteria, which do not export glutamine synthetase. Although the inhibitor of glutamine synthetase may target additional extracellular proteins, our report provides strong evidence for the concept that targeting extracellular proteins of pathogenic mycobacteria and perhaps other pathogens is a feasible strategy for developing new antibiotics.
Materials and Methods
Bacterial Cultures.
E. coli DH5α, L. pneumophila Philadelphia 1, the M. tuberculosis strains Erdman (35801; American Type Culture Collection [ATCC]), H37Rv (25618; ATCC), and H37Ra (25177; ATCC), M. bovis (19210; ATCC), M. bovis BCG (bacille Calmette-Guérin [19274; ATCC]), M. phlei (11758; ATCC), M. smegmatis (14468; ATCC), and Mycobacterium avium (25291; ATCC) were cultured as described (5).
Assays of Glutamine Synthetase Activity In Vitro.
M . tuberculosis Erdman extracellular and intracellular glutamine synthetase was purified as described (5) or by chromatography on Affi-Gel Blue 100–200 mesh (Bio-Rad Labs.) and size fractionation on Superdex 75 (Pharmacia Biotech, Inc.). Active fractions were dialyzed against 15 mM imidazole, 2.2 mM MnCl2, pH 7, stored at 4°C, and assessed for enzyme activity by biosynthetic and transfer assay as described (8). Proteins in the active fractions were analyzed by denaturing PAGE and stained with Coomassie brilliant blue R or silver nitrate. The NH2-terminal sequence of the extra- and intracellular M. tuberculosis glutamine synthetase (TEKTPDD) was presented in our earlier report (5). In that report, we showed that this NH2 terminus of active glutamine synthetase corresponds exclusively to the DNA sequence of the glnA1 gene. Although the M. tuberculosis genome contains four genes with domains homologous with other bacterial glutamine synthetases, the glnA1 gene has the highest overall homology, and it is not known whether the other genes are transcribed or expressed (9). Protein concentrations were determined by incubation with bicinchoninic acid reagent (Pierce Chemical Co.).
The amount and activity of extracellular glutamine synthetase released by M. tuberculosis or other microorganisms over their entire growth period (16 h–6 wk) was determined by assaying aliquots of cell-free culture supernates, taken at hourly, daily, or weekly intervals, for enzyme activity by the γ-glutamyltransferase assay (8). The theoretical possibility of leakage of cytoplasmic glutamine synthetase from dead or dying M. tuberculosis cells was assessed by monitoring the activity of the cytoplasmic marker protein lactate dehydrogenase during the 6-wk growth period, both in the culture supernate and in the cell pellet, using a commercially available diagnostic kit (Sigma Chemical Co.). Extracellular lactate dehydrogenase activity never exceeded 0.5% of intracellular activity.
Activity and inhibition profiles of M. tuberculosis Erdman, E. coli W (Sigma Chemical Co.), and sheep brain glutamine synthetase (Sigma Chemical Co.) in the presence and absence of l-methionine-S-sulfoximine and d,l-phosphinothricin (a gift from David Eisenberg, University of California at Los Angeles, Los Angeles, CA) were determined for 1 U of each enzyme in 50 mM MgCl2 as described, using the biologically relevant biosynthetic reaction for all assays (8). K m and K i values for the three enzymes were calculated as described (10, 11). K i values were determined by plotting reciprocal values for the reaction velocity versus substrate concentration for concentrations of the l-glutamate substrate ranging from 2.5 to 100 mM and for concentrations of the inhibitor ranging from 2 to 200 μM in the enzyme assays. K i values, determined in triplicate assays, were expressed as K i = K m × [I] / (K m − K m(app)). Direct analyses of cell pellets and culture supernates for glutamine synthetase activity were performed using the transfer reaction. The recombinant M. tuberculosis glutamine synthetase, which was cloned in the E. coli/mycobacterial shuttle vector pNBV-1 as a genomic DNA fragment containing the structural gene plus extensive flanking regions (12), was expressed in M. smegmatis and exported into the extracellular milieu (12). Recombinant glutamine synthetase was purified, and its enzymatic activity and inhibition profile were determined as described above for the endogenous enzymes.
Inhibition of Bacterial Cultures by l-Methionine-S-Sulfoximine and d,l-Phosphinothricin.
Broth cultures of bacteria were inoculated at a density of 1–5 × 105 cells/ml and grown until stationary phase was reached (overnight–6 wk). Various amounts of l-methionine-S-sulfoximine; d,l-phosphinothricin; the standard antituberculous antibiotics amikacin, ethambutol, isoniazid, pyrazinamide, and rifampin (Sigma Chemical Co.); or l-glutamate/ glutamine were added at time points specified in the figure legends. Viable cells in each culture were determined by removing culture aliquots, plating washed bacteria on appropriate growth media, and enumerating colonies after standard incubation periods (overnight–2 wk).
Inhibition of Mycobacterial Growth in Human Monocytes.
THP-1 cells, a human acute monocytic leukemia line (TIB 202; ATCC), were seeded at 107 cells/ml, differentiated with 100 nM PMA (Sigma Chemical Co.), infected with freshly grown M. tuberculosis Erdman or M. avium bacteria at a multiplicity of 1 for 90 min (thereby infecting 6–11% of the monocytes, based on a bacterial count 3 h after infection), and cultured for up to 5 d in the presence of various concentrations of l-methionine-S-sulfoximine. At various time points, THP-1 cell cultures were lysed by the addition of 0.1% SDS or by vortexing, and serial dilutions of the lysates were plated on agar medium for the enumeration of viable mycobacterial colonies (13).
Determination of Poly-l-Glutamate/Glutamine and d,l-Alanine in the Cell Walls of l-Methionine-S-Sulfoximine–treated and –untreated M. tuberculosis Bacteria.
M. tuberculosis broth cultures in 7H9 or Sauton's medium (Difco Labs.) were inoculated at a density of 1–5 × 105 cells/ml and grown for 6 wk until stationary phase was reached. l-methionine-S-sulfoximine was added, either at the time of inoculation (0.2 and 2 μM final concentration) or after an initial 2-wk growth period (20 and 200 μM final concentration), to duplicate cultures. A second and third set of duplicate cultures were inoculated in parallel and treated with amikacin (0.06–1 μg/ ml) or rifampin (0.0125–0.2 μg/ml) after 2 wk of cell growth. The isolation of poly-l-glutamate/glutamine and the peptidoglycan portion of the bacteria's cell walls followed a procedure described in an earlier report (6). In brief, the procedure involves (i) treatment of disrupted cells with trypsin and chymotrypsin, (ii) delipidation of cell walls by acetone and chloroform/methanol extractions, (iii) acid hydrolysis of the cell walls, and (iv) precipitation of the poly-l-glutamate/glutamine with hydrochloric acid and redissolution in sodium hydroxide, a process that is repeated several times before the final precipitate is washed with water and lyophilized to obtain a pure white powder. The pellet after the first redissolution in sodium hydroxide contains the peptidoglycan chain. Both fractions, poly-l-glutamate/glutamine and peptidoglycan, were analyzed for their amino acid content after complete acid hydrolysis.
Results
M. tuberculosis Glutamine Synthetase Is One to Two Orders of Magnitude More Sensitive to the Inhibitors l-Methionine-S-Sulfoximine and d,l-Phosphinothricin than a Representative Mammalian Glutamine Synthetase.
l -Methionine-S -sul f oximine and d,l-phosphinothricin are well characterized inhibitors of prokaryotic and eukaryotic glutamine synthetases (10, 11, 14). Preparatory to studying their effect on growth of M. tuberculosis and other mycobacteria, we characterized their effect on purified M. tuberculosis glutamine synthetase. Additionally, we compared the sensitivity of M. tuberculosis glutamine synthetase with a representative bacterial glutamine synthetase, E. coli glutamine synthetase, and a representative mammalian glutamine synthetase, sheep brain glutamine synthetase.
We have previously reported the purification and characterization of glutamine synthetase from the highly virulent Erdman strain of M. tuberculosis (5). The homogeneous enzyme is made up of 12 identical M r ∼56–58,000 subunits and active in the biosynthetic assay, with an apparent K m value for l-glutamate of 2.7 ± 0.2 mM, a specific activity of 110 μmol Pi/min/mg enzyme, and a turnover number of ∼70,000 (product/min/mol enzyme). Based on its molecular mass, structure, and biochemical features, the M. tuberculosis glutamine synthetase appears very similar to other bacterial glutamine synthetases (15, 16).
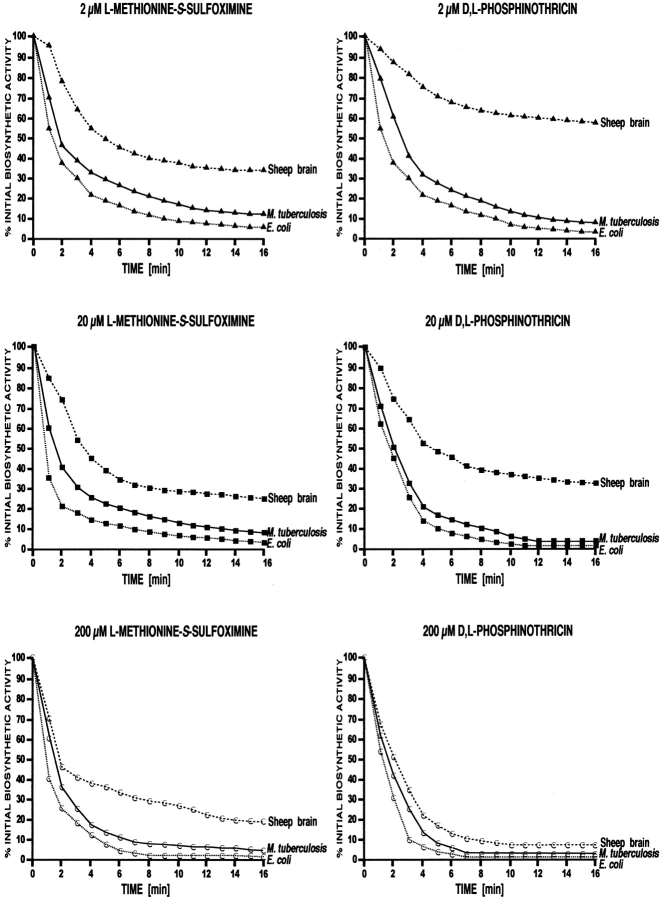
To investigate the effect of glutamine synthetase inhibitors on purified M. tuberculosis, E. coli, and sheep brain glutamine synthetases, we first preincubated the enzymes in l-glutamate–free reaction buffer for various times with l-methionine-S-sulfoximine and d,l-phosphinothricin at final concentrations of 2, 20, and 200 μM. We then assayed the remaining biosynthetic activity of the enzyme over a 2-h period. The prokaryotic and eukaryotic glutamine synthetases exhibited clear differences in sensitivity to the inhibitors (Fig. 1). In the presence of both inhibitors, the prokaryotic glutamine synthetases rapidly lost their enzymatic activity; they retained <10% of initial activity at 2 and 20 μM l-methionine-S-sulfoximine and at 2 μM d,l-phosphinothricin, and they were completely inactivated (≤0.5% initial activity) at higher concentrations of the inhibitors. In contrast, sheep brain glutamine synthetase lost activity much more slowly, retained a higher proportion of initial activity throughout the incubation, and was not completely inactivated at even the highest dose of each inhibitor. For example, at inhibitor concentrations of 2 μM, the bacterial enzymes retained only 3–10% of their initial activity, whereas the sheep brain enzyme retained ∼40% of its initial activity in the presence of l-methionine-S-sulfoximine and 60% in the presence of d,l-phosphinothricin. These results thus confirm earlier reports that eukaryotic glutamine synthetases are much more resistant to these two inhibitors than bacterial enzymes (10, 11).
Figure 1.
Inactivation of various glutamine synthetases by l-methionine-S-sulfoximine and d,l-phosphinothricin. The enzymes (1 U each) were preincubated in the presence of ATP and MgCl2 but in the absence of l-glutamate, with each inhibitor at the concentration indicated on each panel. Aliquots in duplicate were removed every minute, diluted 100-fold in standard reaction buffer, and assayed for remaining enzyme activity in the biosynthetic reaction (8). Glutamine synthetase activity, plotted vs. time, is presented as a percentage of the uninhibited control. In the absence of inhibitors, the three enzymes had similar levels of activity (releasing ∼1.5 μmol inorganic phosphate/120 min/10 mU enzyme).
Inactivation of all three glutamine synthetases was strictly dependent on the presence of ATP and magnesium ions in the incubation mix. The addition of l-glutamate to the incubation mix at a final concentration of 100 mM substantially protected the three enzymes, most likely by competing with the inhibitors for the same binding site (10, 11, 14, 17). In this regard, it is noteworthy that M. tuberculosis exports large amounts of ATP into the extracellular milieu; the detectable ATP concentration in a logarithmically growing culture is ∼150–170 μM (5). However, even in the presence of l-glutamate with very prolonged incubation (30, 60, and 120 min), the bacterial enzymes showed a significant decrease in activity: to ∼40–50% of the initial activity at 2 μM, ∼20% at 20 μM, and ∼7.5% at 200 μM l-methionine- S-sulfoximine and d,l-phosphinothricin. Under the same conditions, the sheep brain enzyme retained ∼75% of its initial activity at 2 μM, ∼50% at 20 μM, and ∼25% at 200 μM l-methionine-S-sulfoximine and d,l-phosphinothricin.
To further compare the effect of the inhibitors on bacterial and mammalian glutamine synthetase, we determined the K m and K i values for all three glutamine synthetases for l-glutamate concentrations between 2.5 and 100 mM (Table I). The K m results, ranging from 2.4 to 4.0 mM, confirmed our previous findings (5) and those described for the E. coli and sheep brain enzymes (10, 11). The K i values for M. tuberculosis and E. coli glutamine synthetases in the presence of the irreversible inhibitor l-methionine-S-sulfoximine (1.1 and 1.3 μM, respectively) were two orders of magnitude lower than the K i value for sheep brain glutamine synthetase (110.5 μM). The precision of the K i values per se was limited somewhat by the variation of ∼15% around the calculated mean value for V max in the inhibition curves. However, the data still allowed a valid comparison between the bacterial and eukaryotic enzymes and confirmed that the eukaryotic enzyme is much more tolerant to the two inhibitors than the bacterial enzymes.
Table I.
Biochemical Characteristics of M. tuberculosis, E. coli, and Sheep Brain Glutamine Synthetase
| Species | K m a l-Glu | K i b l-Ms | K i c d,l-Ppt | |||
|---|---|---|---|---|---|---|
| mM | μM | μM | ||||
| M. tuberculosis | 2.4 | 1.1 | 0.6 | |||
| E. coli | 2.7 | 1.3 | 0.8 | |||
| Sheep brain | 4.0 | 110.5 | 16.4 |
a K m determined for l-glutamate (l-Glu) in the biosynthetic reaction. b K i for l-methionine-S-sulfoximine (l-Ms) determined in the biosynthetic reaction. c K i for d,l-phosphinothricin (d,l-Ppt) determined in the biosynthetic reaction and corrected for the active l-enantiomer. K i values were determined by plotting reciprocal values for the reaction velocity versus l-glutamate concentration between 2.5 and 100 mM for all three inhibitor concentrations assayed (2, 20, and 200 μM) and expressed as K i = K m × [I]/K m − K m(app). The SD for all presented values was ≤10%.
Glutamine Synthetase Inhibitors Block the Growth of M. tuberculosis.
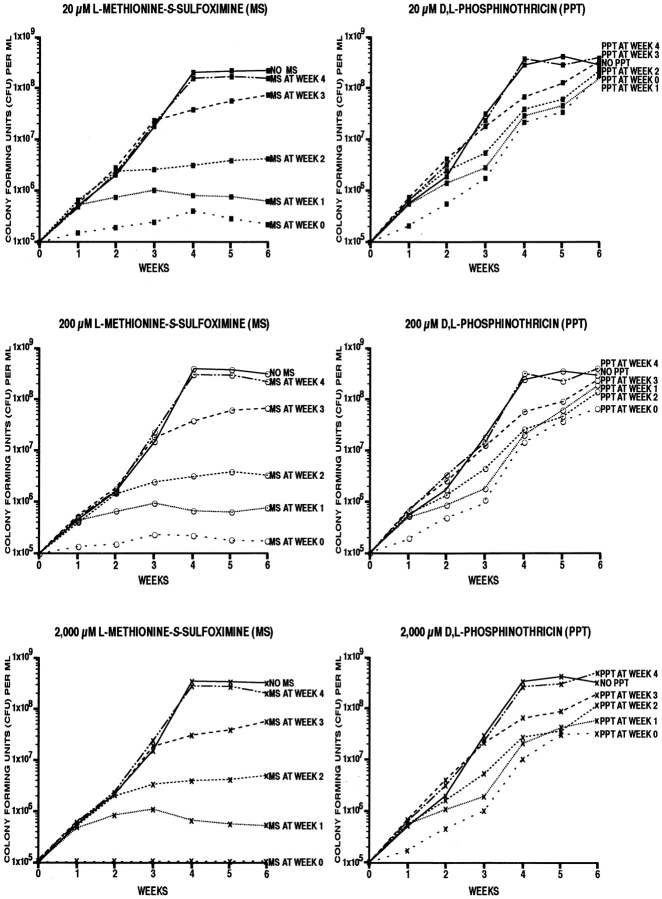
Having determined that M. tuberculosis glutamine synthetase is sensitive to the inhibitors l-methionine-S-sulfoximine and d,l-phosphinothricin, we examined the effect of the two inhibitors on the growth of M. tuberculosis. We first added the inhibitors at final concentrations of 20, 200, and 2,000 μM to broth cultures of M. tuberculosis Erdman strain (Fig. 2). The inhibitors had a profound effect on the growth of the organism. l-methionine-S-sulfoximine at all three concentrations blocked cell growth almost immediately, whether added at the time the culture was inoculated or 1–3 wk later. The effect of d,l-phosphinothricin was less pronounced and not sustained; the cell density of cultures treated with this inhibitor from the time the culture was inoculated lagged the uninhibited cell cultures by 0.5–1.5 log units. As l-methionine-S-sulfoximine had such a sustained and apparently irreversible effect on cell growth, we focused our subsequent investigations on this inhibitor.
Figure 2.
Effect of l-methionine-S-sulfoximine and d,l-phosphinothricin on the growth of M. tuberculosis Erdman in broth culture. Bacterial cultures in triplicate at an initial density of 105 bacteria/ml supplemented 7H9 medium were incubated for 6 wk at 37°C in a 5% CO2 atmosphere. The glutamine synthetase inhibitors l-methionine-S-sulfoximine and d,l-phosphinothricin were added at the times and concentrations indicated on each panel. For the enumeration of viable bacteria in each culture, aliquots were removed weekly, diluted, plated on solid medium, and incubated for 2 wk at 37°C in a 5% CO2 atmosphere.
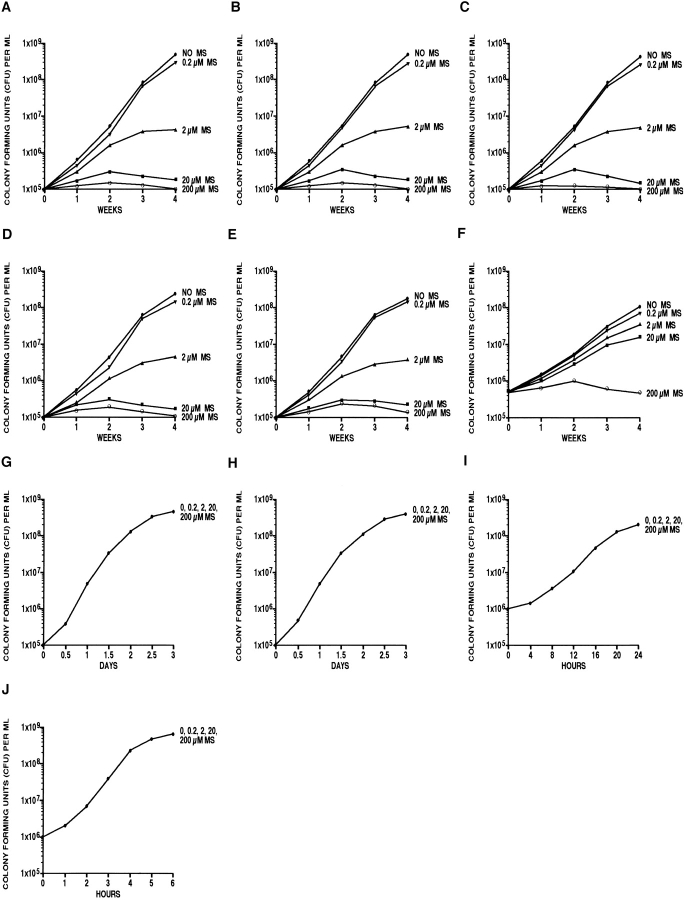
To determine the minimal inhibitory concentration of l-methionine-S-sulfoximine on M. tuberculosis cultures, we incubated the bacteria for 4 wk with 0, 0.2, 2, 20, and 200 μM of the inhibitor (Fig. 3 A). At the highest doses of inhibitor, 20 and 200 μM, growth of M. tuberculosis was completely inhibited. At 2 μM of inhibitor, growth was strongly inhibited, reaching its plateau at ∼2 log units below that of the control cultures. At 0.2 μM of inhibitor, growth was minimally inhibited, lagging the untreated control cultures by ∼0.1–0.2 log units.
Figure 3.
Effect of l-methionine-S-sulfoximine (MS) on the growth of various bacteria in broth culture. The bacterial strains indicated were inoculated into an appropriate broth at an initial cell density of 105 cells/ml, except M. avium (5 × 105 cells/ml) and L. pneumophila and E. coli (106 cells/ ml). Mycobacteria were cultured in supplemented 7H9 medium, L. pneumophila in yeast extract medium, and E. coli in Luria-Bertani medium. All bacterial cultures were grown in the presence of various concentrations of l-methionine-S-sulfoximine, as indicated, at 37°C and, except for E. coli, in a 5% CO2 atmosphere. M. tuberculosis, M. bovis, and M. avium were grown for 4 wk; M. smegmatis and M. phlei for 3 d; L. pneumophila for 24 h; and E. coli for 6 h. For the enumeration of viable bacteria in each culture, aliquots were removed weekly (M. tuberculosis, M. bovis, and M. avium), daily (M. smegmatis and M. phlei), every 4 h (L. pneumophila), or hourly (E. coli), plated on solid medium, and incubated at 37°C in a 5% CO2 atmosphere for 2 wk (M. tuberculosis, M. bovis, and M. avium), 3 d (M. smegmatis and M. phlei), 24 h (L. pneumophila), or 12 h (E. coli). All cultures were in duplicate. For each time point, the variation in the colony count averaged ∼5% and never exceeded 10%. (A) M. tuberculosis Erdman; (B) M. tuberculosis H37Rv; (C) M. tuberculosis H37Ra; (D) M. bovis strain 19210; (E) M. bovis strain BCG; (F) M. avium strain 25291; (G) M. smegmatis strain 1-2c; (H) M. phlei strain 11758; (I) L. pneumophila Philadelphia 1; (J) E. coli DH5α.
The results of the studies presented thus far suggested that the inhibitory effect of l-methionine-S-sulfoximine on M. tuberculosis growth may be due, at least in part, to the inhibitor's effect on the bacterial glutamine synthetase. Studies in which the racemic inhibitors d,l-methionine- S,R-sulfoximine or l-glutamine were added to M. tuberculosis broth cultures were consistent with this hypothesis. Of the four racemic forms of the inhibitor, only l-methionine- S-sulfoximine is active against glutamine synthetase (14). A comparison of the inhibitory effect on M. tuberculosis growth of l-methionine-S-sulfoximine and the racemate d,l-methionine-S,R-sulfoximine showed that 4–5 times higher concentrations of the racemate were required to achieve the same growth-inhibitory effect as l-methionine-S-sulfoximine at concentrations of 2, 20, and 200 μM. The addition of l-glutamine at concentrations 10–100-fold greater than l-methionine-S-sulfoximine reversed the bacteriostatic effect of the inhibitor. At inhibitor concentrations of 0.2 and 2 μM, the reversal of growth inhibition was almost complete, but at inhibitor concentrations of 20 and 200 μM, bacterial growth was only partially restored, lagging uninhibited cultures by 0.4–1.3 log units.
The Glutamine Synthetase Inhibitor l-Methionine-S-Sulfoximine Selectively Blocks the Growth of Bacteria that Export Glutamine Synthetase.
Our finding that the glutamine synthetase inhibitor l-methionine-S-sulfoximine strongly inhibits growth of M. tuberculosis, a pathogenic mycobacterium that releases large quantities of glutamine synthetase extracellularly, suggested the possibility that bacterial sensitivity to the inhibitor is correlated with enzyme release. We first tested this hypothesis by studying the inhibitory effect of l-methionine-S-sulfoximine on the growth of several other mycobacterial strains and subsequently extended the studies to include nonmycobacterial strains as well (Fig. 3). Consistent with this hypothesis, all pathogenic mycobacteria studied, including M. tuberculosis Erdman and H37Rv, M. bovis, and M. bovis BCG, all of which release large amounts of glutamine synthetase extracellularly, were highly sensitive to l-methionine-S-sulfoximine, whereas all nonpathogenic mycobacteria studied, including M. smegmatis and M. phlei, and the nonmycobacterial species L. pneumophila Philadelphia 1 and E. coli DH5α, none of which release glutamine synthetase extracellularly, were insensitive to the inhibitor (Fig. 3). For the pathogenic mycobacteria, the patterns of growth inhibition were very similar to those of M. tuberculosis. M. avium, an avian mycobacterium that is an opportunistic pathogen for immunocompromised but not immunocompetent humans, and that releases small quantities of glutamine synthetase extracellularly, was intermediate between the highly pathogenic and nonpathogenic mycobacteria in its sensitivity to l-methionine-S-sulfoximine (Fig. 3 F).
Extracellular Glutamine Synthetase Is a Prime Target of the Inhibitor l-Methionine-S-Sulfoximine.
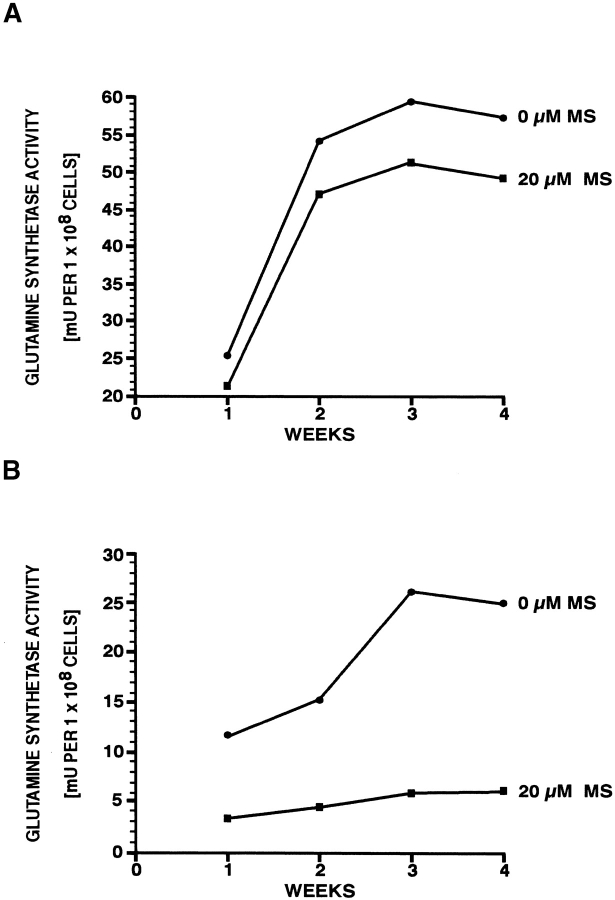
The experiments described above did not reveal whether the growth-inhibitory effect of l-methionine-S-sulfoximine on M. tuberculosis was correlated with inhibition of intracellular or extracellular glutamine synthetase or both forms of the enzyme. To determine the site of the inhibitor's effect, we incubated M. tuberculosis Erdman cultures with l-methionine-S-sulfoximine at 20 μM, a concentration that completely inhibits M. tuberculosis growth over 4 wk, and assayed the levels of intra- and extracellular glutamine synthetase activity by transfer assay at weekly intervals (Fig. 4). In the absence of inhibitor, the total detectable glutamine synthetase activity was 58 mU of cell-associated vs. 23 mU of extracellular enzyme activity per 108 cells, a ratio of ∼2.5:1, nearly identical to our previously reported results (64 mU of cell-associated vs. 29 mU of extracellular enzyme activity per 108 cells, a ratio of 2.2:1 [5]). In the presence of inhibitor, the level of cell-associated glutamine synthetase activity was minimally affected; activity plateaued at ∼50 mU, a 14% decrease from the uninhibited level (Fig. 4 A). In contrast, the level of extracellular glutamine synthetase activity was reduced from the uninhibited level by 80%, from ∼23 mU to ∼4.5 mU after 4 wk of growth (Fig. 4 B).
Figure 4.
Effect of l-methionine-S-sulfoximine (MS) on intra- (A) and extracellular (B) glutamine synthetase activity of cultures of M. tuberculosis Erdman strain. Standard broth cultures of M. tuberculosis at an initial density of 105 bacteria/ml supplemented 7H9 medium, in triplicate, were incubated in the presence or absence of the glutamine synthetase inhibitor l-methionine-S-sulfoximine for 4 wk at 37°C in a 5% CO2 atmosphere. Aliquots of the cultures, containing at least 108 bacteria to allow for reliable enzyme activity assays, were removed weekly and separated into a cell pellet and cell supernatant fraction. Bacterial cell extracts were obtained by treating the pellet with lysozyme/Triton X-100 and crystalline alumina beads (Fisher Scientific). Glutamine synthetase activity was determined in both fractions by transfer assay (8) and expressed in mU/108 cells. For each point, the maximum variation was ≤15%.
l-methionine-S-sulfoximine reduced the activity of extracellular glutamine synthetase but had no detectable effect on the quantity of glutamine synthetase protein exported by M. tuberculosis. Coomassie blue–stained polyacrylamide gels showed no reduction in the amount of extracellular or cell-associated glutamine synthetase in the presence of inhibitor (data not shown). To confirm that l-methionine- S-sulfoximine did not have a general effect on protein export, we analyzed its effect on the export and activity of another large, multimeric, leaderless protein, M. tuberculosis superoxide dismutase (18). Mycobacterial cultures treated with 20 μM l-methionine-S-sulfoximine exhibited no reduction in activity or quantity of extracellular superoxide dismutase compared with untreated cultures, as calculated on a per-cell basis. Moreover, on Coomassie blue–stained polyacrylamide gels, none of the 12 major M. tuberculosis extracellular proteins were reduced in the culture supernate in the presence of inhibitor (data not shown).
l-methionine-S-sulfoximine also selectively inhibited extracellular glutamine synthetase activity of a recombinant strain of M. smegmatis harboring a plasmid that allowed the expression and export of recombinant M. tuberculosis glutamine synthetase. In the absence of inhibitor, export of endogenous glutamine synthetase by the parent and recombinant M. smegmatis strain was near the level of detection (<1 mU), whereas export of M. tuberculosis glutamine synthetase by the recombinant strain was 69 mU/108 cells; the recombinant and endogenous glutamine synthetases were readily differentiated by NH2-terminal amino acid analysis. In the presence of 200 μM l-methionine-S-sulfoximine, the level of extracellular glutamine synthetase activity of the recombinant strain decreased 89%, whereas the level of cell-associated glutamine synthetase activity (sum of endogenous and recombinant enzymes) decreased only 17%; the growth rate of the parent and recombinant M. smegmatis strain was unaffected by the inhibitor.
Inhibitory Effect of l-Methionine-S-Sulfoximine on Mycobacterial Growth in Human Monocytes.
Before studying the effect of the glutamine synthetase inhibitor on M. tuberculosis growing intracellularly in human monocytes, we first evaluated its effect on uninfected host cells. These studies showed that l-methionine-S-sulfoximine at concentrations of 20 and 200 μM had no effect on the morphology, doubling time, or viability of differentiated THP-1 human monocytes. This result was consistent with the relative insensitivity of eukaryotic glutamine synthetases to inhibitors, as demonstrated for sheep brain glutamine synthetase (Table I).
We next analyzed the effect of l-methionine-S-sulfoximine on two different mycobacterial species, M. tuberculosis Erdman and M. avium (25291; ATCC), growing intracellularly in THP-1 cells (Fig. 5, A and B). The enumeration of viable intracellular mycobacteria during 5 d of culture showed that, at 200 μM l-methionine-S-sulfoximine, growth of the bacteria was strongly inhibited. However, little if any killing of bacteria occurred. At 2 μM of inhibitor, growth of M. tuberculosis was slowed, but growth of M. avium was uninhibited. The greater sensitivity to l-methionine-S-sulfoximine in human monocytes of M. tuberculosis compared with M. avium parallels the greater sensitivity to this inhibitor under cell-free growth conditions of M. tuberculosis compared with M. avium. This pattern of sensitivity in turn correlates with the finding that M. tuberculosis releases ∼3–4-fold more glutamine synthetase extracellularly than M. avium.
Figure 5.
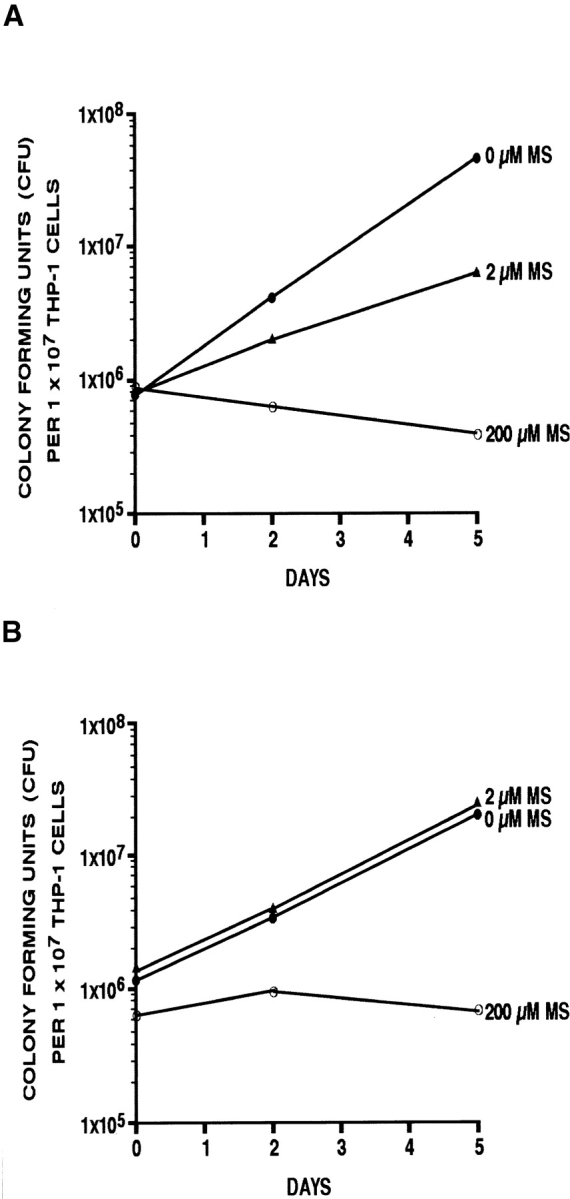
Effect of l-methionine-S-sulfoximine (MS) on mycobacterial growth in human monocytes. THP-1 cells were grown in RPMI 1640 (Irvine Scientific) and differentiated just before the formation of a confluent monolayer. The monocytes were washed, bovine serum was replaced with human serum (Irvine Scientific), and the monolayers were infected with M. tuberculosis Erdman (A) or M. avium 25291 (B) at a multiplicity of infection of 1. Infection was allowed to proceed for 90 min, after which cells were washed thoroughly and incubated under standard conditions for the next 3 h. At that time point, one set of replicate cultures was lysed and plated on agar medium to establish an initial intracellular bacterial count. All other replicate cultures were incubated further for 2 or 5 d in the presence of 0, 2, or 200 μM l-methionine-S-sulfoximine before monocytes were lysed and viable intracellular mycobacteria enumerated. The standard deviation for triplicate cultures was always ≤15%.
l-Methionine-S-Sulfoximine Treatment Decreases the Amount of Poly-l-Glutamate/Glutamine in the M. tuberculosis Cell Wall but Does Not Affect the Peptidoglycan Chain.
Pathogenic, but not nonpathogenic, mycobacteria release abundant amounts of glutamine synthetase and contain a large amount of poly-l-glutamate/glutamine in the bacterial cell wall. The high correlation between glutamine synthetase export and the presence of poly-l-glutamate/glutamine in the bacterial cell wall suggests the possibility that extracellular glutamine synthetase is involved in construction of the poly-l-glutamate/glutamine cell wall structure. If this is true, then inhibition of extracellular glutamine synthetase might reduce the amount of this structure. We investigated this hypothesis by assaying the amount of this cell wall structure in M. tuberculosis organisms grown in the presence of various amounts of the glutamine synthetase inhibitor l-methionine-S-sulfoximine. The mycobacteria were grown for 2 wk without inhibitor (to insure a sufficient number of cells for analysis) and then for 4 wk in the presence of 0, 0.2, 2, 20, and 200 μM inhibitor, after which the amount of the poly- l-glutamate/glutamine structure in the cell walls was analyzed (6). The purity of the isolated polymer ranged from 90 to 95%. The inhibitor caused a marked reduction in the amount of poly-l-glutamate/glutamine, which decreased in a dose-dependent fashion from 46 μg/1010 cells in the absence of inhibitor to 3.2 μg/1010 cells in the presence of 200 μM inhibitor (Table II). Because the isolation procedure results in the hydrolysis of glutamine, we could not determine the exact ratio of glutamate/glutamine residues. For comparison, we studied the effect of the inhibitor on the amount of alanine in the peptidoglycan fraction, which contains one d- and one l-alanine per peptidoglycan monomer. The purified (>90%) peptidoglycan fraction contained from 21 μg d,l-alanine/1010 cells at 0 μM inhibitor to 16.6 μg at 200 μM of inhibitor. Thus, in contrast to the pronounced effect of the inhibitor on the amount of poly- l-glutamate/glutamine in the cell wall, the inhibitor had only a minimal effect on the amount of alanine in the cell wall.
Table II.
Comparison of the Effect of the Glutamine Synthetase Inhibitor l-Methionine-S-Sulfoximine and Two Standard Antibiotics on the M. tuberculosis Cell Wall Components Poly-l-Glutamate/Glutamine and Peptidoglycan
| l-Methionine-S-Sulfoximine | Amikacin | Rifampin | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μM | μg/ml | μg/ml | ||||||||||||||||||||
| Inhibitor/antibiotic concentration | 0 | 0.2 | 2 | 20 | 200 | 0.06 | 0.25 | 1 | 0.0125 | 0.05 | 0.2 | |||||||||||
| Poly-l-Glutamate/glutamine (μg/1010 Cells) | 46 | 18.2 | 9.8 | 6.1 | 3.2 | 49.9 | 43.2 | 26.0a | 54.2 | 41.6 | 40.0a | |||||||||||
| d,l-Alanine (μg/1010 Cells) | 21 | 21.2 | 17.7 | 17.8 | 16.6 | 29.1 | 26.3 | 12.0a | 23.6 | 25.1 | 20.0a | |||||||||||
aAt high concentrations, amikacin (1 μg/ml) and rifampin (0.2 μg/ml) were lytic for M. tuberculosis. Consequently, the values shown for poly- l-glutamate/glutamine and d,l-alanine at these concentrations of antibiotic are calculated on the basis of <1–5 × 107 cells for 1 μg/ml amikacin and 107 cells for 0.2 μg/ml rifampin. The SD for all presented values was ≤15%.
To confirm that the effect of l-methionine-S-sulfoximine on the poly-l-glutamate/glutamine content of the mycobacterial cell wall was not a nonspecific consequence of inhibition of bacterial growth, we studied the effect of two bactericidal antibiotics, amikacin and rifampin, on the mycobacterial cell wall. As shown in Table II, these antibiotics had little influence on the content of either poly-l-glutamate/ glutamine or d,l-alanine, confirming that their mode of action is quite different from that of l-methionine-S-sulfoximine and that they do not target, directly or indirectly, these two structures in the mycobacterial cell wall.
The Glutamine Synthetase Inhibitor l-Methionine-S-Sulfoximine Acts Synergistically with Several Conventional Antibiotics Used in the Treatment of Tuberculosis.
The finding that l-methionine-S-sulfoximine markedly reduces the amount of a cell wall structure that comprises 8–10% of the M. tuberculosis cell wall suggested the possibility that the glutamine synthetase inhibitor affects the integrity of the cell wall. To investigate this idea, we studied the influence of the inhibitor on the sensitivity of M. tuberculosis to conventional antibiotics. We cultured M. tuberculosis in the presence of subinhibitory concentrations of l-methionine-S-sulfoximine (0.02, 0.2, or 2 μM) and subinhibitory concentrations of each of four antibiotics: isoniazid, rifampin, pyrazinamide, or ethambutol. In all cases, bacterial growth in the presence of the combination of l-methionine-S-sulfoximine and antibiotic was significantly less than in the presence of either inhibitor alone. The most pronounced effect on bacterial growth was observed for isoniazid (Fig. 6 A) and rifampin (Fig. 6 B) at one-tenth their minimal inhibitory concentrations in combination with 0.2 μM l-methionine-S-sulfoximine. The antibiotics had a negligible effect on intracellular or extracellular glutamine synthetase activity. This result is consistent with the hypothesis that the inhibitory effect of l-methionine- S-sulfoximine on the extracellular glutamine synthetase affects the integrity of the M. tuberculosis cell wall so as to allow antibiotics greater access to the bacterial cytoplasm.
Figure 6.
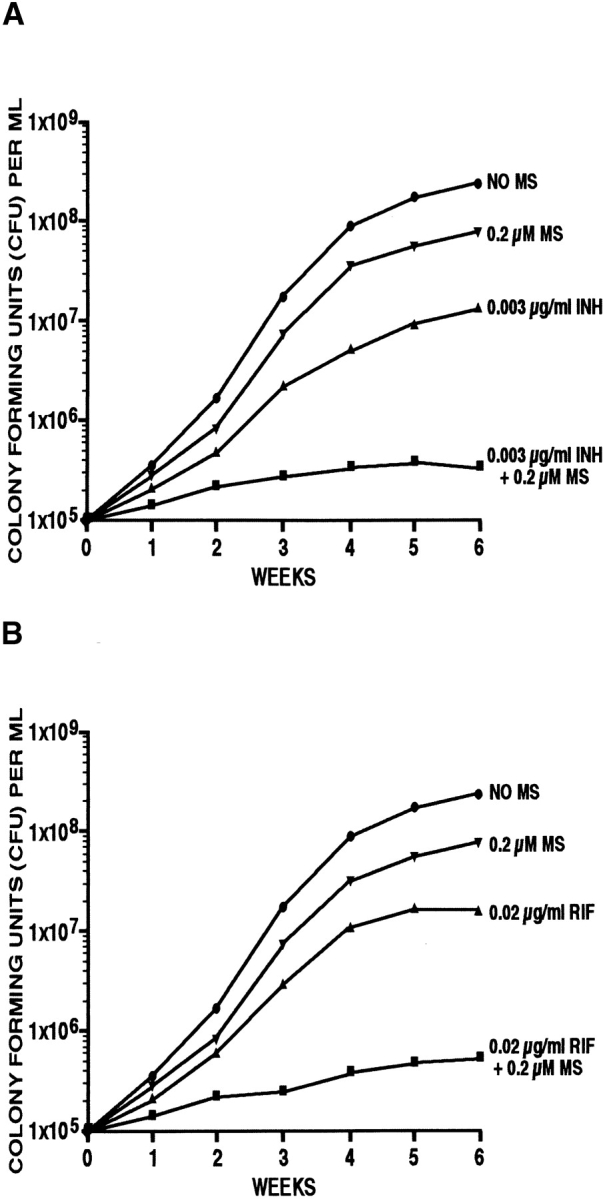
Influence of l-methionine-S-sulfoximine (MS) on the growth of M. tuberculosis in the presence of subinhibitory concentrations of the antibiotics (A) isoniazid (INH) and (B) rifampin (RIF). M. tuberculosis Erdman cultures at an initial density of 105 cells/ml medium were incubated at 37°C in a 5% CO2 atmosphere for 6 wk in the presence or absence of either isoniazid or rifampin and/or the glutamine synthetase inhibitor l-methionine- S-sulfoximine. The chosen concentrations of the antibiotics and the enzyme inhibitor corresponded to approximately one-tenth of their minimal inhibitory concentrations. For the enumeration of viable bacteria in each culture, aliquots were removed weekly, diluted, plated on solid medium, and incubated for 2 wk at 37°C in a 5% CO2 atmosphere in the absence of any inhibitor. The SD for triplicate cultures was always ≤15%.
Discussion
Our study demonstrates that inhibitors of extracellular glutamine synthetase block the growth of M. tuberculosis and other pathogenic mycobacteria. Remarkably, the inhibitors are selective for pathogenic mycobacteria, which export glutamine synthetase and contain the poly-l-glutamate/glutamine cell wall structure. The correlation between export of glutamine synthetase and the presence of this cell wall structure revealed in our previous study led us to hypothesize that exported glutamine synthetase is involved in the synthesis of this major cell wall component, the function of which remains elusive. The study here, demonstrating that inhibition of extracellular glutamine synthetase is associated with a marked diminution in the cell wall structure, lends further support to this hypothesis. That this in turn is correlated with bacteriostasis suggests that the poly-l-glutamate/glutamine structure plays an important role in the homeostasis of pathogenic mycobacteria.
Extracellular glutamine synthetase is clearly a prime target of the irreversible glutamine synthetase inhibitor l-methionine-S-sulfoximine. The inhibitor reduced glutamine synthetase activity in the extracellular milieu of M. tuberculosis cultures by 80% but had little effect on cell-associated glutamine synthetase. Indeed, given the formidable barrier presented by the lipid-rich cell wall of M. tuberculosis, the small amount of cell-associated enzyme that is affected by l-methionine-S-sulfoximine may be an enzyme that has in fact cleared the bacterial cell membrane but not yet been released by the bacterium.
That the glutamine synthetase inhibitor l-methionine- S-sulfoximine selectively blocks the growth of mycobacteria that export a large proportion of their glutamine synthetase suggests that inhibition of glutamine synthetase may be directly responsible for the bacteriostatic effect of the inhibitor. However, inactivation of additional extracellular targets by l-methionine-S-sulfoximine, such as enzymes involved in the synthesis and cell wall anchoring of the heteropolymer poly-l-glutamate/glutamine, may contribute to the observed bacteriostatic effect of this inhibitor.
In addition to inhibiting glutamine synthetase, l-methionine-S-sulfoximine inhibits γ-glutamylcysteine synthetase, which catalyzes a chemical reaction very similar to the one catalyzed by glutamine synthetase. However, the inhibitor's effect on this enzyme is reversible, in contrast to the case with glutamine synthetase (19). Whether this typically intracellular enzyme is exported by M. tuberculosis is not known. However, if it is, the amount exported must be very small compared with glutamine synthetase as, unlike glutamine synthetase, γ-glutamylcysteine synthetase is not one of the 12 most abundant proteins exported by M. tuberculosis.
New antibiotics are desperately needed to combat the rapidly emerging strains of M. tuberculosis that are resistant to conventional antibiotics. However, M. tuberculosis presents a formidable challenge to the design of antibiotics. First, the antibiotic must penetrate the host cell and reach the organism within its unique intracellular compartment, a specialized, membrane-bound phagosome (3). Second, the antibiotic must reach its molecular target, generally within the organism, in which case the thick waxy coat of the mycobacterium presents an additional major obstacle. Our study provides an approach to circumventing the second major obstacle, targeting an extracellular enzyme crucial to bacterial cell growth. Specifically, our study demonstrates that treatment of M. tuberculosis with a drug that inactivates extracellular glutamine synthetase inhibits mycobacterial growth. Hence, drugs functionally analogous to l-methionine-S-sulfoximine, but perhaps with even greater specificity for the M. tuberculosis enzyme relative to the mammalian enzyme, have great potential as antibiotics against this pathogen. Our demonstration that l-methionine-S-sulfoximine inhibits M. tuberculosis growing within its host cell, the human mononuclear phagocyte, underscores the feasibility of this approach.
More generally, our study suggests that other extracellular enzymes of M. tuberculosis and other pathogenic mycobacteria, and even nonmycobacterial pathogens, may serve as readily accessible targets of antibiotics. In this regard, an obvious mycobacterial target is the 30/32-kD protein complex, the most abundant proteins released by M. tuberculosis (2, 20). These unique extracellular enzymes of pathogenic mycobacteria, which have been reported to have mycolyl transferase activity (21), presumably serve an essential role in microbial physiology and pathogenicity and represent highly specific targets for antibiotics.
Acknowledgments
We thank Barbara Jane Dillon for expert technical assistance and Bai-Yu Lee for advice with THP-1 cell cultures. We also thank David Eisenberg for sharing his knowledge on the structure and function of glutamine synthetases with us and for his encouragement of these studies.
This work was supported by grants AI 31338 and AI 42925 from the National Institutes of Health.
References
- 1.Pablo-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, Cohn DL, Lambregts van Weezenbeek CSB, Kim SJ, Chaulet P, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg MG, Belisle JT. Definition of Mycobacterium tuberculosisculture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosisphagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of . Mycobacterium tuberculosis Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harth G, Clemens DL, Horwitz MA. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci USA. 1994;91:9342–9346. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wietzerbin J, Lederer F, Petit J-F. Structural study of the poly-l-glutamic acid of the cell wall of Mycobacterium tuberculosis var. hominis, strain brévannes. Biochem Biophys Res Commun. 1975;62:246–252. doi: 10.1016/s0006-291x(75)80130-6. [DOI] [PubMed] [Google Scholar]
- 7.Hirschfield GR, McNeil M, Brennan PJ. Peptidoglycan-associated polypeptides of . Mycobacterium tuberculosis J Bacteriol. 1990;172:1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolfolk CA, Shapiro B, Stadtman ER. Regulation of glutamine synthetase. Purification and properties of glutamine synthetase from Escherichia coli. . Arch Biochem Biophys. 1966;116:177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- 9.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, et al. Deciphering the biology of Mycobacterium tuberculosisfrom the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 10.Logusch EW, Walker DM, McDonald JF, Franz JE. Substrate variability as a factor in enzyme inhibitor design: inhibition of bovine brain glutamine synthetase by α- and γ-substituted phosphinothricins. Biochemistry. 1989;28:3043–3051. doi: 10.1021/bi00433a046. [DOI] [PubMed] [Google Scholar]
- 11.Logusch EW, Walker DM, McDonald JF, Franz JE, Villafranca JJ, DiIanni CL, Colanduoni JA, Li B, Schineller JB. Inhibition of Escherichia coliglutamine synthetase by α- and γ-substituted phosphinothricins. Biochemistry. 1990;29:366–372. doi: 10.1021/bi00454a009. [DOI] [PubMed] [Google Scholar]
- 12.Harth G, Horwitz MA. Expression and efficient export of enzymatically active Mycobacterium tuberculosis glutamine synthetase in Mycobacterium smegmatisand evidence that the information for export is contained within the protein. J Biol Chem. 1997;272:22728–22735. doi: 10.1074/jbc.272.36.22728. [DOI] [PubMed] [Google Scholar]
- 13.Lee B-Y, Horwitz MA. Identification of macrophage and stress-induced proteins of . Mycobacterium tuberculosis J Clin Invest. 1995;96:245–249. doi: 10.1172/JCI118028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning JM, Moore S, Rowe WB, Meister A. Identification of l-methionine-S-sulfoximine as the diastereomer of l-methionine-S,R-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969;8:2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- 15.Nakano Y, Tanaka E, Kato C, Kimura K, Horikoshi K. The complete nucleotide sequence of the glutamine synthetase gene (glnA) of . Bacillus subtilis FEMS Microbiol Lett. 1989;57:81–86. doi: 10.1016/0378-1097(89)90151-1. [DOI] [PubMed] [Google Scholar]
- 16.Almassy RJ, Janson CA, Hamlin R, Xuong N-H, Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. Nature. 1986;323:304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- 17.Liaw S-H, Pan C, Eisenberg D. Feedback inhibition of fully unadenylylated glutamine synthetase from Salmonella typhimuriumby glycine, alanine, and serine. Proc Natl Acad Sci USA. 1993;90:4996–5000. doi: 10.1073/pnas.90.11.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harth G, Horwitz MA. Export of recombinant M. tuberculosissuperoxide dismutase is dependent upon both information in the protein and mycobacterial export machinery. A model for studying export of leaderless proteins by pathogenic mycobacteria. J Biol Chem. 1999;274:4281–4292. doi: 10.1074/jbc.274.7.4281. [DOI] [PubMed] [Google Scholar]
- 19.Richman PG, Orlowski M, Meister A. Inhibition of γ-glutamylcysteine synthetase by l-methionine-S-sulfoximine. J Biol Chem. 1973;248:6684–6690. [PubMed] [Google Scholar]
- 20.Harth G, Lee B-Y, Wang J, Clemens DL, Horwitz MA. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of . Mycobacterium tuberculosis Infect Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. S. cience. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]