Abstract
During HIV/SIV infection, there is widespread programmed cell death in infected and, perhaps more importantly, uninfected cells. Much of this apoptosis is mediated by Fas–Fas ligand (FasL) interactions. Previously we demonstrated in macaques that induction of FasL expression and apoptotic cell death of both CD4+ and CD8+ T cells by SIV is dependent on a functional nef gene. However, the molecular mechanism whereby HIV-1 induces the expression of FasL remained poorly understood. Here we report a direct association of HIV-1 Nef with the ζ chain of the T cell receptor (TCR) complex and the requirement of both proteins for HIV-mediated upregulation of FasL. Expression of FasL through Nef depended upon the integrity of the immunoreceptor tyrosine-based activation motifs (ITAMs) of the TCR ζ chain. Conformation for the importance of ζ for Nef-mediated signaling in T cells came from an independent finding. A single ITAM motif of ζ but not CD3ε was both required and sufficient to promote activation and binding of the Nef-associated kinase (NAK/p62). Our data imply that Nef can form a signaling complex with the TCR, which bypasses the requirement of antigen to initiate T cell activation and subsequently upregulation of FasL expression. Thus, our study may provide critical insights into the molecular mechanism whereby the HIV-1 accessory protein Nef contributes to the pathogenesis of HIV.
Keywords: Jurkat, immunoreceptor tyrosine-based activation motif, Nef-associated kinase, activation-induced cell death, apoptosis
In HIV/simian immunodeficiency virus (SIV)1 infection, the nef gene plays a key role in viral replication and progression of disease. This is based on studies in macaques and humans, who remain asymptomatic or long-term nonprogressing when infected with an SIV mutant lacking a nef gene or HIV with multiple nef deletions, respectively (1–3). More recently, a study using a transgenic mouse model has demonstrated that Nef harbors a major determinant for HIV-induced pathogenicity (4). Despite the considerable importance of Nef for HIV/SIV pathogenesis, its function at the molecular level is poorly understood. At least three in vitro effects of Nef have been described. Nef downregulates the surface receptors CD4 and MHC I (5, 6), increases viral infectivity (7), and stimulates T cell signaling pathways (8–10).
The major consequence of HIV infection is the depletion of T cells leading to immunoparesis characteristic of AIDS. This is likely due to the widespread programmed cell death (apoptosis) induced by HIV (11–13). Several different apoptotic pathways have been proposed in HIV infection, including Fas/FasL (14), TNF/TNFR (15), and an interaction between TNF-related apoptosis-inducing ligand (TRAIL) and its receptors (16). In HIV-infected patients, there is upregulation of both Fas and FasL as well as an increased susceptibility of CD4+ and CD8+ cells to Fas-mediated killing (17–21). Recently, we have demonstrated in macaques that induction of FasL expression and apoptotic cell death of both CD4+ and CD8+ T cells by SIV is dependent on a functional nef gene (22). However, a molecular mechanism integrating this observation into other documented effects of Nef is lacking.
The concept of Nef interfering with early events emanating from the TCR could explain its dual effects on T cell activation and FasL expression, since both functions are regulated by the TCR complex (23–25). Here we report that HIV-mediated upregulation of FasL in T cells is dependent on the association of Nef with the TCR ζ chain. By demonstrating that Nef directly targets the TCR of the infected cell, we provide novel insight into the molecular function of Nef in HIV infection.
Materials and Methods
Cell Lines and Antibodies.
Generation of Jurkat cell lines constitutively expressing CD8 tag or CD8-Nef chimeras was described recently (8, 26). Jurkat, J.CaM.1 (Lck−), J.45.01 (CD45−), and J.RT3-T3.5 (TCR−) cells were provided by Arthur Weiss (University of California, San Francisco, CA [27]). J.RT3-T3.5 cells expressing various CD16ζ constructs or coexpressing CD8-Nef were generated by electroporation using puromycin and neomycin for selection. Stable clones were enriched for protein expression by magnetic anti-CD16/anti-CD8 beads. The mAbs against the AU-5/ AU-1 epitope and FasL (NOK-1) were purchased from Hiss Diagnostics and PharMingen.
Plasmid Constructions.
Generation of the CD8-Nef (SF2) and CD16ζ/ε chimeras as well as COOH-terminal–tagged Nef (AU-1) was described previously (8, 26, 28). Fusion proteins between CD16 and individual ζ ITAMs (ITAM 1, amino acids [aa] 1–70; ITAM 2, aa 70–110; ITAM 3, aa 110–141) were generated as described previously (28). The mutations in CD16ζ as well as in Nef/CN.94 were generated by a two-step PCR procedure and cloned into the pRcCMV expression vector (Invitrogen). In CD16ζmu, the tyrosine residues in three ITAM motifs (two tyrosine residues in each ITAM) were mutated to alanines. In CN.94PXmu/Nef.PXmu, the FPVR motif of Nef (aa 72–75) was mutated to VRIT. Construction of the proviral clone NL4-3, containing the SF2 nef gene (NL4-3.SF2Nef), as well as the Nef-negative construct (NL4-3ΔNef), was described previously (26). For the generation of recombinant baculoviruses, the “bac to bac” system was used (Bio-Rad Laboratories).
Protein Expression Assays.
Transfections into 293T cells, metabolic labeling with 35S-Translabel, immunoprecipitation, Western blot, and in vitro kinase assays were performed as described previously (8, 26). The immunoprecipitates were washed three times (wash buffer: 1% NP-40, 450 mM NaCl, 50 mM Tris-HCl [pH 8], 1 mM EDTA). To show an interaction between Nef and ζ, extraction and washing buffers contained 1% Brij instead of 1% NP-40. FasL promoter activity was tested as described previously (29) by cotransfection of pFasL-Luc (provided by Xiangdong Liu, Department of Virus and Cancer, Aarhus, Denmark) with CD8-Nef constructs or pCTax as positive control (provided by Ralph Grassman, Institute of Virology, Erlangen, Germany). All transfections were performed in duplicate by mixing 6 μl of liposome reagent (DMRIE-C; GIBCO BRL) and 2 μg of each plasmid for 2 × 106 Jurkat or Jurkat mutant cells.
In Vitro HIV Infection of Jurkat or Jurkat Mutant Cell Lines.
Cells (5 × 106) were superinfected with 1 ml of HIV IIIB (1.6 × 104 cpm/ml, reverse transcriptase [RT] activity), NL4-3.SF2.Nef (2.4 × 104 cpm/ml, RT activity), or NL4-3ΔNef (2.5 × 104 cpm/ml, RT activity) for 2 h. After infection, cells were washed and adjusted to a concentration of 106/ml and incubated for an additional 48 h. Cell culture supernatants were collected on day 5 for analysis of p24 by ELISA or RT activity by Quan-T-RT kit (Amersham Pharmacia Biotech).
Analysis of FasL Expression by Flow Cytometry and Immunoprecipitation.
To assess cell surface FasL expression on HIV-infected cells or transiently transfected Jurkat TAg cells, the metalloprotease inhibitor BB2116 (British Biotech [30]) was added to the medium 4–6 h before the assay to enhance cell surface FasL expression. In brief, cells were stained with 20 μl of biotin-conjugated anti–human FasL mAb (NOK-1; PharMingen) followed by 5 μl of PE-conjugated streptavidin (Sigma). Labeled cells were analyzed on a FACScan™ (Becton Dickinson). Isotype-specific mAbs of irrelevant specificity were used as negative controls (Dako Diagnostics). To assess expression of whole FasL protein, 35S-labeled cells (5 × 106) were immunopreciptatied for FasL using anti-FasL–specific mAb (NOK-1) as described previously (30).
Results
Requirement of the TCR ζ Chain for Binding of Nef-associated Kinase (p62) to Nef.
As shown previously, Nef associates with a serine kinase, termed p62 or Nef-associated kinase (NAK [31]). The Nef–NAK interaction is complex: Nef stimulates the phosphorylation/activation of NAK, and it is only in this activated form that NAK can bind Nef (32). This suggests that Nef must act upstream of NAK to promote NAK activation. Our previous results showing that Nef interfered with early signals emanating from the TCR suggested it may interact with a component of the TCR signaling complex. This prompted us to study Nef-mediated NAK/p62 activation in cell lines with TCR signaling defects. CD8-Nef chimeras (CD8-Nef), containing the extracellular domain of CD8α fused to Nef, were stably transfected into wild-type Jurkat and a variety of Jurkat mutant cell lines lacking either Lck (J.CaM.1), CD45 (CD45−), or the entire TCR signaling complex (RT3.T3.5). Expression of CD8-Nef in these cell lines was verified by metabolic labeling and immunoprecipitation (Fig. 1 B). The Nef chimeras from these transfectants were immunoprecipitated and subjected to an in vitro kinase assay. NAK/p62 association was observed in all cell lines except the TCR− cells (Fig. 1 A). The latter result was confirmed in a second, independently transfected cell clone (data not shown).
Figure 1.
Requirement of the TCR ζ chain for binding of NAK/p62 to Nef. (A) In vitro kinase assay after immunoprecipitation (IP) of CD8-Nef chimeras using the CD8 tag from stably transfected wild-type and mutant Jurkat cell lines lacking Lck, TCR, or CD45. Lane 1, control (Cont.) Jurkat transfected with the CD8 tag. (B) Control immunoprecipitation showing expression of 35S-labeled CD8-Nef. (C) In vitro kinase assay after immunoprecipitation of CD8-Nef to study NAK/p62 association/phosphorylation in wild-type (Cont., lane 1) and TCR− Jurkat cell lines (lane 2), and after coexpression (stable transfection) of CD8-Nef with either CD16ε, CD16ζ2 (ITAM 2), CD16ζ3 (ITAM 3), or CD16ζmu (mutation of all three ITAMs) in the TCR− cell line (lanes 3–6). (D) Control immunoprecipitation of 35S-labeled CD8-Nef from cell lines shown in C. (E) In vitro kinase assay after immunoprecipitation of AU-1–tagged Nef from transiently transfected 293T cells. Lane 1, transfection with Nef alone; lanes 2–7, cotransfection with increasing amounts (0.5 and 1 μg) of CD16ε, CD16ζ, or CD16ζmu. (F) The nitrocellulose filter shown in E was blotted (WB) with an anti-Nef (AU-1) antibody to verify comparable Nef expression.
Next we asked whether NAK binding could be restored in cells lacking the TCR complex by stable transfection with TCR-ζ or CD3ε fused to the extracellular domain of CD16 (CD16ζ and CD16ε). These TCR subcomponents contain signaling motifs (immunoreceptor tyrosine-based T cell activation motifs [ITAMs]), which are required and sufficient for T cell activation (28, 33). After obtaining single cell clones, expression of the chimeras was verified by metabolic protein labeling and FACS® analysis (data not shown). In several attempts, we were unable to coexpress CD8-Nef (CN) with CD16ζ in TCR− cells. Cell clones that were obtained either showed no detectable CN or CD16 expression or died rapidly. The effect resembled activation-induced cell death (AICD) by Nef as reported previously (8). Coexpression of CN and CD16ε was achieved; however, the obtained cell clones had a low CN as well as CD16 surface expression (Fig. 1 D, lane 3). Therefore, we constructed ζ chimeras containing the three individual ζ ITAMs in isolation (CD16ζ1, 2, or 3; see Materials and Methods for details). In a seperate construct, the tyrosine residues in all three YXXL motifs of CD16ζ were mutated to alanines (CD16ζmu). We failed to coexpress the first ζ ITAM with Nef. However, NAK/p62 binding to Nef was reestablished in the TCR− cells by coexpression with the second or third ITAM of ζ (Fig. 1 C, lanes 4 and 5). In these latter cell lines, expression of CD16ζ1 and 2 as well as CD8-Nef decreased significantly over time (data not shown), indicating that coexpression of both proteins was not favorable. The difficulties regarding the coexpression of the individual ζ ITAMs with CD8-Nef may be explained by studies published by Combadiere et al. (34) showing that in particular the first ζ ITAM but much less the second and third are capable of inducing apoptosis when activated. The signaling-defective ζ chain (CD16ζmu) expressed well, but NAK binding to Nef was greatly reduced (lane 6). No NAK/p62 binding was observed by coexpression of CD3ε (lane 3). Since NAK binding to Nef was not completely negative with CD16ζmu, the Nef–ζ complex may recruit additional signaling molecules to the plasma membrane which are important for NAK activation. Assuming that the effects of the first ζ ITAM would be similar to ITAM 2 and 3, it appeared that at least one functional ITAM of the CD3 ζ chain was required for binding of p62/NAK to Nef.
The functional link between Nef, ζ, and NAK was confirmed by transient transfection assays in a heterologous system. As shown in Fig. 1 E, cotransfection of CD16ζ (lanes 4 and 5) but not CD16ε (lanes 2 and 3) significantly increased binding of p62/NAK to Nef. A minimal increase was seen after cotransfection of CD16ζmu (lanes 6 and 7), which paralleled the small effect seen in Fig. 1 C, lane 6. Thus, no other T cell–specific components except the functional ITAM(s) from the TCR ζ chain, were required for NAK activation and NAK/Nef association in 293T cells.
Direct Association of Nef with the TCR ζ Chain.
Full-length CD8-Nef when expressed at the cell membrane promotes AICD. Upon stable transfection, cell clones are preferentially selected in which Nef is predominantly expressed in the cytoplasm, where it does not exert such a detrimental effect on cell survival. In contrast, NH2-terminal fragments of Nef are expressed at high levels at the plasma membrane where TCR-ζ is located (8). These NH2-terminal fragments can recruit a complex of proteins to form the NH2-terminal kinase complex, which binds between amino acids 20 and 35, and may contribute to T cell activation (26). The NH2 terminus also has a conserved domain containing a proline-rich motif (PxxP, aa 73–82) known to associate with SH3 domains of tyrosine kinases (35). We reasoned that these domains/motifs could interact with and bind TCR-ζ. To prove a direct interaction, coimmunoprecipitation experiments were performed using stable cell lines with different CD8-Nef chimeras (Fig. 2, A and B). The only interaction was seen with a construct expressing the NH2-terminal 94 amino acids of Nef containing the PxxP motif (lane 4). Underscoring the importance of the PxxP motif for ζ binding, we found that point mutations in the PxxP motif of the 94–amino acid Nef construct (CN.94.PXmu) almost completely abolished ζ binding (lane 5).
Figure 2.
Association of membrane-associated Nef with the TCR ζ chain. (A) Anti-Nef (CD8) immunoprecipitation, then anti-ζ Western blot (WB). (B) Anti-ζ immunoprecipitation, then anti-Nef Western blot from wild-type Jurkat cells stably transfected with CD8 tag (Cont.), and CD8-Nef chimeras containing full-length Nef expressed in the cytoplasm (CD8.Nef.cyt.), the NH2-terminal 49 amino acids of Nef (CD8-Nef.49), the NH2-terminal 94 amino acids (CD8-Nef.94), and CD8-Nef.94PXmu in which the PxxP motif has been mutated. (C) Control immunoprecipitation of CD8.Nef chimeras from 35S-labeled cells to show a comparable protein expression (*). (D) Nef–ζ association after baculovirus coinfection of Hi5 cells. Control anti-Nef Western blot (WB) after anti-Nef (AU-1) immunoprecipitation from Hi5 cells infected with wild-type Nef or Nef with a mutated PxxP motif (lanes 1 and 2). Immunoprecipitated Nef from Hi5 cells ran, in addition to monomers (Nef-m), as dimers (Nef-d) or higher order multimers (arrows), which may be important for Nef function. Hi5 cells were then coinfected with wild-type Nef and ζ fused to CD16 (CD16ζ; lanes 3–6). CD16ζ was immunoprecipitated using an AU-5 tag, and the immunoprecipitates were blotted for Nef (AU-1). Two immunoprecipitations were performed in the presence of cytoplasmic lysates from Jurkat (ζ-positive; lane 4) or TCR-ζ–negative cells (lane 5). The positions of the antibody heavy (*) and light (**) chains are indicated. (E) Control Western blot. The nitrocellulose filter shown in D was stripped and blotted with an antibody recognizing the CD16ζ construct (AU-5).
Further evidence for an interaction between Nef and ζ was obtained by coimmunoprecipitation of Nef and ζ from Hi5 insect cells coinfected with recombinant baculoviruses (Fig. 2 D). CD16ζ was found to coprecipitate with Nef (Fig. 2 D, lane 3), but not with Nef.PXmu (lane 6). To confirm the specificity of the interaction, aliquots of the anti-ζ immunoprecipitates were incubated with Jurkat (ζ-containing) or the TCR− (ζ-lacking) cytoplasmic lysates. Wild-type Jurkat competed for Nef binding (lane 4), whereas the TCR-ζ–negative cytoplasmic lysates did not (lane 5). The reduced Nef signal in lane 5 may be explained by the reduced amount of immunoprecipitated ζ (Fig. 2 E, lane 5). Additional evidence for the interaction of both proteins was obtained by coimmunoprecipitation after transient transfection into COS cells and subsequent in vitro kinase assay (data not presented).
Upregulation of FasL Expression by HIV Requires Both Intact Nef and TCR ζ Chain.
We have previously shown that the upregulation of FasL in SIV infection requires an intact nef gene (22). In general, the level of cell surface FasL expression is quite low when analyzed by FACS® even when metalloproteinase inhibitors are used which block cleavage of FasL from the cell surface. In view of this difficulty, we used additional experimental approaches to analyze Nef-mediated FasL expression (see below). Since stimulation of TCR-ζ effectively upregulates FasL expression (34, 36), we speculated that the interaction of Nef with TCR-ζ would lead to a similar effect. First, to show that HIV-Nef is required for FasL upregulation, we infected Jurkat with wild-type HIV (NL4-3.SF2Nef) or a mutant lacking the nef gene (NL4-3ΔNef) (Fig. 3). Little if any FasL is seen on cells infected with Nef-deleted HIV, thus confirming our previous results with SIV. The level of viral replication in Jurkat cells was comparable, as determined by RT activity (NL4-3.SF2Nef, 4.6 × 103 cpm; NL4-3ΔNef, 5.8 × 103 cpm). Upregulation of FasL by HIV is also lost in mutant Jurkat cells lacking the TCR complex, whereas cells reconstituted with ζ but not with the ζ mutant restored the FasL expression upon HIV infection as determined by both immunoprecipitation (Fig. 4 A) and FACS® analysis (Fig. 4 B). Viral replication assessed by p24 assay indicated that these cell lines were comparably infected (wild-type, 3.5 ± 0.5; TCR−, 3.8 ± 1.0; CD16ζ, 3.8 ± 0.5; CD16ζmu, 3.1 ± 0.25 ng/ml).
Figure 3.
Nef is required for FasL upregulation. Jurkat cells were infected with (A) wild-type HIV-1 (NL4-3.SF2Nef) or (B) SF2 lacking the nef gene (NL4-3ΔNef). After 48 h, FasL expression (solid line) was assessed by flow cytometry and compared with staining with a control mAb (dashed line). The level of viral replication in Jurkat cells was comparable as determined by RT activity (NL4-3.SF2Nef, 4.6 × 103 cpm; NL4-3ΔNef, 5.8 × 103 cpm).
Figure 4.

FasL upregulation by HIV requires the TCR ζ chain. FasL expression was assessed 48 h after infection with HIV (IIIB strain) by immunoprecipitation using an anti-FasL mAb (A) or by flow cytometry (B). Wild-type (WT) and TCR− Jurkat are compared with the TCR− cells stably transfected with CD16ζ or CD16ζmu (signaling-defective CD16ζ). The level of viral replication in Jurkat cells was comparable as determined by p24 assay (wild-type, 3.5 ± 0.5; TCR−, 3.8 ± 1.0; CD16ζ, 3.8 ± 0.5; CD16ζmu, 3.1 ± 0.25 ng/ml).
Nef Can Induce FasL Expression in the Presence of TCR-ζ.
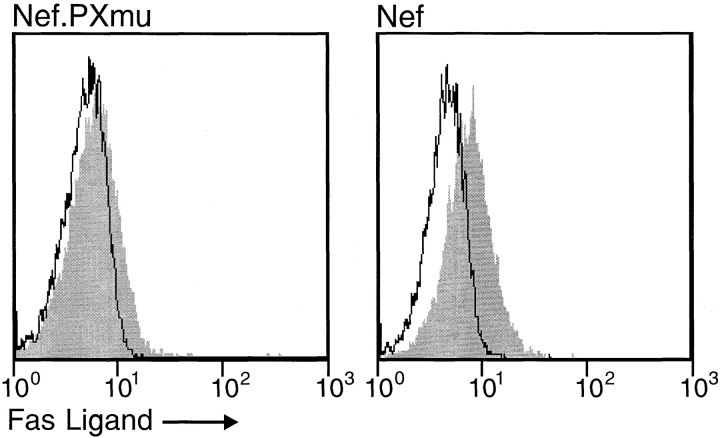
To investigate a direct upregulation of FasL by Nef, a CD8-Nef construct not capable of binding ζ (Nef.PXmu; see Fig. 2 B) and CD8-Nef were transiently expressed in Jurkat cells and analyzed for FasL upregulation. CD8-Nef but not the Nef mutant led to a significant cell surface expression of FasL (Fig. 5). Next, FasL upregulation was studied in Jurkat and TCR mutant cell lines using a FasL promotor/luciferase reporter construct. The latter has been shown to be stimulated in transient assays by the HTLV I Tax protein (29). Nef stimulated the FasL promotor in Jurkat and TCR− mutant cells reconstituted with the TCR ζ chain. No effect was seen using the Nef.PXmu construct or the TCR− Jurkat cell line (Fig. 6). These assays confirmed that a functional Nef protein and the TCR ζ chain were both required and sufficient to upregulate FasL in T cells.
Figure 5.
Induction of FasL expression by Nef or a Nef mutant (Nef.PXmu). Jurkat cells were transfected with CD8-Nef or CD8-Nef.PXmu (no binding to TCR-ζ) construct by electroporation and kept under neomycin selection for 2 wk. Outgrowing cells were selected for CD8 surface expression by Dynabeads and analyzed for FasL expression by FACS® as described above.
Figure 6.

Upregulation of FasL by Nef requires TCR-ζ. Transient cotransfection of a FasL promotor/luciferase reporter with an empty vector (cont.; negative control), Tax (positive control), Nef, and Nef.PXmu into Jurkat (WT), TCR− Jurkat (TCR−), and TCR− cells reconstituted with CD16-ζ (TCR−/Zeta). The expression of the reporter gene was determined as described in Materials and Methods. Bars, the mean ± SD of three experiments.
Discussion
In general, the interaction between Fas and FasL plays a important role in the homeostatic regulation of normal immune responses (37). Stimulation of the TCR–CD3 complex in T cells causes upregulation of FasL and eventually leads to AICD or apoptosis (23–25). A key molecule in this process is the TCR ζ chain and the three ITAMs contained therein. Cross-linking of the ζ chain or constructs containing individual ζ ITAMs alone were found to be sufficient to induce T cell activation and Fas-mediated apoptosis (28, 35, 36). In agreement with these findings, we have shown here that TCR-ζ as well as the functional integrity of the ITAM signaling motifs of ζ were required for HIV-mediated upregulation of FasL. However, these findings further implied that HIV targets the TCR ζ chain directly through a viral protein.
To date, several lines of evidence indicated that the Nef protein exerted such a role. First, Nef-mediated activation of T cells has been demonstrated in a number of reports (8– 10). Second, expression of Nef in the cytoplasm of T cells interferes with early T cell signaling events emanating from the TCR–CD3 complex, including hypophosphorylation of TCR-ζ, whereas expression of a plasma membrane– associated form of Nef causes AICD in Jurkat cells (8). Third, a very aggressive form of Nef from SIV, SIV-YE-Nef, basically functions like an ITAM domain of TCR-ζ (38). Finally, SIV-induced upregulation of FasL in T cells depends on the expression of an intact Nef protein (22), and Nef from a lethal SIV strain (smmPBj14) alone can directly cause FasL upregulation (39). Thus, it appeared very likely that Nef acted at the level of the TCR. Indeed, our study confirms this assumption by showing that Nef can directly interact with the TCR ζ chain.
Strong evidence for the interaction of Nef with ζ came from a second, surprising finding. In Jurkat cells lacking the TCR, binding of the Nef-associated serine kinase p62/NAK was abolished. Conversely, reconstitution of these cells with the ζ ITAM 2 and 3 restored binding of p62/NAK with Nef. Furthermore, the integrity of the ITAM motif appeared to be important, since mutation of the ζ ITAMs greatly reduced the effect. As shown previously, the p62/NAK kinase has to be activated in order to bind to Nef (32). These results suggest a dynamic interaction of Nef with the ζ ITAMs, ultimately resulting in the activation of p62/NAK, which in turn binds to Nef. In view of our and other studies, it is likely that activation of p62/NAK is part of Nef-mediated stimulation of T cell signaling pathways; however, at this point it is not clear whether p62/NAK has a role in the Nef-mediated upregulation of FasL. Notably, Nef binds to p62/NAK in cells lacking a TCR (31; e.g., COS cells). In these cells, the TCR ζ chain may be functionally replaced by other receptors, possibly containing ITAMs. This would explain why Nef has effects in cells usually not infected by HIV (40; e.g., NIH 3T3 cells).
More recently, Howe et al. showed that Nef from SIV or HIV-2 associated with the TCR ζ chain but failed to show an interaction with HIV-1 Nef (41). Our study differs from that of Howe et al. in at least two respects. First we made constructs to target Nef to the plasma membrane where the TCR is located. Second, we have established functional consequence of the Nef–ζ interation which may have relevence to the pathogenesis of HIV interaction.
Induction of cell death by HIV could be mediated by different viral proteins. Cross-linking of CD4 by HIVgp120 in the presence of Tat protein can induce FasL expression and apoptosis of uninfected T cells (42). Additionally, interaction of HIVgp120 with chemokine receptor CXCR4 on macrophages leads to death of CD8+ T cells mediated by TNF–TNFRII interaction (15). In this study, we report an additional important mechanism of HIV-mediated apoptosis by demonstrating that Nef directly interacts with TCR-ζ and that both molecules are required for HIV-mediated upregulation of FasL. The interaction between Nef and TCR-ζ forms a signaling complex, bypassing the requirement for TCR ligation by antigen, and allowing HIV/SIV to activate T cells and upregulate FasL expression on the infected cells.
Thus, upregulation of FasL by Nef on HIV- or SIV- infected cells may, like FasL expression at sites of immune privilege and on some tumors, allow infected cells to evade the immune response. In addition, the effect of immune evasion is enhanced by Nef-mediated downregulation of surface MHC class I and CD4 expression (5, 6, 22, 43–46; see Fig. 7). Taken together, our results provide additional insights into the molecular mechanism whereby the HIV accessory protein Nef regulates T cell activity and contributes to the pathogenesis of HIV.
Figure 7.
Model describing mechanisms of immune evasion mediated by the HIV nef gene. Nef is expressed in the early viral life cycle and, after myristoylation, associates with the plasma membrane where several protein interactions take place. Nef interacts with ζ, which leads to the activation of p62/ NAK, which in turn causes the binding of p62/NAK to Nef. These events ultimately stimulate FasL expression, which may protect infected cells from CTL attack by killing Fas+ viral-specific CTLs in the process (1). Nef can also downregulate MHC class I expression and protect the infected cells against killing by CTLs (2), or CD4 expression leading to loss of CD4 T cell function (2).
Acknowledgments
We thank Drs. Tao Dong for help in virus infection and p24 assay, Xiangdong Liu for the pFasL-luc construct, Ralph Grassman for the pCTax construct, and Arthur Weiss for Jurkat mutant cell lines.
This study was supported in part by the Commonwealth AIDS grant (Australia), the Medical Research Council and the Wellcome Trust (UK), and the Deutsche Forschungsgemeinschaft (Germany).
Abbreviations used in this paper
- aa
amino acid(s)
- AICD
activation- induced cell death
- ITAM
immunoreceptor tyrosine-based activation motif
- mu
mutant
- NAK
nef-associated kinase
- RT
reverse transcriptase
- SIV
simian immunodeficiency virus
Footnotes
X.-N. Xu, B. Laffert, and G.R. Screaton contributed equally to this work.
References
- 1.Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nefgene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 2.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 3.Kirchoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Absence of intact nef sequences in a long-term survivor with non-progressive HIV-1 infection. N Engl J Med. 1995;332:228–230. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 4.Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 5.Lu X, Yu H, Liu S, Brodsky FM, Peterlin M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 6.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 7.Trono D. HIV accessory proteins: leading roles for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 8.Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C, Peterlin BM. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 9.Du Z, Lang SM, Sasseville VG, Lackner AA, Ilyinskii PO, Daniel MD, Jung JU, Desrosiers RC. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 10.Alexander L, Du Z, Rosenzweig M, Jung JU, Desrosiers RC. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. JVirol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameisen JC. Programmed cell death and AIDS: from hypothesis to experiment. Immunol Today. 1992;13:388–391. doi: 10.1016/0167-5699(92)90086-M. [DOI] [PubMed] [Google Scholar]
- 12.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 13.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 14.Shearer G. HIV-induced immunopathogenesis. Immunity. 1998;9:587–593. doi: 10.1016/s1074-7613(00)80656-1. [DOI] [PubMed] [Google Scholar]
- 15.Herbein G, Mahlknecht U, Batliealla F, Gregersen P, Pappas T, Butler J, O'Brien W, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 16.Katsikis PD, Garciaojeda ME, Torresroca JF, Tijoe IM, Smith CA, Herzenberg LA, Herzenberg LA. Interleukin-1β converting enzyme–like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis- inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus–infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra D, Steiner M, Lynch DH, Staiano-Coico L, Laurence J. HIV-1 upregulates Fas ligand expression in CD4+ T cells in vitro and in vivo: association with Fas-mediated apoptosis and modulation by aurintricarboxylic acid. Immunology. 1996;87:581–585. doi: 10.1046/j.1365-2567.1996.510589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YL, Liu ZH, Ware CF, Ashwell JD. A cysteine protease inhibitor prevents activation-induced T-cell apoptosis and death of peripheral blood cells from human immunodeficiency virus-infected individuals by inhibiting upregulation of Fas ligand. Blood. 1997;89:550–557. [PubMed] [Google Scholar]
- 20.Bahr GM, Capron A, Dewulf J, Nagata S, Tanaka M, Bourez JM, Mouton Y. Elevated serum level of Fas ligand correlates with the asymptomatic stage of human immunodeficiency virus infection. Blood. 1997;90:896–898. [PubMed] [Google Scholar]
- 21.Badley AD, Dockrell DH, Algeciras A, Ziesmer S, Landay A, Lederman MM, Connick E, Kessler H, Kuritzkes D, Lynch DH, et al. In vivo analysis of Fas/ FasL interactions in HIV-infected patients. J Clin Invest. 1998;102:79–87. doi: 10.1172/JCI2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu XN, Screaton GR, Gotch FM, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, et al. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus–infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju, S.T., D.J. Panka, H. Cui, R. Ettinger, M. el Khatib, D.H. Sherr, B.Z. Stanger, and A. Marshak-Rothstein. 1995. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 373:444–448. [DOI] [PubMed]
- 24.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 25.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:4338–4444. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 26.Baur AS, Sass G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin BM. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 27.Weiss A. Molecular and genetic insights into T cell antigen receptor structure and function. Annu Rev Genet. 1991;25:487–510. doi: 10.1146/annurev.ge.25.120191.002415. [DOI] [PubMed] [Google Scholar]
- 28.Romeo C, Amiot M, Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992;68:889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Zachar V, Zdravkovic M, Guo M, Ebbesen P, Liu X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J GenVirol. 1997;78:3277–3285. doi: 10.1099/0022-1317-78-12-3277. [DOI] [PubMed] [Google Scholar]
- 30.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawai ET, Baur A, Struble H, Peterlin BM, Levy JA, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X, Wu X, Plemenitas A, Yu H, Sawai ET, Abo A, Peterlin BM. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 33.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 34.Combadiere B, Freedman M, Chen L, Shores EW, Love P, Lenardo MJ. Qualitative and quantitative contributions of the T cell receptor zeta chain to mature T cell apoptosis. J Exp Med. 1996;183:2109–2117. doi: 10.1084/jem.183.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO (Eur Mol Biol Organ) J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 38.Luo W, Peterlin BM. Activation of the T-cell receptor signaling pathway by Nef from an aggressive strain of simian immunodeficiency virus. JVirol. 1997;71:9531–9537. doi: 10.1128/jvi.71.12.9531-9537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge S, Novembre F, Whetter L, Gelbard HA, Dewhurst S. Induction of Fas ligand expression by an acutely lethal simian immunodeficiency virus, SIVsmmPBj14. Virology. 1998;252:354–363. doi: 10.1006/viro.1998.9477. [DOI] [PubMed] [Google Scholar]
- 40.Du Z, Ilyinskii PO, Sasseville VG, Newstein M, Lackner AA, Desrosiers RC. Requirements for lymphocyte activation by unusual strains of simian immunodeficiency virus. JVirol. 1996;70:4157–4161. doi: 10.1128/jvi.70.6.4157-4161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howe AYM, Jung JU, Desrosiers RC. Zeta chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. JVirol. 1998;72:9827–9834. doi: 10.1128/jvi.72.12.9827-9834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 43.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 44.Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells. A mechanism of immune evasion? . Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 45.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 46.Johnson RP. Upregulation of Fas ligand by simian immunodeficiency virus: a nef-arious mechanism of immune evasion? . J Exp Med. 1997;186:1–5. doi: 10.1084/jem.186.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]