Abstract
Mice immunized with optimal doses of autologous tumor–derived gp96 resist a challenge with the tumor that was the source of gp96. Immunization with quantities of gp96 5–10 times larger than the optimal dose does not elicit tumor immunity. This lack of effect is shown to be an active, antigen-specific effect, in that immunization with high doses of tumor-derived gp96, but not normal tissue–derived gp96, downregulates the antitumor immune response. Furthermore, immunization with fractionated doses of gp96 elicits the same kind and level of response as elicited by a single dose equivalent to the total of the fractionated doses. This is true of the tumor-protective doses as well as the high downregulatory doses of gp96. The downregulatory activity can be adoptively transferred by CD4+ but not CD8+ T lymphocytes from mice immunized with high doses of gp96. These observations indicate that immunization with gp96 induces a highly regulated immune response that, depending upon the conditions of immunization, results in tumor immunity or downregulation.
Keywords: intradermal, suppressive T cell, downregulation, autoimmunity, heat shock protein
Immunization of mice with preparations of heat shock proteins (HSPs)1 isolated from tumors or virus-infected cells has been shown to elicit specific protective immunity against the tumor or the virus-infected cells used as the source of the HSP. This phenomenon has been shown to be general, in that specific immunogenicity of tumor-derived HSP preparations has been demonstrated in the case of hepatomas (1), fibrosarcomas (2–8), lung carcinoma (9), prostate cancer (10), spindle cell carcinoma (9), colon carcinoma (9), and melanoma (9) in mice and rats of different haplotypes. These tumors include chemically induced (1–8, 10), UV- induced (11), and spontaneous tumors (9), and efficacy has been demonstrated in prophylactic (1–8, 10, 11) as well as therapeutic (9) models. In the case of viral models, HSP preparations from cognate cells have been shown to elicit virus-specific cellular immune response against influenza virus (12), SV40 (13), vesicular stomatitis virus (14), and lymphocytic choriomeningitis virus (15). HSP preparations from cells transfected with model antigens such as β-galactosidase have been shown to elicit antigen-specific CTLs against β-galactosidase (16). The structural basis of this broad phenomenon lies in the fact that HSP preparations isolated from a given cell are associated with the range of peptides, including self and antigenic peptides, generated within that cell and that HSP–peptide complexes are highly immunogenic (17).
In earlier studies demonstrating the specific immunogenicity of the cognate tumor–derived HSP–gp96 (2), it was noted that the activity of gp96 was dose-restricted, i.e., very low doses of gp96 did not immunize, higher doses immunized effectively, and even higher doses failed to immunize at all. Although trivial reasons for this observation (i.e., high protein quantity, salts, etc.) were ruled out, this interesting and important phenomenon has not been examined since. Studies reported here explore that observation and shed light on the dual nature (immunogenic and downregulatory) of immunogenicity of gp96–peptide complexes. These results have implications for downregulation of antigen-specific T cell immune response.
Materials and Methods
Mice.
Female BALB/cJ mice (6–8 wk of age) bought from The Jackson Laboratory were maintained at the Fordham University (Bronx, NY) vivarium.
Purification of gp96.
Meth A cells, grown in ascites, and livers from naive BALB/cJ mice served as the source of gp96. Purification of gp96 was performed as described earlier (2).
Immunization.
Two doses of gp96 were administered 1 wk apart and mice were challenged with tumor cells 7 d after the last immunization. Injections were performed using a 1-cm3 insulin syringe (Becton Dickinson) in a volume of 200 μl. Subcutaneous injections were administered under the loose skin fold in the cervical region, dorsally, and intradermal injections were given in the skin on the ventral aspect of the trunk. During intradermal injection, care was taken to ensure that the inoculum was in the intradermal compartment without extravasation into the subcutaneous area. This estimation was made by the presence of a raised bleb confirming intradermal inoculation. Intravenous immunization was given retro-orbitally into the venous plexus. Oral immunization was administered by feeding the mice 200 μl of gp96 solution using the nozzle of a syringe. Intramuscular vaccination was performed in the muscle of the thigh. gp96 was injected intraperitoneally by inserting the needle subcutaneously for a length of 2 mm and then turning at right angles to the long axis of the body to penetrate the muscle and peritoneum. After injecting the requisite amount of inoculum, the needle was withdrawn in the same order. This method ensured that there was no extravasation of the inoculum from the peritoneal cavity, as the entry points in the skin and the peritoneum did not line up.
Tumor Rejection Assays.
Meth A and CMS5 lines, derived from antigenically distinct, chemically induced murine sarcomas, were used. Tumor challenges comprised 100,000 live cells (Meth A or CMS5), administered intradermally on the shaved dorsal aspect of the mouse. Tumor growth was recorded twice per week using vernier calipers measuring both the longitudinal and the transverse diameter. Average diameters of the two axes were plotted.
Isolation and Adoptive Transfer of T Cell Subsets.
Spleens were harvested from donor mice and RBCs were removed by incubation of the total cells with a filtered solution containing ammonium chloride and Tris base, pH 7.2. The residual cells were labeled with MACS® antibodies for CD4+ or CD8+ cells (Miltenyi Biotec) and loaded onto MACS® VS+ separation columns (Miltenyi Biotec). After repeated washings, the cells were eluted off the columns and counted. FACS® analysis confirmed >90% purity of the cells. As control donors, cells from mice immunized with buffer and the same age as the recipients were used. Cells were tested for >95% viability, suspended in 200 μl plain RPMI, and injected intravenously via the retro-orbital venous plexus of recipient mice.
Results
Dose Restriction of Immunogenicity of gp96.
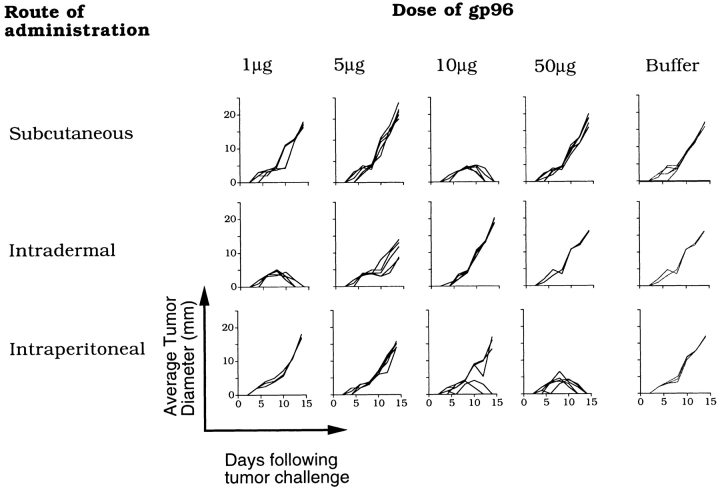
The data in Fig. 1 show the dose-restricted nature of immunogenicity of gp96. BALB/c mice were immunized subcutaneously with Meth A–derived gp96 with 1, 5, 10, or 50 μg per injection (twice, 1 wk apart) and were challenged with 100,000 Meth A cells 1 wk after the last challenge. In accord with a previous report, immunization with 10 μg gp96 was effective at eliciting tumor rejection, whereas lower (1- and 5-μg) and higher (50-μg) doses were ineffective (Fig. 1, top). Doses lower than 1 μg Meth A–derived gp96 were also tested and found to be ineffective (data not shown). Doses between 10 and 50 μg were also tested in independent experiments, and a gradual diminution of activity was observed at higher doses in this range: immunization with 20, 30, or 50 μg of gp96 led to tumor take in 1/5, 3/5, and 4/5 mice, respectively. Thus, the dose restriction was consistent, reproducible, and titratable. In a demonstration of specificity of gp96-elicited immunity, immunization with gp96 derived from normal liver was found ineffective at eliciting immunity to Meth A, and mice immunized with Meth A–derived gp96 remained sensitive to challenges with a syngeneic and antigenically distinct fibrosarcoma CMS5 (Fig. 2).
Figure 1.
Dose restriction of immunogenicity of tumor-derived gp96. BALB/c mice were immunized with Meth A gp96 in the quantities and routes indicated. Immunizations were carried out twice per week, and the doses indicated represent the quantities administered at each immunization. All mice were challenged with 100,000 Meth A cells 1 wk after the last immunization. Each line shows the kinetics of tumor growth in a single mouse.
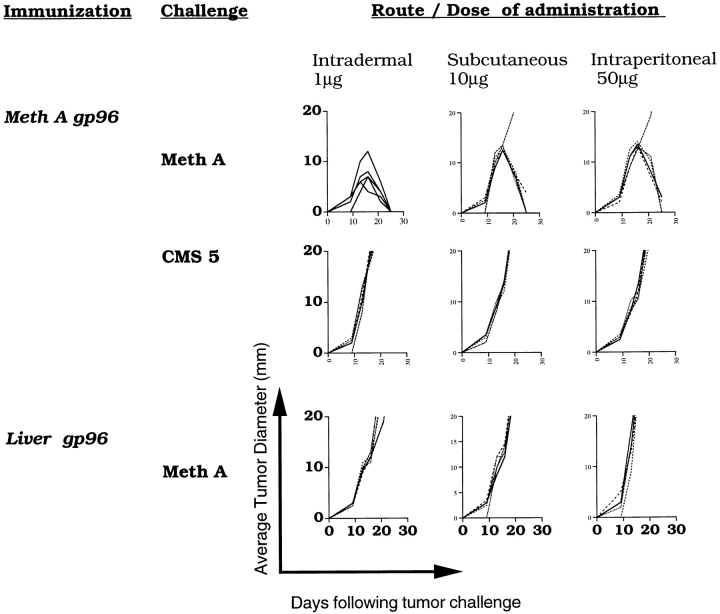
Figure 2.
Specificity of gp96-elicited tumor immunity, regardless of the route of immunization. Mice were immunized with Meth A gp96 or liver gp96 administered intradermally, subcutaneously, or intraperitoneally and were challenged with either Meth A or CMS5 sarcomas. Experimental details are the same as in the Fig. 1 legend.
When mice were immunized with Meth A–derived gp96 by various routes at various doses (1, 5, 10, and 50 μg per dose, two doses given 1 wk apart), several novel aspects emerged (Fig. 1). As little as 1 μg of gp96 administered intradermally imparted tumor protection, whereas a minimum of 10 μg was needed subcutaneously and 50 μg intraperitoneally to elicit corresponding levels of protection. Thus, intradermal immunization was more efficient than subcutaneous, and subcutaneous more efficient than the intraperitoneal route, on a per-microgram basis. Immunization with any dose of gp96 by intramuscular, oral, or intravenous routes showed no protection from tumor challenge (data not shown). Furthermore, although the subcutaneous and intradermal routes were observed to vary quite significantly, dose restriction of activity was observed in both routes (Fig. 1). In the case of intradermal immunization, <1 μg gp96 was ineffective, 1 μg was the optimal dose, and 10 μg did not elicit protective immunity; in the case of subcutaneous immunization, <10 μg gp96 was ineffective, 10 μg was the optimal dose, and 50 μg did not elicit protective immunity.
Other parameters of immunity elicited by immunization with gp96 by various routes also showed common patterns. Immunity elicited by all routes was exquisitely tumor specific, and mice immunized by all routes developed a memory response. Mice immunized intradermally or subcutaneously with Meth A–derived or liver-derived gp96 were parked for 3 mo following the last immunization and were challenged at the end of that period with live Meth A cells. Meth A–derived gp96, given intradermally, intraperitoneally, or subcutaneously, elicited specific protection from subsequent challenge with Meth A and failed to protect from CMS5 challenge (Fig. 2). Liver-derived gp96, delivered by any route, failed to protect from tumor challenge (Fig. 2, bottom).
Lack of Immunogenicity of High Doses of gp96 Is Antigen Specific.
As higher than optimal doses of gp96 did not immunize, it was difficult to assess the antigen specificity of this nonresponse. However, a method of testing this question was developed based on the phenomenon of concomitant immunity (18). It has been observed previously that if mice are challenged with a given tumor at one site in the body, and if this tumor is allowed to grow, the mice show resistance to challenge with the same tumor at another anatomical site. Thus, although the animal succumbs to a tumor at one location, it resists the same tumor at another location. This phenomenon is only observed during a narrow time window of 6–9 d after primary tumor transplantation. North and Bursuker (18) have elegantly demonstrated that concomitant immunity results from the fact that progressively growing tumors elicit an antitumor immune response, which gets rapidly downregulated after a certain period. In the period before downregulation has occurred, mice show systemic protective immunity to challenges with the same tumor as used in the primary challenge but not to other, antigenically distinct tumors (18).
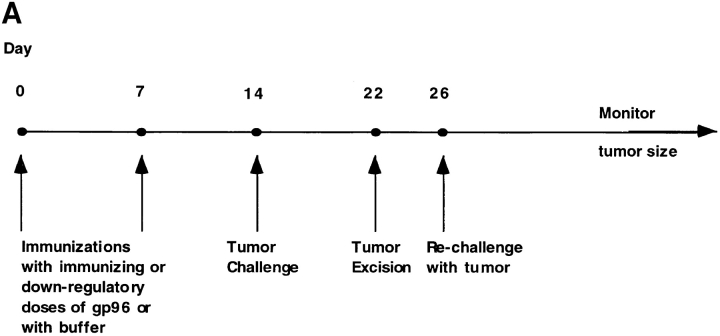
In the present study, we sought to test whether prior immunization of mice with higher than optimal doses of Meth A–derived or unrelated gp96 would or would not abrogate concomitant immunity. The design of this experiment is shown in Fig. 3 A. Mice were preimmunized with gp96 in either tumor-protective doses (1 μg intradermal [i.d.] or 10 μg s.c.) or higher doses (10 μg i.d. or 100 μg s.c.) and subsequently challenged with live tumor cells. 8 d after tumor challenge, the growing tumors were excised. (As shown earlier, all mice develop tumors that grow equally and at a consistent rate for the first 5–10 d, after which they either regress or continue to grow depending upon the immunizing dose.) Mice were allowed to recover for 4–7 d after surgery and were then rechallenged with Meth A tumor cells. The kinetics of tumor growth in each group were monitored.
Figure 3.
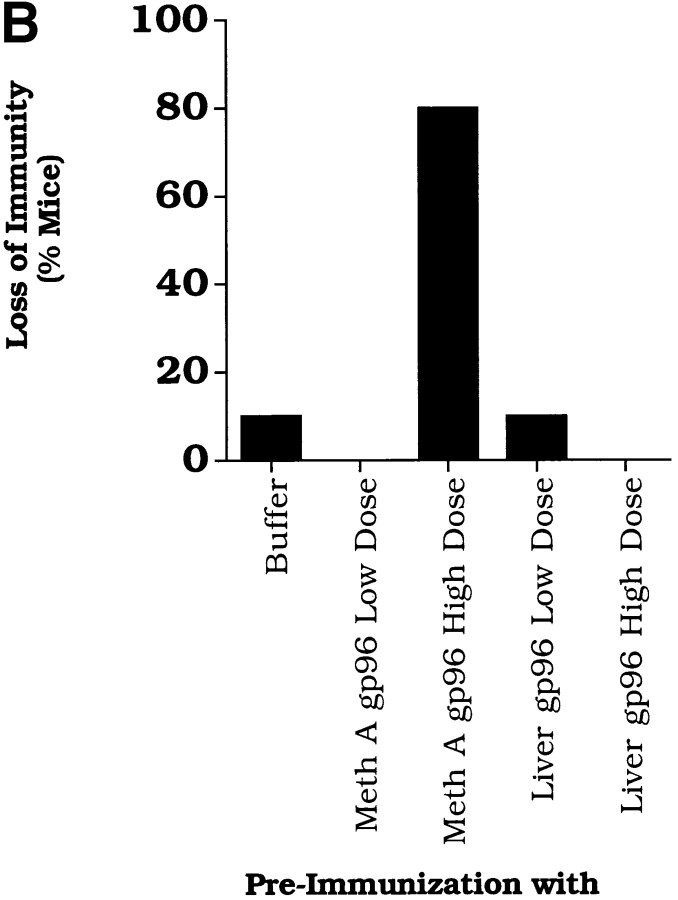
Antigen specificity of downregulation of immune response elicited by immunization with high doses of gp96. (A) The experimental plan used to measure the effect of immunization with gp96 on concomitant immunity; see text for details. (B) Immunization with high doses (10 μg i.d.) of Meth A gp96 but not liver gp96 elicits loss of concomitant immunity to Meth A sarcoma. Corresponding experiments were also carried out with subcutaneous administrations of gp96 with similar results (data not shown).
It was observed (Fig. 3 B) that mice that had been preimmunized with the larger doses of Meth A gp96 showed a loss of concomitant immunity to the second tumor challenge. Previous immunization with buffer alone or with optimal or larger doses of liver-derived gp96 did not show abrogation of immunity. These observations were noted in mice immunized by the subcutaneous or intradermal routes. The results show that the larger than optimal doses of cognate gp96 elicit an antigen-specific downregulatory influence on immune response.
Downregulation of Immune Response by High Doses of gp96 Can Be Adoptively Transferred by CD4+ T Lymphocytes.
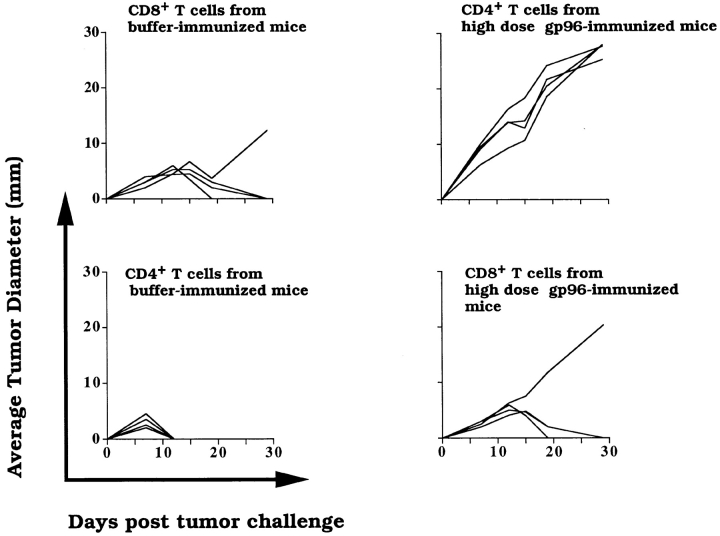
As the loss of tumor immunity elicited by high doses of gp96 would appear similar to immunization with buffer, an indirect assay had to be devised to measure the activity of high doses of gp96. Mice were first immunized with doses of gp96 that elicit tumor immunity. These mice then received sera or T cell subsets from other mice that had been previously immunized with high doses of gp96. Preliminary analysis suggested that a cellular and not a humoral component could transfer downregulation (data not shown). Furthermore, splenic T lymphocytes from mice immunized with high doses of Meth A–derived gp96 were fractionated into CD4+ and CD8+ populations as described in Materials and Methods. The lymphocytes were adoptively transferred to mice that had been previously immunized with the effective dose of gp96. All mice were challenged with Meth A cells, and the kinetics of tumor growth were monitored. It was observed (Fig. 4) that tumor immunity was abrogated in mice that received CD4+ T lymphocytes from high-dose gp96–immunized mice; in contrast, mice that received CD8+ T lymphocytes from the high-dose gp96–immunized group remained protected, as did the mice that received CD4+ or CD8+ T lymphocytes from buffer-immunized mice. In other control experiments, mice that received buffer or CD4+ or CD8+ T lymphocytes from low-dose gp96–immunized mice remained protected from tumor challenge (data not shown).
Figure 4.
Role of CD4+ T lymphocytes in mediating downregulation of immune response. All (recipient) mice shown were immunized with 1 μg of Meth A gp96 i.d. CD4+ or CD8+ T lymphocytes from mice immunized with buffer or high-dose Meth A gp96 (10 μg i.d.) were adoptively transferred (1.25 × 107 CD4+ T lymphocytes/mouse; 3.7 × 106 CD8+ T lymphocytes/mouse) into the recipient mice, which were challenged with 100,000 Meth A cells. Each line represents the kinetics of tumor growth in a single mouse. As controls, CD4+ or CD8+ T lymphocytes from mice immunized with 1 μg Meth A gp96 were also transferred into the recipient mice; however, such transfer had no influence on the tumor immunity in the recipient mice (data not shown).
Immunization with Fractionated Doses of gp96 and Summation of Response.
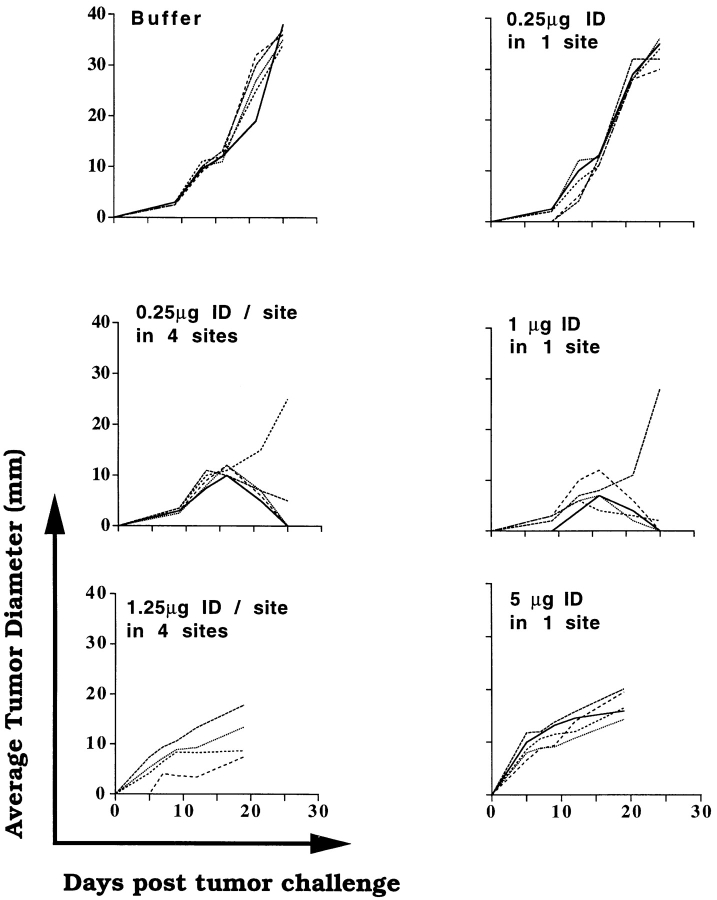
Intradermal immunization with 1 μg Meth A gp96 given in divided doses at different sites (0.25 μg/site in four sites, targeting different regional lymph nodes) was found to elicit protective tumor immunity. The 0.25-μg dose administered at one site only did not elicit immunity (Fig. 5). The degree of protection conferred by immunization with 0.25 μg/site at four sites was comparable to that elicited by 1 μg given at a single site or as a single dose. The higher dose of gp96 (5 μg i.d. at a single site), which mediated active downregulation of immune response when administered at one site in one large dose, also mediated downregulation when given in four divided doses of 1.25 μg each, intradermally, at multiple sites (Fig. 5). These observations indicate that gp96-induced immunity shows spatial summation and suggest the existence of a substantial degree of cross-talk between lymph nodes.
Figure 5.
Effect on fractionation of immunizing and downregulating doses of gp96. Mice were immunized, as indicated, with single-site injections (1 or 5 μg i.d., each injection), or fractionated four-site (two in each flank, so as to target the axillary and the inguinal group of lymph nodes) injections, where the total quantity of gp96 used for immunization was the same as used in single-site injections. All mice were challenged and monitored as indicated in the Fig. 1 legend.
Discussion
Studies reported here indicate that immunization with gp96 preparations elicits a complex and highly regulated immune response, which, depending upon the route and the quantity used for the immunization, results in antitumor immune response or its downregulation. The lack of tumor-protective responses by high doses of gp96 is not a null event but an active process that can downregulate tumor immunity elicited by immunization with a growing tumor or by the optimal dose of the cognate gp96. This active process includes generation of a downregulatory CD4+ T lymphocyte population. Although there is precedent for CD4+ T cell populations that can downregulate immune response to cancers and infectious agents (19, 20), the mechanisms through which they implement their action have not been elucidated in the present or previous systems. This must await cloning and structural characterization of the downregulatory cells and the cytokines elaborated by and responded to by them.
As APCs play the central role in immunogenicity of gp96 preparations (5), the log scale differences among the efficiencies of the various routes suggest that the differences may reflect the nature and density of the relevant APC populations at various sites. Thus, Langerhans cells, assorted subcutaneous macrophages, and peritoneal macrophages might be involved in processing of gp96–peptide complexes in the intradermal, subcutaneous, and intraperitoneal sites, respectively. Differences in efficiencies of such APCs have indeed been recorded (21). The primary events of elicitation or downregulation of immune response presumably occur at the level of gp96–APC interaction. Two possibilities may be envisaged. First, activation of an APC by different quantities of gp96 may lead to qualitatively different types of signals, one leading to stimulation of CD8+ T cells and the other to generation of a downregulatory CD4+ T cell population. This possibility, although attractive, is weakened by the observation that stimulation of macrophage in vitro with gp96–peptide complexes over a wide range of quantities leads to uniform stimulation of CD8+ T cells (14). The second possibility is that a larger quantity of gp96 molecules might interact with a larger number of APCs, leading to generation of an amplified signal, such as a particular cytokine or combination of cytokines, that stimulates CD8+ T cells at lower levels and an inhibitory CD4+ T cell population at higher levels. Presently, we favor the second possibility. It has been postulated that gp96 molecules interact with APCs through a receptor (22). Inherent in the second possibility is the prediction that the expression level of the putative gp96 receptor on APCs is quite low and limiting, leading to the rather narrow window of the effective immunizing dose of gp96. Interestingly, when mice are immunized with increasing doses of irradiated, intact Meth A tumor cells ranging from 10 million cells to 200 million cells, no restriction of immunizing activity is observed at the higher doses (data not shown). This result provides further support to our previous studies, in which it was shown that the mechanisms through which intact cells elicit immunity are distinct from the mechanism through which gp96 isolated from the same cells becomes immunogenic (5). The observation that immunogenicity of gp96 is exquisitely sensitive to the abrogation of the function of APCs, whereas immunogenicity of whole cells is not (5), further points to the APC as the site most likely to be responsible for the dual nature of the specific immunological activity of gp96. Our results with gp96 are also reminiscent of earlier studies describing high-zone tolerance induced by immunization with large quantities of soluble proteins (23). As gp96 molecules chaperone antigens instead of being antigenic themselves (17), the differences between the mechanisms of tolerance induction in the two instances would be interesting and instructive.
The observations that administration of several fractions of doses is as effective in eliciting or downregulating immune response as administration of a single dose appear to suggest that both events, the immune response and its downregulation elicited by gp96, require a threshold that can be met by events at one microenvironment or collectively at several. The observation that immunization with several suboptimal doses can help meet the threshold to an active immune response is understandable with relative ease. In contrast, the observation that several immunizations, each with an optimal immune-stimulating dose, can lead to a systemic downregulated immune response is surprising. It suggests that even though an immune response at a given site may have a given consequence, it may be overridden by independent events occurring at other sites. Collectively, these results argue for a level of cross-talk among different immunological microenvironments that appears surprising in light of other studies that show a high degree of autonomy for individual lymph nodes (24).
Our observations have a significant implication for therapy. Autologous cancer–derived gp96 preparations have been used for immunizing cancer patients in a pilot clinical trial, and other such trials are underway. The quantity of gp96 to be used in the trials requires careful calibration such that it does not become immune inhibitory. Similar but converse concerns must be kept in mind in considering possible applications of the use of gp96 or other HSPs toward therapy of autoimmune conditions.
Abbreviation used in this paper
- HSPs
heat shock proteins
Footnotes
R.Y. Chandawarkar's present address is Department of Surgery, Akron General Medical Center, 400 Wabash Ave., Akron, OH 44307.
References
- 1.Srivastava PK, Das MR. Serologically unique surface antigen of a rat hepatoma is also its tumor-associated transplantation antigen. Int J Cancer. 1984;33:417–422. doi: 10.1002/ijc.2910330321. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palladino MA, Srivastava PK, Oettgen HF, DeLeo AB. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. I. Detection by a cloned cytotoxic T cell line. Cancer Res. 1987;47:5074–5079. [PubMed] [Google Scholar]
- 4.Udono H, Srivastava PK. Relative immunogenicities of heat shock proteins gp96, hsp90 and hsp70 against chemically induced tumors. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- 5.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldweg AF, Srivastava PK. Molecular heterogeneity of the tumor rejection antigen/heat shock protein gp96. Int J Cancer. 1995;63:310–314. doi: 10.1002/ijc.2910630227. [DOI] [PubMed] [Google Scholar]
- 7.Udono H, Srivastava PK. Heat shock protein 70–associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullrich SJ, Robinson EA, Law LW, Willingham M, Appela EA. Mouse tumor-specific antigen is a heat shock-related protein. Proc Natl Acad Sci USA. 1986;83:3121–3125. doi: 10.1073/pnas.83.10.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of metastatic lung cancer by heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari RK, Li G, Osborne MP, Daou M, Srivastava PK. Cancer derived heat shock protein, gp96, elicits protective immunity in a prostate cancer model. Proc Am Assoc Cancer Res. 1996;37:3240. . (Abstr.) [Google Scholar]
- 11.Janetzki S, Blachere NE, Srivastava PK. Generation of tumor-specific CTLs and memory T cells by vaccination with tumor-derived heat shock protein preparation. J Immunother. 1998;21:269–276. doi: 10.1097/00002371-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Heikema A, Agsteribbe E, Wilschut J, Huckriede A. Generation of heat shock protein-based vaccines by loading of gp96 with antigenic peptides. Immunol Lett. 1997;57:69–74. doi: 10.1016/s0165-2478(97)00048-5. [DOI] [PubMed] [Google Scholar]
- 13.Blachere NE, Udono H, Janetzki S, Li Z, Heike M, Srivastava PK. Heat shock protein vaccines against cancer. J Immunother. 1993;14:352–356. doi: 10.1097/00002371-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 15.Ciupitu AM, Petersson M, O'Donnell CL, Williams K, Jindal S, Kiessling R, Welsh RM. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J Exp Med. 1998;187:685–691. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold D, Faath S, Ramensee HG, Schild H. Cross-priming of minor histocompatibility antigen–specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–177. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 18.North RJ, Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. J Exp Med. 1984;159:1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakhmilevich AL, North RJ, Dye ES. Presence of CD4+T suppressor cells in mice rendered unresponsive to tumor antigens by intravenous injection of irradiated tumor cells. Int J Cancer. 1993;55:338–343. doi: 10.1002/ijc.2910550226. [DOI] [PubMed] [Google Scholar]
- 20.Hill JO, Awwad M, North RJ. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. . J Exp Med. 1989;159:1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhardwaj N, Bender A, Gonzalez N, Bui LK, Garrett MC, Steinman RM. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+T cells. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 23.Mitchison NA. The dosage requirements for immunological paralysis by soluble proteins. Immunology. 1968;15:509–530. [PMC free article] [PubMed] [Google Scholar]
- 24.Brichard VG, Warnier G, Van Pel A, Morlighem G, Lucas S, Boon T. Individual differences in the orientation of the cytolytic T cell response against mouse tumor P815. Eur J Immunol. 1995;25:664–671. doi: 10.1002/eji.1830250306. [DOI] [PubMed] [Google Scholar]