Abstract
The activity of interleukin (IL)-9 on B cells was analyzed in vivo using transgenic mice that constitutively express this cytokine. These mice show an increase in both baseline and antigen-specific immunoglobulin concentrations for all isotypes tested. Analysis of B cell populations showed a specific expansion of Mac-1+ B-1 cells in the peritoneal and pleuropericardial cavities, and in the blood of IL-9 transgenic mice. In normal mice, the IL-9 receptor was found to be expressed by CD5+ as well as CD5− B-1 cells, and repeated injections of IL-9 resulted in accumulation of B-1 cells in the peritoneal cavity, as observed in transgenic animals. Unlike other mouse models, such as IL-5 transgenic mice, in which expansion of the B-1 population is associated with high levels of autoantibodies, IL-9 did not stimulate the production of autoantibodies in vivo, and most of the expanded cells were found to belong to the B-1b subset (IgM+Mac-1+CD5−). In addition, we found that these IL-9–expanded B-1b cells do not share the well-documented antibromelain-treated red blood cell specificity of CD5+ B-1a cells. The increase of antigen-specific antibody concentration in immunized mice suggests that these B-1 cells are directly or indirectly involved in antibody responses in IL-9 transgenic mice.
Keywords: cytokines, transgenic mice, B-1 lymphocytes, autoimmunity
Interleukin (IL)-9 is a Th2 cytokine originally identified as a mouse T cell growth factor. Additional in vitro targets include mast cells, B cells, and hematopoietic progenitors (1), and in vivo studies have pointed to a role for IL-9 hyperexpression in allergies and lymphoma development. A link between dysregulated IL-9 production and lymphoid malignancies has been suggested by the observation of constitutive IL-9 expression in lymph nodes from patients with Hodgkin's disease and large cell anaplastic lymphomas (2). Moreover, mice overexpressing IL-9 show a high susceptibility to the development of T cell lymphomas (3), which further supports the view that IL-9 is a tumor-promoting factor.
The implication of IL-9 in the complex pathogenesis of asthma was also proposed recently. In humans, the IL-9 gene is located on chromosome 5q31-q33, in a region for which a linkage has been demonstrated with asthma or its risk factors (4, 5). Interestingly, a linkage for asthma and broncho-hyperresponsiveness has also been reported for the long-arm pseudoautosomal regions of the X and Y chromosomes, where the IL-9 receptor gene is located (6). In the mouse, in addition to a genetic linkage, comparison between bronchial hyporesponsive C57BL/6 and hyperresponsive DBA/2 mice has pointed to significant differences in IL-9 production (7). In line with these observations, IL-9 transgenic mice show a broncho-hyperresponsiveness as well as mast cell and eosinophil lung accumulation (8, 9).
A role for IL-9 in Ig production has also been suggested based on in vitro experiments (10–12). Most of these experimental models focused on IgE production, and it is unclear whether or not IL-9 has a preferential effect on this class of Ig. The mechanism of action of IL-9 also remains unclear. Surprisingly, although no detectable IL-9 binding has been found on normal mouse B cells (13), IL-9 appears to be active on purified B cells (10).
In this report, we describe a significant increase of serum Ig in IL-9 transgenic mice, associated with an expansion of B lymphocytes from the B-1 subset (Mac-1+IgM+). In normal mice, this population represents only 1% of total B cells and is limited to the peritoneal and pleuropericardial cavities. In IL-9 transgenic mice, B-1 cells not only accumulate in these cavities, but are also detected in the blood. Further analysis showed that the IL-9 receptor is expressed by both B-1a and B-1b cells. However, most of the in vivo– expanded B cells belong to the B-1b subpopulation (IgM+ Mac-1+CD5−) and do not have the antibromelain-treated RBC specificity described for the B-1 lymphocytes.
Materials and Methods
Transgenic and Other Strains of Mice.
Transgenic mice overexpressing the IL-9 gene were generated in the FVB/N background using a construct consisting of an IL-9 genomic fragment linked to the promoter of the murine pim-1 gene, including the TATA box and the cap site, followed by two copies of the Eμ enhancer and one copy of the mMLV LTR, as described previously (3). Although this construct should be preferentially expressed in the lymphoid lineage, transgenic animals express large amounts of IL-9 message in all organs, and high levels of biologically active IL-9 (± 1 μg/ml) have been detected in the serum of transgenic but not control mice. We used females of 8–12 wk of age, except in the kinetics experiment, where we used animals of 3, 6, 12, 25, or 48 wk, or older than 18 mo. Mice were maintained in a specific pathogen–free environment. Five independent transgene-positive lines (Tg5, Tg54, Tg83, Tg25, and Tg95) were obtained. For most experiments, the results obtained with the Tg5 line are shown, as these mice give results that are representative for the other IL-9 transgenic lines.
Animals carrying the IL-9 transgene and deficient in IL-5 were obtained by crossing IL-5 knockout C57BL/6 mice (14) and Tg5 or Tg54 mice. F1 mice were backcrossed with IL-5–deficient mice. 50% of the F2 mice were homozygous for the targeted IL-5 gene, and 50% of these expressed the IL-9 transgene. All of the IL-9 transgenic F2 mice expressed similar IL-9 concentrations in serum (mean of 0.2 μg/ml). Normal 8-wk-old C57BL/6 mice were used for the IL-9 injection experiment.
In Vivo Treatments.
Groups of five mice (FVB/N or C57BL/6) received recombinant murine IL-9 (1 μg/d i.p.), which was produced and purified in our laboratory as described previously (13). Control groups of mice received the same volume of buffer (PBS plus 1% mouse serum) used for the cytokine injection. The treatment was performed for 3, 7, 14, or 21 consecutive days. For the LPS injection, 10 FVB and Tg5 females were injected with 50 μg i.p. LPS (Re 595 from Salmonella minnesota; Sigma Chemical Co.) 3 times, at 1-wk intervals, and blood samples were collected 1 wk after the last injection.
Cell Preparations and FACS® Analysis.
Peritoneal and pleural cells were obtained by washing the cavities with 3–5 ml of NaCl 0.9% or medium containing 20 U/ml heparin (Leo Pharmaceuticals). Heparinized blood samples were centrifuged on a Ficoll layer and incubated for 5 min in 0.15 M NH4Cl in order to lyse the RBCs. Spleen cells were treated similarly, to remove RBCs.
Double labeling of cells was performed with biotinylated rat mAbs against Mac-1 (M1/70, rat IgG1) followed by PE-conjugated streptavidin (Becton Dickinson) and FITC-conjugated anti-IgM (LOMM9; provided by H. Bazin, University of Louvain) or FITC-conjugated anti–Mac-1 (Cedarlane Labs., Ltd.) plus PE-conjugated anti-CD5 (PharMingen). Three-color analysis was also performed with FITC-conjugated anti-IgM, PE-conjugated anti-CD5, and biotinylated anti–Mac-1 followed by RED670 (GIBCO BRL)–conjugated streptavidin. Negative controls for double labeling were cells incubated with the respective single labeling. PE-conjugated IgG2a isotype (PharMingen) was used as control of specificity of CD5 staining. After staining, cells were fixed in paraformaldehyde 1.25%, and fluorescence intensity was measured on 104 cells/sample on a FACScan™ apparatus (Becton Dickinson).
An IL-9–Ig fusion protein was produced as follows. The murine IL-9 cDNA was amplified by PCR using a mutated antisense primer that introduced a BclI restriction site just before the stop codon: 5′-TCGGCTGATCAGCCTTTGCATCTCTGT-3′. The region comprising the hinge, CH2, and CH3 domains of the murine IgG3 isotype heavy chain was amplified by PCR using cDNA from the IgG3 anti-TNP hybridoma C3110 as a template with the following primers (15): 5′-AAGACTGAGTTGATCAAGAGAATCGAGCCTAGA-3′ (sense), and 5′-AATGTCTAGATGCTGTTCTCATTTACC-3′ (antisense) containing BclI and XbaI sites for cloning. After amplification, both PCR products were digested with the appropriate restriction enzymes and cloned into the pCDNA/Amp plasmid (Invitrogen). Clones with the correct insert were transiently transfected into COS7 cells, and supernatants were collected after 3 d. For FACS® staining, cells were incubated with 10% COS cell supernatant as the first step and with PE- or FITC-labeled polyclonal anti-IgG3 (Southern Laboratories) as the second step. To assess the specificity of the IL-9 receptor staining, we performed the labeling in the presence or absence of an excess of free IL-9 (103 U).
Spontaneous and Specific Ig Secretion.
Groups of 20 animals of 8–12 wk were used for measuring antibody secretion. For antigen-specific responses, 20 females were immunized with 100 μg i.p. of KLH or TNP-Ficoll in PBS buffer, and boosted 15 d later with the same dose of antigen always without adjuvant. Sera were obtained 15 d after the first immunization and 15 d after the boost. Sera were conserved at 4°C with azide (±10 mM) before antibody measurements.
Antigen-specific antibody measurements were performed as described (16). Microtiter plates (Immunoplates; Nunc, Inc.) were coated with the antigen (20 μg/ml KLH [Calbiochem-Behring Corp.] or 10 μg/ml TNP-Ficoll) in glycine (20 mM)–buffered NaCl (30 mM), pH 9.2, and incubated overnight at room temperature. After washing in 0.15 M NaCl plus Triton 0.01%, serial dilutions of samples were added and plates were incubated for 2–3 h at 37°C. After incubation, plates were washed as before, then soaked for 7 min in saline plus NP-40 1% (Fluka AG) before further incubation. Bound Ig was detected using rabbit antibodies specific for each mouse IgG subclass and by peroxidase-conjugated goat anti–rabbit IgG, following the same washing and incubation steps. The assay was developed by adding o-phenylenediamine dihydrochloride (OPD) as a substrate. Calibration was obtained by referring to the binding of equivalent affinity anti-DNP IgG1, IgG2a, IgG2b, and IgG3 mAbs to DNP-BSA–coated plates.
Spontaneous Ig concentrations were determined as described (17). Plates were coated with goat anti–rabbit IgG followed by rabbit antibodies specific for each mouse IgG subclass or for mouse IgM (provided by J.-P. Coutelier, from our laboratory) and a rat anti–mouse IgE (LO-ME-3; provided by H. Bazin). Incubations of serial dilutions of samples or standards were followed by donkey antibodies specific for mouse IgG (or a rabbit anti– mouse IgM), both conjugated to peroxidase, and for IgE determination a biotinylated rat anti–mouse IgE (LO-ME-2) plus streptavidin-peroxidase (Sigma Chemical Co.) was used. Assays were performed as described above for antigen-specific ELISA.
Analysis of Autoantibody Production.
Titration of rheumatoid factor (RF)1 was performed in serum by latex agglutination as described (18). RF titers were defined as the highest serum dilution resulting in a >10% agglutination of carboxylated polystyrene particles (0.8-μm diameter; Rhône-Poulenc) coated as described with purified mAbs obtained in our laboratory: IgG2a (A7203C11.1) or IgG1 (B8703B8.M). All sera were decomplemented before the agglutination test by heating at 56°C for 30 min and centrifuged for 5 min at 10,000 rpm. In brief, equal volumes (25 μl) of the agglutinator (serum samples, or standards: mAbs anti-IgG2a A7104B7.M and anti-IgG1 B101C1.M, both from 129/Sv mice from our laboratory) and a 0.05% (wt/vol) latex suspension were incubated for 30 min in a shaking water bath at 37°C. The agglutination of IgG-coated particles in this mixture was measured by the particle-counting immunoassay (PACIA), after 100× dilution, by counting the residual nonagglutinated particles.
Antibromelain-treated RBC activity was measured as described (19). Purified RBCs from FVB mice were obtained by retroorbital puncture on heparin and centrifugation on Lymphoprep layer for 20 min at 1,700 rpm. RBCs were incubated as a 50% suspension with bromelain (Sigma Chemical Co.) at a final concentration of 20 mg/ml in PBS for 45 min at 37°C, according to the method of Cunningham (20). RBCs were washed three times, and 25 μl of a suspension of 20 × 106 cells/ml was incubated with the same volume of serum dilutions for 30 min at 4°C. After washing and centrifugation, the bromelain-treated RBCs were resuspended in Hank's medium (with 3% decomplemented FCS and 0.01 M azide), incubated with rat mAb anti-IgM LO-MM9 conjugated to fluorescein (5 μg/ml), and analyzed on the FACScan™ apparatus. Results are given as the mean fluorescence intensity for each sample.
Antithymocyte activity was detected by incubating 20 μl of a suspension of FVB thymocytes (25 × 106 cells/ml) with 50 μl of diluted serum for 30 min at 4°C in microplates. After washing, the resuspended thymocytes were treated, as described for bromelain-treated RBCs, with rat mAb anti-IgM LO-MM9 (provided by H. Bazin) conjugated to fluorescein (5 μg/ml). Results are given as the mean fluorescence intensity for each sample as measured on a FACScan™ apparatus.
Rosetting of B Cells with Bromelain-treated RBCs.
Peritoneal cell suspensions were adjusted to 107/ml with cold Hank's medium supplemented with 3% decomplemented FCS, before addition of 1/10 volume of a 10% bromelain-treated RBC suspension, as described (21). Cells were centrifuged for 5 min at 1,600 rpm, and resuspended on a roller at 4°C for 15 min. Enrichment of rosette-forming cells was performed on a discontinuous Percoll gradient composed of 2 ml of 70% solution for the lower layer and 2 ml of 30% solution for the upper layer. Cell mixtures were gently overlaid on top of the gradient. The tubes were then centrifuged for 15 min at 2,000 rpm, and the pellets containing free RBCs and rosettes were washed; RBCs were removed by NH4Cl treatment followed by washes with Hank's medium, and FACS® staining was performed as described above.
Statistical Analyses.
Statistical analyses were performed by Mann-Whitney U statistical test for unpaired values using Instat software.
Results
Increased Spontaneous and Specific Ig Production in IL-9 Transgenic Mice.
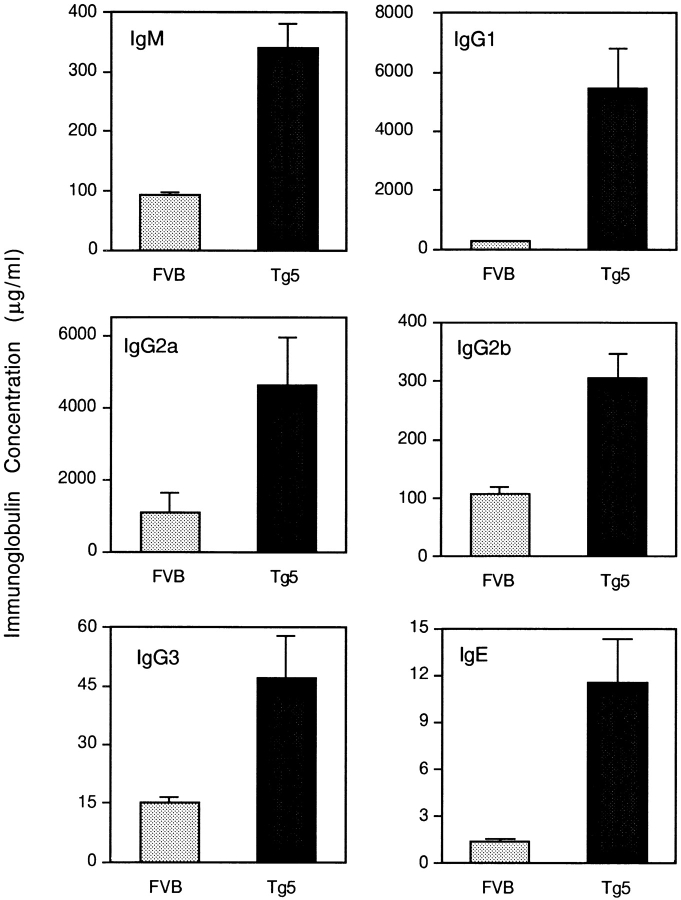
The spontaneous production of Ig was analyzed in the progeny of five independent transgene-positive mice. As reported previously, IL-9 transgenic animals have >1 μg/ml of circulating IL-9 in the serum, whereas IL-9 is undetectable (<100 pg/ml) in normal mice. In individuals of all transgenic lines bred under specific pathogen–free conditions, serum concentrations of IgM, IgG1, IgG2a, IgG2b, IgG3, and IgE were significantly increased, as shown for a representative line (Tg5) in Fig. 1 (P < 0.0001 for IgM, IgG1, IgG2a, IgG2b, and IgG3; P = 0.0015 for IgE, Mann-Whitney test). The most prominent were a 20- and a 9-fold enhancement in IgG1 and IgE, respectively; IgM, IgG2a, IgG2b, and IgG3 levels showed a 3–4-fold increase. A similar picture was found with the other IL-9 transgenic lines.
Figure 1.
Increase of spontaneous serum Ig isotypes in IL-9 transgenic mice compared with FVB control mice. Ig isotypes are measured by ELISA in sera of 20 individual female FVB and Tg5 12-wk-old mice, as described in Materials and Methods. Results are expressed in μg/ml as mean ± SEM.
To investigate the influence of IL-9 on antibody production against foreign antigens, wild-type control FVB and IL-9 transgenic mice were immunized with TNP-Ficoll or KLH. The specific antibody response in the serum was measured by ELISA 14 d after a primary injection or 14 d after a boost injection. The result of such an immunization against TNP-Ficoll is shown in Fig. 2. For every IgG subclass (IgG1, IgG2a, IgG2b, and IgG3), IL-9 induced a modest (two- to fivefold) but significant increase in TNP-specific antibodies (P < 0.05 in the primary response for all IgG subclasses; P < 0.002 in the secondary response, Mann-Whitney test). The results obtained with KLH were the same as with the antigen (not shown).
Figure 2.
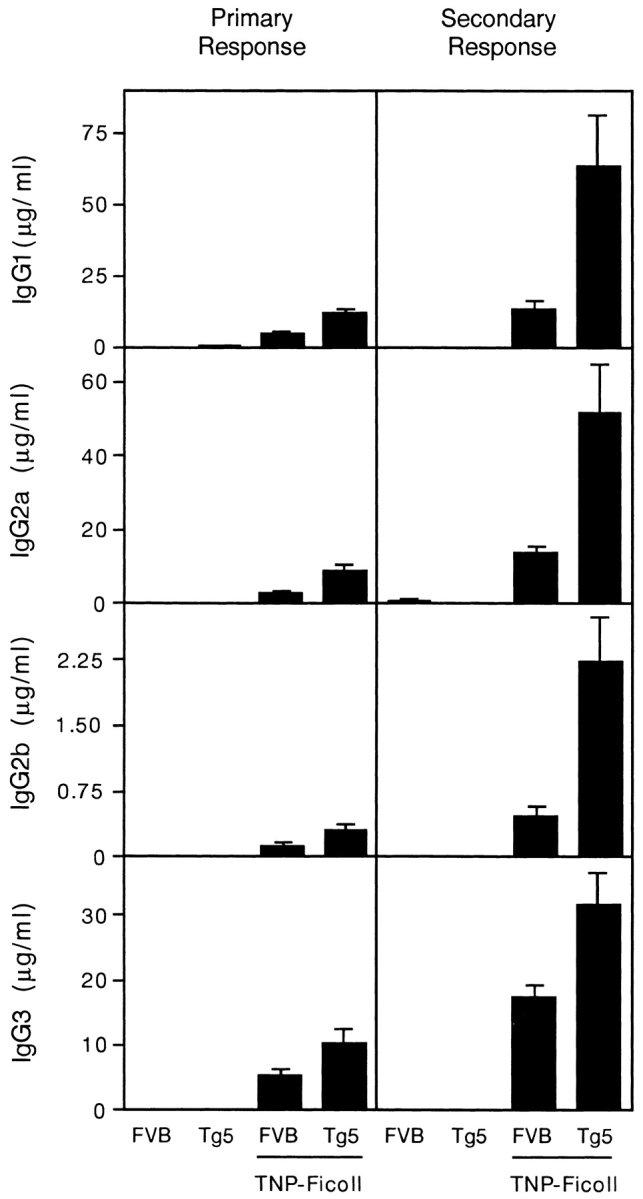
Increase of specific IgG isotypes in serum of FVB and IL-9 transgenic mice immunized against TNP-Ficoll. Primary and secondary responses were shown for nonimmunized mice and mice immunized twice with 100 μg TNP-Ficoll without adjuvant. Antibody concentrations are given as the mean ± SEM for 10 individual measurements.
Increased B Lymphocyte Numbers in IL-9 Transgenic Mice.
We next addressed the possibility that the increase of spontaneous Ig secretion could be associated with a modification in B lymphocyte populations. Total cell counts and FACS® analysis with anti-IgM antibodies were first performed with blood cells from control and Tg5 mice. As shown in Table I, peripheral blood numbers of nucleated cells were tripled in transgenics, and B cell numbers rose from 0.6 × 106/ml in control mice to 4.0 × 106/ml in IL-9 transgenics, indicating a significant expansion of circulating B cells. By contrast, in the spleen, the total number of cells was only mildly increased (1.5–2-fold) in IL-9 transgenic mice, without preferential upregulation of B lymphocyte numbers. This observation indicates that splenic B lymphocytes are not specifically expanded in IL-9 transgenic mice and that their number simply reflects a global spleen enlargement. In line with these data, total cell and B lymphocyte numbers were only marginally increased in mesenteric lymph nodes of IL-9 transgenic animals.
Table I.
Total Cell and B Lymphocyte Numbers from Normal FVB and IL-9 Transgenic Mice
| Peripheral blood cells | Spleen cells | Mesenteric lymph node cells | Peritoneal cells | Pleuropericardial cells | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 106/ml | 106/mouse | 106/mouse | 106/mouse | 106/mouse | ||||||
| FVB | ||||||||||
| Total cells | 4.6 ± 1.2 | 155 ± 25 | 34 ± 14 | 2.7 ± 0.8 | 0.6 ± 0.5 | |||||
| IgM+ | 0.6 ± 0.2 | 59 ± 10 | 8 ± 3 | 0.8 ± 0.2 | 0.13 | |||||
| Tg5 | ||||||||||
| Total cells | 12.2 ± 3.4 | 233 ± 37 | 46 ± 6 | 19.5 ± 4.8 | 13.2 ± 4.7 | |||||
| IgM+ | 4.0 ± 1.6 | 96 ± 17 | 16 ± 3 | 11.8 ± 4.2 | 7.3 |
Cells were obtained as described in Materials and Methods, and numbers of sIgM+ cells were determined by FACS® analysis. Numbers of cells per mouse are given as the mean for groups of 5–10 individuals (±SD), except for the sIgM+ pleuropericardial cells, for which a pool of cells from five animals was analyzed. Number of blood sIgM+ is given as the mean per milliliter of blood.
To complete our analysis, we performed washouts of the peritoneal and the pleuropericardial cavities. In these compartments, we observed a 7- and 22-fold increase, respectively, in total numbers of cells in Tg5 compared with FVB control mice. Labeling of these cells with anti-IgM antibodies revealed an expansion for the B cell population in IL-9 transgenic mice, by a factor of 15 and 56 for peritoneal and pleuropericardial cells, respectively (Table I). These observations indicate that the absolute number of B cells in the peritoneal and pleuropericardial cavities of an IL-9 transgenic mouse is equivalent to 21% of the number of splenic B cells, whereas this ratio is only 1.5% in a normal FVB mouse. Similar observations were made with three other independent IL-9 transgenic lines (data not shown).
Preferential B-1 Cell Expansion in the Peritoneal Cavity of IL-9 Transgenic Mice.
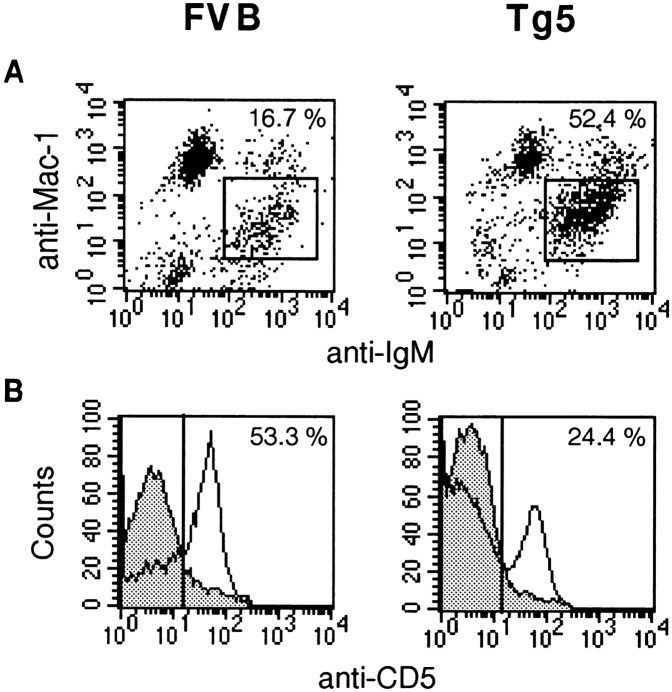
To determine in more detail the phenotype of the expanded B cell population in the peritoneal cavity of IL-9 transgenics, FACS® analysis was performed with anti-IgM, anti–Mac-1, and anti-CD5 antibodies. The peritoneal and pleuropericardial cavities are indeed known to be the preferential locations of B-1 lymphocytes, and these three cell surface antigens allow us to discriminate the major B lymphocyte subpopulations of the peritoneal cavity (22, 23). B-1 lymphocytes can be distinguished from conventional B cells (B-2 lymphocytes) by the expression of an intermediate level of Mac-1, a marker expressed at higher levels by macrophages. The result of FACS® staining of peritoneal cells is shown in Fig. 3. This confirmed the predominant expansion of IgM+ cells in IL-9 transgenic mice. Interestingly, these IgM+ cells expressed an intermediate level of Mac-1, thereby corresponding to the B-1 lineage. Although in normal mice the majority of peritoneal cells consisted of macrophages that express high levels of Mac-1 and no IgM, most of the IL-9 transgenic peritoneal cells consisted of B-1 lymphocytes with expression of both surface IgM and Mac-1 (Fig. 3 A).
Figure 3.
Three-color FACS® staining of FVB and IL-9 transgenic peritoneal cells. Peritoneal cells were labeled as described in Materials and Methods with anti–Mac-1, anti-IgM, and anti-CD5. Results of analysis of 5 × 104 cells are shown for one representative individual of FVB and Tg5 mice. (A) Mac-1/IgM labeling. (B) CD5 labeling of IgM+Mac-1+ cells. Percentages in B correspond to the proportion of CD5+ cells among IgM+Mac-1+ cells.
Within B-1 cells, two subpopulations are defined on the basis of CD5 expression: B-1a lymphocytes are IgM+Mac-1+CD5+, and B-1b lymphocytes are IgM+Mac-1+CD5−. A three-color labeling of peritoneal cells showed that both populations of B-1 lymphocytes were expanded, but that the major increase in the peritoneal populations concerned the B-1b cells (IgM+Mac-1+CD5−) in IL-9 transgenics compared with FVB control mice (Fig. 3 B, and Table II).
Table II.
Numbers of B-1a (IgM+Mac-1+CD5+) and B-1b (IgM+Mac-1+CD5−) Cells in Peritoneal Populations of FVB Normal and Tg5 Mice
Washouts of peritoneal cavities were performed for 10 12-wk-old mice, and cells were three color labeled with anti–Mac-1, anti-IgM, and anti-CD5 as described in Materials and Methods. Results are expressed as millions of cells per mouse (mean ± SD).
P < 0.0001, Mann-Whitney test. In Tg5 animals, B-1b cells outnumbered B-1a cells (P < 0.0001, Mann-Whitney test).
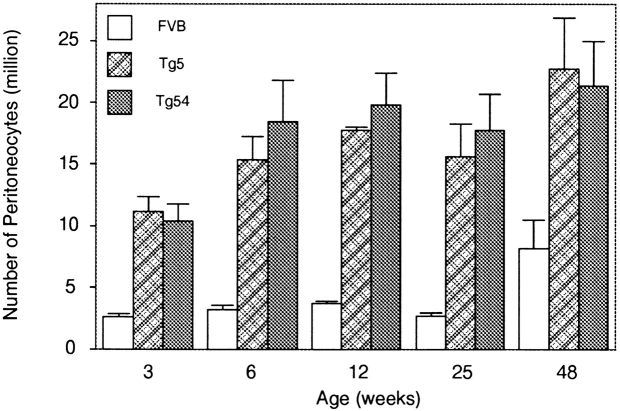
Fig. 4 shows the kinetics of accumulation of peritoneal cells in two IL-9 transgenic lines (Tg5 and Tg54). At 3 wk of age, there was already a significant difference between the number of peritoneal cells found in the peritoneal cavity of transgenic mice compared with control mice (P < 0.008, Mann-Whitney test). This difference reached five- to sevenfold after 6–12 wk, and peritoneal numbers remained stable at this level throughout the life of the individuals. Animals of >18 mo presented a similarly enlarged cell population as younger individuals (data not shown). In aging FVB control mice, peritoneal cell numbers increased slightly compared with young individuals.
Figure 4.
Kinetics of accumulation of peritoneal cells in IL-9 transgenic (Tg5 and Tg54) and FVB mice. Peritoneal cells were counted from groups of 5 mice of 3, 6, 9, and 12 wk of age and 10 mice of 24 and 48 wk of age. Results are expressed as cell numbers per mouse ± SD. The difference between transgenic and control mice was statistically significant for all ages tested (Mann-Whitney test).
B-1 Cell Expansion in Other Lymphoid Compartments of IL-9 Transgenic Mice.
In common strains, the B-1 cell population constitutes a predominant fraction of the peritoneal and pleuropericardial B cell population, but is rare in spleen and lymph nodes and absent in the peripheral blood lymphocytes of adult animals. We checked the other B cell compartments for the presence of B-1 cells by double labeling, with anti-IgM and anti–Mac-1 antibodies, of cells from different locations in IL-9 transgenic and control animals.
As shown in Fig. 5, in every location where we found an increase in B cell numbers (peritoneal and pleuropericardial cavities, and blood), IgM+Mac-1+ cells were clearly expanded. This is particularly remarkable in blood, where IgM+Mac-1+ cells were completely absent in normal mice but represented up to 4.1% of total blood cells and 15% of blood B cells in IL-9 transgenic mice. In spleen, although Mac-1 staining of IL-9 transgenic B cells was too weak to clearly distinguish a positive and negative population, increased Mac-1 staining was observed in IgM+ cells (mean fluorescence intensity 41 in Tg5 vs. 25 in FVB mice), suggesting the presence of Mac-1+ B cells in Tg5 spleen.
Figure 5.
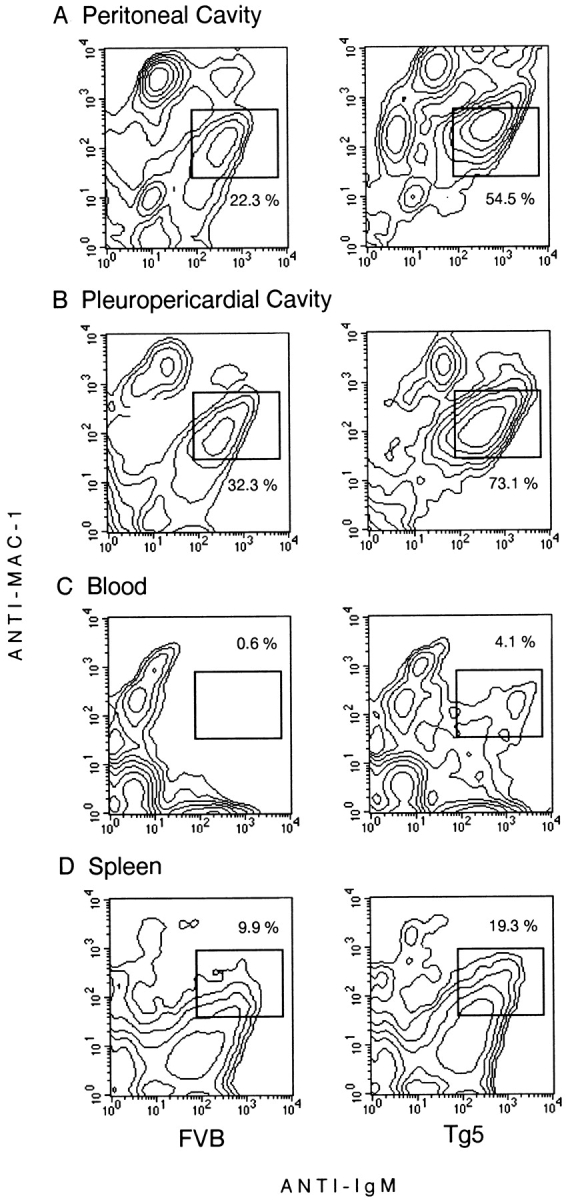
Mac-1/IgM FACS® staining of cells from different locations in FVB and IL-9 transgenic mice. Cells obtained from peripheral blood, spleen, mesenteric lymph nodes, and peritoneal and pleuropericardial cavities were double stained as described in Materials and Methods. Labeling was shown as a contour plot obtained from analysis of 104 cells of one representative mouse for control or transgenic mice.
Peritoneal Cell Expansion Induced by Exogenous IL-9 in Normal Mice.
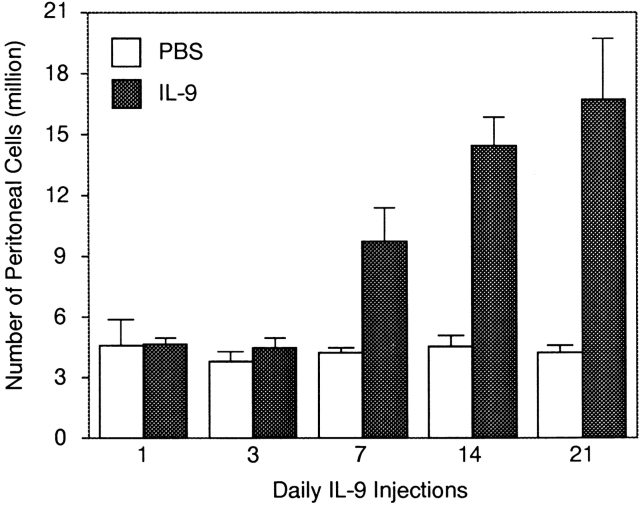
The IL-9 transgenic mice used in this study show elevated levels of this cytokine before birth (Renauld, J.-C., unpublished data). To assess whether the effect of IL-9 on B-1 lymphocytes required early and prolonged overexpression, we administered, to 8-wk-old FVB mice, daily intraperitoneal injections of mouse recombinant IL-9 (1 μg/d/mouse) for 1, 3, 7, 14, or 21 d. Total counts of peritoneal cells are given in Fig. 6. Seven injections of IL-9 were sufficient to observe a twofold increase in peritoneal cell numbers. Seven additional injections allowed a further increase to reach a plateau after 2–3 wk of daily injections (P < 0.008, Mann-Whitney test).
Figure 6.
Accumulation of peritoneal cells upon daily injection of IL-9 to FVB control mice. Groups of three to five mice were injected daily with 1 μg IL-9 or PBS as described in Materials and Methods. The numbers of cells/mouse are shown as mean ± SD. The difference between IL-9–injected and PBS-injected animals was statistically significant for 14 and 21 injections (P < 0.008, Mann-Whitney test).
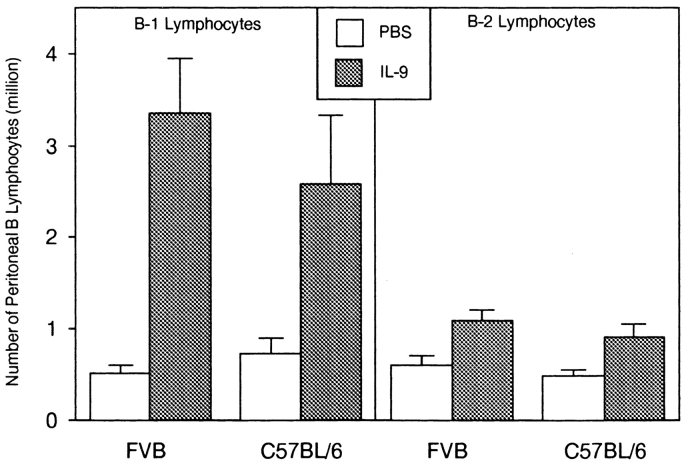
Double labeling with anti-IgM and anti–Mac-1 was performed to confirm B-1 cell expansion in mice that received 21 daily injections of 1 μg of IL-9. Fig. 7 shows the preferential increase of the B-1 population in treated FVB mice as well as in another strain, C57BL/6. Total numbers of IgM+ Mac-1+ cells increased by a factor of 6.8 for FVB (P = 0.016, Mann-Whitney test) and 3.3 for C57BL/6 mice (P = 0.05). By contrast, IgM+Mac-1− (B-2) cells were not significantly affected by IL-9.
Figure 7.
Preferential accumulation of peritoneal B-1 (IgM+Mac-1+) lymphocytes in mice injected with IL-9. Numbers of B-1 lymphocytes are shown for FVB and C57BL/6 mice injected daily with PBS or with IL-9 for 21 d. Double labeling of cells allowed us to differentiate B-1 (IgM+Mac-1+) and B-2 (IgM+Mac-1−) lymphocytes. The numbers of cells/mouse are shown as mean ± SD for a group of five individuals.
IL-9 Mediates an Increase in the Peritoneal B-1 Population in the Absence of IL-5.
Many reports, including the analysis of IL-5 transgenic mice, point to IL-5 as a key factor for the B-1 population (24–26). Although IL-5 is a potent growth factor for B-1 cells, it does not seem to be indispensable, since IL-5 knockout mice show a delay in B-1 population development and a weak reduction of peritoneal B-1 cell numbers in adults (14, 27).
To assess whether the activity of IL-9 is mediated by IL-5 or whether IL-9 can contribute to the development of B-1 cells in the absence of IL-5, we crossed IL-5–deficient mice with IL-9 transgenics (Tg5 or Tg54), and F1 mice were backcrossed with IL-5–deficient mice. Thus, 50% of the F2 hybrids have one copy of the normal IL-5 gene, and 50% of the mice express the IL-9 transgene. We performed B-1 cell numbering and determination by three-color analysis of the ratio between the B-1a (IgM+Mac-1+CD5+) and B-1b (IgM+Mac-1+CD5−) cells in the peritoneal washouts of individuals belonging to the four groups obtained.
In F2 mice having one copy of the normal IL-5 gene, overexpression of IL-9 resulted in a significant increase in the number of the B-1 population, and this expansion concerned mainly the B-1b population, as expected from the results described above (Fig. 8 A). Interestingly, IL-9 had the same effect on the number of CD5− B-1 cells in the IL-5–deficient animals (Fig. 8 B). Similar results were obtained with Tg54 hybrids (not shown). In these experiments, the IL-5 deficiency resulted in a 50% decrease in B-1 numbers, but irrespectively of IL-9 expression. Taken together, these observations demonstrate that, although IL-5 plays a role in B-1 cell development, it does not mediate the activity of IL-9 on this cell type.
Figure 8.
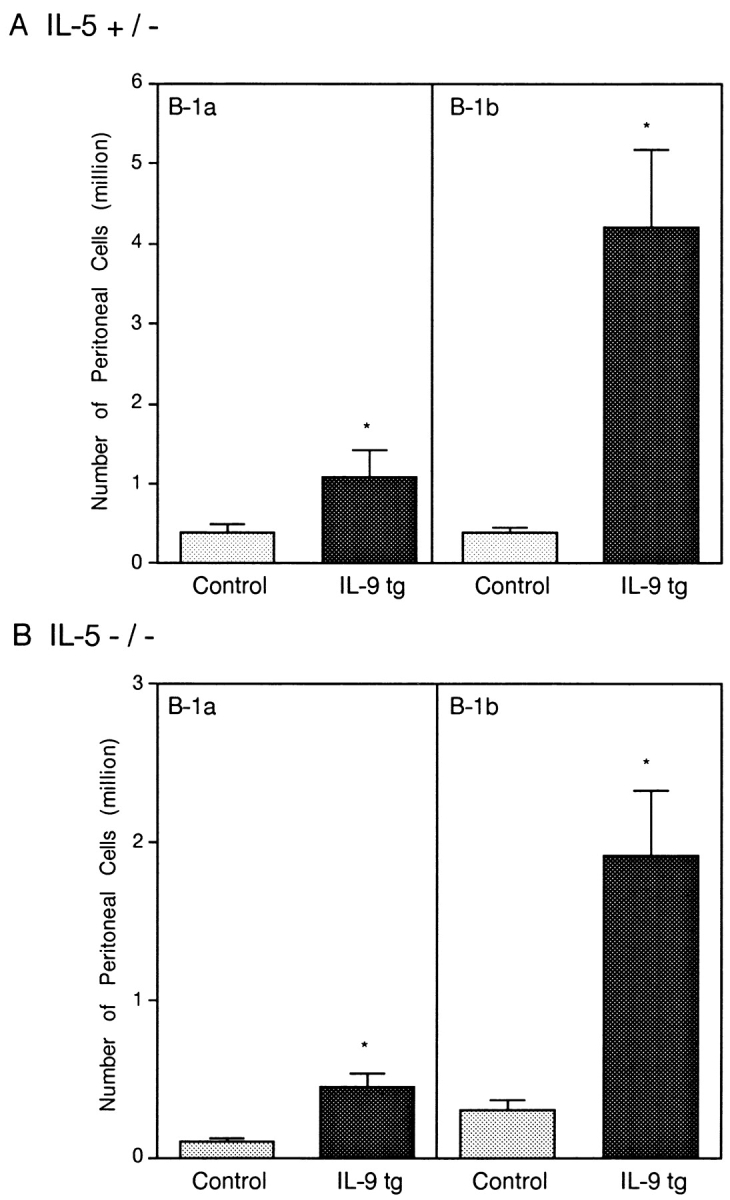
Accumulation of B-1b (IgM+Mac-1+CD5−) cells in IL-9 transgenic mice normal for IL-5 (A) or in mice deficient in IL-5 (B). Three-color staining of peritoneal cells for F2 hybrids was performed for groups of five individuals. Results are expressed in 106 cells/mouse, mean ± SEM, and are given for B-1a (IgM+Mac-1+CD5+) and B-1b cells (IgM+Mac-1+CD5−). *P < 0.05, Mann-Whitney test. tg, transgenic.
Preferential Expression of the IL-9 Receptor by Peritoneal B-1 Cells.
A simple explanation for the expansion of B-1 cells by IL-9 could be a B-1–specific expression of the IL-9 receptor. To analyze IL-9 receptor expression, peritoneal cells from normal FVB mice were stained with a fusion protein consisting of IL-9 fused to the Fc fragment of mouse IgG3. The binding of this protein was detected by FACS®, using a secondary antibody specific for IgG3. The specificity of the binding was demonstrated by its reversion in the presence of an excess of IL-9. As shown in Fig. 9 A, a fraction (∼34%) of peritoneal IgM+Mac-1+ cells were stained with this fusion protein. The labeling with anti-CD5 antibodies showed that both B-1a and B-1b populations expressed the IL-9 receptor. By contrast, the IL-9 receptor was barely detectable at the surface of IgM+ B cells from the spleen (Fig. 9 B).
Figure 9.
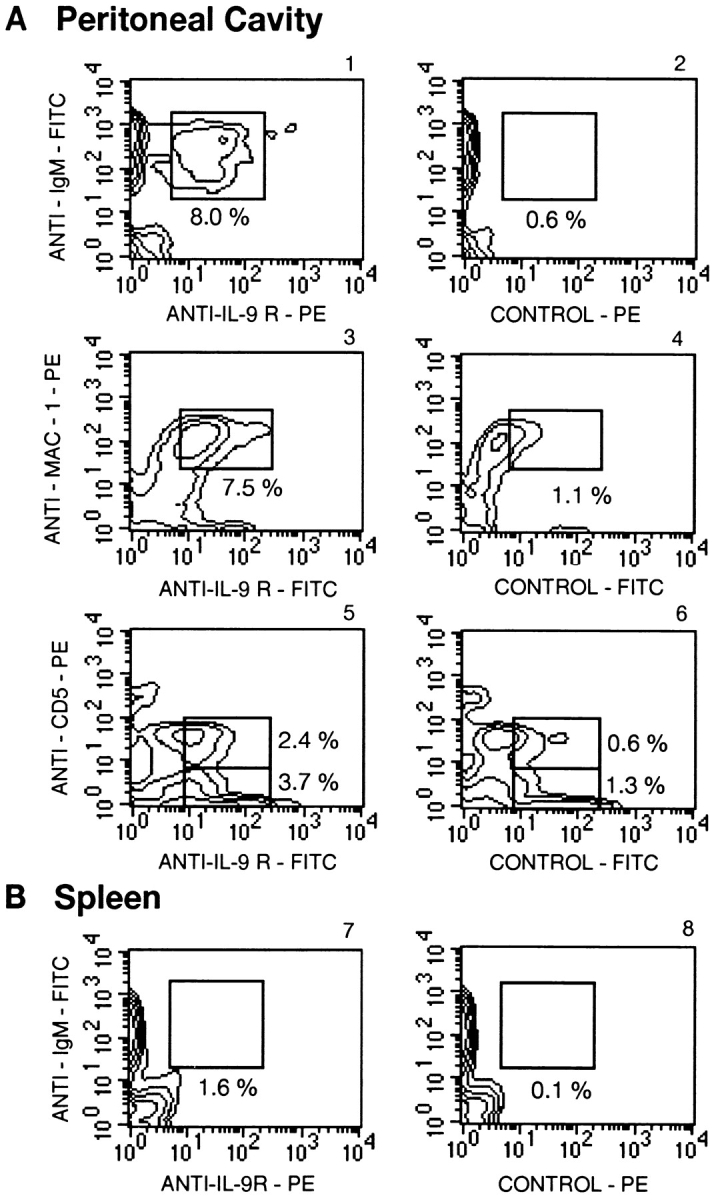
Expression of the IL-9 receptor (IL-9R) by peritoneal B-1 cells from normal FVB mice. Labeling was shown as a contour plot obtained by analysis of 104 cells from a pool of 10 individual mice. (A) Peritoneal cells from normal FVB mice were stained with the mIL-9–Ig fusion protein and anti-IgM (1 and 2), anti–Mac-1 (3 and 4), or anti-CD5 (5 and 6) antibodies as described in Materials and Methods. Controls consisted of cells labeled in the presence of an excess of free IL-9, as a competitor. (B) Splenic cells were similarly stained with the mIL-9–Ig fusion protein and anti-IgM (7 and 8) antibodies.
Distinct Specificities between B-1a and B-1b Cells in IL-9 Transgenic Mice.
Numerous studies have demonstrated a role for B-1 cells in the secretion of autoantibodies such as anti-RBC, antithymocyte, or RF (28–30). In particular, IL-5 transgenic mice showed elevated levels of anti-DNA antibodies in the serum, concomitant with the increased frequency of B-1 cells (25). The best-described specificity of the autoantibodies secreted by B-1 cells is directed against an antigen presented by RBCs treated by the proteolytic enzyme bromelain.
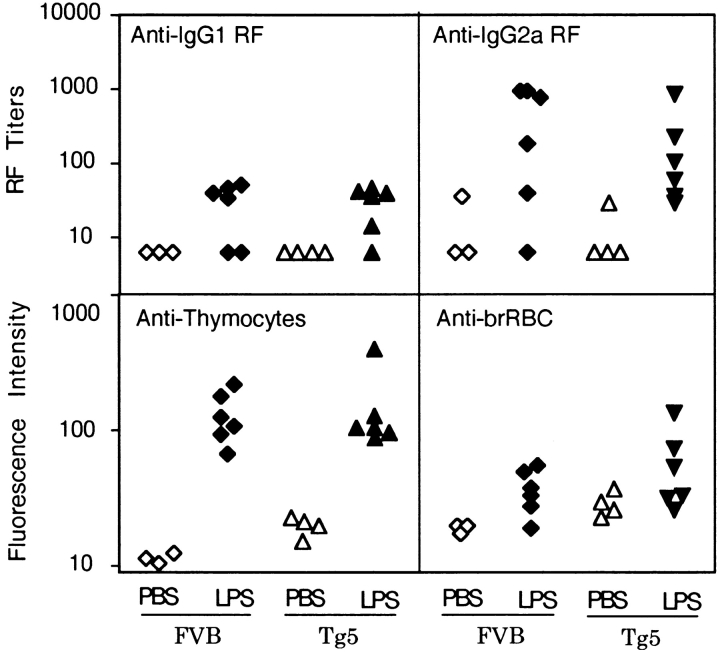
The concentrations of autoantibodies were measured in the serum of Tg5 or FVB mice that either had or had not received three in vivo injections of LPS, since it was known that this treatment upregulates autoantibody production (31). As shown in Fig. 10, we failed to detect any difference between IL-9 transgenic and FVB control mice concerning basal levels of anti-IgG1 or anti-IgG2a RFs, antithymocyte antibodies, or antibromelain-treated RBC autoantibodies. As expected, LPS injection increased the production of these antibodies but with the same intensity in IL-9 transgenic mice as in normal mice.
Figure 10.
Autoantibody concentrations in mice treated with LPS in vivo. Mice were treated three times at 1-wk intervals with PBS or 50 μg LPS i.p., and sera were taken 1 wk after the third injection. Sera were tested for concentrations of anti-IgG1 or anti-IgG2a RF as described in Materials and Methods. Values were given for each individual as an arbitrary titer giving 10% agglutination. Sera were also tested for antithymocyte activity and antibromelain-treated RBCs (Anti-brRBC). Autoantibodies fixed on thymocytes and RBCs were detected by staining with a fluorescent anti-IgM; results are given for each individual (three to four controls versus six LPS-treated mice) as mean fluorescence intensity.
Antibromelain-treated RBC specificity is considered to be a unique characteristic of a significant proportion of B-1 cells (19). Thus, the lack of difference in anti-RBC antibody production between IL-9 transgenic and FVB mice, even after LPS induction, suggests that IL-9–induced B-1 cells have a distinct specificity compared with the classical B-1 population. To check this hypothesis, we analyzed directly the specificity of peritoneal B cells by testing their ability to form rosettes with bromelain-treated RBCs.
Peritoneal cells from FVB or Tg5 mice were incubated with bromelain-treated RBCs, and rosetting cells were separated from free peritoneocytes on a Ficoll layer. The resulting RBC-specific B cells were subsequently stained with anti–Mac-1 and anti-CD5 fluorescent antibodies. The results of two independent experiments are shown in Table III. In both FVB and IL-9 transgenic mice, enrichment in RBC-specific cells was paralleled by an increase in the proportion of CD5+Mac-1+ cells, reflecting the high frequency of anti-RBC lymphocytes in this population. In IL-9 transgenic mice, selection of anti-RBC resulted in a significant drop in the percentage of Mac-1+CD5− cells, indicating that this specificity is much less frequent in this population. Thus, this experiment demonstrates that IL-9–induced B-1b cells do not exhibit the same autoimmune specificity as B-1a cells.
Table III.
Enrichment of B-1a Lymphocytes in Peritoneal Rosette-forming Cells from FVB and Tg5 Mice
| FVB | Tg5 | |||||||
|---|---|---|---|---|---|---|---|---|
| Percent in total | Percent in RFCs | Percent in total | Percent in RFCs | |||||
| Experiment 1 | ||||||||
| B-1a | 14 | 57 | 16 | 63 | ||||
| B-1b | 18 | 21 | 52 | 24 | ||||
| Experiment 2 | ||||||||
| B-1a | 9 | 57 | 16 | 33 | ||||
| B-1b | 11 | 10 | 50 | 18 | ||||
Peritoneal cells from FVB and Tg5 that formed rosettes with bromelain-treated RBCs were labeled with anti–Mac-1 and anti-CD5 as described in Materials and Methods. Results of two different experiments are shown as percentages of Mac-1+CD5+ (B-1a) and Mac-1+CD5− (B-1b) in total cells and in rosette-forming cells (RFCs).
Discussion
In this paper, we report that IL-9 induces a specific expansion of B-1 lymphocytes in vivo. In normal mice, this population constitutes only 1% of the total B cells and resides mainly in the peritoneal and pleuropericardial cavities. In IL-9 transgenics, we found a 15–20-fold increase in B-1 cell numbers at these locations. In addition, although B-1 lymphocytes were absent in the blood of normal animals, they represented up to 15% of blood B cells of IL-9 transgenics, based on Mac-1 and IgM expression.
Another characteristic of the peritoneal B-1 population found in IL-9 transgenics is the ratio between B-1a and B-1b cells in favor of the B-1b subset, contrasting with the more balanced situation between B-1a and B-1b subpopulations found in the FVB background. In addition, the B-1b expansion in IL-9 transgenic mice might be underestimated, since B-1 cells are supposed to lose Mac-1 in the spleen (22, 23). Therefore, additional criteria are critically needed to study the B-1b population in spleen and lymph nodes.
In most models, expansion of the B-1 population is associated with high levels of autoantibody secretion. In NZB and (NZB × NZW)F1 mice, CD5+ B cells accumulate in the peritoneal cavity and in the spleen, and produce autoantibodies against single-stranded DNA, thymocytes, or bromelain-treated mouse erythrocytes (28, 32). In another in vivo model, increased CD19 expression also resulted in an expansion of CD5+ B cells within the peritoneum and spleen, correlating with high levels of anti-DNA antibodies and RF (33). Other models resulting from mutation in the SHP1 gene (34) or targeting of genes involved in signal transduction, such as Btk (35) or vav (36, 37), also support the link between B-1 cell development and levels of autoantibodies.
In the IL-9 transgenics, we failed to detect any increase in the production of RF, antithymocyte, or antibromelain-treated RBC antibodies. In particular, antibromelain-treated RBC specificity was mainly found in peritoneal CD5+ B-1 cells, as demonstrated in the experiments with rosette-forming cells with bromelain-treated RBCs. This is in line with a previous report indicating that the antibromelain-treated RBC specificity is restricted to CD5+ cells (38). However, we cannot formally exclude that the activity of IL-9 could be selective within the B-1b population and that expanded cells derive from a subset of B-1b cells which has properties distinct from B-1b cells from normal animals.
In our model, IL-9 production induced an increase in all spontaneous Ig isotypes. The most prominent were a 20- and a 9-fold enhancement in IgG1 and IgE, respectively; IgM, IgG2a, IgG2b, and IgG3 levels showed only a 3–4-fold increase. This is again different from other models with expansion of B-1 cells, such as mice homozygous for the viable mutation motheaten me v, where all B cells are CD5+, and IgM, IgG3, and IgA serum concentrations are 10–50 times greater than in control mice but IgG1 levels remain unaffected (39). This difference might also be related to the fact that B-1b cells are more expanded than B-1a cells in IL-9 transgenic mice. Although conventional B cells did not seem to express the IL-9 receptor to the same extent as B-1 cells and were not expanded in response to IL-9, we could not rule out the possibility that they also contribute to this increase in Ig production. For example, other IL-9–responsive cells, such as mast cells, T cells, or even the B-1 cells, could mediate an indirect effect of IL-9 on conventional B cells through the production of other cytokines. In this respect, B-1 cells have been reported to be an important source of IL-10 (40). However, we failed to detect any increase in IL-10 production in IL-9 transgenic mice (our unpublished data).
The observation that B-1 cell numbers were increased by IL-9 in the absence of IL-5 ruled out the possibility that the effect of IL-9 is mediated by this cytokine. IL-5 is a potent activator of B-1 cells, and mice overexpressing IL-5 are also characterized by an increase in B-1 cells. However, in contrast to IL-9 transgenics, the cellular expansion exclusively concerned the B-1a population of the spleen (25). The serum IgG1 levels were not modified, but IgM and IgA levels were increased by eight- and fourfold, respectively, in IL-5 transgenics.
Surprisingly, the IL-9 receptor appeared to be expressed at similar levels in many normal B-1a and B-1b lymphocytes, whereas the B-1b population is preferentially expanded in vivo. These data suggest that distinct homeostasis mechanisms control these subpopulations. The fact that the number of CD5+ B cells is endogenously controlled has been reported, but the mechanisms remain unclear (41). In addition, despite the high level of IL-9 receptor at the surface of B-1 cells, we failed to demonstrate in vitro proliferative responses of these cells to IL-9 (our unpublished data), suggesting that the actual role of IL-9 on B-1 cells might be related to other features, such as differentiation or survival.
In this report, the increase of antigen-specific Ig concentrations in TNP-Ficoll– or KLH-immunized IL-9 transgenic mice and the specific expansion of the B-1 populations suggest that these cells could actively participate in the immune response against various proteic antigens. Future experiments will be needed to determine their actual role in more physiological responses and particularly in allergic responses, in which a role for IL-9 was recently demonstrated (7).
Acknowledgments
The authors thank Ms. Marceline Bosefe and Ms. Véronique Lefèvre for their technical assistance, and Drs. Cesar Cambiaso and Jean-Paul Coutelier for their help with measurement of antibody concentrations.
This work was supported in part by the Belgian Federal Service for Scientific, Technical and Cultural Affairs, and the Opération Télévie. J.-C. Renauld is a research associate with the Fonds National de la Recherche Scientifique, Belgium.
Abbreviation used in this paper
- RF
rheumatoid factor
References
- 1.Renauld, J.-C., and J. Van Snick. 1998. Interleukin-9. In The Cytokine Handbook, 3rd ed. A. Thompson, editor. Academic Press, Inc., New York. 313–331.
- 2.Merz H, Houssiau FA, Orscheschek K, Renauld J-C, Fliedner A, Herin M, Noel H, Kadin M, Mueller-Hermelink HK, Van Snick J, Feller AC. IL-9 expression in human malignant lymphomas: unique association with Hodgkin's disease and large cell anaplastic lymphoma. Blood. 1991;78:1311–1317. [PubMed] [Google Scholar]
- 3.Renauld J-C, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994;9:1327–1332. [PubMed] [Google Scholar]
- 4.Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, Ehrlich-Kautzky E, Schou C, Krishnaswamy G, Beaty TH. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum IgE concentrations. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 5.Postma DS, Beecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma-bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 6.Holroyd KJ, Martinati LC, Trabetti E, Scherpbier T, Eleff SM, Boner AL, Pignatti PF, Kiser MB, Dragwa CR, Hubbard F. Asthma and bronchial hyperresponsiveness linked to the XY long arm pseudoautosomal region. Genomics. 1998;52:233–235. doi: 10.1006/geno.1998.5445. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaides N, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, et al. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci USA. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, Renauld J-C. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J Immunol. 1998;160:3989–3996. [PubMed] [Google Scholar]
- 9.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dugas B, Renauld J-C, Pène J, Bonnefoy JY, Petit-Frère C, Braquet P, Bousquet J, Van Snick J, Mencia-Huerta J-M. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–1692. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 11.Petit-Frère C, Dugas B, Braquet P, Mencia-Huerta J-M. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–151. [PMC free article] [PubMed] [Google Scholar]
- 12.Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gretener D, Bonnefoy JY. Interleukin-7 (IL-7) enhances class switching to IgE in the presence of T cells via IL-9 and sCD23. Blood. 1998;91:1355–1361. [PubMed] [Google Scholar]
- 13.Druez C, Coulie P, Uyttenhove C, Van Snick J. Functional and biochemical characterization of mouse P40/IL-9 receptors. J Immunol. 1990;145:2494–2499. [PubMed] [Google Scholar]
- 14.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 15.Gajewski TF, Fallarino F, Uyttenhove C, Boon T. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor. Superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156:2909–2917. [PubMed] [Google Scholar]
- 16.Coutelier JP, Van Roost E, Lambotte P, Van Snick J. The murine antibody response to lactate dehydrogenase-elevating virus. J Gen Virol. 1986;67:1099–1108. doi: 10.1099/0022-1317-67-6-1099. [DOI] [PubMed] [Google Scholar]
- 17.Coutelier J-P, Coulie PG, Wauters P, Heremans H, van der Logt J. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol. 1990;64:5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Snick J, Masson P. Age-dependent production of IgA and IgM autoantibodies against IgG2a in a colony of 129/Sv mice. J Exp Med. 1979;149:1519–1530. doi: 10.1084/jem.149.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy RR, Carmack CE, Shinton SA, Ribblet RJ, Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 20.Cunningham AJ. Large numbers of cells in normal mice produce antibody components of isologous erythrocytes. Nature. 1974;252:749–751. doi: 10.1038/252749a0. [DOI] [PubMed] [Google Scholar]
- 21.Poncet P, Huetz F, Marcos M-A, Andrade L. All VH11 genes expressed in peritoneal lymphocytes encode anti-bromelain-treated mouse red blood cell autoantibodies but other VH gene families contribute to this specificity. Eur J Immunol. 1990;20:1583–1589. doi: 10.1002/eji.1830200726. [DOI] [PubMed] [Google Scholar]
- 22.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 23.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 24.Wetzel GD. Interleukin 5 regulation of peritoneal Ly-1 B lymphocyte proliferation, differentiation and autoantibody secretion. Eur J Immunol. 1989;19:1701–1707. doi: 10.1002/eji.1830190926. [DOI] [PubMed] [Google Scholar]
- 25.Tominaga A, Takaki S, Koyama N, Katoh S, Matsumoto R, Migita M, Hitoshi Y, Hosoya Y, Yamauchi S, Kanai Y, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hitoshi Y, Yamaguchi N, Mita S, Sonoda E, Takaki S, Tominaga A, Takatsu K. Distribution of IL-5 receptor positive B cells. Expression of IL-5 receptor on Ly-1 (CD5)+ B cells. J Immunol. 1990;144:4218–4225. [PubMed] [Google Scholar]
- 27.Bao S, Beagley KW, Murray AM, Caristo V, Matthaei KI, Young IG, Husband AJ. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology. 1998;94:181–188. doi: 10.1046/j.1365-2567.1998.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD, Herzenberg LA. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayakawa K, Carmack CE, Hyman R, Hardy RR. Natural autoantibodies to thymocytes: origin, VH genes, fine specificities, and the role of Thy-1 glycoprotein. J Exp Med. 1990;172:869–878. doi: 10.1084/jem.172.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;329:81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 31.Izui S, Eisenberg RA, Dixon FJ. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979;122:2096–2102. [PubMed] [Google Scholar]
- 32.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal, immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- 34.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 35.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Müller S, Kantor AB, Herzenberg LA, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 36.Tarakhovsky A, Turner M, Shaal S, Mee PJ, Duddy LP, Rajewsky K, Tybulewicz V. Defective antigen receptor-mediated proliferation of B and T cells in the absence of vav. . Nature. 1995;373:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Alt FW, Davidson L, Orkin SH, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto- oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 38.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+(CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidman CL, Shultz LD, Hardy RR, Hayakawa K, Herzenberg LA. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986;232:1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- 40.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 41.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]