Abstract
To localize the immunoglobulin (Ig)-binding regions of the human Fcα receptor (FcαRI, CD89) and the bovine Fcγ2 receptor (bFcγ2R), chimeric receptors were generated by exchanging comparable regions between these two proteins. FcαRI and bFcγ2R are highly homologous and are more closely related to each other than to other human and bovine FcRs. Nevertheless, they are functionally distinct in that FcαRI binds human IgA (hIgA) but not bovine IgG2 (bIgG2), whereas bFcγ2R binds bIgG2 but not hIgA. FcαRI and bFcγ2R possess extracellular regions consisting of two Ig-like domains, a membrane-distal extracellular domain (EC1), a membrane-proximal EC domain (EC2), a transmembrane region, and a short cytoplasmic tail. Chimeras constructed by exchanging complete domains between these two receptors were transfected to COS-1 cells and assayed for their ability to bind hIgA- or bIgG2-coated beads. The results showed that the Ig-binding site of both FcαRI and bFcγ2R is located within EC1. Supporting this observation, monoclonal antibodies that blocked IgA binding to FcαRI were found to recognize epitopes located in this domain. In terms of FcR–Ig interactions characterized thus far, this location is unique and surprising because it has been shown previously that leukocyte FcγRs and FcεRI bind Ig via sites principally located in their EC2 domains.
Keywords: Fc receptor, CD89, bovine Fcγ2 receptor, immunoglobulin A, myeloid
Immunoglobulin (Ig) Fc receptors (FcRs) expressed on phagocytic cells provide a crucial link between the humoral and cellular branches of the immune system. Ligation of FcRs by antigen-bound Ig leads to cellular activation and triggering of powerful effector mechanisms (1, 2). In humans and other mammals, IgA predominates in mucosae and, furthermore, comprises a substantial proportion of the circulating Ig pool. At mucosal surfaces IgA provides a first-line protective function, termed immune exclusion, whereby it inhibits microbial colonization on epithelial cells and penetration of harmful antigens. In addition, the protective function of IgA both in mucosa and in the circulation may be reinforced by interaction of IgA-complexed antigens with the myeloid FcαRI (CD89)(3, 4). FcαRI is expressed on monocytes, macrophages, polymorphonuclear granulocytes, and eosinophils, and its cross-linking triggers a variety of immunological effector functions, including phagocytosis, antibody-dependent cellular cytotoxicity, and release of inflammatory mediators and cytokines (3–6).
Among FcRs characterized until now, FcαRI is most closely related to the bovine Fc receptor for IgG2 (bFcγ2R)1 expressed on monocytes and granulocytes. In fact, these two FcRs are more closely related to each other than to any known human or bovine FcRs (7). More recently, it has been shown that FcαRI and bFcγ2R are members of a new gene family that apparently evolved from a common ancestral gene. Other human genes belonging to this family include the natural killer cell inhibitory receptors (KIRs), the Ig-like transcripts (ILTs), the leukocyte and monocyte/ macrophage Ig-like receptors (LIRs, MIRs), LAIR-1, and HM18 (8–13). These genes are located close to the FcαRI gene within the so-called leukocyte receptor complex on chromosome 19q13.4 (13–15). Several murine members of the same gene family, gp49B1 (a structural homologue of human KIRs), and the paired Ig-like receptors A and B (PIR-A and PIR-B), have also been described (16–18).
FcαRI and bFcγ2R are both transmembrane glycoproteins composed of two extracellular (EC) Ig-like domains (EC1 and EC2), a transmembrane region containing a charged arginine residue, and a short cytoplasmic tail devoid of signaling motifs (7, 19). Signal transduction via FcαRI is mediated via the FcR γ chain, which associates with FcαRI through the charged arginine residue within its transmembrane domain but does not affect its affinity for IgA (20–23). Despite the high level of amino acid identity (41%) within the EC and transmembrane regions of FcαRI and bFcγ2R, these two receptors are functionally quite distinct in that FcαRI binds IgA but not bIgG2, whereas bFcγ2R binds bIgG2 but not IgA. Therefore, to map the ligand-binding domains of these two FcRs we utilized their high degree of identity and exchanged homologous regions between them. Based on knowledge of interactions between other two-domain FcRs (FcγRII, FcγRIII, and FcεRI) with their respective ligands (IgG or IgE), we expected the Ig-binding sites to be located within the membrane-proximal EC2 domain (24– 30). Surprisingly, however, our results demonstrated that the ligand-binding region of both FcαRI and bFcγ2R is located in their membrane-distal EC1 domain. In part, this finding probably reflects the evolutionary development of FcαRI and bFcγ2R from an ancestral gene distinct from the putative FcγR/FcεR precursor.
Materials and Methods
Cell Culture.
COS-1 cells were maintained in DMEM (BioWhittaker) supplemented with 10% FCS, 1 mM L-glutamine, and 50 μg/ml gentamycin (Life Technologies, UK). The murine IIA1.6 B cell line that coexpresses FcαRI and the FcR γ chain has been described previously (21).
cDNAs and Construction of Chimeric FcRs.
cDNAs encoding the complete FcαRI coding region and a mutant cDNA encoding a soluble form of FcαRI were gifts from Dr. C. Maliszewski (Immunex Corp., Seattle, WA) (19, 31). cDNA for bFcγ2R has been described previously (7). Chimeric cDNAs were constructed by overlap extension PCR (21). Although the genomic structure of bFcγ2R is unknown, the high homology to FcαRI allowed us to infer intron–exon boundaries because amino acid residues that link the FcαRI exons are identical to those at comparable positions in bFcγ2R (Fig. 1). Primers were thus designed to allow the fusion of exons at these residues. To construct the bEC1(1–50)-FcαRI mutant, primers were designed to allow the fusion of the first 50 amino acids of bFcγ2R to FcαRI at isoleucine 50; both FcαRI and bFcγ2R have isoleucine residues at this position, which lies almost exactly in the middle of the EC1 domains. The integrity of all chimeric cDNAs was confirmed by sequence analysis. Chimeric FcR cDNAs were cloned into the pCDNA3 mammalian expression vector (Invitrogen, The Netherlands) before transfection. The pCMV-GFP plasmid, which directed the expression of green fluorescent protein (GFP), was constructed by inserting the CMV promoter region from pCDNA3 into the multiple cloning site of the pEGFP-1 vector (Clontech).
Figure 1.
Alignment of amino acid sequences of FcαRI (X54150) and bFcγ2R (Z37506). S1 exons are shown from the methionine initiation codons. Because the gene structure of bFcγ2R is unknown, intron–exon boundaries are shown only for FcαRI. Amino acids that link two FcαRI exons are underlined, and the exon designation is shown above the sequence. Note that exon-linking amino acids are conserved between FcαRI and bFcγ2R. The first 19 amino acids of both sequences are considered to represent NH2-terminal signal peptides, which are removed before cell surface expression. Thus, glutamine (Q) 22 is proposed to be the first amino acid of both mature proteins and therefore is designated +1. Isoleucine (I) 50 is also underlined. Residues of bFcγ2R identical to those of FcαRI are designated (*), and gaps that have been inserted to line up the sequences are designated (–) (also see reference 7).
Transfections.
COS-1 cells were transiently transfected with 2 μg chimeric FcR cDNA constructs by means of Fugene 6 transfection reagent (Boehringer Mannheim, Germany) according to the manufacturer's instructions. In some experiments, 1 μg pCMV-GFP was cotransfected together with the FcR constructs. Cells were incubated at 37°C in a humidified CO2 atmosphere for 48 h before harvesting.
Ig-binding Assays.
Uncoated magnetic M-450 Dynabeads (Dynal, Norway) were coated, according to the manufacturer's instructions, with either human serum IgA (hIgA) or bovine IgG2 (bIgG2), which were purified as previously described (7, 32). Due to low transfection efficiency of some DNA constructs (see Results), transfected COS-1 cells were first enriched for those becoming positive for gene expression by cotransfection of the FcR and pCMV-GFP constructs. Experiments showed that most fluorescent (GFP+) cells had also taken up both plasmids, thus expressing the chimeric FcR together with GFP (see Results). Therefore, binding assays were performed as follows: 5 × 104 GFP+ COS-1 cells (which had also been cotransfected with an FcR construct) were purified in a FACSVantage® cell sorter (Becton Dickinson) and mixed with Ig-coated Dynabeads in a final volume of 50 μl per well in V-bottomed microtiter plates. After a 15-min incubation at room temperature, the plate was spun at 50 g for 1 min and incubated for an additional 45 min at room temperature. Cells and beads were resuspended and examined for the presence of rosettes, using a combination of light and fluorescent microscopy, in a Nikon Eclipse E800 microscope. Rosettes were defined as GFP+ cells binding four or more Ig-coated beads and at least 200 GFP+ COS-1 cells were counted for each determination. For blocking studies, cells were incubated with either mAb My43 (50 μl culture supernatant) or CC-G24 (50 μl ascites fluid diluted 1:4) for 30 min at room temperature before the addition of Ig-coated beads.
Production and Purification of Recombinant Soluble FcαRI.
A cDNA that encodes a soluble form of FcαRI was expressed in Chinese hamster ovary cells by means of the pEE14 expression system (Lonza, UK), and the protein was isolated from the culture supernatant by affinity chromatography with Sepharose-bound human IgA (van Zandbergen, G., and C. van Kooten, unpublished data).
Monoclonal Antibodies.
The previously described FcαRI mAbs My43 (murine IgM), A3, A59, A62, and A77 (all murine IgG1) were used in this study (33, 34). My43 and A62 were gifts from Dr. Li Shen (Dartmouth Medical School, Lebanon, NH) and Dr. Max Cooper (University of Alabama, Birmingham, AL), respectively. A77 was supplied by Medarex Europe (The Netherlands), and A3 and A59 were purchased from Immunotech (France) and Research Diagnostics Inc. respectively. The bFcγ2R mAb CC-G24 (murine IgM) was generated by immunizing mice with bFcγ2R protein purified from cattle leukocytes, and the specificity was confirmed by staining COS-7 cells transfected with cDNA encoding the bFcγ2R or bovine FcγRII (Howard, C.J., unpublished data). To obtain new FcαRI mAbs, female BALB/C mice were immunized with purified soluble FcαRI. Splenocytes isolated from immunized animals were fused with myeloma cells (SP20) in the presence of 50% polyethylene glycol. The cell suspension was diluted in IMDM supplemented with 10% FCS, hypoxanthine (100 μmol), aminopterin (0.4 μM), thymidine (16 μM), 500 pg/ml IL-6, 100 U/liter penicillin, and 100 μg/ml streptomycin. Cells producing antibodies to FcαRI were subcloned by limiting dilution. Five clones producing mAb to FcαRI were expanded and the specificity was determined by FACS® analysis (see below). To define the capacity of these new mAbs to inhibit binding of IgA to FcαRI, blocking studies were performed as follows: FcαRI mAbs or control mAbs of the same isotype were diluted in FACS buffer and incubated together with FcαRI-transfected IIA1.6 cells for 15 min at 4°C. Purified human serum IgA, which had previously been heat-aggregated for 1 h at 63°C (aIgA), was then added for 1 h at 4°C. Cells were washed and bound aIgA was detected by incubation with a goat anti– human IgA F(ab)2-PE polyclonal antibody conjugate (Southern Biotechnology Associates, Inc.) in FACS® analysis. Isotype control antibodies for murine IgG1 were purchased from Becton Dickinson, and those for murine IgM were provided by Dr. Robert Burns (Scottish Agricultural Science Agency, Edinburgh, UK).
FACS® Analysis.
Cells (5 × 105) were washed twice with FACS buffer (PBS/0.5% BSA/0.02% azide) and incubated with either FcαRI mAb (murine IgM or IgG1) culture supernatant or the appropriate isotype control supernatant for 1 h at 4°C. Cells were then washed twice with FACS buffer and incubated for 1 h at 4°C with either goat anti–mouse (GAM) IgM-FITC conjugate (1:150 final dilution), or a GAM IgG1-PE conjugate (1:150 final dilution) (both from Southern Biotechnology Associates, Inc.). In experiments where GFP+ cells were analyzed for chimeric FcR expression, a GAM IgG1 Tricolor secondary reagent (1:200 final dilution) (Caltag Labs.) was used. After washing twice with FACS buffer, cells were fixed in PBS-buffered 1% (wt/vol) paraformaldehyde at 4°C and analyzed on a FACScan®. Data acquisition was conducted with Lysis II software (Becton Dickinson), and data analysis was performed using WinMDI software (available from The Scripps Research Institute, La Jolla, CA).
Results
The EC1 Domains of FcαRI and bFcγ2R Mediate Ligand Binding.
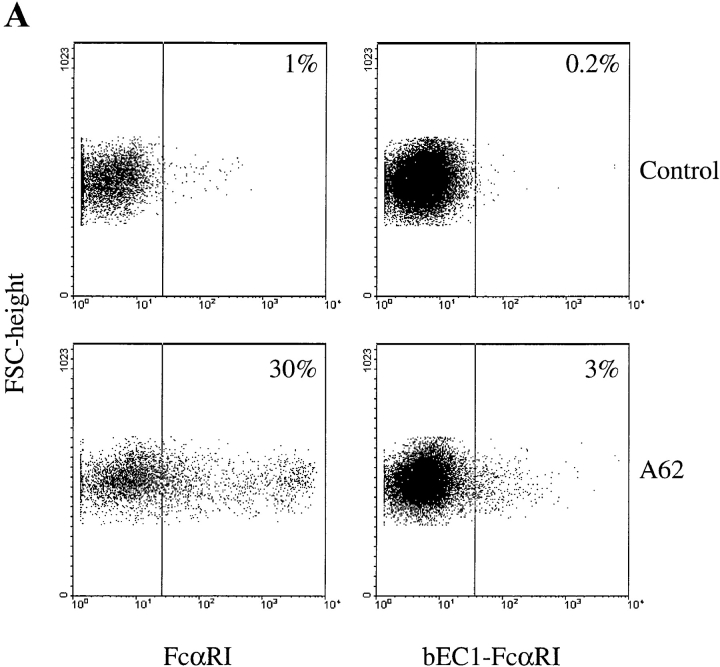
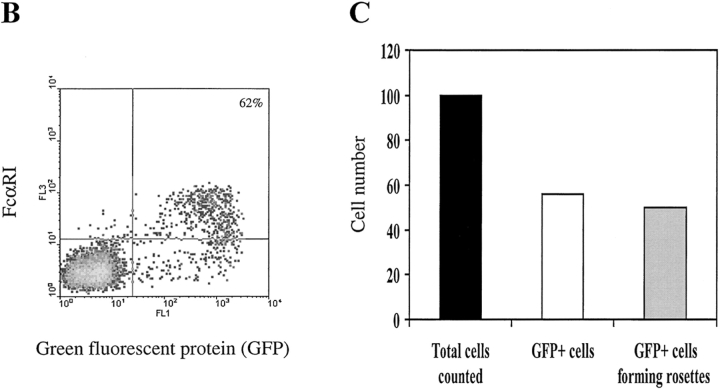
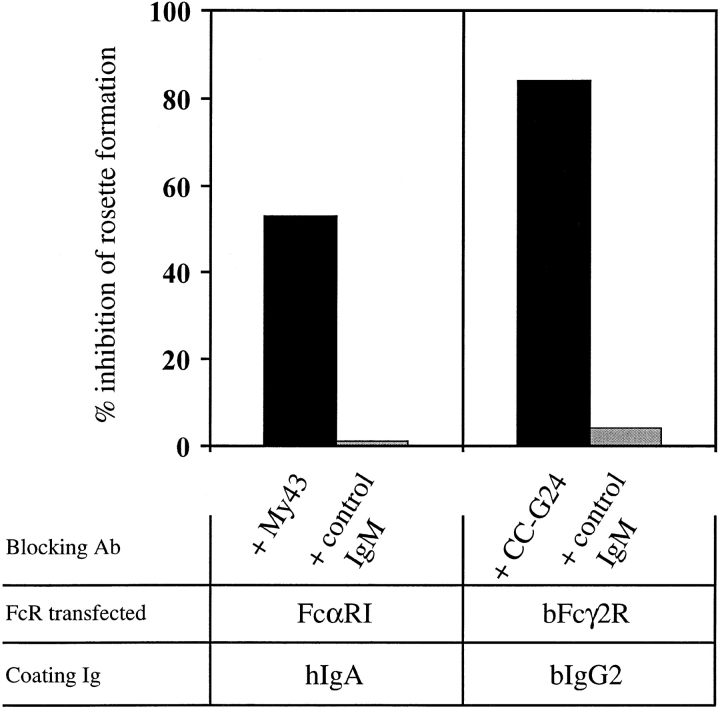
To map the ligand-binding domains of FcαRI and bFcγ2R, we generated five chimeric receptors as follows: hEC-bFcγ2R, consisting of the two EC domains of FcαRI fused to the transmembrane/cytoplasmic (TM/C) domain of bFcγ2R; bEC-FcαRI, the two bovine EC domains fused to the TM/C domain of FcαRI; hEC1-bFcγ2R, the EC1 domain of FcαRI fused to the EC2 TM/C region of bFcγ2R; bEC1-FcαRI, the EC1 domain of bFcγ2R joined to the EC2 TM/C region of FcαRI; and bEC1(1–50)-FcαRI, the first 50 amino acids of bFcγ2R fused at isoleucine 50 to FcαRI (Fig. 1). Together with wild-type FcαRI and bFcγ2R cDNAs, individual chimeric FcR constructs were transfected to COS-1 cells, and their cell surface expression was assessed by FACS® analysis and by a specific binding assay using Ig-coated beads (see Materials and Methods). Initial experiments revealed the transfection efficiency of individual constructs to be quite variable. The most efficient construct directed expression of FcαRI on the surface of ∼30% of COS-1 cells, whereas the least efficient (bEC1-FcαRI) was expressed by only 3% of the transfectants (Fig. 2 A). Therefore, we developed a method to selectively enrich for transiently transfected cells by cotransfecting a plasmid that directed expression of GFP (visualized by green fluorescence) together with the chimeric FcR constructs. Most GFP+ COS-1 cells cotransfected with FcαRI were recognized by mAb A62 (Fig. 2 B), and formed rosettes with hIgA-coated beads (Fig. 2 C). By this procedure we obtained an approximately twofold enrichment of chimeric FcR-expressing cells, which in the case of FcαRI resulted in >60% of cells being reactive with FcαRI mAb and further able to form rosettes with hIgA coated beads (Fig. 2, B and C). It should be noted that GFP− COS-1 almost never formed rosettes, most likely because transfectants expressing FcαRI alone accounted for only ∼1% of the total cells (Fig. 2 B). We, furthermore, demonstrated the specificity of our binding assay by using blocking mAbs specific for FcαRI or bFcγ2R to inhibit rosette formation (Fig. 3). The finding that the inhibition obtained with My43 was only partial (∼50%) was most likely explained by the use of culture supernatant other than a higher concentration of purified antibody which was not available.
Figure 2.
Expression of chimeric FcR by COS-1 cells. (A) Cos-1 cells were transfected with the indicated constructs 2 d before harvesting and FACS® analysis. Cells were stained with either FcαRI EC2-specific mAb A62 (mouse IgG1) (bottom panels) or an appropriate isotype control (top panels), followed by a GAM IgG1 Tricolor reagent. Numbers in the top right corners of the plots refer to the percentage of positive cells. (B) Enrichment of FcαRI expression in COS-1 cells cotransfected with GFP. Cells transfected with both FcαRI and GFP were stained with FcαRI EC2-specific mAb A62 followed by GAM IgG1 Tricolor reagent and analyzed by FACS®. More than 60% of the GFP+ cells also express FcαRI. (C) Rosette formation by COS-1 cells cotransfected with both FcαRI and GFP and exposed to hIgA-coated beads. Rosettes were quantified as specified in Materials and Methods. Black bar, total number of cells counted; white bar, total number of GFP+ cells assessed by fluorescent microscopy; gray bar, number of GFP+ cells forming rosettes. Results shown are representative of three separate experiments.
Figure 3.
Specificity of the bead rosetting assays. COS-1 cells were cotransfected with the indicated FcR construct together with pCMV-GFP. GFP+ cells were purified as described in Materials and Methods and incubated with the relevant murine IgM-blocking mAb or an irrelevant murine IgM control mAb for 30 min before addition of Ig-coated beads. Results are shown as percentage of inhibition of rosette formation when compared with transfectants that were not incubated with mAb before rosetting analysis. Results shown are representative of two experiments.
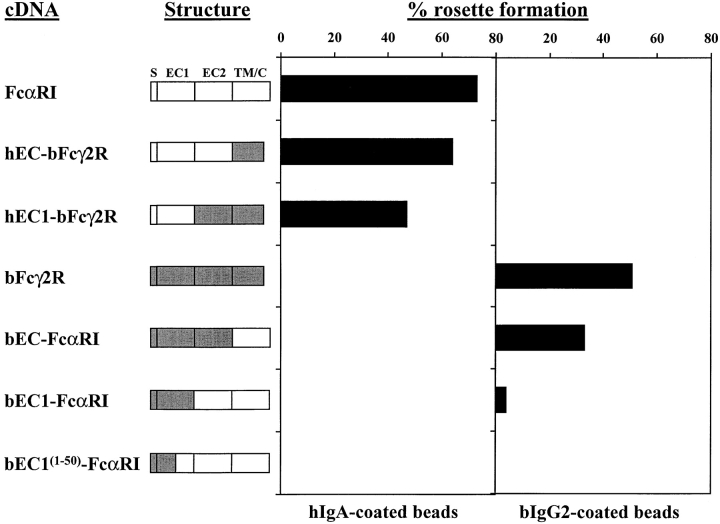
FcαRI does not bind bIgG2, and bFcγ2R does not bind hIgA (reference 7 and this paper); accordingly, we neither observed rosettes when hIgA-coated beads were mixed with bFcγ2R transfectants, nor when bIgG2-coated beads were mixed with FcαRI transfectants (Fig. 4). Binding studies with COS-1 cells enriched for FcR expression as described above, showed not unexpectedly that wild-type FcαRI and bFcγR2 transfectants produced the highest levels of rosette formation with hIgA- and bIgG2-coated beads, respectively (Fig. 4). Transfectants expressing chimeras coding for the entire EC portions of the receptors (hEC-bFcγ2R and bEC-FcαRI) bound their respective Ig-coated beads efficiently, although at a slightly lower level than their wild-type counterparts (Fig. 4). The fact that the hEC1-bFcγ2R chimera retained IgA-binding capacity demonstrated that the binding site of FcαRI lies within the membrane-distal EC1 domain. Furthermore, because this chimera did not form rosettes with bIgG2-coated beads, our finding further suggested that the binding site for bIgG2 in bFcγ2R was not located within the EC2 domain of this receptor. Conversely, the bEC1-FcαRI chimera did form rosettes with bIgG2-coated beads, but not with beads coated with hIgA.
Figure 4.
Rosette formation by FcR/GFP cotransfected COS-1 cells. Schematic representation of wild-type and chimeric FcRs. Unshaded regions are derived from FcαRI, shaded regions from bFcγ2R. S, signal peptide; EC1, extracellular domain 1; EC2, extracellular domain 2; TM/C, transmembrane/cytoplasmic tail. GFP+ transfectants were purified from COS-1 cells cotransfected with GFP and FcR constructs as described. Ig-binding to FcRs coexpressed with GFP was assessed by rosetting with either hIgA- or bIgG2-coated beads. More than 200 cells were counted for each determination, and the number of cells binding four or more Ig-coated beads is expressed as percentage rosette formation. Results are representative of three separate experiments.
Altogether these results showed that, in common with FcαRI, the ligand-binding site of bFcγ2R appeared to lie within the EC1 domain. It should be noted, however, that the level of binding obtained with the two EC1 chimeras (hEC1-bFcγ2R and bEC1-FcαRI) was reduced when compared with the wild-type receptors, and the EC chimeras (hEC-bFcγ2R and bEC-FcαRI) (Fig. 4). Futhermore, to better localize the Ig-binding sites of these two receptors, a further chimera was constructed in which the first 50 amino acids of the EC1 domain were from bFcγ2R, while the remaining EC1 (49 amino acids) and the rest of the receptor were from FcαRI [bEC1(1–50)-FcαRI]. Although this chimera was expressed at the cell surface and could be recognized by the majority of FcαRI mAb, it bound neither hIgA- nor bIgG2-coated beads.
EC1-specific Antibodies Block FcαRI Binding of IgA.
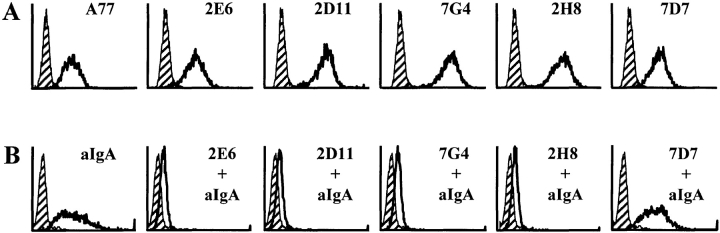
To confirm that the IgA-binding site of FcαRI lies within the EC1 domain, we mapped the specific epitopes for a number of blocking and nonblocking mAbs. Of the previously described FcαRI mAbs, only My43 (murine IgM) was able to block the binding of hIgA to FcαRI (33). Four others (A3, A59, A62, and A77, all murine IgG1) did not inhibit binding (34). We also included a number of new FcαRI mAbs (2E6, 2D11, 7G4, 2H8, and 7D7, all murine IgG1), raised against a soluble form of FcαRI. These mAbs were shown to be specific for FcαRI by reaction with IIA1.6 cells expressing this receptor (Fig. 5 A). They were next assayed for ability to block binding of heat-aggregated hIgA to FcαRI: mAbs 2E6, 2D11, 7G4, and 2H8 produced such inhibition while 7D7 did not (Fig. 5 B). Accordingly, we presumed that the blocking mAbs would prove to be EC1-specific, whereas the nonblocking ones would be EC2 specific.
Figure 5.
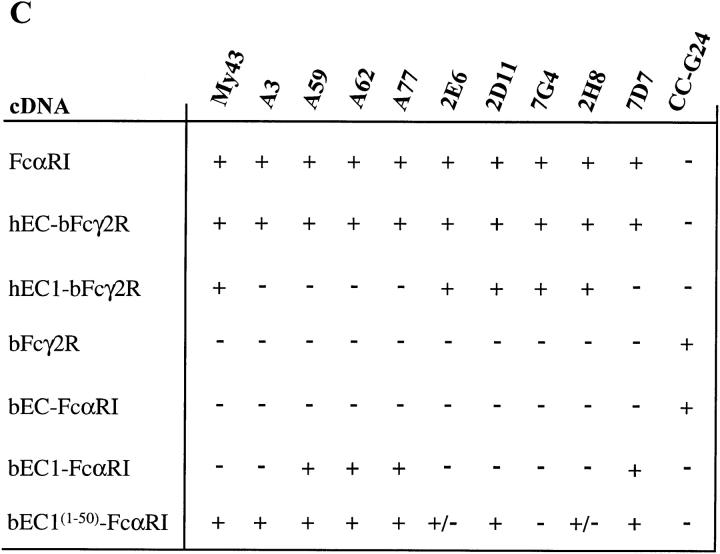
(A) FACS® analysis of newly produced mAbs to FcαRI compared with mAb A77 of similar specificity. FcαRI-expressing murine B cells (IIA1.6) were incubated with mAbs as indicated (white peaks) or with concentration and isotype-matched control mAbs (hatched peaks), followed by a GAM IgG1–PE conjugate. (B) FcαRI-expressing IIA1.6 cells were incubated without (left panel) or with mAbs as indicated, followed by heat-aggregated hIgA (aIgA). After 1 h at 4°C, cells were washed and bound hIgA was detected by incubation with goat anti–human IgA F(ab)2-PE conjugate (white peaks). Cells incubated with only the secondary reagent served as negative controls (hatched peaks). (C) Reactivity of chimeras (left) with a panel of FcαR and bFcγ2R mAb (top) as measured by FACS® analysis. Binding was graded as follows: +, strong binding; +/−, weak binding; −, no binding.
To test this hypothesis, we screened the reactivity of all mAbs against the panel of chimeric FcR expressed in COS-1 cells by FACS® analysis. Indeed, all mAbs capable of blocking the binding of heat-aggregated hIgA to FcαRI (Fig. 5 B), mapped to the EC1 domain (Fig. 5 C). Also, all nonblocking mAbs were directed against the EC2 domain, except for mAb A3 that apparently recognized an epitope depending on parts of both domains. Unfortunately, because only one mAb against bFcγ2R was available, a similar detailed study could not be performed for this receptor. We showed that mAb CC-G24 only recognized wild-type bFcγ2R and the bEC-FcαRI chimera (Fig. 5 C). Thus, like mAb A3, it is most likely directed against a conformational epitope depending on both EC1 and EC2.
Discussion
By means of a panel of chimeric FcRs, we identified conclusively for the first time the ligand-binding sites of FcαRI and bFcγ2R. Surprisingly, these sites were found to be located in the EC1 domains. FcαRI and bFcγ2R are highly homologous both at the protein and nucleotide level (41 and 56% identity, respectively), but show much less homology with other human and bovine FcRs (7). This suggests that FcαRI and bFcγ2R evolved from a common ancestral gene (also shared by KIR, ILT, MIR, LIR, LAIR-1, HM18, PIR, and gp49B1 genes) and not shared by other human or bovine FcRs (7). Mapping of the bFcγ2R gene to bovine chromosome 18, which corresponds to human chromosome 19, further supports this notion (35).
Our finding that the Ig-binding sites of both FcαRI and bFcγ2R are located within their EC1 domains, was based on the fact that rosetting with Ig-coated beads was only seen for the corresponding FcαRI or bFcγ2R EC1 chimera. In both cases, however, a reduction in binding activity was seen compared with that obtained for the wild-type receptors and for the comparable EC chimeras (Fig. 4). Although these differences in part may be attributed to the expression levels of individual constructs (especially for the bEC1-FcαRI chimera, see above), it is also possible that the EC2 domains and membrane-proximal regions of the receptors contribute either directly (by forming “secondary” contact sites) or indirectly (by preserving three- dimensional structure) to the affinity and stability of the ligand interactions. This would be analogous to the activity of other two-domain FcRs, namely FcγRII, FcγRIII, and FcεRI in which the ligand-binding sites are located in the membrane-proximal EC2 domains, whereas structures within the EC1 domains contribute to the binding process (29, 30).
It should also be noted that the region of hIgA interacting with FcαRI has recently been mapped to the Cα2/ Cα3 boundary (36). This is in contrast to the region of human IgG responsible for interaction with FcγRs, which is proposed to lie much closer to the hinge (30). Therefore, in terms of the evolution of Ig/FcR interactions, it should be interesting to map the region of bIgG2 that binds to bFcγ2R. Because recombinant bIgG2 is available, experiments can readily be designed to determine whether this region lies close to the hinge region as in human IgG or in a position analogous to that of hIgA (37).
To substantiate our observation that hIgA binds to the EC1 domain of FcαRI, we mapped the epitopes for a panel of blocking and nonblocking FcαRI mAbs that bound equally to the EC parts of wild-type FcαRI and the hEC-Fcγ2R chimera. The nonblocking mAbs (A59, A62, A77, and 7D7) were shown to react with the membrane-proximal EC2 domain because they bound only to wild-type FcαRI and the hEC-bFcγ2R, bEC1-FcαRI, and bEC1(1–50)-FcαRI chimeras. The only exception was the nonblocking mAb A3 that bound only to wild-type FcαRI and the hEC-bFcγ2R and bEC1(1–50)-FcαRI chimeras, suggesting that its epitope is conformational and depends on regions of both EC1 and EC2 (similar to the bFcγ2R mAb CC-G24; see Results section). In contrast, all blocking mAbs (My43, 2E6, 2D11, 7G4, and 2H8) were shown to react with the EC1 domain of FcαRI because their binding activity was retained with the hEC1-bFcγ2R chimera. The epitopes recognized by My43 and 2D11 were further localized to the region of EC1 directly adjacent to EC2, because they were shown to bind the bEC1(1–50)-FcαRI chimera. 2E6 and 2H8 on the other hand, bound only weakly to this chimera, whereas 7G4 did not bind at all. Similar mapping studies with blocking mAbs have previously been used to localize the IgG-binding sites of FcγRII and FcγRIII to their EC2 domains (25, 27).
A number of FcαRI mRNAs have been isolated and shown to encode splice variants of the receptor (38–41). One such report described cell surface expression of an FcαRI variant that lacked the complete EC2 domain, and suggested the EC1 domain to be involved in hIgA binding (40). However, in contrast to our results, mAb My43 was proposed to react with EC2, and mAb A59 with EC1. We believe that observation to be spurious either due to incorrect cell surface expression of the splice variant and/or aberrant receptor structure caused by lack of the complete EC2 domain. This possibility was supported by our attempts to express various FcαRI splice variants in COS cells with no success (38). Additionally, chimeras constructed between FcαRI and FcγRII were not expressed efficiently (Morton, H.C., and J.G.J. van de Winkel, unpublished observations), possibly reflecting a degree of structural incompatibility between these two FcRs. In fact, our unsuccessful experience with those approaches led us to construct chimeras between FcαRI and bFcγ2R as reported here, because their levels of homology (and hence presumably their overall structure) are more similar than for other FcRs. Thus swapping of highly homologous regions should have minimal affect on the overall structural integrity of the resultant chimeras. Therefore, we feel that the present approach is more physiological than previous attempts to this end.
The surprising difference seen between the ligand-binding sites of FcαRI and bFcγ2R versus those of other leukocyte FcγRs and FcεRI may have interesting implications in terms of Ig interactions. As mentioned above, this disparity could simply reflect the proposed evolution of FcαRI and bFcγ2R from an ancestral gene distinct from that giving rise to other FcγRs and FcεRI. This notion is supported by the observation that residues within the membrane-distal domain of two KIR proteins determine their ability to bind to their respective ligands, the two groups of HLA-C allotypes (42). Moreover, due to their high levels of homology, the three-dimensional structure of FcαRI and bFcγ2R might more closely resemble that of the KIR proteins than that of more distantly related FcRs (43). Indeed, more detailed mutational analysis, directed by modeling studies using the recently published three dimensional structure of the p58 KIR as a template for the protein backbones of FcαRI and bFcγ2R, are currently underway in our laboratory to further localize the Ig-binding sites within these two receptors.
An alternative evolutionary explanation possibly applicable at least for FcαRI might be that its ligand-binding site developed to ensure interaction with all molecular forms of IgA: monomeric IgA, dimeric IgA (including J chain), and secretory IgA (including J chain and secretory component). FcαRI is reported to bind all these ligand variants (44, 45). Therefore, because the site of interaction with FcαRI at the Cα2/Cα3 boundary appears to be accessible to the receptor in all these forms of IgA, FcαRI could have evolved to accomplish this interaction via its EC1 domain to avoid potential problems of steric hindrance of a more membrane-proximal binding site in relation to large IgA polymers.
In conclusion, we have shown that the closely related FcαRI and bFcγ2R bind their ligands via sites located in their membrane-proximal EC1 domains. The difference in the Ig-binding sites of these two receptors versus other leukocyte FcγRs and FcεRI, may reflect the proposed divergent evolutionary pathway from a distinct genetic precursor, or (at least in the case of FcαRI) a specific adaptation for efficient interaction with large molecular forms of IgA.
Acknowledgments
We would like to thank Drs. Li Shen for My43, Max Cooper for A62, and Charles Maliszewski and Immunex for FcαRI cDNAs. We also gratefully acknowledge the technical staff of LIIPAT, specifically Bjørg Simonsen, Marie Johannesen, and Inger Johanne Ryen, for expert laboratory assistance. We further thank Gøril Olsen for help with cell sorting, and Dr. Finn-Eirik Johansen (LIIPAT) for helpful discussions and provision of the pCMV-GFP plasmid.
Abbreviations used in this paper
- b
bovine
- EC
extracellular
- GAM
goat anti–mouse
- GFP
green fluorescent protein
- KIR
natural killer cell inhibitory receptor
- TM/C
transmembrane/cytoplasmic
Footnotes
Note added in proof. A recent report by Wines et al. (J. Immunol. 1999. 162:2146–2153) likewise identified the EC1 domain of FcαRI to be responsible for ligand binding.
References
- 1.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 2.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 3.Morton HC, van Egmond M, van de Winkel JG. Structure and function of human IgA Fc receptors (FcαR) Crit Rev Immunol. 1996;16:423–440. [PubMed] [Google Scholar]
- 4.Shen L. Receptors for IgA on phagocytic cells. Immunol Res. 1992;11:273–282. doi: 10.1007/BF02919133. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro RC, Kubagawa H, Cooper MD. Cellular distribution, regulation, and biochemical nature of an Fcα receptor in humans. J Exp Med. 1990;171:597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro RC, Hostoffer RW, Cooper MD, Bonner JR, Gartland GL, Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993;92:1681–1685. doi: 10.1172/JCI116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, Young JR, Tregaskes CA, Sopp P, Howard CJ. Identification of a novel class of mammalian Fcγ receptor. J Immunol. 1995;155:1534–1541. [PubMed] [Google Scholar]
- 8.Dupont B, Selvakumar A, Steffens U. The killer cell inhibitory receptor genomic region on human chromosome 19q13.4. Tissue Antigens. 1997;49:557–563. doi: 10.1111/j.1399-0039.1997.tb02802.x. [DOI] [PubMed] [Google Scholar]
- 9.Samaridis J, Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol. 1997;27:660–665. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- 10.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu ML. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 11.Borges L, Hsu ML, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J Immunol. 1997;159:5192–5196. [PubMed] [Google Scholar]
- 12.Arm JP, Nwankwo C, Austen KF. Molecular identification of a novel family of human Ig superfamily members that possess immunoreceptor tyrosine-based inhibition motifs and homology to the mouse gp49B1 inhibitory receptor. J Immunol. 1997;159:2342–2349. [PubMed] [Google Scholar]
- 13.Wagtmann N, Rojo S, Eichler E, Mohrenweiser H, Long EO. A new human gene complex encoding the killer cell inhibitory receptors and related monocyte/macrophage receptors. Curr Biol. 1997;7:615–618. doi: 10.1016/s0960-9822(06)00263-6. [DOI] [PubMed] [Google Scholar]
- 14.Kremer EJ, Kalatzis V, Baker E, Callen DF, Sutherland GR, Maliszewski CR. The gene for the human IgA Fc receptor maps to 19q13.4. Hum Genet. 1992;89:107–108. doi: 10.1007/BF00207054. [DOI] [PubMed] [Google Scholar]
- 15.Wilson M, Torkar M, Trowsdale J. The leukocyte receptor complex on human chromosome 19q13.4. Immunology. 1997;92:75. . (Abstr.) [Google Scholar]
- 16.Arm JP, Gurish MF, Reynolds DS, Scott HC, Gartner CS, Austen KF, Katz HR. Molecular cloning of gp49, a cell-surface antigen that is preferentially expressed by mouse mast cell progenitors and is a new member of the immunoglobulin superfamily. J Biol Chem. 1991;266:15966–15973. [PubMed] [Google Scholar]
- 17.Wang LL, Mehta IK, LeBlanc PA, Yokoyama WM. Mouse natural killer cells express gp49B1, a structural homologue of human killer inhibitory receptors. J Immunol. 1997;158:13–17. [PubMed] [Google Scholar]
- 18.Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maliszewski CR, March CJ, Schoenborn MA, Gimpel S, Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990;172:1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfefferkorn LC, Yeaman GR. Association of IgA-Fc receptors (FcαR) with FcεRI γ2 subunits in U937 cells. Aggregation induces the tyrosine phosphorylation of γ2. J Immunol. 1994;153:3228–3236. [PubMed] [Google Scholar]
- 21.Morton HC, van den Herik-Oudijk IE, Vossebeld P, Snijders A, Verhoeven AJ, Capel PJ, van de Winkel JG. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR γ chain. Molecular basis for CD89/FcR γ chain association. J Biol Chem. 1995;270:29781–29787. doi: 10.1074/jbc.270.50.29781. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, Suzuki K, Matsuda H, Okumura K, Ra C. Physical association of Fc receptor γ chain homodimer with IgA receptor. J Allergy Clin Immunol. 1995;96:1152–1160. doi: 10.1016/s0091-6749(95)70200-8. [DOI] [PubMed] [Google Scholar]
- 23.Reterink TJ, van Zandbergen G, van Egmond M, Klar-Mohamad N, Morton HC, van de Winkel JG, Daha MR. Size-dependent effect of IgA on the IgA Fc receptor (CD89) Eur J Immunol. 1997;27:2219–2224. doi: 10.1002/eji.1830270915. [DOI] [PubMed] [Google Scholar]
- 24.Hulett MD, McKenzie IF, Hogarth PM. Chimeric Fc receptors identify immunoglobulin-binding regions in human FcγRII and FcεRI. Eur J Immunol. 1993;23:640–645. doi: 10.1002/eji.1830230310. [DOI] [PubMed] [Google Scholar]
- 25.Ierino FL, Hulett MD, McKenzie IF, Hogarth PM. Mapping epitopes of human FcγRII (CDw32) with monoclonal antibodies and recombinant receptors. J Immunol. 1993;150:1794–1803. [PubMed] [Google Scholar]
- 26.Hulett MD, Witort E, Brinkworth RI, McKenzie IF, Hogarth PM. Multiple regions of human FcγRII (CD32) contribute to the binding of IgG. J Biol Chem. 1995;270:21188–21194. doi: 10.1074/jbc.270.36.21188. [DOI] [PubMed] [Google Scholar]
- 27.Tamm A, Schmidt RE. The binding epitopes of human CD16 (FcγRIII) monoclonal antibodies. Implications for ligand binding. J Immunol. 1996;157:1576–1581. [PubMed] [Google Scholar]
- 28.Tamm A, Kister A, Nolte KU, Gessner JE, Schmidt RE. The IgG binding site of human FcγRIIIB receptor involves CC′ and FG loops of the membrane-proximal domain. J Biol Chem. 1996;271:3659–3666. doi: 10.1074/jbc.271.7.3659. [DOI] [PubMed] [Google Scholar]
- 29.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan M, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- 31.Maliszewski CR, VandenBos T, Shen L, Schoenborn MA, Kubagawa H, Beckmann MP, Monteiro RC. Recombinant soluble IgA Fc receptor: generation, biochemical characterization, and functional analysis of the recombinant protein. J Leukocyte Biol. 1993;53:223–232. doi: 10.1002/jlb.53.3.223. [DOI] [PubMed] [Google Scholar]
- 32.Brandtzaeg P, Fjellanger I, Gjeruldsen ST. Human secretory immunoglobulins. I. Salivary secretions from individuals with normal or low levels of serum immunoglobulins. Scand J Haematol Suppl. 1970;12:3–83. [PubMed] [Google Scholar]
- 33.Shen L, Lasser R, Fanger MW. My 43, a monoclonal antibody that reacts with human myeloid cells inhibits monocyte IgA binding and triggers function. J Immunol. 1989;143:4117–4122. [PubMed] [Google Scholar]
- 34.Monteiro RC, Cooper MD, Kubagawa H. Molecular heterogeneity of Fcα receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992;148:1764–1770. [PubMed] [Google Scholar]
- 35.Klungland H, Vage DI, Lien S. Linkage mapping of the Fcγ2 receptor gene to bovine chromosome 18. Mamm Genome. 1997;8:300–301. doi: 10.1007/s003359900425. [DOI] [PubMed] [Google Scholar]
- 36.Carayannopoulos L, Hexham JM, Capra JD. Localization of the binding site for the monocyte immunoglobulin (Ig) A Fc receptor (CD89) to the domain boundary between Cα2 and Cα3 in human IgA1. J Exp Med. 1996;183:1579–1586. doi: 10.1084/jem.183.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight KL, Suter M, Becker RS. Genetic engineering of bovine Ig. Construction and characterization of hapten-binding bovine/murine chimeric IgE, IgA, IgG1, IgG2, and IgG3 molecules. J Immunol. 1988;140:3654–3659. [PubMed] [Google Scholar]
- 38.Morton HC, Schiel AE, Janssen SW, van de Winkel JG. Alternatively spliced forms of the human myeloid Fcα receptor (CD89) in neutrophils. Immunogenetics. 1996;43:246–247. doi: 10.1007/BF00587311. [DOI] [PubMed] [Google Scholar]
- 39.Patry C, Sibille Y, Lehuen A, Monteiro RC. Identification of Fcα receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J Immunol. 1996;156:4442–4448. [PubMed] [Google Scholar]
- 40.Pleass RJ, Andrews PD, Kerr MA, Woof JM. Alternative splicing of the human IgA Fc receptor CD89 in neutrophils and eosinophils. Biochem J. 1996;318:771–777. doi: 10.1042/bj3180771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyabe S, Kuwano Y, Takeda K, Uchiyama M, Abo T. IgA nephropathy-specific expression of the IgA Fc receptors (CD89) on blood phagocytic cells. Clin Exp Immunol. 1997;110:226–232. doi: 10.1111/j.1365-2249.1997.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winter CC, Long EO. A single amino acid in the p58 killer cell inhibitory receptor controls the ability of natural killer cells to discriminate between the two groups of HLA-C allotypes. J Immunol. 1997;158:4026–4028. [PubMed] [Google Scholar]
- 43.Fan QR, Mosyak L, Winter CC, Wagtmann N, Long EO, Wiley DC. Structure of the inhibitory receptor for human natural killer cells resembles haematopoietic receptors. Nature. 1997;389:96–100. doi: 10.1038/38028. [DOI] [PubMed] [Google Scholar]
- 44.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Zandbergen G, van Kooten C, Mohamad NK, Reterink TJ, de Fijter JW, van de Winkel JG, Daha MR. Reduced binding of immunoglobulin A (IgA) from patients with primary IgA nephropathy to the myeloid IgA Fc-receptor, CD89. Nephrol Dial Transplant. 1998;13:3058–3064. doi: 10.1093/ndt/13.12.3058. [DOI] [PubMed] [Google Scholar]