Abstract
Members of the tumor necrosis factor (TNF) family induce pleiotropic biological responses, including cell growth, differentiation, and even death. Here we describe a novel member of the TNF family, designated BAFF (for B cell activating factor belonging to the TNF family), which is expressed by T cells and dendritic cells. Human BAFF was mapped to chromosome 13q32-34. Membrane-bound BAFF was processed and secreted through the action of a protease whose specificity matches that of the furin family of proprotein convertases. The expression of BAFF receptor appeared to be restricted to B cells. Both membrane-bound and soluble BAFF induced proliferation of anti-immunoglobulin M–stimulated peripheral blood B lymphocytes. Moreover, increased amounts of immunoglobulins were found in supernatants of germinal center–like B cells costimulated with BAFF. These results suggest that BAFF plays an important role as costimulator of B cell proliferation and function.
Keywords: tumor necrosis factor, B lymphocytes, T lymphocytes, B cell growth, immunoglobulin G
Members of the TNF cytokine family are critically involved in the regulation of inflammation, of the immune response to infections, and of tissue homeostasis (1). The family members are type II membrane proteins that can act in a membrane-bound form or as proteolytically processed, soluble cytokines in an autocrine, paracrine, or endocrine manner (1). Binding of the ligands to their respective receptors induces oligomerization, initiating downstream signaling events.
Signaling pathways stimulated by TNF ligand members are diverse, including the activation of caspases, the translocation of nuclear factor (NF)-κB,1 or the activation of mitogen-activated protein kinases such as c-Jun NH2-terminal kinase (JNK) or extracellular signal regulatory kinase (ERK) (1). Thus, TNF-related ligands can lead to apoptosis, differentiation, or proliferation. Presently 16 members of the TNF-cytokine family have been described, several among them having important regulatory roles in function and development of the immune system. For instance, TNF acts as an inflammatory cytokine coordinating host defenses in response to infection (2). The lymphotoxin (LT) system is crucial in the development of peripheral lymphoid organs and the organization of splenic architecture (3, 4). Fas ligand (FasL, CD95L), TNF, and CD30L are responsible for TCR-mediated apoptosis of T cells and of immature thymocytes (5–7). Several of the TNF members and their receptors, in conjunction with TCR stimulation, also enhance T cell proliferation. Therefore, upregulation of TNF cytokine members and their receptors by TCR-induced signals can provide an autocrine costimulatory mechanism to increase the lymphocyte's own proliferation after stimulation with the antigen. However, upregulation of TNF-related ligands on T cells is also important for the activation and stimulation of neighboring cells. For example, CD40L is important for B cell survival, proliferation, Ig isotype switch, and differentiation (8), and the interaction of OX40 with OX40L is necessary for the differentiation of activated B cells into high Ig-producing cells (9).
Here we characterize the structural and functional properties of a new ligand of the TNF cytokine family. The new ligand, termed BAFF (B cell activating factor belonging to the TNF family), appears to be expressed by T cells and dendritic cells for the purpose of B cell costimulation, and may therefore play an important role in the control of B cell function.
Materials and Methods
Materials.
The anti-Flag M2 mAb, biotinylated anti-Flag M2 antibody, and the anti-Flag M2 antibody coupled to agarose were purchased from Sigma Chemical Co. Cell culture reagents were obtained from Life Sciences and BioWhittaker. Flag-tagged soluble human APRIL (a proliferation inducing ligand; residues K110– L250) was produced in 293 cells as described (10, 11). FITC- labeled anti-CD4, anti-CD8, and anti-CD19 antibodies were purchased from PharMingen. Goat F(ab′)2 specific for the Fc5μ fragment of human IgM were purchased from Jackson ImmunoResearch Laboratories. Secondary antibodies were obtained from either PharMingen or Jackson ImmunoResearch Laboratories and were used at the recommended dilutions.
Cells.
Human embryonic kidney 293 T cells (12) and fibroblast cell lines (see Table I) were maintained in DMEM containing 10% heat-inactivated FCS. Human embryonic kidney 293 cells were maintained in DMEM-nutrient mix F12 (1:1) supplemented with 2% FCS. T cell lines, B cell lines, and macrophage cell lines (see Table I) were grown in RPMI supplemented with 10% FCS. Molt-4 cells were cultivated in Iscove's medium supplemented with 10% FCS. Epithelial cell lines were grown in MEM-α medium containing 10% FCS, 0.5 mM nonessential amino acids, 10 mM Na-Hepes, and 1 mM Na pyruvate. Human umbilical vein endothelial cells were maintained in M199 medium supplemented with 20% FCS, 100 μg/ml of epithelial cell growth factor (Collaborative Research, Inotech), and 100 μg/ml of heparin sodium salt (Sigma Chemical Co.). All media contained penicillin and streptomycin antibiotics.
Table I.
Binding of sBAFF to Various Cell Lines
| Cell type | Cell lines | BAFF binding | Specific details | |||
|---|---|---|---|---|---|---|
| Epithelial-like | HT-29 | − | Colon adenocarcinoma | |||
| A375 | −/+ | Melanoma | ||||
| MCF-7 | − | Breast adenocarcinoma | ||||
| Me260 | − | Melanoma | ||||
| Cos | + | Monkey kidney cells | ||||
| Fibroblasts | WI-38 | − | Lung | |||
| Hs-68 | − | Foreskin | ||||
| Hs-27 | − | Foreskin | ||||
| Endothelial cells | HUVEC | − | Umbilical vein | |||
| Macrophages/monocytes | THP-1 | −/+ | Monocyte | |||
| T cell lines | Molt-4 | − | Lymphoblastic leukemia | |||
| Hut-78 | − | Cutaneous lymphoma | ||||
| Jurkat | − | Lymphoblastic leukemia | ||||
| B cell lines | BJAB | +++ | Burkitt lymphoma | |||
| Namalawa | ++ | Burkitt lymphoma | ||||
| Daudi | +/− | Burkitt lymphoma EBNA+ VCA+ | ||||
| Ramos | ++ | Burkitt lymphoma EBV− | ||||
| Raji | +++ | Burkitt lymphoma | ||||
| JIYOYE | + | Burkitt lymphoma | ||||
| SKW.64 | ++ | IgM secreting EBV+ | ||||
| RPMI 1788 | +++ | Peripheral blood, IgM secreting | ||||
| IM-9 | +++ | Lymphoblast Ig secreting | ||||
| NC-37 | +++ | Lymphoblast EBV+ | ||||
| Mouse cell lines | WEHI-231 | − | B cell lymphoma | |||
| A20 | − | B cell lymphoma |
PBLs were isolated from heparinized blood of healthy adult volunteers by Ficoll-Paque (Amersham Pharmacia Biotech) gradient centrifugation and cultured in RPMI, 10% FCS.
T cells were obtained from nonadherent PBLs by rosetting with neuraminidase-treated sheep red blood cells and separated from nonrosetting cells (mostly B cells) by Ficoll-Paque gradient centrifugation. Purified T cells were activated for 24 h with phytohemagglutinin (1 μg/ml; Sigma Chemical Co.), washed, and cultured in RPMI, 10% FCS, 20 U/ml of IL-2. CD14+ monocytes were purified by magnetic cell sorting using anti-CD14 antibodies, goat anti–mouse-coated microbeads, and a Minimacs™ device (Miltenyi Biotech), and cultivated in the presence of GM-CSF (800 U/ml, Leucomax®; Essex Chemie) and IL-4 (20 ng/ml; Lucerna Chem) for 5 d, then with GM-CSF, IL-4, and TNF-α (200 U/ml; Bender) for an additional 3 d to obtain a CD83+, dendritic cell–like population.
Human B cells of >97% purity were isolated from peripheral blood or umbilical cord blood using anti-CD19 magnetic beads (M450; Dynal) as described (13).
Northern Blot.
Northern blot analysis was carried out using Human Multiple Tissue Northern Blots I and II (7760-1 and 7759-1; Clontech). The membranes were incubated in hybridization solution (50% formamide, 2.5× Denhardt's, 0.2% SDS, 10 mM EDTA, 2× SSC, 50 mM NaH2PO4, pH 6.5, 200 μg/ml sonicated salmon sperm DNA) for 2 h at 60°C. Antisense RNA probe containing the nucleotides corresponding to amino acids (aa) 136–285 of human BAFF (hBAFF) was heat denatured and added at 2 × 106 cpm/ml in fresh hybridization solution. The membrane was hybridized 16 h at 62°C, washed once in 2× SSC, 0.05% SDS (30 min at 25°C), once in 0.1× SSC, 0.1% SDS (20 min at 65°C), and exposed at −70°C to x-ray films.
Characterization of BAFF cDNA.
A partial sequence of hBAFF cDNA was contained in several expressed sequence tag (EST) clones (sequence data available from EMBL/GenBank/DDBJ under accession nos. T87299 and AA166695) derived from fetal liver and spleen and ovarian cancer libraries. The 5′ portion of the cDNA was obtained by 5′ rapid amplification of cDNA ends (RACE) PCR (Marathon-Ready cDNA; Clontech) with oligonucleotides AP1 and JT1013 (5′-ACTGTTTCTTCTGGACCCTGAACGGC-3′) using the provided cDNA library from a pool of human leukocytes as template, as recommended by the manufacturer. The resulting PCR product was cloned into PCR-0 blunt (Invitrogen) and subcloned as EcoRI-PstI fragment into pT7T3 Pac vector (Amersham Pharmacia Biotech) containing EST clone T87299. Therefore, full-length hBAFF cDNA was obtained by combining 5′ and 3′ fragments using the internal PstI site of BAFF. The sequence has been assigned EMBL/GenBank/ DDBJ accession no. AF116456.
A partial 617-bp sequence of murine BAFF was contained in two overlapping EST clones (EMBL/GenBank/DDBJ accession nos. AA422749 and AA254047). A PCR fragment spanning nucleotides 158–391 of this sequence was used as a probe to screen a mouse spleen cDNA library (Stratagene, Inc.). The sequence has been assigned EMBL/GenBank/DDBJ accession no. AF119383.
Expression of Recombinant BAFF.
Full-length hBAFF was amplified using oligos JT1069 (5′-GACAAGCTTGCCACCATGGATGACTCCACA-3′) and JT637 (5′-ACTAGTCACAGCAGTTTCAATGC-3′). The PCR product was cloned into PCR-0 blunt and resubcloned as HindIII-EcoRI fragment into PCR-3 mammalian expression vector. A short version of soluble BAFF (sBAFF/short, aa Q136–L285) was amplified using oligos JT636 (5′-CTGCAGGGTCCAGAAGAAACAG-3′) and JT637. A long version of sBAFF (sBAFF/long, aa L83–L285) was obtained from full-length BAFF using internal PstI site. sBAFFs were resubcloned as PstI-EcoRI fragments behind the hemagglutinin signal peptide and Flag sequence of a modified PCR-3 vector, and as PstI-SpeI fragments into a modified pQE16 bacterial expression vector in frame with an NH2-terminal Flag sequence (14). Constructs were sequenced on both strands.
The establishment of stable 293 cell lines expressing the short soluble form or full-length BAFF, and the expression and purification of recombinant sBAFF from bacteria and mammalian 293 cells were performed as described (14, 15).
Reverse Transcriptase PCR.
Total RNA extracted from T cells, B cells, in vitro–derived immature dendritic cells, 293 wild-type (wt) and 293-BAFF (full-length) cells was reverse transcribed using the Ready to Go system (Amersham Pharmacia Biotech) according to the manufacturer's instructions. BAFF and β-actin cDNAs were detected by PCR amplification with Taq DNA polymerase (steps of 1 min each at 94°C, 55°C, and 72°C for 30 cycles) using specific oligonucleotides: for BAFF, JT1322 5′-GGAGAAGGCAACTCCAGTCAGAAC-3′ and JT1323 5′-CAATTCATCCCCAAAGACATGGAC-3′; for IL-2 receptor α chain, JT1368 5′-TCGGAACACAACGAAACAAGTC-3′ and JT1369 5′-CTTCTCCTTCACCTGGAAACTGACTG-3′; and for β-actin, 5′-GGCATCGTGATGGACTCCG-3′ and 5′-GCTGGAAGGTGGACAGCGA-3′.
Gel Permeation Chromatography.
293 T cells were transiently transfected with the short form of sBAFF and grown in serum-free Optimem medium for 7 d. Conditioned supernatants were concentrated 20 times, mixed with internal standards catalase and OVA, and loaded onto a Superdex-200 HR10/30 column. Proteins were eluted in PBS at 0.5 ml/min, and fractions (0.25 ml) were precipitated with TCA and analyzed by Western blotting using anti-Flag M2 antibody. The column was calibrated with standard proteins: ferritin (440 kD), catalase (232 kD), aldolase (158 kD), BSA (67 kD), OVA (43 kD), chymotrypsinogen A (25 kD), and ribonuclease A (13.7 kD).
PNGase F Treatment.
Samples were heated in 20 μl of 0.5% SDS, 1% 2-ME for 3 min at 95°C, then cooled and supplemented with 10% NP-40 (2 μl), 0.5 M sodium phosphate, pH 7.5 (2 μl), and Peptide N-glycanase F (PNGase F; 125 U/μl, 1 μl, or no enzyme in controls). Samples were incubated for 3 h at 37°C before analysis by Western blotting.
Edman Sequencing.
293 T cells were transiently transfected with the long form of sBAFF and grown in serum-free Optimem medium for 7 d. Conditioned supernatants were concentrated 20 times, fractionated by SDS-PAGE, and blotted onto polyvinylidene difluoride membrane (Bio-Rad Laboratories) as described previously (16), and then sequenced using a gas phase sequencer (ABI 120A; Perkin Elmer) coupled to an analyzer (ABI 120A; Perkin Elmer) equipped with a phenylthiohydantoin C18 2.1 × 250 mm column. Data were analyzed using ABI 610 software (Perkin Elmer).
Antibodies.
Polyclonal antibodies were generated by immunizing rabbits (Eurogentec) with recombinant sBAFF/long. Spleens of rats immunized with the same antigen were fused to x63Ag8.653 mouse myeloma cells, and hybridomas were screened for BAFF-specific IgGs. One of these mAbs, 43.9, is an IgG2a that specifically recognizes hBAFF.
FACS®.
Cells were stained in 50 μl of FACS buffer (PBS, 10% FCS, 0.02% NaN3) with 50 ng (or the indicated amount) of Flag-tagged short soluble hBAFF for 20 min at 4°C, followed by anti-Flag M2 (1 μg) and secondary antibody. Anti-BAFF mAb 43.9 was used at 40 μg/ml. For two-color FACS® analysis, peripheral blood lymphocytes were stained with Flag-tagged sBAFF/long (2 μg/ml), followed by biotinylated anti-Flag M2 (1:400) and PE-labeled streptavidin (1:100), followed by FITC-labeled anti-CD4, anti-CD8, or anti-CD19.
PBL Proliferation Assay.
PBLs were incubated in 96-well plates (105 cells/well in 100 μl RPMI supplemented with 10% FCS) for 72 h in the presence or absence of 2 μg/ml of goat anti–human μ chain antibody (Sigma Chemical Co.) or control F(ab′)2 and with the indicated concentration of native or boiled sBAFF/long. Cells were pulsed for an additional 6 h with [3H]thymidine (1 μCi/well) and harvested. [3H]Thymidine incorporation was monitored by liquid scintillation counting. In some experiments, recombinant sBAFF was replaced by 293 cells stably transfected with full-length BAFF (or 293 wild-type [wt] as control) that had been fixed for 5 min at 25°C in 1% paraformaldehyde. Assay was performed as described (17). In further experiments, CD19+ cells were isolated from PBLs with magnetic beads, and the remaining CD19− cells were irradiated (3,000 rads) before reconstitution with CD19+ cells. Proliferation assay with sBAFF was then performed as described above.
B Cell Activation Assay.
Purified B cells were activated in the EL-4 culture system as described (13). In brief, 104 B cells mixed with 5 × 104 irradiated murine EL-4 thymoma cells (clone B5) were cultured for 5–6 d in 200 μl medium containing 5% vol/vol of culture supernatants from human T cells (106/ml) which had been activated for 48 h with PHA (1 μg/ml) and PMA (1 ng/ml). B cells were then reisolated with anti-CD19 beads and cultured for another 7 d (5 × 104 cells in 200 μl, duplicate or triplicate culture in flat-bottomed 96 well plates) in medium alone or in medium supplemented with 5% T cell supernatants, or with 50 ng/ml IL-2 (a gift from the former Glaxo Institute for Molecular Biology, Geneva) and 10 ng/ml each IL-4 and IL-10 (PeproTech), in the presence or absence of sBAFF. The anti-Flag M2 antibody was added at a concentration of 2 μg/ml and had no effect by itself.
Results
BAFF Is a Novel Ligand of the TNF Family.
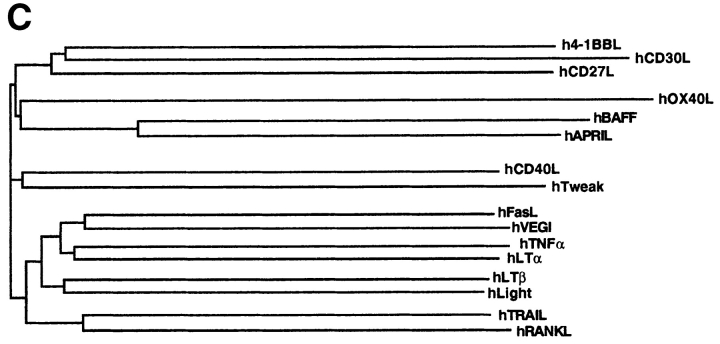
hBAFF was identified by sequence homology as a possible novel member of the TNF ligand family while we screened public databases using an improved profile search (18). A cDNA encoding the complete protein of 285 aa was obtained by combining EST clones (covering the 3′ region) with a fragment (5′ region) amplified by PCR. The absence of a signal peptide suggested that BAFF was a type II membrane protein that is typical of the members of the TNF ligand family. The protein has a predicted cytoplasmic domain of 46 aa, a hydrophobic transmembrane region, and an extracellular domain of 218 aa containing two potential N-glycosylation sites (Fig. 1 A). The sequence of the extracellular domain of BAFF shows highest homology with APRIL (33% aa identity, 48% homology), whereas the identity with other members of the family, such as TNF, FasL, LTα, TRAIL (TNF-related apoptosis-inducing ligand), or RANKL (receptor activator of NF-κB ligand) is <20% (Fig. 1, B and C). The mouse BAFF (mBAFF) cDNA clone isolated from a spleen library encoded a slightly longer protein (309 aa) due to an insertion between the transmembrane region and the first of several β strands which constitute the receptor binding domain in all TNF ligand members (19). This β strand–rich ectodomain is almost identical in mBAFF and hBAFF (86% identity, 93% homology), suggesting that the BAFF gene has been highly conserved during evolution (Fig. 1 A).
Figure 1.
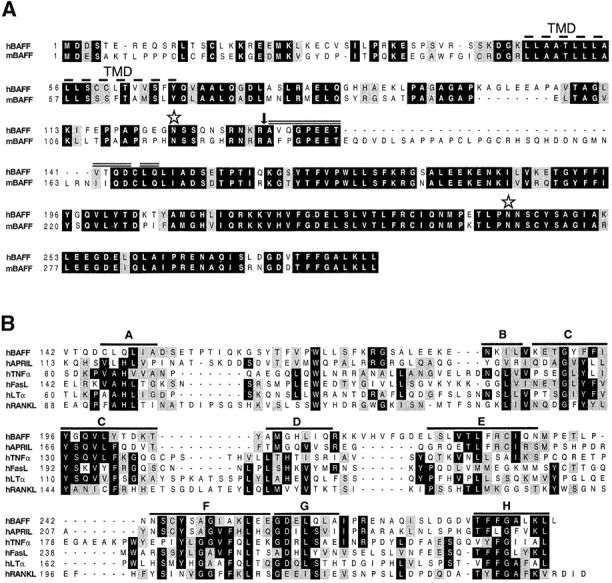
(A) Predicted aa sequence of human and mouse BAFF. The predicted transmembrane domain (TMD, dashed line), the potential N-linked glycosylation sites (stars), and the natural processing site of hBAFF (arrow) are indicated. The double line above hBAFF indicates the sequence obtained by Edman degradation of the processed form of hBAFF. (B) Comparison of the extracellular protein sequence of BAFF and some members of the TNF ligand family. Identical and homologous residues are represented in black and shaded boxes, respectively. (C) Dendrogram of TNF family ligands.
BAFF Is Processed and Secreted.
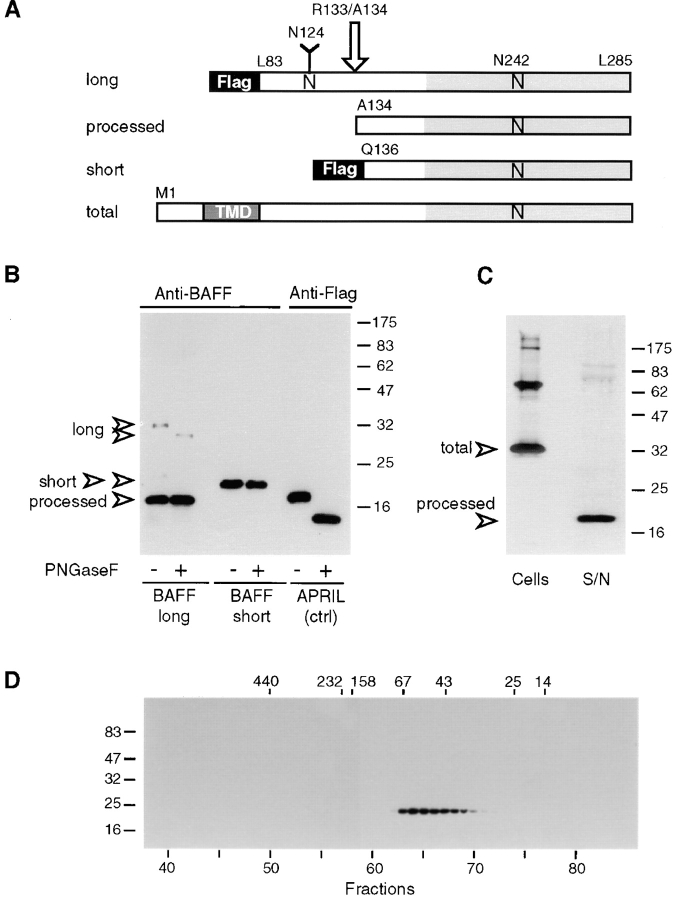
Although TNF family members are synthesized as membrane-inserted ligands, cleavage in the stalk region between transmembrane and receptor binding domains is frequently observed. For example, TNF and FasL are readily cleaved from the cell surface by metalloproteinases (20, 21). While producing several forms of recombinant BAFF in 293 T cells, we noticed that a recombinant soluble 32-kD form of BAFF (aa 83–285, sBAFF/long), containing the complete stalk region and an NH2-terminal Flag tag in addition to the receptor binding domain, was extensively processed to a smaller 18-kD fragment (Fig. 2, A and B). Cleavage occurred in the stalk region since the fragment was detectable only with antibodies raised against the complete receptor interaction domain of BAFF but not with anti-Flag antibodies (data not shown). This experiment also revealed that only N124 (located in the stalk) but not N242 (located at the entry of the F-β sheet) was glycosylated, since the molecular mass of the nonprocessed sBAFF/long was reduced from 32 to 30 kD upon removal of the N-linked carbohydrates with PNGase F, whereas the 18-kD cleaved form was insensitive to this treatment. Peptide sequence analysis of the 18-kD fragment indeed showed that cleavage occurred between R133 and A134 (Fig. 1 A). R133 lies at the end of a polybasic region that is conserved between human (R-N-K-R) and mouse (R-N-R-R) BAFF. To test whether cleavage was not merely an artifact of expressing soluble, nonnatural forms of BAFF, membrane-bound full-length BAFF was expressed in 293 T cells (Fig. 2 C). The 32-kD complete BAFF and some higher molecular mass species (probably corresponding to nondissociated dimers and trimers) were readily detectable in cellular extracts, but >95% of BAFF recovered from the supernatant corresponded to the processed 18-kD form, indicating that BAFF was also processed when synthesized as a membrane-bound ligand.
Figure 2.
Characterization of recombinant BAFF. (A) Schematic representation of recombinant BAFF constructs. Soluble recombinant BAFFs starting at Leu83 and Gln136 are expressed fused to an NH2-terminal Flag tag and a 6–amino acid linker. The long form is cleaved between Arg133 and Ala134 (arrow) in 293 T cells, to yield a processed form of BAFF. Asn124 and Asn242 belong to N-glycosylation consensus sites. N-linked glycan present on Asn124 is shown as a Y. TMD, transmembrane domain. (B) PNGase F treatment of recombinant BAFF. Concentrated supernatants containing Flag-tagged BAFFs and APRIL were deglycosylated and analyzed by Western blotting using polyclonal anti-BAFF antibodies or anti-Flag M2, as indicated. All bands except processed BAFF also reacted with anti-Flag M2 (data not shown). (C) Full-length BAFF is processed to a soluble form. 293 T cells were transiently transfected with full-length BAFF. Transfected cells and their concentrated supernatants (S/N) were analyzed by Western blotting using polyclonal anti-BAFF antibodies. Supernatants corresponding to 10 times the amount of cells were loaded onto the gel. (D) Size exclusion chromatography of sBAFF on Superdex-200. Concentrated supernatants containing sBAFF/short were fractionated on a Superdex-200 column, and the eluted fractions were analyzed by Western blotting using anti-Flag M2 antibody. The migration positions of the molecular mass markers (in kD) are indicated on the left-hand side for SDS-PAGE and at the top of the figure for size exclusion chromatography.
Therefore, we engineered an sBAFF (Q136–L285, sBAFF/short) whose sequence started 2 aa downstream of the processing site (Fig. 1 B). As predicted, the Flag tag attached to the NH2 terminus of this recombinant molecule was not removed (data not shown), which allowed its purification by an anti-Flag affinity column. To test its correct folding, the purified sBAFF/short was analyzed by gel filtration where the protein eluted at an apparent molecular mass of 55 kD (Fig. 2 D). We conclude that sBAFF/short correctly assembles into a homotrimer (3 × 20 kD) in agreement with the quaternary structure of other TNF family members (19). Finally, unprocessed sBAFF/long was readily expressed in bacteria, indicating that the cleavage event was specific to eukaryotic cells.
Expression and Chromosomal Localization of BAFF.
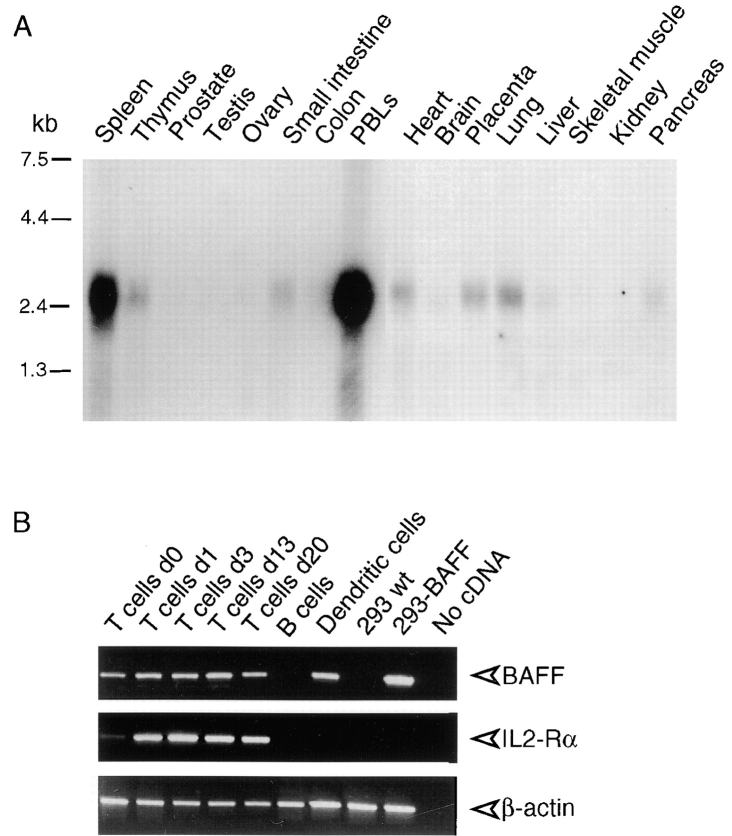
Northern blot analysis of BAFF revealed that the 2.5-kb BAFF mRNA was abundant in the spleen and PBLs (Fig. 3 A). Thymus, heart, placenta, small intestine, and lung showed weak expression. This restricted distribution suggested that cells present in lymphoid tissues were the main source of BAFF. Through PCR analysis, we found that BAFF mRNA was present in T cells and peripheral blood monocyte–derived dendritic cells but not in B cells (Fig. 3 B). Even naive, nonstimulated T cells appeared to express some BAFF mRNA.
Figure 3.
Expression of BAFF. (A) Northern blots (2 μg poly A+ RNA per lane) of various human tissues were probed with BAFF antisense mRNA. (B) Reverse transcriptase amplification of BAFF, IL-2 receptor α chain (IL2-Rα), and actin from RNA of purified blood T cells at various time points of PHA activation, E-rosetting–negative blood cells (mostly B cells), in vitro–derived immature dendritic cells, 293 cells, and 293 cells stably transfected with full-length BAFF (293-BAFF). Control amplifications were performed in the absence of added cDNA. IL-2 receptor α chain was amplified as a marker of T cell activation.
A sequence-tagged site (STS, SHGC-36171) was found in the database which included the hBAFF sequence. This site maps to human chromosome 13, in a 9-cM interval between the markers D13S286 and D13S1315. On the cytogenetic map, this interval corresponds to 13q32-34. Of the known TNF ligand family members, only RANKL (Trance) has been localized to this chromosome (22) though quite distant to BAFF (13q14).
BAFF Receptor Is Expressed on B Cells.
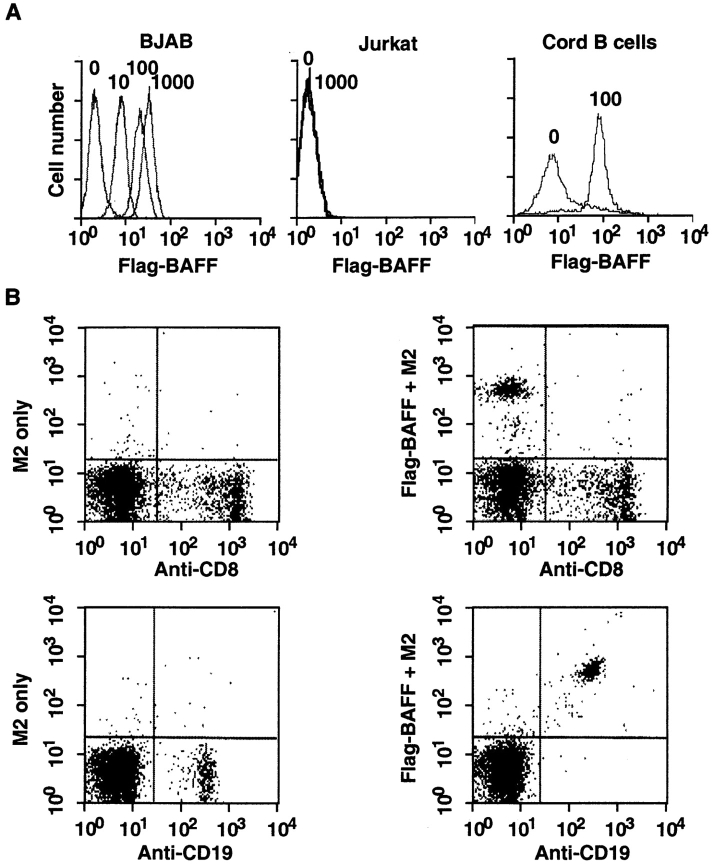
For the ligand to exert maximal biological effects, it was likely that the BAFF receptor (BAFF-R) would be expressed either on the same cells or on neighboring cells present in lymphoid tissues. Using the recombinant sBAFF as a tool to specifically determine BAFF-R expression by FACS®, we indeed found high levels of receptor expression in various B cell lines, such as the Burkitt lymphomas Raji and BJAB (Fig. 4 A, and Table I). In contrast, cell lines of T cell, fibroblastic, epithelial, and endothelial origin were all negative. Very weak staining was observed with the monocyte line THP-1, which, however, could be due to Fc receptor binding. Thus, BAFF-R expression appears to be restricted to B cell lines. The two mouse B cell lines tested were negative using the hBAFF as a probe, although weak binding was observed on mouse splenocytes (data not shown). The presence of BAFF-R on B cells was corroborated by analysis of umbilical cord and peripheral blood lymphocytes. While CD8+ and CD4+ T cells lacked BAFF-R (Fig. 4 B, and data not shown), abundant staining was observed on CD19+ B cells (Fig. 4, A and B), indicating that BAFF-R is expressed on all blood B cells, including naive and memory B cells. No evidence was obtained for a CD19+, BAFF-R− population.
Figure 4.
BAFF binds to mature B cells. (A) Binding of sBAFF to BJAB and Jurkat cell lines, and to purified CD19+ cells of cord blood. Cells were stained with the indicated amount (in ng/50 μl) of Flag-BAFF and analyzed by flow cytometry. (B) Binding of sBAFF to PBLs. PBLs were stained with anti-CD8–FITC or with anti-CD19–FITC (x axis) and with Flag-BAFF plus M2-biotin and avidin-PE (y axis). Flag-BAFF was omitted in controls.
BAFF Can Costimulate B Cell Growth.
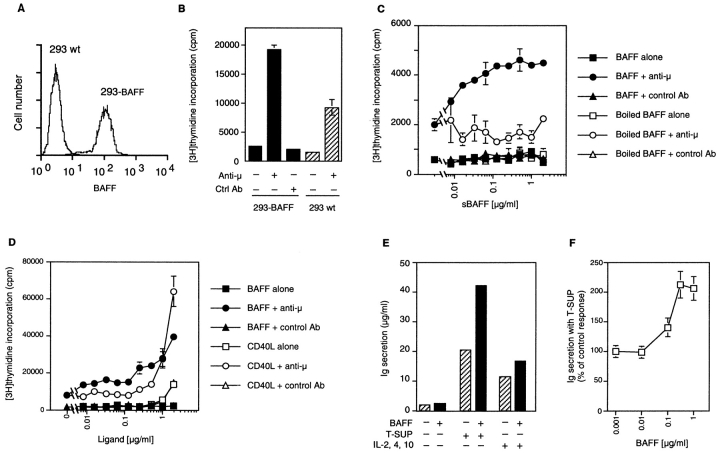
Since BAFF bound to blood-derived B cells, experiments were performed to determine whether the ligand could deliver growth-stimulatory or growth-inhibitory signals. PBLs were stimulated with anti-IgM (μ) antibodies together with fixed 293 cells stably expressing surface BAFF (Fig. 5 A). The levels of [3H]thymidine incorporation induced by anti-μ alone were not altered by the presence of control cells but were increased twofold in the presence of BAFF-transfected cells (Fig. 5 B). A dose-dependent proliferation of PBLs was also obtained when BAFF-transfected cells were replaced by purified sBAFF (Fig. 5 C), indicating that BAFF does not require membrane attachment to exert its activity. In this experimental setup, proliferation induced by sCD40L required concentrations >1 μg/ml, but was less dependent on the presence of anti-μ than that mediated by BAFF (Fig. 5 D). When purified CD19+ B cells were cocultured with irradiated autologous CD19− PBLs, costimulation of proliferation by BAFF was unaffected, demonstrating that [3H]thymidine uptake was mainly due to B cell proliferation and not to an indirect stimulation of another cell type (data not shown). The observed B cell proliferation in response to BAFF was entirely dependent on the presence of anti-μ antibodies, indicating that BAFF functioned as costimulator of B cell proliferation.
Figure 5.
BAFF costimulates B cell proliferation. (A) Surface expression of BAFF in stably transfected 293 cells. 293-BAFF and 293 wt cells were stained with anti-BAFF mAb 43.9 and analyzed by flow cytometry. (B) Costimulation of PBLs by 293-BAFF cells. PBLs (105/well) were incubated with 15,000 paraformaldehyde-fixed 293 cells (293 wt or 293-BAFF) in the presence or absence of anti-B cell receptor antibody (anti-μ). Fixed 293 cells alone incorporated 100 cpm. (C) Dose-dependent costimulation of PBL proliferation by sBAFF in the presence of anti-μ. Proliferation was determined after 72 h incubation by [3H]thymidine incorporation. Controls include cells treated with BAFF alone, with heat-denatured BAFF, or with an irrelevant isotype-matched antibody in place of anti-μ. (D) Comparison of (co)stimulatory effects of sCD40L and sBAFF on PBL proliferation. Experiment was performed as described in panel C. (E) BAFF costimulates Ig secretion of preactivated human B cells. Purified CD19+ B cells were activated by coculture with EL-4 T cells and activated T cell supernatants for 5–6 d, then reisolated and cultured for another 7 d in the presence of medium only (−) or containing 5% activated T cell supernatants (T-SUP) or a blend of cytokines (IL-2, IL-4, IL-10). The columns represent means of Ig concentrations for cultures with or without 1 μg/ml BAFF. Means of fold increase ± SD were 1.23 ± 0.11 for medium only, 2.06 ± 0.18 with T cell supernatants (four experiments), and 1.45 ± 0.06 with IL-2, IL-4, and IL-10 (two experiments). These were performed with peripheral blood (three experiments) or cord blood B cells (one experiment; 2.3-fold increase with T cell supernatants, 1.5-fold increase with IL-2, IL-4, and IL-10). (F) Dose–response curve for the effect of BAFF in cultures with T cell supernatants, as shown in panel D. Mean ± SD of three experiments.
To investigate a possible effect of BAFF on preplasma, germinal center–like B cells (13), purified peripheral or cord blood B cells were preactivated by coculture with EL-4 T cells in the presence of a cytokine mixture from supernatants of PHA/PMA-stimulated T cells (23). These B cells were reisolated to 98% purity and yielded a twofold increase in secreted Ig during a secondary culture in the presence of BAFF and activated T cell cytokines compared with cytokines alone. No significant effect was seen in the absence of exogenous cytokines, and an intermediate (1.5-fold) effect was observed in the presence of the recombinant cytokines IL-2, IL-4, and IL-10 (Fig. 5, E and F).
Discussion
Here we report the molecular cloning, expression, and biological activity of a new member of the TNF ligand family. The human and mouse sequences exhibit the typical characteristics of this family, i.e., a type II membrane protein organization and the conservation of nine β sheets, which fold into a “jelly-roll” structure that trimerizes to form receptor interacting sites. The biochemical analysis of BAFF is also consistent with the typical homotrimeric structure of TNF family members. In this family of ligands, BAFF exhibits the highest level of sequence similarity with APRIL, which we have recently characterized as a ligand stimulating growth of various tumor cells (11). Unlike TNF and LTα, which are two family members with equally high homology (33% identity) and whose genes are linked on chromosome 6, APRIL and BAFF are not clustered on the same chromosome. APRIL is located on chromosome 17 (our unpublished data), whereas BAFF maps to the distal arm of human chromosome 13 (13q34). Abnormalities in this locus were characterized in Burkitt lymphomas as the second most frequent defect (24) besides the translocation involving the myc gene into the Ig locus (25). Considering the high expression levels of BAFF-R on all Burkitt lymphoma cell lines analyzed (see Table I), this raises the intriguing possibility that some Burkitt lymphomas may have deregulated BAFF expression, thus stimulating growth in an autocrine manner.
B cell growth was efficiently costimulated with recombinant sBAFF lacking the transmembrane domain. This activity is in contrast to several TNF family members that are active only as membrane-bound ligands, such as TRAIL, FasL, and CD40L. Soluble forms of these ligands have poor biological activity that can be enhanced by their cross-linking, thereby mimicking the membrane-bound ligand (15). In contrast, cross-linking Flag-tagged sBAFF with anti-Flag antibodies or the use of membrane-bound BAFF expressed on the surface of epithelial cells did not further enhance the mitogenic activity of BAFF, suggesting that it can act systemically as a secreted cytokine, like TNF does. This is in agreement with the observation that a polybasic sequence present in the stalk of BAFF acted as a substrate for a protease. Similar polybasic sequences are also present at corresponding locations in both APRIL and TWEAK (Apo-3L), and for both of them there is evidence of proteolytic processing (26; Holler, N., and J. Tschopp, unpublished observation). Although the protease responsible for the cleavage remains to be determined, it is unlikely to be the metalloproteinase responsible for the release of membrane-bound TNF, as their sequence preferences differ completely (21). The multibasic motifs in BAFF (R-N-K-R), APRIL (R-K-R-R), and TWEAK (R-P-R-R) are reminiscent of the minimal cleavage signal for furin (R-X-K/R-R), the prototype of a proprotein convertase family (27).
The role of antigen-specific B lymphocytes during the different stages of the immune response is highly dependent on signals and contacts from helper T cells (28) and antigen-presenting cells such as dendritic cells (29). B lymphocytes first receive these signals early on during the immune response when they interact with T cells at the edge of the B cell follicles in lymphoid tissues, leading to their proliferation and differentiation into low-affinity antibody-forming cells (30). At the same time, some antigen-specific B cells also migrate to the B cell follicle and contribute to the formation of germinal centers, another site of B cell proliferation but also affinity maturation and generation of memory B cells and high-affinity plasma cells (31).
Signals triggered by CD40L have been shown to be critical for the function of B lymphocytes at multiple steps of the T cell–dependent immune response (32). However, several studies clearly showed that CD40L–CD40 interaction does not account for all contact-dependent T cell help for B cells. Indeed, CD40L-deficient T cells isolated from either knockout mice or patients with X-linked hyper IgM syndrome have been shown to successfully induce proliferation of B cells and their differentiation into plasma cells (33). Likewise, studies using blocking antibodies against CD40L showed that a subset of surface IgD+ B cells isolated from human tonsils proliferate and differentiate in response to activated T cells in a CD40-independent manner (34). Other members of the TNF family, such as membrane-bound TNF and CD30L, have also been shown to be involved in a CD40- and surface Ig–independent stimulation of B cells (33, 35). Finally, CD40-deficient B cells can be stimulated to proliferate and differentiate into plasma cells by helper T cells as long as the surface B cell receptors are triggered at the same time (36). BAFF as well as CD30L and CD40L is expressed by T cells, but its uniqueness resides in its expression by dendritic cells as well as the highly specific location of its receptor on B cells in contrast to the wider expression patterns of CD40, CD30, and the TNF receptors (37). Hence, BAFF may uniquely affect B cells.
In support of a role for BAFF in T cell– and/or dendritic cell–induced B cell growth and potential maturation, we found that BAFF costimulates proliferation of blood- derived B cells concomitantly with cross-linking of the B cell receptors. Moreover, using CD19+ B cells differentiated in vitro into preplasma, germinal center–like B cells (13), we observed a costimulatory effect of BAFF on Ig production by these B cells in the presence of cytokines from activated T cells. Thus, BAFF can induce signals in both naive B cells and germinal center–committed B cells in vitro. Whether this observation will translate during a normal immune response or not will have to be addressed by proper in vivo experiments.
The biological responses induced in B cells by BAFF are distinct from that of CD40L, since proliferation triggered by CD40L occurred at a lower level independently of an anti-μ costimulus (17; Fig. 5 D). Moreover, CD40L can counteract apoptotic signals in B cells after engagement of the B cell receptor (38), whereas BAFF was not able to rescue the B cell line Ramos from anti-μ–mediated apoptosis, despite the fact that Ramos cells do express BAFF-R (Table I; MacKay, F., unpublished observations). Therefore, it is likely that CD40L and BAFF fulfill distinct functions. In this respect, it is noteworthy that BAFF did not interact with any of 16 recombinant receptors of the TNF family tested, including CD40 (Schneider, P., and J. Tschopp, unpublished observations).
Several obscure zones remain in our understanding of an immune response. For instance, little is known about the mechanisms governing the differentiation of a B cell into a plasma cell versus a germinal center B cell. Similarly, aside from the possible involvement of the CD40 pathway shown in vitro (39), we have very little information about the signals deciding the differentiation of a germinal center B cell into a memory B cell or a plasma cell. It will be very interesting to investigate whether or not BAFF has any unique role to play in these critical checkpoint decisions.
Acknowledgments
We thank S. Hertig (University of Lausanne), L. Scarpellino (University of Lausanne), and S. Foley (Biogen, Inc.) for technical assistance, Richard Tizard (Biogen, Inc.) and Brittney Colemen (Biogen, Inc.) for DNA sequencing, and Ralph Budd (University of Lausanne) for reading the manuscript.
This work was supported by grants from the Swiss National Science Foundation (to J. Tschopp) and the Swiss Federal Office of Public Health (to P. Schneider and J. Tschopp).
Abbreviations used in this paper
- aa
amino acid(s)
- APRIL
a proliferation inducing ligand
- BAFF
B cell activating factor belonging to the TNF family
- EST
expressed sequence tag
- LT
lymphotoxin
- NF
nuclear factor
- PNGase F
peptide N-glycanase F
- RANKL
receptor activator of NF-κB ligand
- TRAIL
TNF-related apoptosis-inducing ligand
- wt
wild-type
Footnotes
P. Schneider and F. MacKay contributed equally to this work.
References
- 1.Smith CA, Farrah T, Goodwin RG. The TNF-receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 3.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 4.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 5.Amakawa R, Hakem A, Kundig TM, Matsuyama T, Simard JJ, Timms E, Wakeham A, Mittruecker HW, Griesser H, Takimoto H, et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- 6.Russell JH, Rush B, Weaver C, Wang R. Mature T cells of the autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 8.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 9.Stuber E, Strober W. The T cell–B cell interaction via OX40–OX40L is necessary for the T cell–dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider P, Bodmer JL, Holler N, Mattmann C, Scuderi P, Terskikh A, Peitsch MC, Tschopp J. Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J Biol Chem. 1997;272:18827–18833. doi: 10.1074/jbc.272.30.18827. [DOI] [PubMed] [Google Scholar]
- 11.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 13.Grimaitre M, Werner-Favre C, Kindler V, Zubler RH. Human naive B cells cultured with EL-4 T cells mimic a germinal center-related B cell stage before generating plasma cells. Concordant changes in Bcl-2 protein and messenger RNA levels. Eur J Immunol. 1997;27:199–205. doi: 10.1002/eji.1830270130. [DOI] [PubMed] [Google Scholar]
- 14.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 15.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 17.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 18.Bucher P, Karplus K, Moeri N, Hofmann K. A flexible search technique based on generalized profiles. Comp Chem. 1996;20:3–23. doi: 10.1016/s0097-8485(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 19.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor- human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 21.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 22.Wong R, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett F, Frankel W, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 23.Kindler V, Zubler RH. Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J Immunol. 1997;159:2085–2090. [PubMed] [Google Scholar]
- 24.Berger R, Le Coniat M, Derre J, Vecchione D. Secondary nonrandom chromosomal abnormalities of band 13q34 in Burkitt lymphoma-leukemia. Genes Chromosomes Cancer. 1989;1:115–118. doi: 10.1002/gcc.2870010202. [DOI] [PubMed] [Google Scholar]
- 25.Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/s0065-230x(08)60470-4. [DOI] [PubMed] [Google Scholar]
- 26.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgkin PD, Basten A. B cell activation, tolerance and antigen-presenting function. Curr Opin Immunol. 1995;7:121–129. doi: 10.1016/0952-7915(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 29.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 31.MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 32.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 33.Armitage RJ, Alderson MR. B-cell stimulation. Curr Opin Immunol. 1995;7:243–247. doi: 10.1016/0952-7915(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard D, Gaillard C, Hermann P, Banchereau J. Role of CD40 antigen and interleukin-2 in T cell- dependent human B lymphocyte growth. Eur J Immunol. 1994;24:330–335. doi: 10.1002/eji.1830240209. [DOI] [PubMed] [Google Scholar]
- 35.Shanebeck KD, Maliszewski CR, Kennedy MK, Picha KS, Smith CA, Goodwin RG, Grabstein KH. Regulation of murine B cell growth and differentiation by CD30 ligand. Eur J Immunol. 1995;25:2147–2153. doi: 10.1002/eji.1830250805. [DOI] [PubMed] [Google Scholar]
- 36.Schrader CE, Stavnezer J, Kikutani H, Parker DC. Cognate T cell help for CD40-deficient B cells induces c-myc RNA expression, but DNA synthesis requires an additional signal through surface Ig. J Immunol. 1997;158:153–162. [PubMed] [Google Scholar]
- 37.Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 38.Tsubata T, Wu J, Honjo T. B-cell apoptosis induced by antigen receptor crosslinking is blocked by a T-cell signal through CD40. Nature. 1993;364:645–648. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- 39.Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]