The Th1/Th2 paradigm is a cornerstone for our understanding of T cell responses 1. It conveniently subdivides T cell immune responses into those specialized for defense against intracellular pathogens including viruses and some bacteria (Th1), and a second for defense against large extracellular pathogens such as helminths. Th1 responses depend on IL-12 and IFN-γ to mediate a range of biological effects designed for antiviral immunity. In contrast, Th2 responses employ the cytokines IL-5, IL-4, and IL-13, which promote the mobilization of eosinophils and cause other inflammatory processes designed to expel large parasites. The Th1/Th2 paradigm also has particular relevance for certain inflammatory diseases, as asthma is essentially a Th2 response gone awry, and many autoimmune diseases depend on a Th1 response to autoantigens. However, this neat scheme had one troubling aspect: where do helper cells for antibody production fit in? T cell help for antibody production has long been considered a Th2 property 2 3, although certain Ig class switching relies on IFN-γ.
As acceptance of the Th1/Th2 paradigm increased among immunologists, so too has the effort to identify Th1- or Th2-associated genes or molecules. Numerous transcription factors/signal transducers such as T-bet, signal transducer and activator of transcription (Stat)-6, GATA-3, and Stat-4 were identified that determined Th1 or Th2 differentiation and function 4 5 6. In mice deficient in Stat-6, a Th2 transcription factor, IL-4 signaling is abolished and Th2 responses are absent. Nevertheless, these mice are still capable of mounting T cell–dependent antibody responses (although not IgE, an isotype for mast cell activation). The discovery of the various Th1- and Th2-related transcription factors cemented the validity of the Th1/Th2 paradigm, but what remained unclear was the precise role of Th1 cytokine–producing, or Th2 cytokine–producing cells in different immune responses.
Cell surface molecules that reliably mark Th1 or Th2 cells were also sought, and to date the chemokine receptors are probably the most convenient (although by no means ideal) markers of functional subsets. Th1 cells preferentially express CC chemokine receptor (CCR)5 and CXC chemokine receptor (CXCR)3, and migrate in response to a select set of chemokines induced by cytokines such as IFN-γ or IL-1, whereas Th2 cells preferentially express CCR3 and CCR4 and migrate to chemokines induced by IL-4 or IL-13 7 8 9 10 11 12. The regulated expression of chemokine receptors by effector T cells relates to the simple concept that migration or positioning of a cell is intimately connected to that cell's function 11. In this issue, two groups have provided one of the best examples of this concept, by identifying CXCR5 as a chemokine receptor responsible for the follicular homing of a unique subset of T helper cells for antibody production 13 14. This subset of T cells could be distinguished from Th1 or Th2 cells, and would thus represent a third class of effector-type T cell.
T and B Cell Positioning in Lymphoid Tissue.
T and B cells continuously recirculate from blood to lymphoid tissue, gaining entry through their expression of L-selectin and the chemokine receptor CCR7. B cells localize in the B cell follicles, whereas T cells colocalize with dendritic cells in the T cell areas. This segregation is necessary because it is important for unprimed T cells to first receive signals from professional APCs (dendritic cells) and not from B cells. A specific sequence of cellular movements then accompanies successful T cell priming 15. Activated T cells migrate to the B cell follicles and position themselves at the edge of the follicle, where they meet antigen-primed B cells that have also specifically migrated outwards. The two cell types then physically interact to accomplish CD40-CD154–dependent B cell activation. Thereafter, antigen-primed T cells become distributed throughout the entire follicle, presumably to egg on the germinal center reaction.
This lymphocyte positioning is tightly controlled by chemokine–chemokine receptor interactions 16. The constant traffic of resting T cells through lymphoid tissue is controlled by “lymphoid” chemokines. T cell activation in the T cell areas results in a loss of responsiveness to lymphoid chemokines and gain of responsiveness to the chemokine CXCL13 (also called B cell–attracting chemokine [BCA]-1/B lymphocyte chemoattractant [BLC]; reference 17). CXCL13 binds to the chemokine receptor CXCR5, and mice deficient in CXCR5 or CXCL13 have disorganized lymphoid architecture and impaired antibody responses 18 19. The directed migration of T cells into the B cell follicles 15, the gain of CXCR5 upon T cell activation, and the expression of CXCL13 by stromal cells in the follicles 17 suggested that CXCR5 marked follicle homing T cells.
Follicular B Helper T Cells.
CXCR5 was originally considered a B cell chemokine receptor. An antibody to CXCR5 was produced that stained all B cells, and also an interesting subset of CD45RO+ (memory) T cells in blood and lymphoid tissue 20. CXCR5+ T cells were found to be almost all CD4+, suggesting a role in T helper cell migration. A more comprehensive examination of these T cells 13 14 revealed an important distinction between blood and tonsils. Inflamed tonsils (but not blood) contained CXCR5+ T cells that were activated and distinct from circulating T cells. For instance, the tonsilar CXCR5+ T cells expressed CD69 and inducible costimulator (ICOS), and were generally L-selectin–negative. Expression of ICOS is particularly noteworthy, as this molecule is a CD28-like family member that facilitates effector T cell responses, rather than primary T cell activation. ICOS probably participates in all effector T cell responses, particularly T cell help for antibody production 21 22 23; however, Th1 responses may rely less on ICOS, as strongly polarized Th1 cells downregulate ICOS 22. ICOS binds to a B7 relative termed B7RP-1, which is expressed abundantly on B cells 21. The other notable feature of CXCR5+ T cells is that they do not produce the typical Th1 or Th2 cytokines, although a proportion do produce IL-2. Thus, CXCR5+ lymphoid T cells have all the hallmarks of effector function, but not Th1 or Th2 function or phenotype.
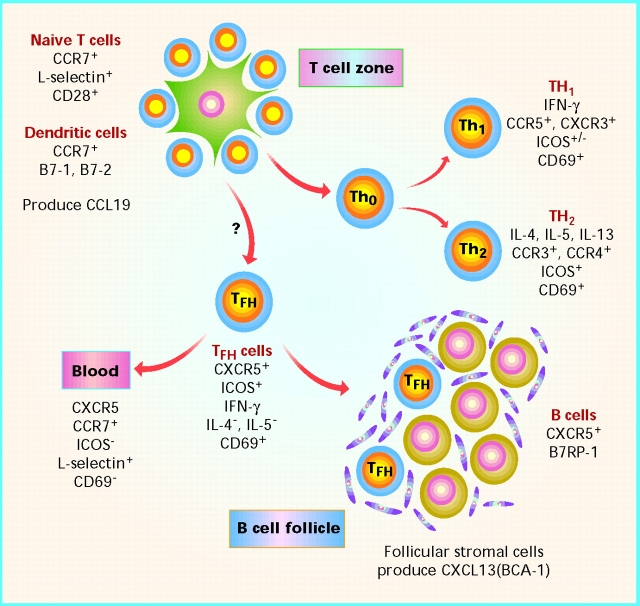
The distinguishing feature of CXCR5+ T cells from lymphoid tissue is that they are capable of providing help for antibody responses 13 14. Whether these T cells produce a B cell helper cytokine is not yet known, although it is conceivable that they deliver help to B cells purely through cell–cell contact and activation through ICOS and other surface molecules. The involvement of CXCR5+ T cells in B cell responses was also suggested by their localization in the mantle and light zone of germinal centers. Indeed all T cells that localize in B cell follicles are CXCR5+. The distinction of a new subset of effector T cells with classic T helper function for Ig production led the two groups to label these cells “follicular B helper T cells” (TFH). In contrast to tonsilar TFH cells, the CXCR5+ T cells in blood do not display effector function and may represent a circulating TFH-type memory cell. These blood CXCR5+ T cells express CCR7, a chemokine receptor for naive and memory lymphocyte recirculation that is often absent from activated effector T cells 24. A cartoon depicting the phenotype and positioning properties of TFH cells in lymphoid tissue is presented in Fig. 1.
Figure 1.
Development and positioning of subsets of effector T cells in lymphoid tissue. Naive T cells and dendritic cells colocalize in the T cell zones, through actions of chemokines such as CCL19 (macrophage inflammatory protein [MIP]-3β). Naive T cell activation is dependent on CD28–B7-1 or –B7-2 interactions. For CD4+ cells, at least three effector subsets may arise: Th1 cells, Th2 cells, or TFH cells. TFH cells can be distinguished by their expression of CXCR5 and their ability to position within B cell follicles, whereas Th1 and Th2 express other chemokine receptors. TFH differ from Th1 or Th2 cells by their lack of cytokine production. Similar to Th2 cells, they express high levels of ICOS, a CD28-like molecule that binds B7RP-1 expressed on B cells. The issue of whether Th1 and Th2 cells localize within B cell follicles is unresolved, although the cytokines they produce certainly influence B cell development. IL-4 stimulates B cells and induces IgE isotype switching, whereas IFN-γ induces IgG isotypes for antiviral defence. The exact relationship of TFH to Th1 and Th2 cells is undetermined, and it is possible that TFH cells derive from either cell type. CXCR5+ T cells in blood (descendents from TFH?) have a phenotype consistent with memory rather than effector function. Phenotypes listed are generalizations.
Relationship of TFH to Th1 and Th2 Cells.
The discovery of a non-Th1, non-Th2 effector T cell for B cell help poses an interesting question: what to make of all the previous data implicating Th2 cells as helper cells for antibody responses? A plausible explanation is that TFH cells are derived from Th2 or Th1 cells. There is no doubt about the profound effects that IL-4, a Th2 cytokine, has on B cell responses, including upregulation of costimulatory molecules (i.e., CD40), stimulation of B cell division and survival, and Ig isotype switching. However, IL-4– or Stat-6–deficient mice still do make antibodies, particularly of the non-IgE isotypes 25 26 27. There is some evidence for a relationship between TFH and Th1 or Th2 cells. Th1 and Th2 cells adoptively transferred to recipients can both migrate to B cell follicles and support antibody production in a CD154-dependent manner 28. Nevertheless Th1 and Th2 cells do show differences in their localization in lymphoid tissue, with Th2 cells being much more follicle centric. In mice, this might relate to expression of CCR7, which keeps Th1 cells closer to the T cell area, whereas its absence allows Th2 cells to drift closer to the B cell follicles 29. The most likely scenario is that TFH cells are a separate effector-type T cell that does not necessarily derive from Th1 or Th2 cells. It could be that the TFH subset expresses cytokines and transcription factors particular only to these cells. B cell activator belonging to the TNF family (BAFF), a newly identified cytokine for B cell responses 30, is one possibility.
There are important implications of a separate TFH subset, capable of facilitating B cell activation in a non-Th1/Th2–related manner. One of these is that aspects of B cell responses can occur independently of Th2 (and Th1) cytokines. This would be consistent with the notion that Th2 responses are primarily designed for immunity against extracellular parasites. The Th1 and Th2 cytokines could be seen as factors that skew the antibody response in one direction or another depending on need for IgE for mast cell activation, or the development of Ig isotypes that help in the clearance of infected cells through opsonization and phagocytosis. Like many aspects of immunology, there is overlap and redundancy, because Th2 cells also show a degree of follicular homing capability, high expression of ICOS, and some helper function for antibody responses. The relationship of TFH to Th1 and Th2 cells, and the conditions for interconversion among these three subsets, are still missing pieces in the story. Irrespective of the many unknown aspects of TFH cells, these cells are an interesting new extension to the Th1/Th2 paradigm.
Acknowledgments
C.R. Mackay is supported by the Glazebrook Trust and the Cooperative Research Center for Asthma.
References
- Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Coffman R.L., Seymour B.W., Lebman D.A., Hiraki D.D., Christiansen J.A., Shrader B., Cherwinski H.M., Savelkoul H.F., Finkelman F.D., Bond M.W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Glimcher L.H., Singh H. Transcription factors in lymphocyte development—T and B cells get together. Cell. 1999;96:13–23. doi: 10.1016/s0092-8674(00)80955-1. [DOI] [PubMed] [Google Scholar]
- Ho I.C., Hodge M.R., Rooney J.W., Glimcher L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Rincon M., Flavell R.A. T-cell subsetstranscriptional control in the Th1/Th2 decision. Curr. Biol. 1997;7:R729–R732. doi: 10.1016/s0960-9822(06)00368-x. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Mackay C.R., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A., Mackay C.R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D'Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B.O., Zanni M.P., Uguccioni M., Loetscher M., Mackay C.R., Pichler W.J., Yawalkar N., Baggiolini M., Moser B. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr. Biol. 1997;7:836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mackay C.R. T cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Uguccioni M., Bordoli L., Baggiolini M., Moser B., Chizzolini C., Dayer J.M. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- Schaerli P., Willimann K., Lang A.B., Lipp M., Loetscher P., Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D., Ohl L., Kremmer E.K., Ellwart J., Sallusto F., Lipp M., Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1551. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P., Ingulli E., Merica R.R., Johnson J.G., Noelle R.J., Jenkins M.K. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., McHeyzer-Williams L.J., Ngo V.N., McHeyzer-Williams M.G., Cyster J.G. In vivo–activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Forster R., Sedgwick J.D., Browning J.L., Lipp M., Cyster J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Forster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Yoshinaga S., Whoriskey J., Khare S., Sarmiento U., Guo J., Horan T., Shih G., Zhang M., Coccia M., Kohno T. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- Coyle A.J., Lehar S., Lloyd C., Tian J., Delaney T., Manning S., Nguyen T., Burwell T., Schneider H., Gonzalo J.A. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- Kopf M., Coyle A.J., Schmitz N., Barner M., Oxenius A., Gallimore A., Gutierrez-Ramos J.C., Bachmann M.F. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Shimoda K., van Deursen J., Sangster M.Y., Sarawar S.R., Carson R.T., Tripp R.A., Chu C., Quelle F.W., Nosaka T., Vignali D.A. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Coyle A.J., Kosco-Vilbois M., Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol. Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Smith K.M., Pottage L., Thomas E.R., Leishman A.J., Doig T.N., Xu D., Liew F.Y., Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J. Immunol. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- Randolph D.A., Huang G., Carruthers C.J., Bromley L.E., Chaplin D.D. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. 1999;286:2159–2162. doi: 10.1126/science.286.5447.2159. [DOI] [PubMed] [Google Scholar]
- Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]