Abstract
Leukocyte traffic through secondary lymphoid tissues is finely tuned by chemokines. We have studied the functional properties of a human T cell subset marked by the expression of CXC chemokine receptor 5 (CXCR5). Memory but not naive T cells from tonsils are CXCR5+ and migrate in response to the B cell–attracting chemokine 1 (BCA-1), which is selectively expressed by reticular cells and blood vessels within B cell follicles. Tonsillar CXCR5+ T cells do not respond to other chemokines present in secondary lymphoid tissues, including secondary lymphoid tissue chemokine (SLC), EBV-induced molecule 1 ligand chemokine (ELC), and stromal cell–derived factor 1 (SDF-1). The involvement of tonsillar CXCR5+ T cells in humoral immune responses is suggested by their localization in the mantle and light zone germinal centers of B cell follicles and by the concomitant expression of activation and costimulatory markers, including CD69, HLA-DR, and inducible costimulator (ICOS). Peripheral blood CXCR5+ T cells also belong to the CD4+ memory T cell subset but, in contrast to tonsillar cells, are in a resting state and migrate weakly to chemokines. CXCR5+ T cells are very inefficient in the production of cytokines but potently induce antibody production during coculture with B cells. These properties portray CXCR5+ T cells as a distinct memory T cell subset with B cell helper function, designated here as follicular B helper T cells (TFH).
Keywords: chemokines, CXC chemokine receptor 5, lymphocytes, B cell follicle, antibodies
Introduction
T cell–dependent immune responses to pathogens are initiated in specialized lymphoid tissues, including LNs, Peyer's patches (PPs), and the spleen. The outcome of these defensive mechanisms relies on the finely tuned traffic of T and B cells as well as antigen-presenting cells, suggesting that chemokines may be involved in the recruitment and proper positioning of leukocytes within these compartments. Chemokines comprise a large family of structurally related chemoattractant proteins that regulate the composition of cellular infiltrates at sites of inflammation or, alternatively, the physiological leukocyte migration during hematopoiesis, antigen sampling in secondary lymphoid tissues, and immune surveillance 1 2 3 4 5 6. This report focuses on a particular subset of T cells with homing selectivity for B cell follicles in secondary lymphoid tissues.
Chemokines shown to be produced in LNs, PPs, or the spleen include secondary lymphoid tissue chemokine (SLC; CCL21) (systematic nomenclature for human chemokines available at http://cytokine.medic.kumamoto-u.ac.jp/), EBV-induced molecule 1 ligand chemokine (ELC; CCL19), B cell–attracting chemokine 1 (BCA-1; CXCL14), stromal cell–derived factor 1 (SDF-1; CXCL12), and monocyte-derived chemokine (MDC; CCL22 6 7 8). In addition, several typical inflammatory chemokines are upregulated in inflamed rather than resting LNs, which may reflect an enhanced T cell activation status. In contrast to SDF-1 and MDC, the role of SLC and ELC in controlling cellular traffic in secondary lymphoid tissues is well established. The two chemokines are produced within T zone areas but are absent in B cell follicles, and their expression is dependent on TNF and/or lymphotoxin (LT)α/β 4 6 7. CC chemokine receptor 7 (CCR7), the selective receptor for SLC and ELC, is present on the bulk of resting T cells in peripheral blood and mature dendritic cells and mediates rapid adhesion to integrin ligands and chemotactic migration 1 2 3 4 6 7. Importantly, mutant mice with defective SLC/ELC production 9 10 11 12 and gene-targeted mice that lack CCR7 13 show severe defects in secondary lymphoid tissue architecture, notably in the proper positioning of T and B cells and in the homing function of mature dendritic cells. Consequently, these mutant mice display markedly delayed kinetics in T cell–dependent immune responses whereas antibody responses remain largely unaffected.
What about the involvement of chemokines in the regulation of humoral responses? BCA-1 is a highly efficient chemoattractant for mature B cells that uniformly express its receptor CXC chemokine receptor 5 (CXCR5 14 15 16). In mice, the lack of CXCR5 or BLC (the murine homologue of human BCA-1) resulted in abnormal lymphoid tissue formation 17 18, notably follicular organization in the spleen and PPs, whereas transgenic mice expressing BLC in pancreatic islets showed secondary lymphoid tissue–like structures at these sites 19, suggesting an important role for BCA-1 in the organization of human B cell follicles. However, CXCR5 deficiency does not prevent murine B cell responses, and occasionally small germinal center–like B cell aggregates are observed 20. In mice, BCA-1 transcripts are detected in follicles and, similar to SLC and ELC, its expression is also dependent on TNF and/or LTα/β 11 15 21 22. In humans, BCA-1 expression was mainly studied in Helicobacter pylori–associated lymphoid tissue and gastric lymphomas 23. In addition to B cells, low numbers of human peripheral blood T cells and a larger fraction of tonsillar T cells express CXCR5 but their function is not known 17 24. Here we report the migration and effector properties of human CXCR5+ T cells present in blood and tonsils. Distinct migration properties and the ability to induce antibody production during coculture with B cells indicate that CXCR5 expression marks a T cell fraction with unique effector function.
Materials and Methods
Antibodies.
Antibodies to human proteins were purchased from the following sources: rat antibodies to IL-5 (TRFK5) and IL-10 (JES3-9D7), and mouse antibodies to CD4 (RPA-T4), CD40 (5C3), CD45RO (UCHL1), CD45RA (HI100), CD62L (DREG-56), CD69 (FN50), CD134 (ACT35), CD152 (BNI3), and CD154 (TRAP-1) were all from BD PharMingen; mouse antibodies to CCR6 (53103.111), CXCR3 (49801.111), CXCR4 (12G5), and goat IgG to BCA-1 (AF470) were from R&D Systems; mouse antibodies to IFN-γ (25723.11), IL-2 (5344.111), IL-4 (3010.211), and IL-13 (JES10-5A2) were from Becton Dickinson; mouse antibody to CD71 (DF1513) was from Sigma-Aldrich; mouse antibodies to CD4 (MT310), CD19 (HD37), CD20 (L26), CD21 (1F8), CD25 (ACT-1), and CD31 (JC/70A), rabbit IgG to CD3 (A0452), and bovine S-100 were from Dako; mouse antibodies to CCR3 (7B11) and CCR5 (5C7) were from LeukoSite, Inc.; and mouse antibody to HLA-DR (L243) was from Research Diagnostics Inc. Secondary and control antibody reagents were from the following sources: goat anti–rabbit IgG (F-1262), mouse IgG1, and goat IgG were from Sigma-Aldrich; biotinylated rabbit anti–rat IgG (E0468) and mouse IgG2a were from Dako; goat anti–mouse MicroBead IgG (484-01) and goat anti–rabbit MicroBead IgG (486-02) were from Miltenyi Biotec; mouse IgG2b was from R&D Systems; rabbit IgG was from Zymed Laboratories; rat IgG was from BD PharMingen; biotinylated donkey anti–goat/sheep Ig (AB360) was from The Binding Site. The following antibodies were from noncommercial sources: rat antibody to CCR7 (3D12; M. Lipp, Max-Delbrück Center for Molecular Medicine, Berlin, Germany); mouse antibody to inducible costimulator (ICOS; F44; R.A. Kroczek, Robert Koch Institute, Berlin, Germany); mouse antibody to CD3 (Tr66; A. Lanzavecchia, Institute for Research in Biomedicine, Bellinzona, Switzerland); and rabbit IgG to CXCR5 23.
Cell Preparation and Cultures.
Human PBLs were isolated from donor blood buffy coats by centrifugation on Ficoll-Paque followed by Percoll 25. For the measurement of intracellular cytokines, CD4+CD45RO+ PBLs were negatively selected by removing CD8+, CD14+, CD16+, CD45RA+, and CD56+ cells using the magnetic cell sorting system from Miltenyi Biotec. The cells obtained were also used to study the induction of CXCR5 expression after removal of the CXCR5+ cells. Tonsils from individuals undergoing tonsillectomy were mechanically dispersed and lymphocytes were isolated by centrifugation on Ficoll-Paque. CD4+CD45RO+CXCR5+ tonsillar cells were isolated by first removing CD8+, CD20+, CD45RA+, and CD56+ cells and then by positive selection of CXCR5+ cells (>97% purity). Lymphocytes were cultured in RPMI 1640 containing 2 mM l-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/ml penicillin/streptomycin, 5 × 10−5 M 2-mercaptoethanol (GIBCO BRL), and 10% heat-inactivated FCS (Inotech/Biological Industries). Cells were stimulated with 5 μg/ml anti-CD3 precoated on tissue plates or 1 μg/ml PHA (Wellcome) in the presence of 20 U/ml IL-2.
T Cell Effector Functions.
For detection of intracellular cytokines, purified CD4+CD45RO+ PBLs were stimulated with 10 ng/ml PMA and 1 μg/ml ionomycin for 6 h, and 10 μg/ml Brefeldin A was added for the last 4 h. Cells were then fixed with 2% paraformaldehyde, permeabilized with 0.2% saponin/2% FCS in PBS, and stained with the appropriate antibodies to cytokines for flow cytometric analysis (FACScan™; Becton Dickinson). Labeling of surface proteins was performed before fixation.
T cell help in antibody production was studied as follows. Tonsillar T cells (CD4+CXCR5+ and CD4+CXCR5−) and B cells (CD4−CD8−CXCR5+) were sorted by FACS® and cocultured in 96-well plates at 105 cells/well each of T and B cells in the absence of IL-2 for 10 d. IgM, IgG, and IgA contents in the culture supernatants were determined by ELISA.
T Cell Chemotaxis.
Cell migration was measured in 48-well chemotaxis chambers (Neuro Probe Inc. 25). In brief, chemokines in Hepes-buffered RPMI 1640 supplemented with 1% pasteurized plasma protein (Swiss Red Cross Laboratory) were added to the lower wells and 100,000 cells in the same medium to the upper wells. Polyvinylpyrrolidone-free polycarbonate membranes (Poretics Corp.) with 3-μm pores coated with type IV collagen were used. After incubation for 1.5 h, the membrane was removed, washed on the upper side with PBS, fixed, and stained. Migrated cells were counted microscopically at 1,000× magnification in five randomly selected fields per well. The assay was performed in triplicates.
Northern Blot Analysis.
Total RNA was extracted from CXCR5+ T cells immediately after isolation or after stimulation with anti-CD3, and 10-μg samples were analyzed by Northern blotting using 32P-labeled receptor DNA fragments (109 cpm/mg DNA) as hybridization probes 26. The following probes were used: a 550-bp BamH1 fragment for CXCR4 27, a 550-bp BstX1 fragment for CXCR5 14, and a 430-bp BstX1 fragment for CCR7 26.
Immunohistochemistry and In Situ Analysis.
Tonsillar tissue sections were either snap frozen in liquid nitrogen or, alternatively, formaldehyde fixed and paraffin embedded. For immunostaining, sections of frozen tissue were fixed with acetone, air dried, and incubated in 10% rabbit or swine serum in Tris-buffered saline (TBS) containing 0.01% Tween 20, and then further processed as the paraffin-embedded sections. Optimal staining for S-100, CD21, CD45RO, and HLA-DR required incubation of paraffin-embedded sections with proteinase (proteinase XXV; Sigma-Aldrich) for 6 min at 37°C, and identification of CD3+ or CD20+ cells was improved by three times boiling of paraffin-embedded sections (microwave, 5 min each time) in 10 mM citrate buffer, pH 6.0, before incubation with primary antibodies in TBS containing 1% rabbit or swine serum plus either 2% Tween 20 (S-100) or 0.01% Tween 20 (others). After overnight incubation, sections were treated by standard alkaline phosphatase anti–alkaline phosphatase (APAAP) or avidin–biotin complex (ABC) techniques (Dako) for mouse or rabbit/goat antibodies, respectively, stained with New Fuchsin solution (New Fuchsin Kit; Dako), counterstained with Mayer's hematoxylin (Fluka), and mounted using Aquamount (BDH Laboratory Supplies). For double immunofluorescence staining, frozen sections were incubated with goat anti–BCA-1 antibody and mouse anti-CD31 antibody as described above, then further processed in TBS/0.5% BSA with biotinylated sheep anti–goat antibody/streptavidin-Cy5–RPE (C0050; Dako) for BCA-1, followed by FITC-goat anti–mouse Ig (F0479; Dako) for CD31, and, finally, mounted with Mowiol (Aventis) plus 0.02% diazabicyclo[2.2.2]octane (DABCO; Fluka) to avoid bleaching. Stained sections were analyzed by light or confocal microscopy (Nikon microscope Eclipse E600 or ZEISS Laser Scanning Microscope LSM410).
For in situ hybridization, digoxigenin (DIG)-labeled RNA probes of 368 nucleotides corresponding to the 5′ region in the BCA-1 DNA 14 were prepared by in vitro transcription using the DIG RNA labeling kit (Roche Diagnostics). Dewaxed/rehydrated and proteinase-treated paraffin-embedded sections were fixed in 4% paraformaldehyde/PBS and acetylated for 10 min with 26.4 mM acetic anhydride in 0.1 M triethanolamine. After prehybridization in 4× SSC, 50% deionized formamide, 2× Denhardt's solution, 250 μg/ml yeast RNA, and 100 μg/ml salmon sperm DNA for 2 h at 50°C, sections were incubated at 50°C overnight with 10 ng of DIG-labeled sense or antisense RNA probes in 4× SSC, 1% dextran sulfate, 50% deionized formamide, 2× Denhardt's solution, 500 μg/ml yeast RNA, and 500 μg/ml salmon sperm DNA. After one washing with 2× SSC at room temperature for 30 min, RNase T1 (Roche Diagnostics) treatment at 100,000 U/ml, and additional washings at 54°C with 2× SSC followed by 0.2× SSC, DIG-labeled RNA was detected by anti-DIG antibody coupled to alkaline phosphatase and color development according to the supplier (Roche Diagnostics).
Results
CXCR5+ T Cells in Blood and Tonsils.
Coexpression of cell surface markers with CXCR5 on human T cells was analyzed by flow cytometry (Table ). In peripheral blood, CXCR5 is present on a subpopulation of memory (CD45RO+) T cells, but absent on naive (CD45RA+) T cells, as reported previously 17 24. Over 90% of CXCR5+ T cells express CD4 and <10% express CD8. About 20% of total CD4+CD45RO+ T cells are CXCR5+, showing that CXCR5 is less frequent on these cells than on B cells, which uniformly express CXCR5 16 17 24 28. Circulating CXCR5+ T cells are in a resting state (CD25−, CD69−, CD71−, HLA-DR−) and lack the expression of costimulatory molecules (CD40L, OX40, ICOS), suggesting that they are not involved in ongoing immune responses. In contrast, CXCR5+ T cells are highly enriched in inflamed tonsillar tissue. Almost all memory T cells (>95%) are CD4+ and stain positive for CXCR5, whereas naive T cells do not express CXCR5. Furthermore, several activation markers (CD69, HLA-DR, ICOS) are found on the majority of CXCR5+ T cells, suggesting that this fraction is engaged in tonsillar immune responses (Table ). Other inducible T cell markers, including alternative costimulatory molecules (CD40L, OX40, CTLA-4) or activation/proliferation markers (CD25, CD71), were not detected.
Table 1.
Phenotypic Markers on CXCR5+ T Cells in Blood and Tonsils
| Marker | Blood | Tonsils |
|---|---|---|
| CD4 | 95 ± 2.1 (8) | 92 ± 4.3 (7) |
| CD8 | 5 ± 1.5 (8) | 8 ± 2.9 (7) |
| CD45RO | 98 ± 1.9 (8) | 97 ± 2.5 (7) |
| CD25 | 1 ± 0.5 (5) | 1 ± 0.8 (5) |
| CD69 | 3 ± 0.8 (5) | 88 ± 4.1 (5) |
| CD71 | 1 ± 0.5 (5) | 2 ± 1.2 (5) |
| HLA-DR | 2 ± 0.9 (5) | 78 ± 9.8 (5) |
| OX40 (CD134) | 1 ± 0.6 (4) | 3 ± 1.4 (4) |
| CD40L (CD154) | 2 ± 1.9 (4) | 2 ± 1.4 (4) |
| CTLA-4 (CD152) | 1 ± 0.6 (4) | 2 ± 0.8 (4) |
| ICOS | 4 ± 1.2 (5) | 65 ± 8.5 (5) |
| CD62L | 82 ± 4.8 (5) | 42 ± 5.8 (5) |
Freshly isolated CXCR5+CD3+ T cells from blood or tonsils were analyzed by three-color flow cytometry.
Modulation of CXCR5 Expression and Migration to BCA-1.
The migration capacity of leukocytes and, consequently, their potential involvement in immune responses, depends largely on the repertoire of chemokine receptors that are functionally expressed. CCR7, which defines homing of circulating T cells to T cell compartments within secondary lymphoid tissues 29 30 31, is coexpressed on virtually all circulating CXCR5+ T cells (Table ). This is in striking contrast to the marked reduction of CCR7 expression on tonsillar CXCR5+ T cells (Fig. 1), suggesting that SLC and ELC, the ligands of CCR7, may have caused its downmodulation 2. Similarly, L-selectin (CD62L) expression is reduced on tonsillar CXCR5+ T cells compared with blood CXCR5+ T cells, implying that the majority of tonsillar CXCR5+ T cells have recently immigrated from circulation. No striking differences between blood and tonsillar CXCR5+ T cells were observed for other chemokine receptors. CXCR4 is unique in its uniform but weak expression on freshly isolated blood and tonsillar T cells (see below). Fractions of similar size stain positive for CXCR3, CCR3, CCR5, and CCR6, whereas other chemokine receptors were not detected.
Table 2.
Chemokine Receptor Profile on CXCR5+CD4+ T Cells in Blood and Tonsils
| Receptor | Blood | Tonsils |
|---|---|---|
| CXCR3 | 25 ± 3.2 (5) | 30 ± 4.3 (5) |
| CXCR4 | 34 ± 15 (5) | 29 ± 12 (5) |
| CCR3 | 3 ± 2.9 (3) | 9 ± 5.0 (3) |
| CCR5 | 4 ± 3.2 (3) | 14 ± 9.4 (3) |
| CCR6 | 32 ± 5.5 (4) | 36 ± 6.8 (4) |
| CCR7 | 97 ± 2.5 (5) | 28 ± 6.2 (5) |
Freshly isolated CXCR5+CD4+ T cells from blood or tonsils were analyzed by three-color flow cytometry for coexpression of chemokine receptors.
Figure 1.
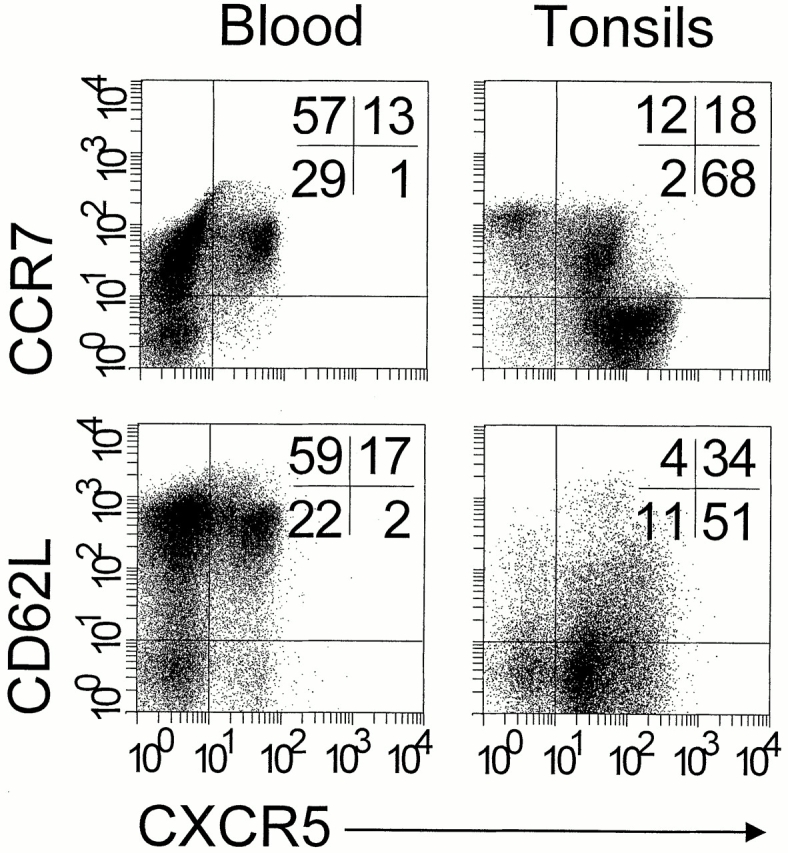
CCR7 and CD62L are downmodulated on tonsillar CXCR5+ T lymphocytes. Freshly isolated blood and tonsillar lymphocytes were triple stained with antibodies to CD4, CXCR5, and CCR7 or CD62L and analyzed by flow cytometry. Dot plots show cells gated for the expression of CD4. Quadrants were set according to the staining of control antibodies and the percentage of cells in each quadrant is indicated. Comparable results were obtained with five additional blood and tonsillar cell preparations.
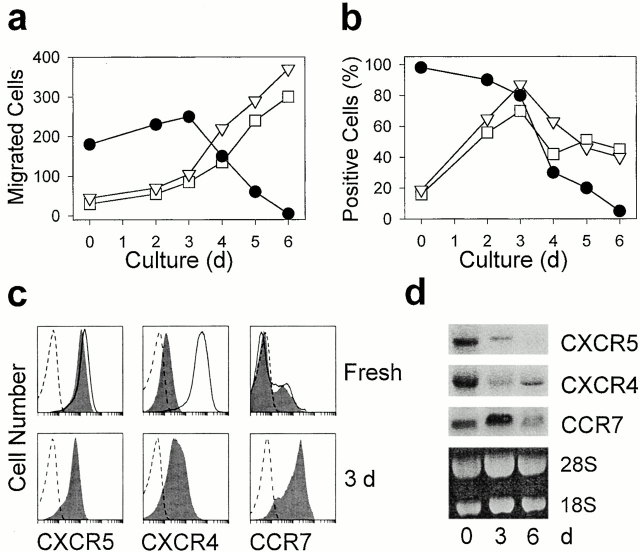
Freshly isolated tonsillar CXCR5+ T cells migrated readily in response to BCA-1, whereas responsiveness to ELC and SDF-1 was routinely low or undetectable (Fig. 2 a). Responsiveness to BCA-1 was moderately enhanced when the cells were treated with anti-CD3 or PHA (not shown). Prolonged activation resulted in complete loss of chemotaxis and receptor expression, and this effect correlates with the appearance of T cell blasts (Fig. 2, a and b). CXCR5 transcripts were readily detected in freshly isolated CXCR5+ T cells and decreased to undetectable levels by day 6 of culture (Fig. 2 d). These observations are in striking contrast to changes in ELC- and SDF-1–mediated responses and in the expression of their receptors. Initially, chemotaxis to ELC and SDF-1 remained low until responsiveness to BCA-1 started to decline, i.e., T cell blast and cell proliferation became evident, and then rapidly increased to levels exceeding the maximal responses observed with BCA-1 (Fig. 2 a). CCR7 protein and mRNA expression increased during the first 3 d of activation, followed by a slow decline. Of note, these changes do not correlate with changes in T cell migration, as marked responses were observed only in the phase of chemokine receptor downmodulation (Fig. 2, a and b). CXCR4 is uniformly but at low levels present on freshly isolated tonsillar CXCR5+ T cells and high level expression is restored by overnight culture under nonstimulatory conditions (Fig. 2 c), suggesting receptor relocation from intracellular stores rather than de novo gene expression 32 33 34. In this respect, CXCR4 expression clearly differs from the expression of CXCR5 and CCR7, which was not affected by overnight culture (Fig. 2 c). However, similar to ELC/CCR7, enhancement of chemotaxis to SDF-1 occurred late in culture and was not related to changes in cell surface CXCR4.
Figure 2.
Inhibition of migration to BCA-1 and CXCR5 expression during culture of tonsillar CXCR5+ T cells. Freshly isolated tonsillar CXCR5+ T cells were activated with immobilized anti-CD3 for up to 6 d and examined for in vitro migration (a); chemokine receptor expression was assessed by flow cytometry (b and c) and Northern blot analysis (d). (a) Chemotaxis in response to 1,000 nM BCA-1 (•), 100 nM SDF-1 (□), and 100 nM ELC (▿; mean number of migrated cells per 5 HPFs in triplicate wells). The data are representative of five independent experiments. (b) The percentage of cells positive for CXCR5 (•), CXCR4 (□), and CCR7 (▿), with gates set at 99% isotype-matched control antibody staining. (c) Expression of CXCR5, CXCR4, and CCR7 (shaded) in freshly isolated cells and in cells treated with anti-CD3 for 3 d. Broken lines represent the staining of isotype-matched control antibodies, and solid lines show chemokine receptor expression after overnight culture under nonstimulatory conditions. (d) Northern blot analysis of CXCR5, CXCR4, and CCR7 transcripts with total RNA from freshly isolated cells and after treatment with anti-CD3 for 3 and 6 d.
Peripheral blood CXCR5+ T cells migrated poorly to BCA-1 (25 ± 5 migrated cells/5 high power fields [HPFs]; n = 3). Similar to tonsillar CXCR5+ T cells, activation with anti-CD3 or PHA led to a transient enhancement of migration, reaching maximal levels by day 3 (92 ± 6 migrated cells/5 HPFs; n = 3), followed by a rapid decline in both BCA-1–mediated migration and CXCR5 expression. CXCR5 expression in naive T cells was not achieved by common stimulation protocols (anti-CD3 with or without anti-CD28) and, thus, may require accessory stimulation provided by antigen-presenting cells. Loss of CXCR5 expression and responsiveness to BCA-1 was routinely observed during in vitro culture of T cells, as evidenced by 10 independent cell lines established from sorted CXCR5+ T cells from blood or tonsils. Also, CXCR5 expression was completely absent in numerous unrelated T cell lines with defined antigen specificity and cytokine production profile.
Cytokine Profile of CXCR5+ T Cells.
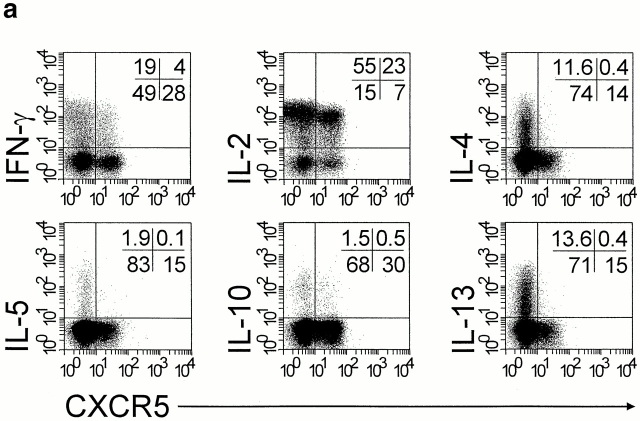
The pattern of cytokine production was studied by flow cytometry in CXCR5+ and CXCR5− peripheral blood T cells after potent polyclonal stimulation 35. Only minor populations within CXCR5+ T cells were capable of producing IFN-γ (11.2 ± 1.5%; mean ± SEM); IL-4, IL-5, IL-10, and IL-13 were not produced (<3%; Fig. 3 a). As expected, both CXCR5+ and CXCR5− T cells synthesized IL-2 equally well (77.2 ± 1.0 and 80.0 ± 1.1%, mean ± SEM, respectively). In contrast, CXCR5− T cells produced more prominently IFN-γ (28.4 ± 0.6%, mean ± SEM), IL-4 (12.0 ± 1.5%, mean ± SEM), and IL-13 (13.5 ± 2.6%, mean ± SEM; Fig. 3 a). Of note, the loss of CXCR5 expression in long-term cultures was accompanied by the acquisition of enhanced cytokine production, as shown in cell lines generated from sorted CXCR5+ T cells. Except for IL-2 and IFN-γ, CXCR5+ T cells from tonsils did not produce cytokines, which fully agrees with the results obtained with blood CXCR5+ T cells.
Figure 3.
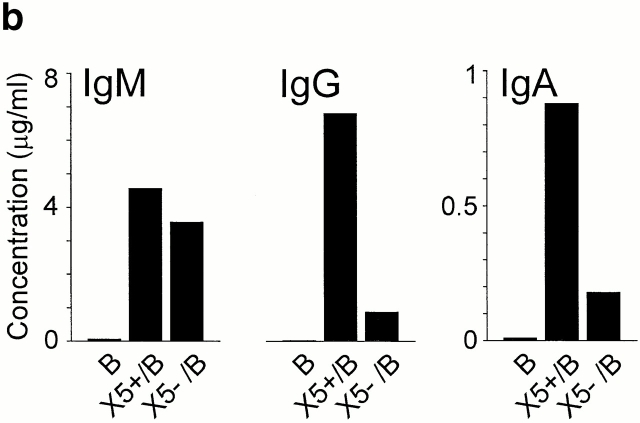
Lack of cytokine production but induction of IgG/IgA synthesis by CXCR5+ T cells. (a) CD4+CD45RO+ T cells were isolated from blood by negative selection. After polyclonal stimulation, CXCR5+ and CXCR5− CD4+ T cells were examined for intracellular production of IFN-γ, IL-2, IL-4, IL-5, IL-10, and IL-13 by three-color flow cytometry. Dot plots show cells gated for CD4. Quadrants were set according to the staining of isotype-matched control antibodies and the percentage of cells in each quadrant is indicated. Results are representative of a total of 11 experiments with memory T cell preparations from different blood and tonsil donors. (b) CXCR5+ T cells induce antibody production in tonsillar B cells. Tonsillar B cells were cultured for 10 d either alone (B) or in the presence of CXCR5+ (X5+/B) or CXCR5− T cells (X5−/B), and IgG, IgA, and IgM concentrations in the culture supernatants were determined by ELISA. A representative example out of four independent experiments is shown.
B Cell Helper Function of CXCR5+ T Cells.
Coculture experiments with tonsillar T and B cells showed that CXCR5+ T cells are potent inducers of antibody production (Fig. 3 b). CXCR5+ T cells enhanced the production of IgG and IgA by a factor of 6.3 ± 2.1 (mean ± SEM, n = 4) and 4.6 ± 1.7 (mean ± SEM, n = 4), respectively, compared with CXCR5− T cells. Both T cell fractions were equally potent in induction of IgM secretion (1.3 ± 0.6–fold [mean ± SEM, n = 4] enhancement by CXCR5+ T cells), and IgM levels were consistently lower than those of IgG, suggesting that CXCR5+ T cells affected B cell Ig switching (IgM to IgG/IgA) and/or memory B cell activation. B cells cultured in the absence of T cells produced marginal levels of antibodies (<60 ng/ml for IgG and IgM, and <30 ng/ml for IgA). In contrast to tonsillar T cells, CXCR5+ T cells from peripheral blood did not support antibody production in B cell cultures in the absence of exogenous stimuli, which correlates with their resting state.
Colocalization of CXCR5+ T Cells and BCA-1 Production in Tonsils.
Prominent attraction by BCA-1 and reduced responsiveness to SLC, ELC, and SDF-1 suggest that tonsillar CXCR5+ T cells are recruited to sites of BCA-1 production. Immunohistochemistry and in situ hybridization of tonsillar tissue sections revealed the highly selective expression of BCA-1 in the follicular mantle zone and, albeit less prominently, in the light zone of germinal centers (Fig. 4, a and b), which markedly contrasts with the T zone–selective expression of SLC and ELC 4 6 7. Positivity in the mantle zone is associated with reticular cells, as demonstrated by the intense staining of dendrite-like cellular extensions (Fig. 4 c, inset). BCA-1–producing cells may represent a distinct class of dendritic cells, as their localization differs from follicular (CD21+) or interdigitating (S-100+) dendritic cells (Fig. 4e and Fig. f). In addition, prominent BCA-1 production is found on high endothelial venules (HEVs) within follicles but not in the T zone, as shown by single staining (Fig. 4 c, arrowheads). This is supported by confocal microscopy with fluorescence-labeled antibodies to BCA-1 (red) and CD31 (green), demonstrating colocalization in follicular HEVs (yellow) (Fig. 4 d; inset shows T zone HEVs). In 20 randomly selected areas in tonsillar sections (0.32 mm2/each), a total of 699 vessels were identified by anti-CD31 staining and 99 out of 101 BCA-1+ vessels were located in the follicular mantle. This selectivity in vascular BCA-1 expression suggests that circulating CXCR5+ cells could be directly recruited into B cell follicles via BCA-1–induced attachment to follicular HEVs.
Figure 4.
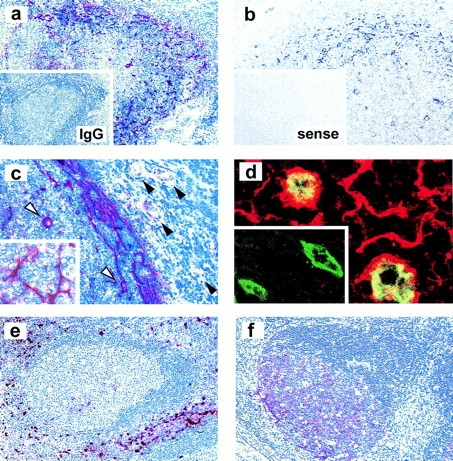
BCA-1 expression in tonsillar follicles. (a and c) Immunostaining of BCA-1 (red) in frozen tonsillar sections; inset in panel a shows control staining with isotype-matched control. (b) In situ hybridization of BCA-1 transcripts using an antisense probe (black) on paraffin sections; no staining is observed with a sense probe (inset in b). Positivity in the mantle zone is associated with reticular cells, as highlighted in the inset of c, and follicular HEVs (white arrowheads in c) but not T zone HEVs (black arrowheads in c). (d) Confocal microscopic analysis of BCA-1 (red) and CD31 (green) expression in follicular HEVs and T zone HEVs (inset in d). Immunostaining of interdigitating dendritic cells with an anti–S-100 antibody (e) and follicular dendritic cells with an anti-CD21 antibody (f) is shown to highlight the nonoverlapping localization of BCA-1–producing reticular cells. Original magnifications: (a, e, and f) ×100; (b and c) ×200; and (d) ×1,600.
Numerous CD3+ T cells are present in the light zone of germinal centers, and similar high numbers of cells at this location stained positive for CD4 (not shown), HLA-DR, CD69, or ICOS (Fig. 5). These data agree with the follicular localization of tonsillar CXCR5+ T cells, which express these activation markers (Table ). Follicular T cells are greatly outnumbered by B cells, which all express CXCR5 (not shown [16, 17, 24, 28]). Follicular mantle zones are areas of intensive cellular interactions, and dense nuclear staining with eosin/hematoxylin greatly diminishes overlapping signals obtained by immunohistochemistry. Nevertheless, positive staining for CD3, CD4, CD45RO, HLA-DR, CD69, and ICOS was clearly detected and, as expected, was much less prominent than staining for CD20 (B cells).
Figure 5.
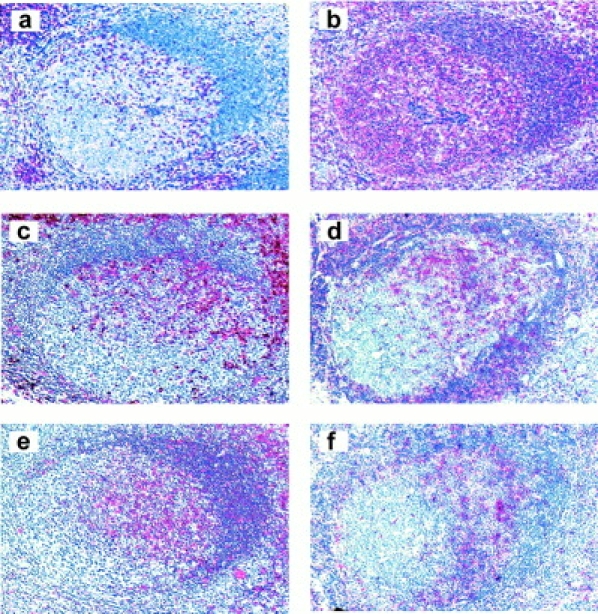
Phenotypic characterization of follicular T cells. Immunostaining (red) on paraffin sections is shown for CD3 (a), CD20 (b), CD45RO (c), and HLA-DR (e), and on frozen sections for CD69 (d) and ICOS (f). Original magnification: ×100.
Discussion
Recruitment of lymphocytes into secondary lymphoid tissue requires the presence of CD62L, which mediates initial adhesive interactions of lymphocytes on HEVs 36, and CCR7, which triggers subsequent firm adhesion through integrin activation 30 31 37 38 39. This mechanism is operative for naive T cells, whose numbers are drastically reduced in secondary lymphoid tissues of CCR7- or SLC-deficient mice 10 13, and may also apply to memory T cells, which give rise to effector cells during recognition of recall antigens. The majority (65–95%; n = 5) of resting/memory T cells in peripheral blood are double positive for CD62L and CCR7, and termed central memory T cells (TCM) to designate antigen-experienced T cells with secondary lymphoid tissue–homing characteristics 29. CXCR5 is present on a subset of circulating CD4+ memory T cells with poor cytokine production capabilities that coexpress CD62L and CCR7. CXCR5 expression is negligible (3%) on CD8+ T cells and is absent on naive T cells. Accordingly, peripheral CXCR5+ T cells represent a subpopulation of TCM and, thus, differ from CCR7− effector memory T cells (TEM), which are proposed to directly participate in immune responses at inflammatory sites 29.
In addition to CCR7, CXCR5+ T cells uniformly express low levels of CXCR4, but there is no evidence for a role of this receptor in T cell traffic through secondary lymphoid tissues. Except for CXCR3, CXCR5+ T cells lack chemokine receptors that are typically involved in recruitment of effector/memory T cells to inflammatory sites in peripheral tissues. This chemokine receptor profile suggests that CXCR5+ T cells home preferentially into secondary lymphoid tissues and, therefore, T cells from tonsils were selected for further studies. Tonsils of patients with pharyngeal inflammation contain a high density of secondary follicles separated by small T zone areas, indicative of intensive B cell activation processes. Therefore, CXCR5+ T cells within this tissue may actively participate in local immune responses. Practically all (>95%) CD4+ memory T cells in tonsils are CXCR5+ and the majority express activation markers (CD69, HLA-DR, ICOS) suggesting their engagement in B cell activation. Drastic reduction in cell surface CCR7 and CD62L indicates that the majority of local CXCR5+ T cells have recently entered the tonsillar tissue (see below).
Tonsillar CXCR5+ T cells readily migrate in response to BCA-1, demonstrating that CXCR5 is functional. However, CXCR5 expression and responsiveness to BCA-1 are sensitive to T cell activation, as shown by polyclonal stimulation of tonsillar and circulating CXCR5+ T cells. This contrasts strikingly with the migration responses to ELC and SDF-1, which are only observed late in CXCR5+ T cell culture, well after enhancement of CCR7 and CXCR4 expression, illustrating that these two chemokine systems are not essential for the localization of CXCR5+ T cells within tonsils. Failure in finding CXCR5− T cell lines and the inability to induce reexpression of this receptor in cell lines derived from sorted CXCR5+ and CXCR5− T cells led us to propose that CXCR5 marks nonproliferating memory T cells. Consequently, CXCR5+ T cells in peripheral blood may represent a subset of memory T cells that have left secondary lymphoid tissues before clonal expansion. The requirements for induction of CXCR5 expression in naive T cells are not defined. Preliminary data indicate that PHA or anti-CD3/CD28 stimulation are not sufficient, suggesting that CXCR5 expression in naive T cells requires contact with antigen-presenting cells. In support, CXCR5 expression during primary responses in mice was shown to depend on sequential signaling by CD28 and OX40 40 41.
How are circulating CXCR5+ T cells recruited into tonsillar follicles? BCA-1 production is highly concentrated in the mantle zone, but is also found in the light zone of germinal centers, marking B cell areas with prominent T cell involvement. In agreement, follicular T cells colocalize with BCA-1–producing cells and express the same range of subset (CD4, CD45RO) and activation markers (CD69, HLA-DR, ICOS) that are present on CXCR5+ T cells freshly isolated from tonsils. Evidently, CXCR5 expression characterizes the subset of tonsillar T cells that are attracted to areas with prominent BCA-1 production. Cellular sources of BCA-1 include reticular cells in the follicular mantle and follicular HEVs. Selective expression of BCA-1 in follicular as opposed to T zone HEVs may represent an important address code for direct recruitment of circulating CXCR5+ T cells into B cell follicles. Similarly, SLC is selectively expressed on T zone (but not follicular) HEVs and is shown to direct the transmigration of CCR7-bearing T cells through induction of firm adhesion and subsequent diapedesis 30 31 38 42. As such, BCA-1 and SLC are nonoverlapping in their sorting function and may therefore contribute to an efficient positioning of T cells with mutually exclusive properties within secondary lymphoid tissues. Peripheral blood B cells differ from the bulk of T cells in their uniform expression of both CXCR5 and CCR7. B cell recruitment into secondary lymphoid tissues of CXCR5- or CCR7-deficient mice is not impaired, suggesting that the absence of either chemokine receptor can be compensated by signaling through the other chemokine receptor 6 13 17 30 43. However, CXCR5 (but not CCR7) is critical for B cell follicle formation in the spleen and PPs 13 17, which fits well with the follicular localization of CXCR5+ T cells.
CXCR5+ memory T cells are poor producers of cytokines. However, expression of ICOS strongly suggests that tonsillar CXCR5+ T cells are engaged in B cell activation. This CD28-related costimulatory molecule is induced during T cells activation and is prominently expressed on T cells in the mantle and light zone of secondary follicles (44; and shown here). The B7-related ICOS ligand is expressed on B cells and macrophages and costimulates, together with TCR signaling, T cell proliferation 45 46 47. Hyperplasia in secondary lymphoid tissues, plasmacytosis, and elevated levels of blood IgG in transgenic mice that constitutively express soluble ICOS ligand point to a prominent role of this costimulatory system in proliferative and B helper responses of follicular T cells 46. A functional link between ICOS and CXCR5 expression is suggested here by the selective production of IgG and IgA during coculture of tonsillar CXCR5+ T cells with B cells. Due to the preactivation status of tonsillar T cells, exogenous stimuli were not required for B cell stimulation, and lack of preferential IgM synthesis suggests that CXCR5+ T cells are mainly active during secondary antibody responses. These effector functions together with the follicular localization demonstrate that CXCR5+ T cells play an important regulatory role in humoral immunity and led us to propose the term follicular B helper T cells (TFH) to designate this particular T cell subset.
Acknowledgments
We thank A. Blaser, R. Stuber, and A. Zehnder for excellent technical assistance, and M. Uguccioni and A. Wetterwald for support in the BCA-1 expression analysis. We are grateful to I. Clark-Lewis for providing chemokines, to R.A. Kroczek for the anti-ICOS antibody, to LeukoSite, Inc. for antibodies to CCR3 and CCR5, to M. Caversaccio for the supply of tonsils, to L. Mazzucchelli for tissue embedding, and to K. Baltensperger for help in confocal microscopy.
This work was supported by grant 31-055996.98 from the Swiss National Science Foundation.
Footnotes
Abbreviations used in this paper: BCA-1, B cell–attracting chemokine 1; CCR7, CC chemokine receptor 7; CXCR5, CXC chemokine receptor 5; DIG, digoxigenin; ELC, EBV-induced molecule 1 ligand chemokine; HEV, high endothelial venule; HPF, high power field; ICOS, inducible costimulator; PP, Peyer's patch; SDF-1, stromal cell–derived factor 1; SLC, secondary lymphoid tissue chemokine; TBS, Tris-buffered saline.
References
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Moser B., Baggiolini M. Chemokines and their receptors in lymphocyte traffic and HIV infection. Adv. Immunol. 2000;74:127–180. doi: 10.1016/s0065-2776(08)60910-4. [DOI] [PubMed] [Google Scholar]
- Murphy P.M., Baggiolini M., Charo I.F., Hebert C.A., Horuk R., Matsushima K., Miller L.H., Oppenheim J.J., Power C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Sallusto F., Mackay C.R., Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokinesa new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Butcher E.C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- Melchers F., Rolink A.G., Schaniel C. The role of chemokines in regulating cell migration during humoral immune responses. Cell. 1999;99:351–354. doi: 10.1016/s0092-8674(00)81521-4. [DOI] [PubMed] [Google Scholar]
- Nakano H., Mori S., Yonekawa H., Nariuchi H., Matsuzawa A., Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–2895. [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L.T., Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo V.N., Korner H., Gunn M.D., Schmidt K.N., Riminton D.S., Cooper M.D., Browning J.L., Sedgwick J.D., Cyster J.G. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva G., Soto H., Zlotnik A., Nakano H., Kakiuchi T., Hedrick J.A., Lira S.A. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R., Schubel A., Breitfeld D., Kremmer E., Renner-Müller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Legler D.F., Loetscher M., Roos R.S., Clark-Lewis I., Baggiolini M., Moser B. B cell–attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M.D., Ngo V.N., Ansel K.M., Ekland E.H., Cyster J.G., Williams L.T. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Bowman E.P., Campbell J.J., Soler D., Dong Z., Manlongat N., Picarella D., Hardy R.R., Butcher E.C. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J. Exp. Med. 2000;191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Forster R., Sedgwick J.D., Browning J.L., Lipp M., Cyster J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Luther S.A., Lopez T., Bai W., Hanahan D., Cyster J.G. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Voigt I., Camacho S.A., de Boer B.A., Lipp M., Forster R., Berek C. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur. J. Immunol. 2000;30:560–567. doi: 10.1002/1521-4141(200002)30:2<560::AID-IMMU560>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Ngo V.N., Ekland E.H., Gunn M.D., Sedgwick J.D., Ansel K.M. Chemokines and B-cell homing to follicles. Curr. Top. Microbiol. Immunol. 1999;246:87–93. doi: 10.1007/978-3-642-60162-0_11. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., McHeyzer-Williams L.J., Ngo V.N., McHeyzer-Williams M.G., Cyster J.G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli L., Blaser A., Kappeler A., Schärli P., Laissue J.A., Baggiolini M., Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Invest. 1999;104:R49–R54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Loetscher P., Seitz M., Baggiolini M., Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willimann K., Legler D.F., Loetscher M., Roos R.S., Delgado M.B., Clark-Lewis I., Baggiolini M., Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Loetscher M., Geiser T., O'Reilly T., Zwahlen R., Baggiolini M., Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- Brandes M., Legler D.F., Sporri B., Schaerli P., Moser B. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 2000;12:1285–1292. doi: 10.1093/intimm/12.9.1285. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Warnock R.A., Campbell J.J., Dorf M.E., Matsuzawa A., McEvoy L.M., Butcher E.C. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J.V., Rot A., Luo Y., Narasimhaswamy M., Nakano H., Gunn M.D., Matsuzawa A., Quackenbush E.J., Dorf M.E., von Andrian U.H. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function–associated antigen 1–mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 2000;191:61–76. doi: 10.1084/jem.191.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo M., Martin-Serrano J., Oberlin E., Pedraza M.A., Serrano A., Santiago B., Caruz A., Loetscher P., Baggiolini M., Arenzana-Seisdedos F., Alcami J. Activation of blood T lymphocytes down-regulates CXCR4 expression and interferes with propagation of X4 HIV strains. Eur. J. Immunol. 1998;28:3192–3204. doi: 10.1002/(SICI)1521-4141(199810)28:10<3192::AID-IMMU3192>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Förster R., Kremmer E., Schubel A., Breitfeld D., Kleinschmidt A., Nerl C., Bernhardt G., Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsetsrapid internalization and recycling upon activation. J. Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- Agace W.W., Amara A., Roberts A.I., Pablos J.L., Thelen S., Uguccioni M., Li X.Y., Marsal J., Arenzana-Seisdedos F., Delaunay T. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr. Biol. 2000;10:325–328. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Picker L.J., Singh M.K., Zdraveski Z., Treer J.R., Waldrop S.L., Bergstresser P.R., Maino V.C. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Hedrick J., Zlotnik A., Siani M.A., Thompson D.A., Butcher E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann K., Tam C., Cyster J.G., Rosen S.D., Williams L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachynski R.K., Wu S.W., Gunn M.D., Erle D.J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin α4β7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 1998;161:952–956. [PubMed] [Google Scholar]
- Flynn S., Toellner K.M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiationthe B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.S.K., Gulbranson-Judge A., Flynn S., Brocker T., Raykundalia C., Goodall M., Förster R., Lipp M., Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5–positive CD4 cells and germinal centers. J. Exp. Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H., Moore A.M., Brown M.J., Hwang S.T. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Goodnow C.C., Cyster J.G. Lymphocyte homingthe scent of a follicle. Curr. Biol. 1997;7:R219–R222. doi: 10.1016/s0960-9822(06)00105-9. [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Swallow M.M., Wallin J.J., Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S.K., Whoriskey J.S., Khare S.D., Sarmiento U., Guo J., Horan T., Shih G., Zhang M., Coccia M.A., Kohno T. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- Ling V., Wu P.W., Finnerty H.F., Bean K.M., Spaulding V., Fouser L.A., Leonard J.P., Hunter S.E., Zollner R., Thomas J.L. Cutting edgeidentification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J. Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]