Recent studies of two newcomers to the TNF cytokine family have revealed an unsuspected link between autoimmunity and cancer. In this issue, Rennert et. al. 1 define the specific receptors for the orphan ligand known as APRIL (a proliferation-inducing ligand), which was previously recognized for its growth stimulating activity for tumors of lymphoid, colon, and thyroid origin 2. APRIL binds cell surface receptors B cell maturation antigen (BCMA) and transmembrane activator and CAML interactor (TACI) (see also reference 3); a soluble decoy form of BCMA that antagonizes APRIL's activity inhibits the growth of tumors that naturally overexpress APRIL. What makes this observation biologically intriguing is that the two receptors, BCMA and TACI, were just very recently recognized as receptors for another TNF family member, BAFF, a B lymphocyte activating factor. BAFF is implicated as a key cytokine sustaining B cell survival and may play a significant role in lupus-like autoimmune nephritis observed in NZB/WF1 mice. The definition of BAFF and APRIL cytokine systems establishes a connection between autoimmunity and cancer that is poised for detailed exploration.
Ligands related to TNF are type 2 (NH2 terminus inside the cell) single transmembrane proteins that assemble in their biologically active form as trimers. In either membrane-anchored or secreted states, the trimeric ligands bind and aggregate their specific cell surface receptors, activating cell death or survival signals 4 5. The amino acid sequence homology that defines the TNF family is limited to the receptor-binding domain, which between APRIL and BAFF (also known as BlyS, THANK, TALL, zTNF4; references 6 7 8 9) is only 33% identical, an insufficient clue as to their closer functional ties. Both ligands are similarly processed by a furin-like proteinase that releases biologically active soluble trimers, which circulates in serum and thus may act systemically 10.
A significant breakthrough came with recent reports by Gross et al. 9 and others 11 12 13 14 15 that the biological functions of BAFF are signaled through two orphans in the TNF receptor family, TACI and BCMA. Both are type 1 single transmembrane glycoproteins with a cysteine-rich extracellular (ecto) domain that identifies a distant relationship to the other cognates in the TNF receptor family 16. BCMA and TACI bind APRIL and BAFF with relatively high affinity (low nanomolar range), as measured by surrogate receptors formed with the receptor's ectodomain fused with the Fc region of IgG1, creating soluble dimeric fusion proteins 1 3. Some selectivity in binding is observed, with TACI–BAFF and BCMA–APRIL being favored partners (Fig. 1). Neither BAFF nor APRIL bind any of the other known members of the TNF receptor family, including herpes virus entry mediator (HVEM) and Fas, which is in contrast to a previous report 17. The shared pairing of APRIL and BAFF and their receptors is now emerging as the rule rather than the exception in the TNF superfamily (e.g., TNF/LTα, LTαβ/LIGHT), yet each cytokine pair identified so far has a unique physiologic purpose. TACI was originally identified as a receptor protein that activates nuclear factor of activated T cells, a transcription factor involved in T cell activation 18, but also stimulates nuclear factor (NF)-κB/Rel and activator protein (AP)-1 families of transcription factors 3, which orchestrate gene expression involved in B cell development, inflammation, and cellular stress responses. By contrast, BCMA was identified in a T cell lymphoma as a chromosomal translocation at t(4;16) at the IL-2 locus 16. The cytoplasmic tails of TACI and BCMA are nonconserved and contain neither a death domain, which is the characteristic signaling domain in Fas, TNFR1, and TRAIL receptors, nor a recognizable TRAF binding motif that is present in LTβR, CD40, CD30, and several other family members. Most if not all of the members of the TNF receptor family activate NF-κB and AP-1 via TRAF family of adaptors, and TACI and BCMA are not exceptions 12 13 14 15. TACI and BCMA are reported to bind a distinct set of TRAFs, BCMA recruits TRAF1, -2, and -3, and TACI interacts with TRAF2, -5, and 6, which suggests that they may activate different pathways. However, a clear link between a particular TRAF adaptor and a phenotype associated with a given receptor is lacking at this time. Nonetheless, the physiologic activities initiated by BCMA and TACI are emerging.
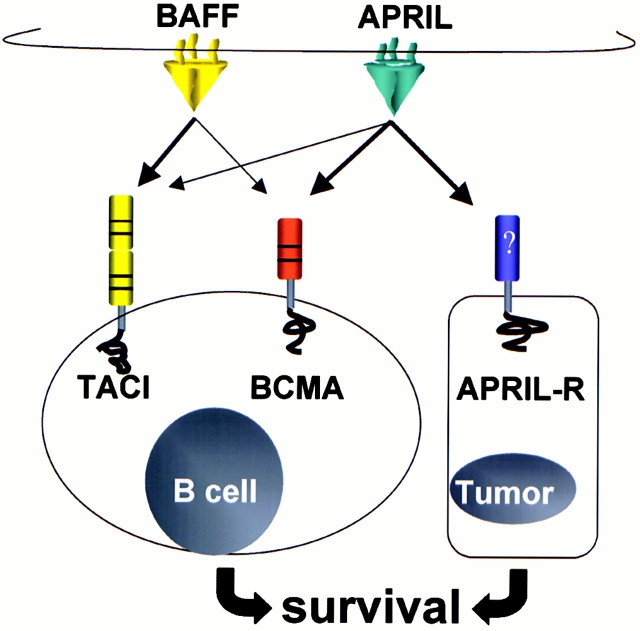
Figure 1.
The BAFF/APRIL cytokine systems. This schematic shows the two TNF-related cytokines, BAFF and APRIL, in their membrane-anchored states binding with their cognate receptors, TACI and BCMA, expressed on B lymphocytes, and a candidate APRIL-specific receptor expressed on epithelial cells. Arrows indicate ligand–receptor binding interactions; and bold arrows indicate the preferred binding partners. TACI and BCMA activate signal transduction pathways that enhance cell survival. Tumor cells also express APRIL and thus may be able to directly activate B cells via TACI or BCMA.
BCMA is tightly restricted in its expression to CD19+ resting mature B cells, as is TACI (although some T cells express TACI), which provided a firm basis for the biological functions induced by BAFF 19. Mice that express BAFF as a transgene 8 9 20 have enlarged spleens and lymph nodes that are engorged with antigen-responsive mature B cells (B220+, surface IgM+, IgD+). The B cells in BAFF-transgenic mice express an activated phenotype with increased expression of MHC class II antigen presenting molecules and the antiapoptotic gene Bcl2. By contrast, the T cell compartment was modestly decreased, although the percentage of effector T cells appeared elevated in some mice. A key observation that established a direct connection to lupus was the presence of antibodies to DNA, a hallmark of lupus, as well as other autoimmune Igs, such as rheumatoid factors in BAFF-transgenic mice 20. As BAFF-transgenic animals age, they display overt signs of lupus nephritis, with protein spilling to the urine, enlarged glomeruli containing fibrin deposits, and cellular infiltrates that precede frank loss of kidney function. Gross et al. 9 showed that NZB/WF1 mice, which are genetically prone to autoimmune nephritis, have BAFF in their sera that corresponds to disease progression. Using a TACI decoy receptor injected into the nephritis prone mice, Gross et al. then showed a reduction in the level of activated B cells with alleviation of nephritis.
The effect of BAFF on B lymphocytes appears to be distinct from that of CD40–CD40L system, already well known for its role in B cell biology 21. CD40L is expressed by activated CD4+ T cells and plays a critical role as a survival factor of activated B cells and acts as a cofactor for B cell effector function. On the other hand, BAFF is constitutively produced by macrophages and possible dendritic cells and activated T cells. Batten et al. 22 show that BAFF allows survival and differentiation of a subset of immature B lymphocytes found in the spleen, the transitional type 2 cell, which are subject to negative selection for removal of autoreactive clones. BAFF overexpression may break tolerance to self by promoting survival of immature B cells. Thus, BAFF is important in B cell maturation and homeostasis, whereas CD40L is a cofactor for B cell activation.
APRIL, in contrast to many other TNF-related ligands, revealed itself as a factor that promotes survival of tumor cells in tissue culture and when human tumors are transplanted into immune-deficient mice 2. Indeed, the expression of APRIL is nearly undetectable in normal tissue but dramatically elevated in tumor tissue. Addition of recombinant APRIL or transfection of tumor lines with APRIL provided a significant growth advantage to lymphoid and nonlymphoid cells. This suggests that APRIL expression may be a consequence of the stress of malignant transformation. However, adenocarcinoma tumor cells lack any detectable expression of BCMA or TACI, implicating the existence of yet another receptor specific for APRIL. NIH3T3 fibroblastic tumor cells bind recombinant APRIL but not BAFF, demonstrating the existence of an as yet uncharacterized APRIL receptor. This result suggests that this other receptor may signal specific effects of APRIL in nonlymphoid cells. Having shown that APRIL binds TACI and BCMA, Rennert et al. 1 take it a step further and demonstrate, again with the versatile soluble BCMA decoy receptor, that the growth of human tumors HT29 colon carcinoma and A549 lung carcinoma is suppressed when transplanted into immune-deficient mice.
The definition of the APRIL/BAFF cytokine systems provides a major advance in understanding molecular mechanisms of B lymphocyte and tumor cell survival pathways. The results raise additional but addressable questions that could provide a clearer picture of fundamental processes in autoimmunity and cancer, as well as new therapeutic targets. Among several questions is whether APRIL-like BAFF plays a significant role in B cell biology and which receptor, TACI or BCMA, is involved in B cell homeostasis and autoimmunity. This question has a practical implication in whether a decoy receptor that targets both cytokines or an antibody specific for a single ligand should be selected as the appropriate antagonist. The favored partnering of APRIL with BCMA and BAFF with TACI suggests that their roles may diverge. BAFF stimulates B cells when the cells are coactivated via their antigen receptors and induces expression of Bcl2, a critical antiapoptotic gene initially described for its oncogenic effect in B cell transformation. These findings indicate that BAFF acts as a survival factor rather than as a true growth factor. APRIL may likewise provide protective signals that help tumor cells survive their hostile microenvironment by arresting apoptosis. APRIL could provide these signals in an autocrine fashion, acting via its specific receptor expressed on the tumor cells. In a more insidious fashion, APRIL could also act in a paracrine mode, stimulating B cells in the microenvironment of the malignant cell. In this latter scenario, epithelial cell transformation with concurrent expression of APRIL could provide the necessary signals, in the presence of a continuous antigenic burden (e.g., in the intestinal mucosa), that breaks immune tolerance leading to autoantibody production. Autoantibody, in turn, could participate in generation of an inflammatory milieu sufficient to sustain continued expression of APRIL. Such a scenario could underlie the increased incidence of tumors in organ-specific and systemic autoimmune diseases. Whether BAFF or APRIL play key roles in human lupus erythematosus or tumorigenesis, as suspected from the animal models, remains to be established but is not far away from being experimentally tested. More importantly, direct intervention targeted at BAFF, APRIL, or both is readily accomplished with the soluble decoys of TACI or BCMA and, presumably, the forthcoming APRIL receptor. This could be the pharmaceutical version of the “two for the price of one.”
References
- Rennert P., Schneider P., Cachero T.G., Thompson J., Trabach L., Hertig S., Holler N., Qian F., Mullen C., Strauch K. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 2000;192:1677–1683. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne M., Kataoka T., Schröter M., Hofmann K., Irmler M., Bodmer J.-L., Schneider P., Bornand T., Holler N., French L.E. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsters S.A., Yan M., Pitti R.M., Haas P.E., Dixit V.M., Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr. Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Farrah T., Goodwin R.G. The TNF receptor superfamily of cellular and viral proteinsactivation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Ware C.F., Santee S., Glass A. Tumor necrosis factor-related ligands and receptors. In: Thompson A., editor. The Cytokine Handbook. Academic Press; San Diego, CA: 1998. pp. 549–592. [Google Scholar]
- Moore P.A., Belvedere O., Orr A., Pieri K., LaFleur D.W., Fengm P., Soppet D., Charters M., Gentz R., Parmelee D. BLySmember of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;9:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., Ni J., Zhai Y., Yu G.-H., Aggarwal B.B. Identification and characterization of a novel cytokine, THANK a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J. Biol. Chem. 1999;274:15978–15981. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]
- Khare S.D., Sarosi I., Xia X.-Z., McCabe S., Miner K., Solovyev I., Hawkins N., Kelley M., Change D., Van G. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc. Natl. Acad. Sci. USA. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.A., Johnston J., Mudri S., Enselman R., Dillon S.R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C. TACI and BCMA are receptors for a TNF homologue implicated in B cell autoimmune disease. Nature. 2000;404:595–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- Schneider P., Mackay F., Steiner V., Hofmann K., Bodmer J.-L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbera H. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Schneider P., Kalled S., Wang L., Lefevre E., Cachero T., Mackay F.B., Zafari S.A., Liu Z., Woodcock S. BAFF binds to the TNF receptor-like molecule BCMA and is important for maintaining the peripheral B cell population. J. Exp. Med. 2000;192:129–136. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou A., Roussel J., Bourgeade M.F., Rogier E., Madry C., Inoue J., Devergne O., Tsapis A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000;165:1322–1330. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- Shu H.B., Johnson H. B cell maturation protein is a receptor for the tumor necrosis factor family member TALL-1. Proc. Natl. Acad. Sci. USA. 2000;97:9156–9161. doi: 10.1073/pnas.160213497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Bressette D., Carrell J.A., Kaufman T., Feng P., Taylor K., Gan Y., Cho Y.H., Garcia A.D., Gollatz E. Tumor necrosis factor receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J. Biol. Chem. 2000;275:35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- Xia X.Z., Treanor J., Senaldi G., Khare S.D., Boone T., Kelley M., Theill L.E., Colombero A., Solovyev I., Lee F. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J. Exp. Med. 2000;192:137–143. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C., Laabi Y., Callebaut I., Roussel J., Hatzoglou A., Le Coniat M., Mornon J.-P., Gerger R., Tsapis A. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int. Immunol. 1998;10:1693–1702. doi: 10.1093/intimm/10.11.1693. [DOI] [PubMed] [Google Scholar]
- Kelly K., Manos E., Jensen G., Nadauld L., Jones D.A. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000;60:1021–1027. [PubMed] [Google Scholar]
- von Bülow G.-U., Bram R.J. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–141. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- Gras P.-P., Laabi Y., Linares-Cruz G., Blondel M.-O., Rigaut J.-P., Brouet J.-C., Leca G., Haguenauer-Tsapis R., Tsapis A. BCMApan integral membrane protein in the golgi apparatus of human mature B lymphocytes. Int. Immunol. 1995;7:1093–1106. doi: 10.1093/intimm/7.7.1093. [DOI] [PubMed] [Google Scholar]
- Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton A., Otipoby K.L., Jiang A., Clark E.A. Signal transduction pathways that regulate the fate of B lymphocytes. Adv. Immunol. 1999;73:79–152. doi: 10.1016/s0065-2776(08)60786-5. [DOI] [PubMed] [Google Scholar]
- Batten M., Groom J., Cachero T.G., Qian F., Schneider P., Tschopp J., Browning J.L., Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 2000;192:1453–1465. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]