Abstract
The goal of tumor immunotherapy is to elicit immune responses against autologous tumors. It would be highly desirable that such responses include multiple T cell clones against multiple tumor antigens. This could be obtained using the antigen presenting capacity of dendritic cells (DCs) and cross-priming. That is, one could load the DC with tumor lines of any human histocompatibility leukocyte antigen (HLA) type to elicit T cell responses against the autologous tumor. In this study, we show that human DCs derived from monocytes and loaded with killed melanoma cells prime naive CD45RA+CD27+CD8+ T cells against the four shared melanoma antigens: MAGE-3, gp100, tyrosinase, and MART-1. HLA-A201+ naive T cells primed by DCs loaded with HLA-A201− melanoma cells are able to kill several HLA-A201+ melanoma targets. Cytotoxic T lymphocyte priming towards melanoma antigens is also obtained with cells from metastatic melanoma patients. This demonstration of cross-priming against shared tumor antigens builds the basis for using allogeneic tumor cell lines to deliver tumor antigens to DCs for vaccination protocols.
Keywords: shared tumor antigens, cross-priming, tumor immunity, tumor vaccine, immunotherapy
Introduction
The induction of tumor immunity is a multistep process that includes: (a) the capture and processing of tumor Ags by APCs; (b) selection and differentiation of antigen-specific T cells; and (c) homing of effector lymphocytes to the tumor site where recognition of restriction elements leads to tumor elimination (for reviews, see references 1–3). Many tumors show some antigenicity, and known tumor-associated Ags (TAAs) include nonmutated, overexpressed, or inappropriately expressed tissue differentiation Ags 4 5 6. However, only a few tumors are associated with effective immune responses 1 2 3 7 8. In this context, dendritic cells (DCs) represent attractive vectors for tumor immunotherapy because of their unique properties, high Ag capture and presenting capacity, which result in extremely efficient induction and maintenance of immune responses 3 9 10 11. Whereas several strategies to deliver TAAs to DCs have been successfully employed to induce TAA-specific immune responses both in vitro and in animal models 12 13 14 15 16 17 18, optimal antigen loading strategies for human trials still remain to be determined. The most commonly used, clinically approved approach is based on loading empty MHC class I molecules with exogenous peptides. However, this is limited by: (a) peptide restriction to a given HLA type; (b) induction of CTL responses only; and (c) limitation of the induced responses to defined TAAs. These TAAs have been identified based on the T cell responses in individual tumor-bearing patients, which raises the question as to whether or not they represent true tumor rejection Ags. Indeed, if judged by the rate and durability of tumor clearance, many immunotherapy protocols in human cancer have shown limited efficacy 19 20, justifying research for improved strategies.
Recent studies demonstrate that DCs capture killed tumor cells overexpressing defined TAAs and present these TAAs to elicit recall CTL responses 21 22. Such an approach offers new possibilities for TAA delivery to DCs because the use of tumor cells as a source of Ags should provide: (a) both MHC class I and class II epitopes leading to a diverse immune response involving many clones of CTLs and CD4 T cells, the latter being able to recruit other effectors such as macrophages and eosinophils 1 23; (b) a broad spectrum of presented TAAs resulting in a broader repertoire of elicited T cell responses; and (c) stable and durable expression of peptide–MHC complexes sufficient for the priming of naive T cells 24 25 26.
We have explored whether loading DCs with killed allogeneic tumor cells, without manipulation of TAA expression, would permit T cell priming and selection of T cells specific for shared TAAs. Melanoma was used as a tumor model because numerous shared TAAs have been identified, possibly including tumor rejection Ags as suggested by tumor regression in animal models and some human trials 27 28. In this study, we demonstrate that DCs loaded with killed allogeneic melanoma cells prime naive T cells to differentiate into CTLs that are specific for a broad spectrum of shared melanoma Ags and are able to kill melanoma cell lines. This demonstration of cross-priming against shared tumor Ags builds the basis for using allogeneic tumor cell lines to deliver tumor Ags to DCs for vaccination protocols.
Materials and Methods
Media and Reagents.
Complete culture medium (CM) consisted of RPMI 1640, 1% l-glutamine, 1% penicillin/streptomycin, 50 mM 2-mercaptoethanol, 1% sodium pyruvate, 1% essential amino acids, and 10% heat-inactivated FCS (GIBCO BRL). For T cell cultures, FCS was replaced by 10% human serum AB (Gemini Bio-Products). GM-CSF (Schering-Plough or Immunex), soluble CD40 ligand (CD40L; Immunex), and IL-7 (Immunex or R&D Systems) were used at the respective concentrations of 100 ng/ml, 200 ng/ml, and 10 IU/ml. IL-4 (Schering-Plough or R&D Systems) was used at 5 ng/ml and IL-2 (Genzyme) was used at 10 IU/ml. Betulinic acid (BA) (Sigma-Aldrich) was used at the concentration of 10 mg/ml.
Synthetic Peptides.
Melan/MART-127–35 (AAGIGILTV), gp100g209-2M (IMDQVPFSV), tyrosinase368–376 (YMDGTMSQV), MAGE-3271–279 (FLWGPRALV), and PSA1141–150 (FLTPKKLQCV) were >70% pure as indicated by HPLC analysis (BioSynthesis). Lyophilized peptides were dissolved in DMSO, diluted to 1 mg/ml in apyrogen water, and stored at −80°C.
Cell Lines.
The Colo829 and SkMel28 melanoma cell lines, K562, LnCAP prostate carcinoma cell line, 1806 breast cancer cell line (established by Drs. A. Gazdar and J. Minna at University of Texas Southwestern Medical Center, Dallas, TX), and T2 cells were from the American Type Culture Collection. The Me275 and Me290 cell lines were established at the Ludwig Cancer Institute in Lausanne. All cell lines were maintained in CM.
Induction of Tumor Cell Death.
ME275 cell death was induced by treatment with 10 μg/ml of BA for 48 h. Colo829 cells were killed by the same method, or by γ-irradiation (150 Gy and then cultured for 48 h in serum-free medium). Cell death was assessed by morphology, externalization of phosphatidylserine using FITC-labeled annexin V (Caltag), and staining with DNA-specific dyes, 7AAD and trypan blue (Sigma-Aldrich).
Generation of Monocyte-derived DCs.
Immature DCs were generated either from Ficoll-separated PBMCs of HLA-A201+ healthy volunteers (Institutional Review Board 097-053) or from patients with stage IV melanoma (enrolled in an unrelated DC vaccination clinical trial, Institutional Review Board 097-053). PBMCs from nonmobilized or G-CSF (Amgen) mobilized blood were suspended in CM and allowed to adhere onto plastic dishes (Falcon 6-well) for 2 h at 37°C. The nonadherent cells were removed, and the adherent cells were cultured in CM for 6 d. GM-CSF and IL-4 were added in the culture every 2 d, and the DC recovery at day 7 as determined by immunofluorescence and FACS® analysis was >90% of CD1a+ cells. To induce DC maturation, soluble CD40L was added in the culture from day 5 to day 7. CD40L can be replaced by Poly I:C to induce DC maturation (data not shown).
T Cell Purification.
Purified CD4+ and CD8+ T cells (autologous to the DCs) were depleted of other cells using purified CD4 (13B8.2), CD8 (B9.11), CD14 (RM052), CD16 (3G8), CD19 (J4,119), anti-CD45RO (UCHL1), CD56 (NKH-1), anti–HLA-DR (B8.12.2), anti-glycophorin A (D2.10), mAbs (Immunotech), and goat anti–mouse IgG Dynabeads (Dynal). For CD8+CD45RA+CD27+ T cells, the CD8+ T cells were stained with both anti-CD45RA–PE and anti-CD27–FITC (Becton Dickinson) and were FACS® sorted.
Flow Cytometry Analysis.
FACS® analysis was performed on a FACSCalibur™ and sorting was done on a FACS Vantage™ (Becton Dickinson). Antibodies used to phenotype or sort the cells were anti-CD1a–FITC labelled (Biosource International), anti-CD14–APC, anti-CD80–PE, anti-CD83–PE, anti-CD86–PE, anti–HLA-DR–PerCP, anti-CD45RA–PE, and anti-CD27–FITC.
Confocal Analysis.
Living cells were allowed to adhere on polylysine-coated slides (Baxter) for 30 min at room temperature and supernatant was then removed. After fixation for 10 min with 4% paraformaldehyde in PBS, cells were washed with 0.1% glycine in PBS, then permeabilized with 0.1% Triton (Sigma-Aldrich) in PBS. After a wash in PBS/0.5% saponin/0.2% BSA/0.2% gelatin, slides were incubated for 30 min at room temperature with the NKI/beteb anti-gp100 antibody (Biodesign) followed by Texas red–conjugated anti–mouse Ab, washed, incubated with 10% mouse serum followed by biotinylated Melan A/MART-1 antibody (A103; a gift from Dr. E. Stockert, Ludwig Institute for Cancer Research at New York, NY), and revealed with FITC-conjugated streptavidin. For double gp100/HLA-DR stainings, loaded or unloaded DCs were labelled with NKI/beteb following the same protocol, and then incubated for 30 min with an anti–HLA-DR, FITC-conjugated antibody after 10 min neutralizing incubation with mouse serum. Confocal microscopy was performed using a Leica TCS-NT SP equipped with argon, krypton, and helium/neon lasers, and a spectrophotometer was used to separate the detection channels of FITC (510–550 nm) and Texas red (580–660 nm).
T Cell Proliferation Assay.
CD1a, FITC-labeled DCs were cocultured for 1 h at 37°C with tumor bodies, and then were sorted based on CD1a expression (purity >95%), and plated in U-bottomed 96-well plates at graded doses. Purified CD4+ T cells or purified CD8+ T cells (105/well/200 μl) were added to the plates. The proliferation assay was carried out in CM supplemented with heat-inactivated 10% human AB serum. Soluble CD40L (200 ng/ml) was added to induce DC maturation and IL-2 (10 U/ml) to support the proliferation of purified CD8+ T cells. After 5 d, tritiated thymidine (NEN Life Science Products) was added at the activity of 1 μCi/well. The plates were harvested 16 h later (Wallac) and incorporated radioactivity was measured.
Generation of Specific CTLs.
DCs loaded with tumor-derived cell bodies and sorted were used as stimulatory cells while autologous purified CD8+ T cells, CD8+CD45RA+CD45RO−, or CD8+CDR45A+CD27+ T cells were used as responders. Cultures were prepared in 24-well plates (Costar) by plating loaded DCs at 105 cells with 106 T cells in a final volume of 1 ml. CM was supplemented with 10% AB serum, soluble CD40L, IL-7 (10 IU/ml week 1), and IL-2 (10 IU/ml in weeks 2 and 3). T cells were restimulated weekly for an additional 2 wk with loaded or unloaded DCs. 6 d after the last stimulation, cells were harvested and their cytotoxic activity as well as the capacity for IFN-γ release was tested.
51Cr Cytotoxicity Assay.
Cytotoxicity was measured in a standard 4-h 51Cr-release assay. In brief, T2 cells were pulsed overnight with 10 μg/ml of the various peptides. Then, the different targets were labeled with 51Cr (NEN Life Science Products) and washed three times with PBS. CTLs were cocultured at 37°C for 4 h with 103 51Cr-labeled target cells in 200 μl of CM supplemented with 10% AB serum in 96-well culture plates. After 4 h, 50 μl of supernatant was collected and the percentage of killed cells was calculated using the formula: % release = 100 × (cpm experiment − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release).
Enzyme-linked Immunospot Assay for IFN-γ Release.
To quantitate antigen-specific, IFN-γ–releasing effector T cells, an enzyme-linked immunospot (ELISPOT) assay was used as recommended by the Mabtech. CD8+ T cells (1–2 × 105 per well) were added in triplicate to nitrocellulose-bottomed 96-well plates (MAHA S4510; Millipore) precoated with the primary anti–IFN-γ mAb (1-D1K; Mabtech) in 50 μl cRPMI per well. For the detection of specific reactive T cells, autologous mature monocyte-derived DCs pulsed with MHC class I–restricted peptides were added at 104 per well (final volume 100 μl/well). After 20 h, wells were washed six times and incubated with biotinylated second mAb to IFN-γ (7 B6-1; Mabtech) for 2 h, washed again, and stained with Vectastain Elite kit (Vector Laboratories).
Results
Melanoma Cell Lines as a Source of Ags.
To determine whether killed allogeneic melanoma cells delivered to DCs would permit selection and expansion of T cells specific for shared melanoma Ags, we identified two melanoma cell lines, Me275 and Colo829. The choice was based on: (a) the expression of HLA-A201 by Me275 cells but not by Colo829 cells, and (b) the expression of “known” melanoma TAAs, both cell lines expressing Melan A/MART-1, gp100, tyrosinase, and MAGE-3 at the RNA and protein level (Fig. 1 A) (29; data not shown).
Figure 1.
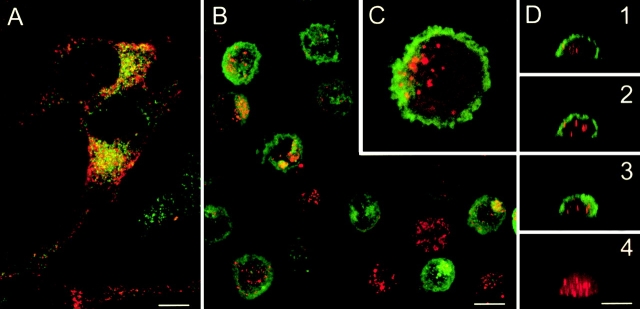
Immature DCs capture killed melanoma cells. (A) Colo829 cells are labelled with mAbs specific for gp100 (red) and Melan A/MART-1 (green). Confocal analysis shows that TAAs are expressed in different vesicular compartments. (B–D) DCs are cultured for 1 h with killed Colo829 cells and sorted according to size and granularity (forward/side scatter), fixed, and labelled with anti-gp100 (red) and anti–HLA-DR (green). Confocal microscopy analysis shows gp100 staining in the cytoplasm of most HLA-DR+ cells. (B) Projection of four xy serial sections. (C) Insert of a single section; original magnification: ×2.5. (D) xz vertical serial sections 1 2 3 and sum of gp100 staining of one cell 4. Representative of several experiments with Colo829 and Me275 cells. Bars, 10 μm.
To generate killed tumor cells, several death-inducing factors were tested including DNA damage and receptor-mediated death, with cell death monitored using the annexin V/propidium iodide staining. Colo829 cells could be killed by γ-irradiation serum starvation (150 Gy, >30% of dead cells). Me275 cells proved resistant to γ-irradiation as well as receptor-mediated death via either Fas ligation, TNF, or TNF-related apoptosis-inducing ligand (TRAIL) (not shown). However, Me275 cells undergo swift death when treated with BA 30 31 32. BA is particularly active against melanoma (as well as malignant brain tumor cells) and induces mitochondria-dependent death through the activation of caspase-8 and caspase-3. Based on initial kinetic and dose–response experiments (not shown), melanoma cells were treated for 48 h with 10 μg/ml BA (>50% dead cells, a combination of apoptosis and necrosis). Because both Colo829 and Me275 grow as adherent cells and detach when dying, we used the nonadherent fraction composed mostly of killed cells for DC loading.
DCs Capture Killed Melanoma Cells and Present Their Ags to Autologous T Cells.
We first determined the capacity of immature monocyte-derived DCs to capture killed melanoma cells. To this end, immature DCs were mixed with killed melanoma cells at a 1:5 ratio and incubated for 1 h at 37°C to allow phagocytosis (conditions were established as optimal in preliminary experiments). Thereafter, DCs were sorted based on forward scatter/side scatter properties reflecting size and granularity. The presence of gp100 melanoma protein within the HLA-DR–labelled DC was determined after subsequent staining with a specific mAb. Confocal microscopy analysis revealed that >90% of DCs internalized the gp100-labelled melanoma cells (Fig. 1B–D). The Melan A/MART-1 epitope recognized with the A103 antibody was lost.
The presentation of Ags captured by DCs was assessed by their ability to induce the proliferation of autologous T cells. CD1a-labelled immature DCs were loaded with killed melanoma cells, sorted based on CD1a expression (>90% purity), and cocultured with purified (>90%) autologous T cells in the presence of CD40L, which induces maturation of both loaded and unloaded DCs as determined by CD83 expression (not shown). The T cells proliferated when cultured with DCs loaded with killed Me275 cells (but not when cultured with killed melanoma cells only) (Fig. 2).
Figure 2.
DCs loaded with killed melanoma cells induce T cell proliferation. (a) Unloaded DCs (UL-DC) and loaded DCs (L-DC) are sorted and cultured with purified autologous CD4+ and (b) CD8+ T cells (with CD40L and IL-2). Thymidine incorporation at day 5. Representative of two experiments.
These results demonstrate that DCs capture killed allogeneic melanoma cells and present their Ags, whether alloantigens or melanoma-associated Ags, to autologous T cells.
DCs Loaded with Killed Melanoma Cells Elicit CTLs Able to Kill Melanoma Cells.
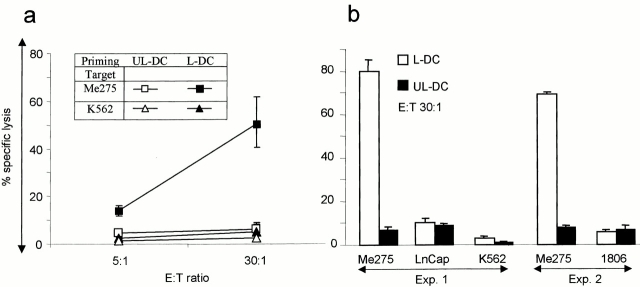
We next determined whether DCs loaded with killed melanoma cells elicit T cells with cytotoxic activity against the melanoma cells used for immunization. The immature HLA-A201+ DCs were loaded with killed Me275, sorted, and used to stimulate purified autologous CD8+ T cells over a 3-wk culture. Soluble CD40L was added to induce DC maturation, as well as IL-7 (10 U/ml week 1) and IL-2 (10 U/ml in weeks 2 and 3) to help T cell proliferation. The T cells were harvested after three stimulation cycles and their cytotoxic activity was determined using Me275, unrelated HLA-A201+ tumor cells, and K562. As shown in Fig. 3, loaded DCs induced differentiation of CTLs able to kill the Me275 melanoma cells, at 50 ± 10% specific lysis (mean ± SE, n = 4; 6 ± 0.5% specific lysis using control T cells cultured with unloaded DCs), but not the unrelated HLA-A201+ LnCAP or 1806 cells or the NK-sensitive K562 cell line.
Figure 3.
Induction of CTLs by DCs loaded with killed melanoma cells. Purified CD8+ T cells are cultured for 3 wk with unloaded DCs (UL-DC) or DCs loaded with Me275 (L-DC). (a) T cells are tested in a 4-h chromium-release assay, using as targets immunizing Me275 cells and K562 cells as a control for NK activity (mean ± SE, n = 4), as well as (b) unrelated HLA-A201+ tumor cell lines, prostate cancer (LnCap) and breast cancer (1806). No CTLs are elicited when T cells are cultured with killed melanoma cells without the DCs (not shown).
These results demonstrate that DCs loaded with killed allogeneic melanoma cells can trigger CD8+ T cells to differentiate into CTLs able to kill the melanoma cells used for immunization.
Loading of DCs with Killed Allogeneic Melanoma Cells Induces CTLs Specific for Multiple Shared Melanoma TAAs.
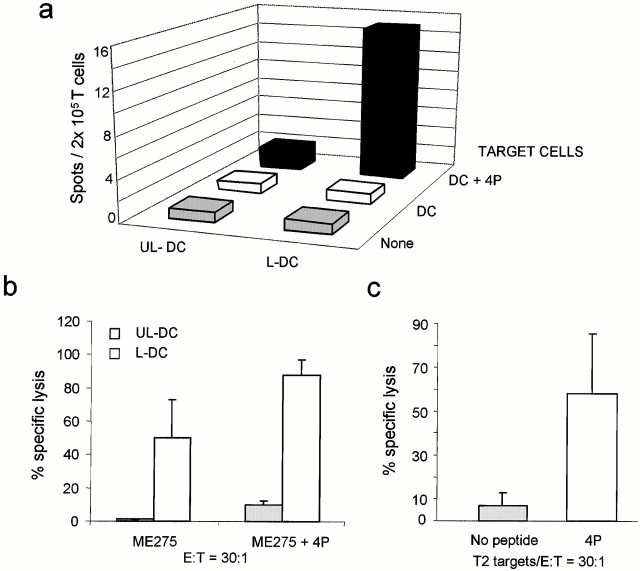
Two assays were developed to establish whether the T cell lines generated with killed melanoma cell–loaded DCs contained T cells specific for melanoma-associated Ags: an overnight IFN-γ ELISPOT assay with the HLA-A201+ DCs pulsed with melanoma peptides Melan A/MART-127–35, tyrosinase368–376, MAGE-3271–279, and mutated gp100g209-2M; and a cytotoxic chromium-release assay using T2 cells pulsed with the four peptides as target cells. As shown in Fig. 4 a, 3-wk CTL lines elicited using DCs loaded with killed Me275 cells contain cells recognizing a combination of the four melanoma peptides: 27 ± 2.9 melanoma-specific spots/2 × 105 T cells (mean ± SE, n = 3, P = 0.03). T cell lines generated with unloaded DCs and peptide-pulsed DCs alone (without the T cells) yielded 3 ± 1 and 4 ± 1.5 spots, respectively. As shown in Fig. 4 b, these T cell lines are able to kill T2 cells pulsed with a combination of the four peptides (56 ± 13% specific lysis; mean ± SE, n = 3), but not T2 cells loaded with a prostate-specific antigen–derived peptide, thus indicating specificity for melanoma TAAs. The ability to induce melanoma-specific CTLs was not restricted to Me275 cells, as the T cells elicited by DCs loaded with the HLA-A201− Colo829 also contained cells that could kill the T2 cells loaded with each of the four melanoma peptides (Table ).
Figure 4.
Induction of melanoma-specific CTLs by killed melanoma cell–loaded DCs. Purified CD8+ T cells are cultured for 3 wk with unloaded (UL-DC) or Me275-loaded DCs (L-DC). (a) IFN-γ ELISPOT using as targets DCs, either unpulsed or pulsed with the four melanoma peptides (4P). Representative experiment from three performed using DCs and T cells from different donors. (b) CTL activity in a 4-h chromium-release assay using unpulsed and melanoma peptide–pulsed (4P; mean values of three experiments) and control peptide PSA-pulsed T2 cells (one experiment).
Table 1.
T Cells Elicited by Killed Melanoma Cell–loaded DCs Kill Melanoma Peptide–pulsed T2 Cells
| No peptide | Tyrosinase | gp100 | MAGE-3 | MART-1 | |
|---|---|---|---|---|---|
| Exp. 1/Me275 | 18 ± 5 | 68 ± 5 | 48 ± 4 | ND | ND |
| Exp. 2/Me275 | 22 ± 2.5 | 60 ± 7 | 40 ± 9.5 | ND | ND |
| Exp. 3/Colo829 | 19 ± 4.5 | 65 ± 9.5 | 34 ± 3.5 | 33 ± 3 | 36 ± 7.5 |
| Mean ± SD | 20 ± 2 | 64 ± 4 | 41 ± 7 | ||
| P = 0.006 | P = 0.03 |
Percent specific lysis, expressed as mean of triplicates ± SD. Paired two-tailed t test on log transformed data comparing the killing of unpulsed and peptide-pulsed T2 cells. Exp., experiment.
Thus, different melanoma cell lines and different methods to kill melanoma cells can be used to load DCs to elicit CTL lines specific for melanoma-associated Ags.
DCs Loaded with Killed Melanoma Cells Prime Naive CD8 T Cells to Differentiate into Melanoma-Specific CTLs.
We wondered whether induction of melanoma-specific CTLs was due to priming of naive T cells. First, CD45RA+CD45RO− naive CD8+ T cells (>80% pure) were cultured with DCs loaded with killed Me275 cells. In two independent experiments, the elicited T cells were able to kill: (a) T2 cells pulsed with the four melanoma peptides (up to 70% specific lysis, Fig. 5 A); (b) T2 cells loaded with a single peptide, either tyrosinase, gp100, or MAGE-3 (Fig. 5 a); and (c) the Me275 cell line used for immunization (not shown).
Figure 5.
DCs loaded with killed melanoma cells prime naive T cells. Naive CD8+CD45RA+CD45RO− T cells are cultured for 3 wk with unloaded DCs (UL-DC) or with DCs loaded with killed melanoma cells (L-DC). T cells are tested in a 4-h chromium-release assay. (a) CTLs elicited by DCs loaded with Me275 bodies kill the T2 cells pulsed with different melanoma peptides (paired two-tailed t test on log transformed data comparing the killing of unpulsed and peptide-pulsed T2 cells). (b) Naive CD27+ CD45RA+CD8+ T cells differentiate into CTLs able to kill the cell line used for immunization, and (c) T2 cells pulsed with melanoma peptides (4P) but not PSA peptide (representative of two experiments).
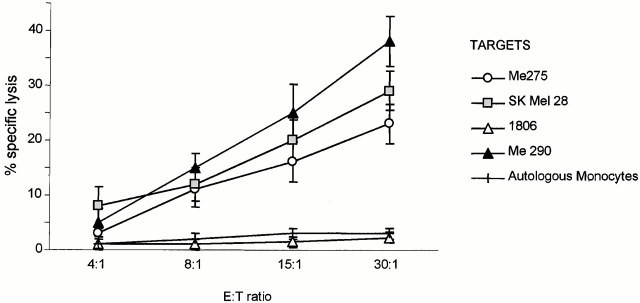
Though mostly comprised of truly naive T cells, the pool of CD8+CD45RA+CD45RO− T cells contains a small fraction of “effector memory” cells that might expand in our culture conditions. Therefore, based on CD27 expression, the pool of CD8+CD45RA+CD45RO− T cells was subdivided to distinguish truly inexperienced T cells (CD45RA+CD27+) from uncommitted effector T cells (CD45RA+CD27−) 33. When cultured with killed melanoma cell–loaded DCs, these CD8+CD45RA+CD27+ T cells yielded melanoma-specific CTLs able to kill both the melanoma cells used for immunization (up to 75% specific lysis, Fig. 5 b) and T2 cells pulsed with melanoma peptides (75% melanoma peptide–specific killing, Fig. 5 c). The induction of melanoma-specific T cells was further confirmed by IFN-γ ELISPOT with melanoma peptide–pulsed, autologous DCs (23 peptide-specific spots/105 T cells versus 5 spots/105 T cells background, not shown). Most importantly, these naive T cells primed by DCs loaded with HLA-A201− Colo829 cells were able to kill HLA-A201+ melanoma targets including Me275, Me290, and SkMel28 cells, thus demonstrating cross-priming (Fig. 6).
Figure 6.
Cross-priming using DCs loaded with killed melanoma cells. Naive CD27+CD45RA+CD8+ T cells are primed by autologous HLA-A201+ DCs loaded with killed HLA-A201− Colo829 cells. The elicited T cells are able to kill HLA-A201+ melanoma targets but not HLA-A201+ breast cancer cells or autologous monocytes.
Taken together, our results demonstrate for the first time that DCs loaded with killed allogeneic melanoma cells can prime naive CD8+ T cells to differentiate into CTLs specific for shared melanoma-associated Ags and are able to kill tumor cells HLA matched to the DCs.
DCs Loaded with Killed Melanoma Cells Elicit Melanoma-specific Responses from Blood T Cells of Patients with Stage IV Melanoma.
The main thrust behind the above experiments is the prospect of vaccinating melanoma patients with DCs loaded with killed melanoma cells. However, these patients may suffer from immune dysfunction that may be reflected in the inability of their blood T cells to mount melanoma-specific immune responses 34. Therefore, we analyzed whether or not CD8+ T cells from patients with metastatic melanoma could be differentiated into effective melanoma-specific CTLs. Purified CD8+ T cells cultured for 3 wk with DCs loaded with killed Me275 cells contained melanoma-specific T cells as determined by IFN-γ ELISPOT using peptide-pulsed DCs (15 spots/2 × 105 T cells; Fig. 7 a, patient 6). As shown in Fig. 6 b, purified CD8+ T cells cultured with killed Me275-loaded DCs generated CTL lines able to kill Me275 cells, either unpulsed (50% specific lysis) or pulsed with the four melanoma peptides (88% specific lysis; Fig. 7 a, patient 1). In two patients, CD45RA+CD45RO−CD8+ T cells cultured for 3 wk with killed melanoma cell–loaded DCs were found to differentiate into melanoma-specific CTLs, as shown by their capacity to kill T2 cells pulsed with a combination of four melanoma peptides (Fig. 7, and data not shown).
Figure 7.
DCs loaded with killed melanoma cells stimulate melanoma-specific CD8+ T cells from the blood of patients with metastatic melanoma. Purified CD8+ T cells are cultured for 3 wk with unloaded (UL-DC) or Me275-loaded DCs (L-DC). (a) IFN-γ ELISPOT using DCs, either unpulsed or pulsed with four melanoma peptides (4P). Results from one patient. (b) T cells are able to kill Me275 used for immunization, and the killing is increased when Me275 cells are pulsed with the four melanoma peptides. Similar results were obtained in two patients. (c) CD8+CD45RA+CD45RO− T cells cultured for 3 wk with DCs loaded with killed Me275 cells, kill T2 cells pulsed with four melanoma peptides.
These results demonstrate that the DCs loaded with killed allogeneic melanoma cell lines can elicit melanoma-specific CTLs using blood T cells and DCs from patients with advanced malignant melanoma.
Discussion
This study is the first demonstration that DCs loaded with killed allogeneic tumor cells can prime naive CD8+ T cells to differentiate into CTLs specific for shared tumor Ags. Indeed, melanoma-specific responses can be elicited from truly inexperienced CD45RA+CD27+CD8+ T cells 33 in a cross-priming setup. HLA-A201+ T cells primed by autologous DCs loaded with HLA-A201− Colo829 cells are able to kill several HLA-A201+ melanoma targets. Importantly, this approach can be used to elicit melanoma-specific CTLs from the blood of patients with stage IV melanoma.
Our results complement and extend recent reports on killed tumor cells that can be provided to DCs for presentation to peptide-specific T cell lines and can detect recall responses in tumor-bearing patients 21 22. In this study, we show that shared TAAs do not need to be transfected for the loaded DCs to induce TAA-specific responses. Indeed, the melanoma model, where numerous TAAs have been identified, indicates that DCs loaded with killed melanoma cells induce: (a) T cells with CTL activity against melanoma cell lines used for immunization, and (b) CTLs that are specific for multiple melanoma Ags, including epitopes of Melan A/MART-1, gp100, tyrosinase, and MAGE-3, as demonstrated in the T2 killing and ELISPOT assays. The implications of these findings are the possibility of inducing responses against multiple tumor Ags (this may permit minimization of tumor escape by loss of a given antigen expression), and the opportunity to identify novel tumor Ags relevant for tumor rejection 2.
A possible explanation for the induced melanoma peptide–specific CTL responses is that peptides are actually formed by the melanoma cells and just transferred to DCs. However, the generation of melanoma TAA-specific HLA-A201–restricted CTLs using the killed HLA-A201− Colo829 cell line suggests antigen processing rather than peptide transfer. Identification of T cells reactive with the MAGE-3271–279 epitope, which is not expressed by melanoma cells because it cannot be generated by a standard proteasome 35, further suggests antigen processing. However, we cannot formally exclude the possibility that killed melanoma cells may display immunoproteasome-like enzymatic activity that could result in the generation of the MAGE-3271–279 epitope.
The range of melanoma TAA specificities reported in this study is likely to be underestimated because we have used peptides representing only four melanoma epitopes and restricted to a given HLA allele (HLA-A201). Thus, the real repertoire of melanoma-specific responses may in fact be much higher as it is likely to incorporate (a) other epitopes presented by other HLA alleles; (b) other defined tumor Ags expressed in melanoma cells such as tyrosinase-related protein 2, her2/neu, and potentially TERT 36 37 38; and (c) “unknown,” i.e., unidentified melanoma TAAs. This may explain the apparent discrepancy between the high killing of melanoma targets and relatively low frequency of T cells specific for the four melanoma peptides in the ELISPOT assay.
There is no consensus as to whether shared tumor Ags or unique tumor-specific Ags would provide the optimal basis for immunotherapy protocols. The current approach based on the use of allogeneic tumor cell lines to induce responses against shared TAAs presents numerous advantages: (a) these relatively well-characterized antigen sources can permit a more rigorous clinical assessment of responses to DC vaccines than the ill-defined autologous tumor preparations that cannot permit standardization and are often limiting in their quantity; (b) applicability to many patients regardless of HLA type; (c) the possibility of identifying novel, shared tumor Ags that may “escape” detection using autologous tumors due to potential immunodominance of unique tumor Ags; and (d) the possibility of mounting tumor-specific CD4 responses, the importance of which has been demonstrated in mouse tumor models, in the development of tumor immunity 1 23. In this context, we are currently analyzing the specificity of induced CD4 T cells.
In conclusion, our study demonstrates that DCs loaded with killed allogeneic melanoma cells can cross-prime CTLs specific for multiple melanoma Ags.
Acknowledgments
The authors would like to thank E.T. Kraus and S. Coquery for their help with flow cytometry and cell sorting, J. Fay, B. Chang, and S. Taquet, as well as L. Pineiro and the Apheresis/Bone Marrow Processing Laboratories at the Baylor University Medical Center (Dallas, TX) for help with the mobilization and apheresis of healthy volunteers and melanoma patients, and S. Narula from Schering-Plough for providing GM-CSF and IL-4, Immunex for providing CD40L, J. Fordtran for his continuous support, and, last but not least, our volunteers and patients.
This work was supported by grants from the Baylor Health Care Systems Foundation, the National Institute of Health, and the Falk Foundation; Bourse Marc Chaptal de Recherche en Dermatologie and Fondation Rene Touraine (to F. Berard); Association pour la Recherche Contre le Cancer and Université Victor Segalen and Centre Hospitalo-Universitaire de Bordeaux (to P. Blanco); Fondation de France and Federation Nationale des Centres de Lutte Contre le Cancer (to E.-M. Neidhart-Berard).
Footnotes
F. Berard and P. Blanco contributed equally to this work.
J. Davoust's present address is Institut Curie, U255, 26 rue d'Ulm, 75005 Paris, France.
Abbreviations used in this paper: BA, betulinic acid; CM, complete culture medium; DC, dendritic cell; ELISPOT, enzyme-linked immunospot; TAA, tumor-associated Ag.
References
- Pardoll D.M. Cancer vaccines. Nat. Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- Sogn J.A. Tumor immunologythe glass is half full. Immunity. 1998;9:757–763. doi: 10.1016/s1074-7613(00)80641-x. [DOI] [PubMed] [Google Scholar]
- Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- Boon T., Coulie P.G., Van den Eynde B. Tumor antigens recognized by T cells. Immunol. Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- Wang R.F., Rosenberg S.A. Human tumor antigens for cancer vaccine development. Immunol. Rev. 1999;170:85–100. doi: 10.1111/j.1600-065x.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R.M., Ehl S., Aichele P., Oehen S., Kundig T., Hengartner H. Antigen localization regulates immune responses in a dose- and time-dependent fashiona geographical view of immune reactivity. Immunol. Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Ochsenbein A.F., Klenerman P., Karrer U., Ludewig B., Pericin M., Hengartner H., Zinkernagel R.M. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc. Natl. Acad. Sci. USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Mayordomo J.I., Zorina T., Storkus W.J., Zitvogel L., Celluzzi C., Falo L.D., Melief C.J., Ildstad S.T., Kast W.M., Deleo A.B. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- Porgador A., Gilboa E. Bone marrow–generated dendritic cells pulsed with a class I–restricted peptide are potent inducers of cytotoxic T lymphocytes. J. Exp. Med. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Mayordomo J.I., Tjandrawan T., DeLeo A.B., Clarke M.R., Lotze M.T., Storkus W.J. Therapy of murine tumors with tumor peptide–pulsed dendritic cellsdependence on T cells, B7 costimulation, and T helper cell 1–associated cytokines. J. Exp. Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczkowski D., Nair S.K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen presenting cells in vitro and in vivo. J. Exp. Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Kong H.L., Carpenter H., Torii H., Granstein R., Rafii S., Moore M.A., Crystal R.G. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht J.M., Wang G., Do M.T., Lam J.S., Royal R.E., Reeves M.E., Rosenberg S.A., Hwu P. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J. Exp. Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields R.C., Shimizu K., Mule J.J. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman J.M., Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999;50:507–529. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- Haigh P.I., Difronzo L.A., Gammon G., Morton D.L. Vaccine therapy for patients with melanoma. Oncology. 1999;13:1561–1574. [PubMed] [Google Scholar]
- Albert M.L., Darnell J.C., Bender A., Francisco L.M., Bhardwaj N., Darnell R.B. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat. Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- Russo V., Tanzarella S., Dalerba P., Rigatti D., Rovere P., Villa A., Bordignon C., Traversari C. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc. Natl. Acad. Sci. USA. 2000;97:2185–2190. doi: 10.1073/pnas.040540197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Lezzi G., Viola A. From TCR engagement to T cell activationa kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Sallusto F. From synapses to immunological memorythe role of sustained T cell stimulation. Curr. Opin. Immunol. 2000;12:92–98. doi: 10.1016/s0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- Nestle F.O., Alijagic S., Gilliet M., Sun Y., Grabbe S., Dummer R., Burg G., Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Thurner B., Haendle I., Roder C., Dieckmann D., Keikavoussi P., Jonuleit H., Bender A., Maczek C., Schreiner D., von den Driesch P. Vaccination with mage-3A1 peptide–pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries T.J., Fourkour A., Wobbes T., Verkroost G., Ruiter D.J., van Muijen G.N. Heterogeneous expression of immunotherapy candidate proteins gp100, MART-1, and tyrosinase in human melanoma cell lines and in human melanocytic lesions. Cancer Res. 1997;57:3223–3229. [PubMed] [Google Scholar]
- Pisha E., Chai H., Lee I.S., Chagwedera T.E., Farnsworth N.R., Cordell G.A., Beecher C.W., Fong H.H., Kinghorn A.D., Brown D.M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- Fulda S., Jeremias I., Steiner H.H., Pietsch T., Debatin K.M. Betulinic acida new cytotoxic agent against malignant brain-tumor cells. Int. J. Cancer. 1999;82:435–441. doi: 10.1002/(sici)1097-0215(19990730)82:3<435::aid-ijc18>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Selzer E., Pimentel E., Wacheck V., Schlegel W., Pehamberger H., Jansen B., Kodym R. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J. Invest. Dermatol. 2000;114:935–940. doi: 10.1046/j.1523-1747.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Hamann D., Baars P.A., Rep M.H., Hooibrink B., Kerkhof-Garde S.R., Klein M.R., van Lier R.A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.P., Yee C., Savage P.A., Fong L., Brockstedt D., Weber J.S., Johnson D., Swetter S., Thompson J., Greenberg P.D. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- Valmori D., Gileadi U., Servis C., Dunbar P.R., Cerottini J.C., Romero P., Cerundolo V., Levy F. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte–defined peptide derived from the tumor antigen MAGE-3. J. Exp. Med. 1999;189:895–906. doi: 10.1084/jem.189.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide R.H., Hahn W.C., Schultze J.L., Nadler L.M. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- Parkhurst M.R., Fitzgerald E.B., Southwood S., Sette A., Rosenberg S.A., Kawakami Y. Identification of a shared HLA-A*0201-restricted T cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–4901. [PubMed] [Google Scholar]
- Rongcun Y., Salazar-Onfray F., Charo J., Malmberg K.J., Evrin K., Maes H., Kono K., Hising C., Petersson M., Larsson O. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J. Immunol. 1999;163:1037–1044. [PubMed] [Google Scholar]