Abstract
A proliferation-inducing ligand (APRIL) is a ligand of the tumor necrosis factor (TNF) family that stimulates tumor cell growth in vitro and in vivo. Expression of APRIL is highly upregulated in many tumors including colon and prostate carcinomas. Here we identify B cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI), two predicted members of the TNF receptor family, as receptors for APRIL. APRIL binds BCMA with higher affinity than TACI. A soluble form of BCMA, which inhibits the proliferative activity of APRIL in vitro, decreases tumor cell proliferation in nude mice. Growth of HT29 colon carcinoma cells is blocked when mice are treated once per week with the soluble receptor. These results suggest an important role for APRIL in tumorigenesis and point towards a novel anticancer strategy.
Keywords: tumor necrosis factor, tumorigenesis, cell survival, apoptosis, cancer therapy
Introduction
TNF-related ligands can induce pleiotropic biological responses. Many of the ligands trigger costimulatory growth signals in primary cells, lead to the activation of nuclear factor (NF)-κB, or even induce cell death. A proliferation-inducing ligand (APRIL) has the unique capacity to stimulate the growth of transformed tumor cell lines 1. The development of cancer is viewed as a multistep process involving mutation and progressive selection for cells with increasing capacity for proliferation, survival, and invasion, even under conditions where the growth factor supply is limited. APRIL allows tumor cells to proliferate at a reasonable rate even in low serum 1. Moreover, APRIL is abundantly expressed in many tumor cells and tumor tissues, in particular gastrointestinal tumors, including rectum, duodenum, colon, stomach, and esophagus 1 2, suggesting that APRIL may function as an autocrine growth factor during tumorigenesis. APRIL is a close sequence homologue of B cell activation factor of the TNF family (BAFF, also known as BLyS/zTNF4/TALL-1) 3 4. BAFF, a costimulator of B lymphocyte survival and proliferation, has two known receptors, B cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI) 4 5 6 7. In this study, we demonstrate that TACI and BCMA also bind to APRIL and that inhibition of APRIL with a soluble form of BCMA interferes with tumor growth in vivo, suggesting an important role of APRIL in carcinogenesis.
Materials and Methods
Recombinant Proteins, Transient Transfections, and Stable Cell Lines.
Receptors–Fc fusion proteins and Flag-tagged ligands (hAPRIL amino acids [aa] 110–250, mAPRIL aa 88–232) were produced in 293T cells essentially as described 1 8. For FACS® stainings, a soluble APRIL was used in which the oligomerization domain of murine adipocyte complement-related protein 30 kD (aa 18–111) was inserted 3′ of the Flag sequence and 5′ of the APRIL sequence. This chimeric protein has the same receptor binding properties as Flag–APRIL and improved background staining by FACS®. Flag-tagged hBAFF and 293–BAFF cells have been previously described 3. A cDNA encoding the fusion protein hBAFF (aa 1–132)–hAPRIL (aa 110–250) was cloned in PCR-3 expression vector and stably transfected into 293 cells following established protocols 8.
Myc-tagged mAPRIL (aa 98–232) cloned into a pPIC9 (Invitrogen) derived vector was expressed in Pichia pastoris strain GS115 grown in BMMY medium (Invitrogen). Pichia supernatant was dialyzed overnight against 10 mM Tris, pH 6.8, and then loaded onto a Sepharose–SP column. The column was washed extensively with 10 mM Tris/HCl, pH 6.8, and eluted with 250 mM NaCl in PBS. A second purification step was performed using a gel filtration column (S300; Amersham Pharmacia Biotech). The purified protein was analyzed by Western blot using the monoclonal 9E10 (anti-Myc) antibody and NH2-terminal sequence determination.
Immunoprecipitations, Receptor Binding ELISA, and Flow Cytometry.
Receptors–Fc (∼500 ng) mixed with Flag ligands (200 ng) in 1 ml of PBS were incubated for 2 h at 4°C on a wheel with 2.5 μl of protein A–Sepharose (Amersham Pharmacia Biotech). Beads were harvested, loaded in empty mini columns, washed three times with 100 volumes of PBS, eluted with 15 μl of 0.1 M citrate-NaOH, pH 2.7, neutralized, and analyzed by Western blotting with anti-Flag M2 antibody. Membranes were subsequently reprobed with goat anti–human IgG antibodies.
Receptor binding ELISA was performed essentially as described 8. In brief, receptors–Fcs were coated to ELISA plates in carbonate buffer. After blocking, serial dilutions of Flag ligands were added, and ligand binding was revealed with anti-Flag M2 antibody.
Staining using the rat IgG2a anti-hBAFF antibody Buffy-1 (formerly 43.9) was performed as described 3. Stable cell lines expressing surface BAFF or surface APRIL were stained with 200 ng hBCMA–Fc, followed by goat anti–human–PE antibodies (1/100) (Southern Biotechnology Associates, Inc.). NIH3T3 cells were stained with Flag–BAFF (100 ng per staining), Flag–APRIL (100 ng per staining), or with Flag–APRIL that had been previously depleted using protein A beads loaded with BCMA–Fc or TNF-related apoptosis-inducing ligand receptor 2 (TRAILR2)–Fc (25 μg). A 500-fold depletion was achieved with BCMA–Fc, as assessed by ELISA on BCMA–Fc. Bound ligands were revealed with biotinylated anti-Flag M2 antibody and PE-coupled streptavidin as previously described 3 4.
Northern Blots.
Total RNA was obtained from ∼107 cells using the RNeasy purification system (QIAGEN). 20 μg of RNA per sample was run on a 1.2% agarose gel containing formaldehyde and transferred to a nylon membrane. The Northern blots were hybridized using random primed radioactive probes in ExpressHyb buffer (CLONTECH Laboratories, Inc.) for ∼3 h and then washed at room temperature for 40 min with several changes of 2× SSC/0.1% SDS followed by 0.1× SSC/0.05% SDS for 40 min at 50°C. The blots were probed with human or mouse BCMA, TACI, or actin.
APRIL-transfected Cell Lines.
The tetracycline-inducible mAPRIL–expressing cell lines were constructed using the Tet-On™ expression system (CLONTECH Laboratories, Inc.). In brief, the soluble extracellular domain of murine APRIL was PCR amplified using primers 5′-TGTGAATTCCGGCCCCACCATGGATTACAAAGACGATGACGATAAAGGGGGCTCAGT-CAGTCAGAGAGCC-3′ and 5′-TGTCTAGATCATAGTTTCACAAACCCCAGG-3′, which allowed directional cloning into the pTRE vector. This APRIL expression vector was stably transfected along with a hygromycin selection plasmid into a cell line that had already been stably transfected, under neomycin selection, with the pTet-on regulatory plasmid. Cell lines derived from this dual selection process were then selected on the basis of induction of APRIL expression in the presence of the tetracycline analogue doxycycline (2 μg/ml; Sigma-Aldrich). Cell extracts of stable clones were analyzed by Western blotting using anti-Flag M2 mAb (Sigma-Aldrich).
For cellular proliferation analyses, the Tet-inducible APRIL expression lines were maintained in 0.5% serum overnight to induce quiescence. Cells were then plated in 96-well plates at a concentration of 105 cells/ml in RPMI/1% serum and were left untreated, received 2 μg/ml doxycycline, or received doxycycline and 20 μg/ml BCMA–Fc fusion protein. Cells were left overnight and then pulsed for 8 h with 0.5 μCi/well [3H]thymidine (Amersham Pharmacia Biotech).
Tumor Models.
Tumor growth was investigated using 6–8-wk-old female nude (Nu/Nu) mice (Harlan Laboratories). Tumor cell lines HT29 and A549 were obtained from American Type Culture Collection and cultured following the supplier's instructions. Cells were trypsinized, washed twice, and resuspended in pyrogen-free PBS at a concentration of 107 cells/ml. Mice were anesthetized using a ketamine/xylazine solution, and 100 μl of cells was carefully implanted in the flank subcutaneously. Mice were injected with purified proteins intraperitoneally just before tumor implantations and weekly thereafter. Dosing was 200 μg BCMA–Fc or control hIgG in 100 μl PBS intraperitoneally. Tumor diameter was measured at various times after implantation using a caliper. Tumor volume was calculated using the formula v = 4/3πR3. Statistical significance was determined using mean equivalence tests.
Results and Discussion
As APRIL is a member of the TNF ligand family, it was likely that it interacted with one of the currently known 25 members of the TNF receptor family, some of which are still orphan 9. When supernatants from 293T cells expressing recombinant soluble Flag-tagged APRIL (aa 110–250, corresponding to the TNF homology region) were incubated with various soluble TNF receptor–Ig-Fc fusion proteins (receptor–Fc), two of them, BCMA–Fc and TACI–Fc, were found to immunoprecipitate APRIL. In contrast, no specific interaction with other receptors, including herpes virus entry mediator (HVEM) and Fas, which have been recently proposed to interact with APRIL 2, was observed (Fig. 1 A). BCMA and TACI are atypical TNF receptor family members 5 6. Both are devoid of a signal sequence and contain one (BCMA) or two (TACI) sets of A and C cysteine-rich modules instead of multiple A and B modules found in most other TNF receptor members 10. Recently, BCMA and TACI have been identified as receptors for BAFF/BLyS/zTNF4/TALL-1 4. BAFF, which promotes B cell survival and proliferation, is the closest sequence homologue of APRIL 3. Thus, both BCMA and TACI can act as receptors for the same two ligands, APRIL and BAFF.
Figure 1.
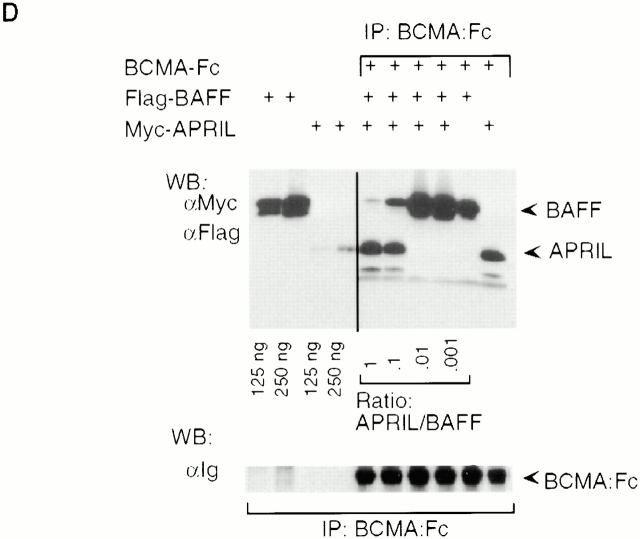
APRIL is a ligand for the TNF receptors BCMA and TACI. (A) Various receptor–Fc fusion proteins of the TNF receptor family (0.5 μg) were mixed with Flag-tagged soluble hAPRIL (0.2 μg), immunoprecipitated with protein-A, and detected by Western blotting with antibodies to human Ig (top panel). Coimmunoprecipitating APRIL was detected with anti-Flag antibodies (bottom panel). (B) Binding of Flag-APRIL and Flag-BAFF to plastic-coated BCMA–Fc, TACI–Fc, or TRAILR2–Fc (control) was assessed by ELISA. Flag–ligands were added at the indicated concentrations, and their binding was revealed using anti-Flag M2 antibody. (C) Binding of BCMA–Fc to 293 cells (ctrl) and to 293 cells stably transfected with full-length hBAFF (BAFF) or with a fusion protein in which the TNF homology domain of BAFF had been replaced by that of APRIL. The same cells were also stained with anti-hBAFF mAb (Buffy-1) to determine the absolute amount of surface expressed ligands (right panel). The epitope recognized by Buffy-1, which is close to the membrane, is retained in the APRIL fusion protein, as shown in the scheme. (D) Mixtures of Flag-tagged BAFF (10 μg) and Myc-tagged APRIL (10, 1, 0.1, or 0.01 μg) were immunoprecipitated with substoechiometric amounts of BCMA–Fc (0.2 μg) and analyzed by Western blotting. Ligands were detected simultaneously with anti-Flag and anti-Myc antibodies (top panel), and BCMA–Fc with antibodies to hIg (bottom panel).
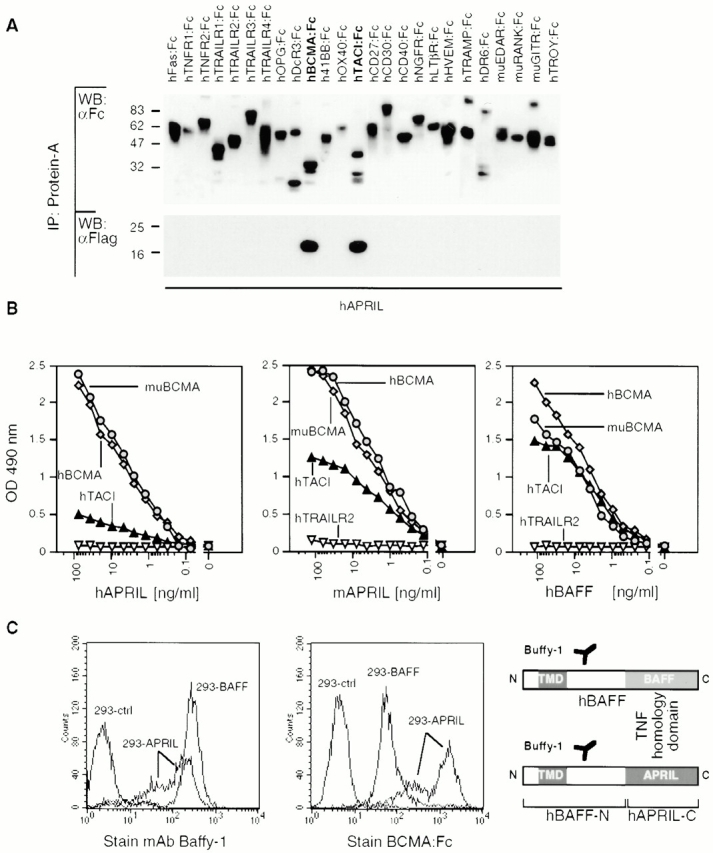
BCMA–Fc and TACI–Fc did not immunoprecipitate any of the 14 other TNF ligand family members tested, confirming that the interactions with APRIL and BAFF were specific (data not shown). Moreover, when human or murine BCMA–Fc was coated to plastic, specific binding of both human and murine APRIL was found in each case (Fig. 1 B), indicating that there is ligand–receptor cross-species reactivity. Binding of human and murine APRIL to human TACI–Fc was weaker in this format (Fig. 1 B). In contrast, human BAFF bound TACI–Fc with similar affinity (Fig. 1 B), in agreement with recent data 4.
Binding of BCMA–Fc was not restricted to soluble ligands but was also observed with membrane-bound APRIL and BAFF. 293HEK cells were stably transfected with a BAFF or with a BAFF (stalk region)–APRIL (TNF homology region) hybrid ligand (Fig. 1 C). Using the anti-BAFF stalk antibody (Buffy-1), similar surface expression levels of BAFF and of the APRIL hybrid construct were demonstrated in stably transfected cell populations. We found that BCMA–Fc bound more strongly to APRIL than to BAFF (Fig. 1 C). Moreover, when equal concentrations of Myc–APRIL and Flag–BAFF were incubated with BCMA–Fc, only APRIL was detected in BCMA–Fc immunoprecipitates (Fig. 1 D). A great excess of BAFF over APRIL was required to inhibit APRIL binding. These data suggest that although BCMA and TACI are both receptors for APRIL and BAFF, some specificity of interaction may be imposed by the apparent preference of BCMA for APRIL and of TACI for BAFF.
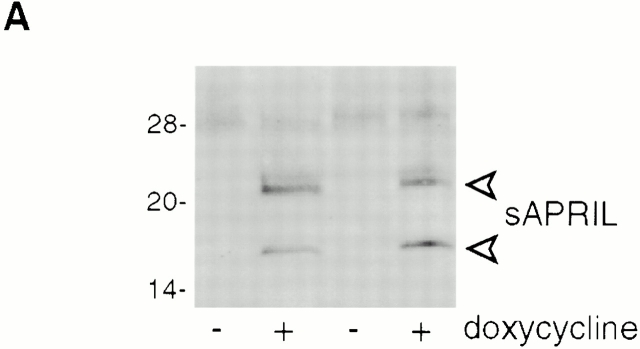
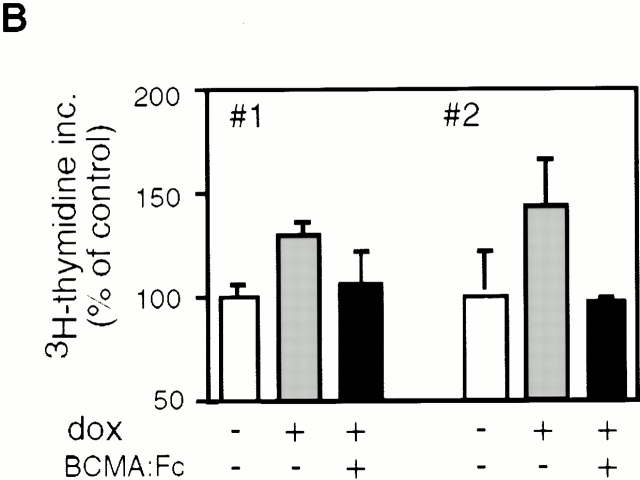
We have previously shown that NIH-3T3 cells stably transfected with full-length human APRIL proliferate faster than mock-transfected cells in vitro and in vivo 1. As membrane-bound APRIL is predicted to be efficiently processed in the extracellular stalk region by a furin to release soluble (s)APRIL 3, we next investigated whether increased proliferation rates were also discernible in cell lines that directly expressed the soluble form. NIH-3T3 clones that expressed soluble murine APRIL under a tetracycline-inducible promotor were generated. sAPRIL expression could be induced by treatment with the tetracycline analogue doxycycline for 16 h (Fig. 2 A). In Western blots, two forms of sAPRIL migrating at 17 and 22 kD were detected that corresponded to the expected sizes of the nonglycosylated and N-glycosylated proteins, respectively. We maintained the tet-inducible APRIL expression clones in low serum to induce quiescence, and the cells were then either left untreated or incubated with doxycycline overnight. When proliferation was assayed using a [3H]thymidine pulse, induced sAPRIL cell lines showed a 30–40% increase in proliferation rate (Fig. 2 B). This increase in growth rate is comparable to that observed with NIH-3T3 clones stably expressing the membrane-bound APRIL 1. In the presence of BCMA–Fc, the increased proliferation observed in sAPRIL-expressing cells was reversed (Fig. 2 B), indicating that the growth advantage was indeed APRIL mediated.
Figure 2.
In vitro proliferation induced by APRIL expression is blocked by soluble hBCMA–Fc fusion protein. (A) Western blot analysis of APRIL expression in the presence or absence of doxycycline (dox), a tetracycline analogue that induces expression. Two independently derived lines are shown. The major APRIL bands at 17 and 22 kD are indicated. (B) [3H]thymidine incorporation in the presence or absence of doxycycline with or without 20 μg/ml of hBCMA–Fc added. Values shown are 3H incorporation (inc.) expressed as percent of control. Two different experiments are shown. P < 0.02; n = 3 wells per data point.
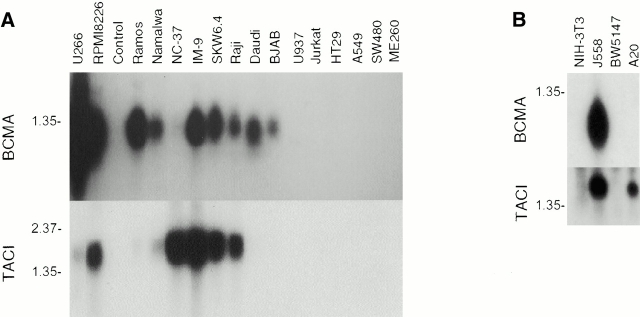
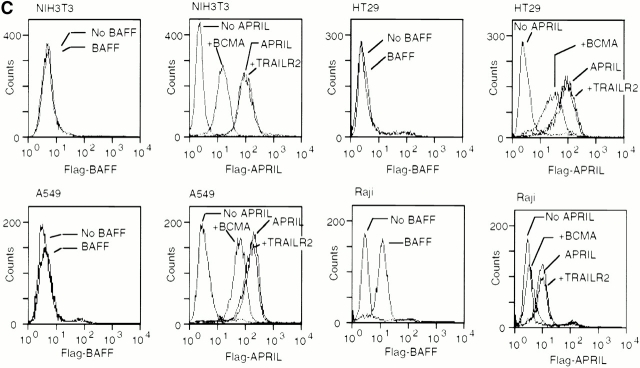
As BCMA and TACI mRNA expression was reported exclusively in B cells or activated T cells 5 6, the proliferative effect of APRIL on the fibroblastic NIH-3T3 cells was difficult to explain. We therefore measured mRNA levels of the two APRIL receptors in a panel of murine and human cell lines and could confirm the previously reported cell type distribution (Fig. 3A and Fig. B). In no case were BCMA or TACI mRNA detected in nonlymphoid cells such as the human adenocarcinoma cell line HT-29, the lung carcinoma cell line A549, or the murine fibroblastic NIH-3T3 cell line, even after prolonged Northern blot exposure. As expected from the Northern blot data, we were unable to stain these cells with BAFF, further supporting their lack of BCMA or TACI expression (Fig. 3 C). In contrast, a strong staining of these cells was observed with Flag–APRIL, which could be partially reduced by prior depletion of Flag–APRIL with BCMA–Fc, but not with the control TRAILR2–Fc (Fig. 3 C). The control Raji cells, which express message for both TACI and BCMA, were positive for both BAFF and APRIL staining as expected. Taken together, these results suggest that NIH-3T3, HT-29, A549, and other fibroblastic and epithelial cell lines (data not shown) express a third yet uncharacterized APRIL receptor that does not cross-react with BAFF (Fig. 3).
Figure 3.
Expression of APRIL receptors. Northern blot showing expression of TACI and BCMA mRNA in various human (A) and murine (B) cell lines. Probing with β-actin gave comparable signals for all cell lines (data not shown). B cell lines: J558, BW5147, A20, U266, RPMI8226, Ramos, Namalwa, NC-37, IM-9, SKW6.4, Raji, Daudi, BJAB; monocytic line: U937; T cell line: Jurkat; adenocarcinoma cell lines: HT29, SW480; lung carcinoma cell line: A549; melanoma cell line: ME260; fibroblast-derived cell line: NIH-3T3. (C) Flow cytometric analysis of NIH-3T3, HT-29, A459, and Raji cells stained with Flag–BAFF (BAFF), Flag–APRIL (APRIL), Flag–APRIL depleted with BCMA–Fc (+BCMA) or, as control, Flag–APRIL depleted with TRAILR2–Fc (+TRAILR2). Bound ligands were detected with anti-Flag mAb.
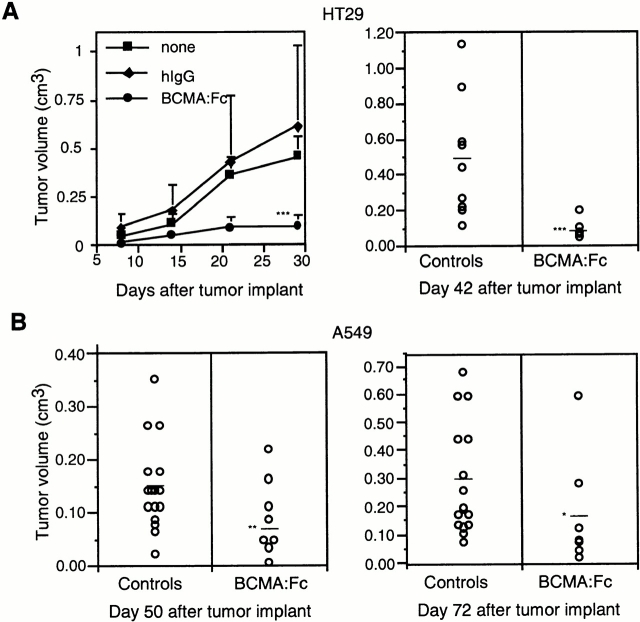
The availability of a soluble high-affinity APRIL receptor (BCMA–Fc) capable of inhibiting APRIL-proliferative effects in vitro allowed us to investigate the role of APRIL in tumorigenesis. When the epithelial tumor cell line HT29 was grown as subcutaneous tumors in nude mice, small palpable tumors were observed 2 wk after injection, and the average tumor volume exceeded 0.5 cm3 after 6 wk. Treatment with human BCMA–Fc (200 μg per mouse every week, the first time immediately before tumor cell injection) almost completely blocked tumor formation, and even 6 wk after injection, tumors were barely detectable. The human lung carcinoma cell line A549 was also grown as a subcutaneous tumor and subjected to the same treatment course. 7 wk after tumor implantation, the average tumor size was 0.15 cm3. Injection of BCMA–Fc retarded tumor growth, resulting in a significant reduction of tumor size to <0.10 cm3 (Fig. 4 B). This difference was still very significant 10 wk after tumor implantation, indicating that BCMA–Fc can modulate tumor growth over a long period of time (Fig. 4 B). Results similar to the A549 case were obtained with SW480 cells and NIH-3T3 cells (data not shown).
Figure 4.
Growth of human adenocarcinoma and lung carcinoma cell lines in nude (Nu/Nu) mice. (A) Growth rate of HT29 tumors implanted subcutaneously. Mice were left untreated or were treated with purified BCMA–Fc (200 μg) or control hIgG (200 μg) on day of inoculation and weekly thereafter. The scattergram showing tumor volume in pooled control mice and BCMA–Fc-treated mice 6 wk after HT29 tumor implantation is shown in the right panel. (B) Scattergrams showing tumor volume in pooled control mice and BCMA–Fc-treated mice 7 wk (left panel) and 10 wk (right panel) after subcutaneous implantation of A549 lung carcinoma cells. *P = 0.06; **P = 0.01; ***P < 0.005.
Several mechanisms could contribute to the retardation of tumor growth in BCMA–Fc-treated animals. Most tumor cell lines investigated in this report express endogenous APRIL (reference 1 and additional Northern blot data not shown), and, because increased levels of APRIL enhance proliferation in vitro, it is possible that BCMA–Fc interferes with autocrine APRIL-mediated growth signals. TACI and BCMA signaling leads to increased activation of the transcription factor NF-κB (and nuclear factor of activated T cells and activator protein [AP]-1 for TACI through CAML) 5, and constitutive NF-κB and AP-1 activation is frequently found in highly tumorigenic cells 11. Therefore, a high local concentration of furin-processed soluble APRIL 3, perhaps together with increased expression of APRIL receptors, will potentially result in conditions that favor neoplastic growth. In this context, it is noteworthy that BCMA was originally identified as a gene fused to the IL-2 locus in a chromosomal translocation from a T lymphoma 12. However, the growth stimulatory effects of APRIL on NIH-3T3 cells are unlikely to be due to the stimulation of the BCMA or TACI signaling pathways, as these cells do not express detectable amounts of these receptors. As strong APRIL binding to NIH-3T3 and other cells is observed, it is reasonable to assume that fibroblasts express an additional APRIL receptor that, in contrast to BCMA and TACI, does not appear to cross-react with BAFF. We are currently attempting to characterize this putative receptor.
High levels of APRIL mRNA expression have been described in carcinomas 1 2, a tumor class in which mutations in the tumor suppressor p53 gene are frequently found 13. As APRIL is localized on chromosome 17p13.1, 130–150 kb telomeric to p53 14, it is conceivable that an increased local transcriptional activity may contribute to augment APRIL expression. We cannot rule out, however, that the action of BCMA–Fc is indirect. BCMA–Fc may interfere with APRIL produced by host cells, which may stimulate tumor growth in a paracrine manner. Moreover, as administration of BCMA–Fc has been demonstrated to partially deplete B cells in mice 4 7, the effect of BCMA–Fc on B cells has to be considered. We feel, however, that this latter possibility is unlikely given that nude mice lack T cells necessary for B cell help.
APRIL's growth-stimulatory capacity on transformed cells together with the observation that the growth of tumor cell lines is suppressed upon interference with APRIL's activity suggests an important role for APRIL in tumorigenesis. Therefore, a potential role for APRIL in supporting the growth of B cell tumors that express high levels of BCMA should also be considered. Indeed, stimulation of BCMA and/or TACI leads to increased Bcl-2 expression in primary B cells 15, and the Raji Burkitt lymphoma cell line has enhanced growth in the presence of APRIL 1. Interestingly, Burkitt lymphoma cells require signals from monocytes for optimal survival and proliferation 16. As APRIL is expressed in monocytes (our unpublished observations), it is possible that APRIL may provide this stimulus. Such a signal may be a critical component in tumorigenesis in a variety of settings. For example, chronic inflammation in inflammatory bowel disease or after Helicobacter pylori infection in humans has been implicated in the pathogenesis of cancers of the gastrointestinal tract, suggesting that tumor cells receive stimulatory signals from inflammatory cells 17.
Cancer is a major public health problem despite impressive advances in the understanding of molecular mechanisms underlying carcinogenesis and in the development of novel anticancer therapies. The recent success of antibodies to epidermal growth factor receptor2 (HER2) in the treatment of breast cancer tumors 18 demonstrates that antibodies or antibody-like molecules can effectively be used to treat cancer patients, complementing classical drugs. BCMA–Fc or other inhibitors interfering with APRIL activity may therefore constitute exciting new tools in cancer therapy.
Acknowledgments
We thank Andreas Tsapis (INSERM U131, Clamart, France) for providing hBCMA cDNA. We thank Mohammad Zafari, Chris Benjamin, and Sarah Bixler (Biogen, Inc.) for sharing their unpublished observations.
This work was supported in part by grants from the Swiss National Science Foundation.
Footnotes
P. Rennert and P. Schneider contributed equally to this work.
References
- Hahne M., Kataoka T., Schroter M., Hofmann K., Irmler M., Bodmer J.L., Schneider P., Bornand T., Holler N., French L.E. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Manos E., Jensen G., Nadauld L., Jones D.A. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000;60:1021–1027. [PubMed] [Google Scholar]
- Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J.L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.A., Johnston J., Mudri S., Enselman R., Dillon S.R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- von Bulow G.U., Bram R.J. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–141. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- Laabi Y., Gras M.P., Brouet J.C., Berger R., Larsen C.J., Tsapis A. The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res. 1994;22:1147–1154. doi: 10.1093/nar/22.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.S., Schneider P., Kalled S.L., Wang L., Lefevre E.A., Cachero T.G., MacKay F., Bixler S.A., Zafari M., Liu Z.Y. BAFF binds to the tumor necrosis factor receptor–like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J. Exp. Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P. Production of recombinant TRAIL and TRAIL receptorsFc chimeric proteins. Methods Enzymol. 2000;322:325–345. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- Kwon B., Youn B.S., Kwon B.S. Functions of newly identified members of the tumor necrosis factor receptor/ligand superfamilies in lymphocytes. Curr. Opin. Immunol. 1999;11:340–345. doi: 10.1016/s0952-7915(99)80054-5. [DOI] [PubMed] [Google Scholar]
- Naismith J.H., Sprang S.R. Modularity in the TNF-receptor family. Trends Biochem. Sci. 1998;23:74–79. doi: 10.1016/s0968-0004(97)01164-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Laabi Y., Gras M.P., Carbonnel F., Brouet J.C., Berger R., Larsen C.J., Tsapis A. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:3897–3904. doi: 10.1002/j.1460-2075.1992.tb05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrum M.A., Vousden K.H. Regulation and activation of p53 and its family members. Cell Death Differ. 1999;6:1162–1168. doi: 10.1038/sj.cdd.4400625. [DOI] [PubMed] [Google Scholar]
- Cousin P., Billotte J., Chaubert P., Shaw P. Physical map of 17p13 and the genes adjacent to p53. Genomics. 2000;63:60–68. doi: 10.1006/geno.1999.6062. [DOI] [PubMed] [Google Scholar]
- Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens J.M., Gordon J., Gregory C.D. Micro-environmental factors in the survival of human B-lymphoma cells. Cell Death Differ. 2000;7:59–69. doi: 10.1038/sj.cdd.4400636. [DOI] [PubMed] [Google Scholar]
- Graham D.Y. Therapy of Helicobacter pyloricurrent status and issues. Gastroenterology. 2000;118:S2–8. doi: 10.1016/s0016-5085(00)70003-5. [DOI] [PubMed] [Google Scholar]
- Baselga J., Tripathy D., Mendelsohn J., Baughman S., Benz C.C., Dantis L., Sklarin N.T., Seidman A.D., Hudis C.A., Moore J. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]