Abstract
The generalized Shwartzman reaction in mice which had been primed and challenged with lipopolysaccharide (LPS) depends on interleukin (IL)-12–induced interferon (IFN)-γ production at the priming stage. We examined the involvement in the priming mechanism of the unique population of Vα14 natural killer T (NKT) cells because they promptly produce IFN-γ after IL-12 stimulation. We report here that LPS- or IL-12–primed NKT cell genetically deficient mice were found to be resistant to LPS-elicited mortality. This outcome can be attributed to the reduction of IFN-γ production, because injection of recombinant mouse IFN-γ, but not injection of IL-12, effectively primed the NKT cell–deficient mice. However, priming with high doses of LPS caused mortality of severe combined immunodeficiency, NKT cell–deficient, and CD1-deficient mice, indicating a major contribution of NKT cells to the Shwartzman reaction elicited by low doses of LPS, whereas at higher doses of LPS NK cells play a prominent role. These results suggest that the numerically small NKT cell population of normal mice apparently plays a mandatory role in the priming stage of the generalized Shwartzman reaction.
Keywords: natural killer T cells, interferon γ, interleukin 12, lipopolysaccharide, Shwartzman reaction
Introduction
The generalized Shwartzman reaction is a lethal shock syndrome of mice after two consecutive injections of LPS. IL-12, IFN-γ, and TNF-α are known to be involved in the pathogenesis 1, but IL-12 is pivotal in the priming phase because it induces IFN-γ production 2 3, which is crucial for its ability to induce large amounts of TNF-α, IL-1, and possibly other inflammatory cytokines. In any event, IFN-γ production in the sensitization phase is essential for the development of postchallenge mortality, because neither TNF-α or IL-1 alone, nor a combination of both of these cytokines, causes mortality 2.
NKT cells are a unique population of T lymphocytes that express the NK1.1 molecule 4. They use an invariant TCR α chain encoded by the Vα14 and Jα281 gene segments and are usually CD4−CD8−, although NKT cells residing in the liver coexpress CD4 5. Stimulation of NKT cells by cross-linking the NK1.1 molecules 6, or incubation with either IL-12 7 or the synthetic ligand α-galactosylceramide 8, promptly induces IFN-γ production.
We considered that the Vα14 subset of NKT cells could be involved in the generalized Shwartzman reaction on the following grounds: (a) IL-12 exerts potent activation of NKT cells and promotes very early IFN-γ production 6 7; (b) inoculation of LPS activates NKT cells in the liver through IL-12 production by Kupffer cells 9; and (c) functional impairment or depletion of NKT cells by Abs to cell surface markers 10 11, or by genetic knockout of either β2-microglobulin 11 or CD7 12 molecules, resulted in resistance against the generalized Shwartzman reaction. However, the impairment of NKT cells in these studies also affected other lymphoid populations or did not separate the LPS priming and elicitation phases. Furthermore, distinct roles of NK and NKT cells were attributed in the LPS-induced liver injury and mortality, respectively 13.
Therefore, it seemed desirable to focus further on the identification of the cellular target, using mice with highly selective depletion of the Vα14 NKT cell subset in the thymus, spleen, lymph nodes, liver, and bone marrow 14.
Materials and Methods
Mice.
C57BL/6, BALB/c, and C.B.-17 SCID mice were purchased from OLAC Ltd. Generation of Jα281-deficient mice has been described previously 14. Mice which lack the Jα281 gene segment are devoid of Vα14+ NKT cells while having the other lymphoid cell lineages intact 14. Homogeneous populations were established by backcrossing heterozygous mice to C57BL/6 or BALB/c mice for more than five generations. The resultant heterozygous mice were bred to obtain homozygotes 14. CD1-deficient mice were purchased from The Jackson Laboratory and backcrossed to C57BL/6 mice for five generations. Mice were fed and kept under specific pathogen-free conditions and used at 8–12 wk of age. In each experiment, age- and sex-matched mice were used.
Induction of the Shwartzman Reaction.
Mice were injected intravenously 11 with 40 μg Escherichia coli–derived, phenol-extracted LPS (Sigma-Aldrich), or intraperitoneally with 0.5 μg recombinant murine IL-12 (specific activity 0.7–2.1 × 107 U/mg; BD PharMingen) or IFN-γ (specific activity 0.3–1 × 108 U/mg; BD PharMingen). Endotoxin levels of recombinant cytokines were <0.1 ng/μg protein. 24 h later, mice were challenged by intravenous injection of 400 μg phenol-extracted LPS 11.
In the experiments reported in Fig. 3, mice were injected intravenously with different doses of two preparations of phenol-extracted LPS, one of which was purified by ion-exchange chromatography (both preparations from Sigma-Aldrich), and were challenged by intravenous injection of 400 μg phenol-extracted LPS. The occurrence of generalized Shwartzman reaction was evaluated by mortality.
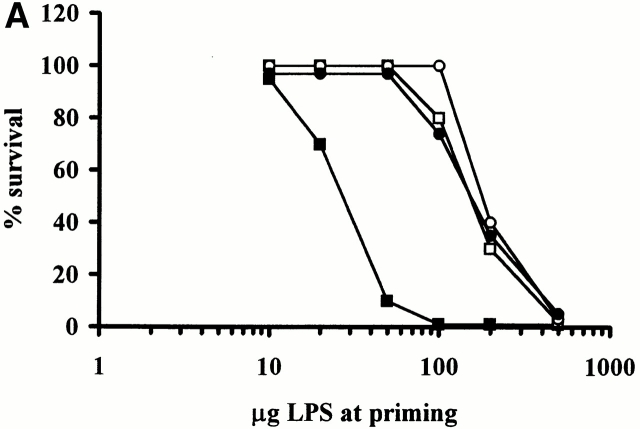
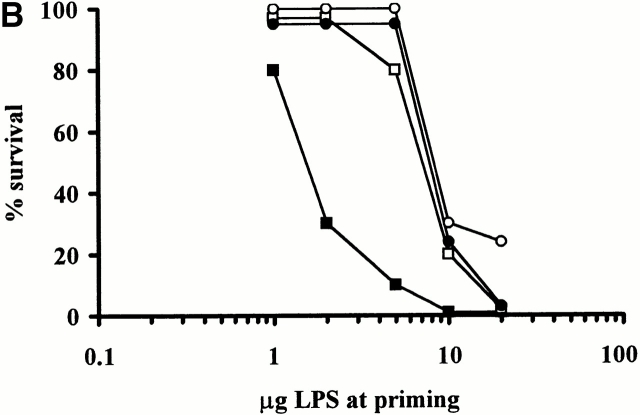
Figure 3.
Induction of the Shwartzman reaction in normal C57BL/6 (▪), SCID (○), NKT-deficient (□), and CD1-deficient (•) mice. Mice were primed by intravenous injection of different doses of phenol-extracted LPS (A), or phenol-extracted and ion-exchange chromatography–purified LPS (B). 24 h later, they were challenged by intravenous injection of 400 μg phenol-extracted LPS.
Assay for Serum Cytokine Levels.
Serum levels of IL-12 and IFN-γ were measured 20 h after priming with LPS or IL-12 11. TNF-α serum levels were measured 1 h after challenge with LPS 11. Cytokine levels were assessed by sandwich ELISA using commercially available (BD PharMingen) pairs of mAbs and recombinant cytokines for setting standard curves.
Histological Analysis.
Mice were killed by cervical dislocation 8–12 h after challenge with LPS. The livers and kidneys were removed immediately, fixed with 10% formalin for 12 h, and embedded in paraffin. Several tissue sections were deparaffinized and stained with hematoxylin and eosin.
Preparation of Liver Mononuclear Cells and Cell Sorting.
Liver mononuclear cells were prepared as described 15. In brief, livers were minced, passed through a mesh, and suspended in complete RPMI 1640 (GIBCO BRL). After washing, the cells were resuspended in 30% Percoll containing 100 U/ml heparin and centrifuged at 2,000 rpm for 20 min at room temperature. The cell pellet was washed once in medium and then resuspended in cold distilled water for 20 s to lyse red blood cells by osmotic shock. After lysis, the cell suspension was diluted with NaCl solution to readjust the osmotic pressure and was then washed with RPMI 1640 supplemented with 10% heat-inactivated FCS. NK1.1+ and NK1.1− cells were sorted by incubating liver mononuclear cells with anti-NK1.1 (PK 136, mouse IgG2a; BD PharMingen) and anti–mouse Ig-coated immunomagnetic beads (Advanced Magnetics), as described previously 16. Purification of cell subsets was routinely >90% as determined by FACS® analysis, and cell viability was also >90% as determined by trypan blue exclusion.
Intracellular Staining for IFN-γ.
Intracellular staining was used to determine IFN-γ production at the single cell level. NK1.1+ and NK1.1− cells were cultured for 5 h with Brefeldin A (Sigma-Aldrich) to accumulate intracellularly new synthesized protein. Cells were harvested and fixed with 4% (wt/vol) paraformaldehyde in PBS for 10 min at room temperature. Fixed cells were suspended and washed twice with permeabilization buffer containing 0.1% saponin (Sigma-Aldrich), 1% heat-inactivated FCS, and 0.1% NaN3 in PBS. The permeabilized cells were then incubated in the presence of saponin with PE-conjugated anti–mouse IFN-γ mAb (XMG1.2, rat IgG1; BD PharMingen) or a PE-conjugated isotype control mAb (R3-34, rat IgG1; BD PharMingen) for 30 min at room temperature. After being washed at room temperature, the cells were analyzed with a FACScan™ flow cytometer (Becton Dickinson). Viable lymphocytes were gated by forward and side scatter and analysis was performed on 10,000 acquired events for each sample.
To identify the phenotype of the IFN-γ–producing cells, surface marker analysis was performed by staining the cells with FITC-conjugated anti–TCR-α/β (H57-597, hamster IgG; BD PharMingen) before paraformaldehyde fixation.
Statistics.
The χ2 and the Student's t tests were used to compare significance of differences between groups.
Results and Discussion
The results obtained after priming and challenge with LPS showed that both C57BL/6 and BALB/c mice had very high 72-h mortality rates of 97% (13/15 mice) and 100% (15/15 mice), respectively, and very high serum levels of IL-12, IFN-γ, and TNF-α (Table ). However, a strikingly high survival of 93% (14/15 mice) NKT-deficient C57BL/6 mice and 100% (15/15) NKT-deficient BALB/c mice was observed (Table ). Moreover, NKT-deficient mice had low serum levels of IFN-γ and TNF-α, whereas serum IL-12 levels were comparable to control mice.
Table 1.
Induction of the Generalized Shwartzman Reaction in Normal and NKT-deficient Mice
| Strain | Priming | Challenge | Deaths/tested | Mortality | IL-12 | IFN-γ | TNF-α |
|---|---|---|---|---|---|---|---|
| n | % | ng/ml | ng/ml | ng/ml | |||
| C57BL/6 | − | LPS | 0/15 | 0 | |||
| LPS | − | 0/15 | 0 | ||||
| LPS | LPS | 13/15 | 93 | 1.2 ± 4 | 7.8 ± 1.4 | 80 ± 10 | |
| C57BL/6 NKT−/− | − | LPS | 0/15 | 0 | |||
| LPS | − | 0/15 | 0 | ||||
| LPS | LPS | 1/15 | 7 | 1.3 ± 0.2 | 0.2 ± 0.05 | 0.35 ± 0.09 | |
| BALB/c | − | LPS | 0/15 | 0 | |||
| LPS | − | 0/15 | 0 | ||||
| LPS | LPS | 15/15 | 100 | 0.9 ± 0.2 | 5.4 ± 0.9 | 68 ± 5 | |
| BALB/c NKT−/− | − | LPS | 0/15 | 0 | |||
| LPS | − | 0/15 | 0 | ||||
| LPS | LPS | 0/15 | 0 | 0.8 ± 0.1 | 0.18 ± 0.05 | 0.25 ± 0.06 |
The histological analysis (Fig. 1) showed areas of focal necrosis/apoptosis and mononuclear cell infiltrates in livers and kidneys of control mice and very poor focal necrosis/apoptosis and mononuclear cell infiltrates in the livers and kidneys of NKT-deficient mice, thus corroborating the observed mortality rates. Altogether, these results clearly demonstrated that the lack of NKT cells protects mice from the generalized Shwartzman reaction accompanied by reduced levels of IFN-γ and TNF-α.
Figure 1.
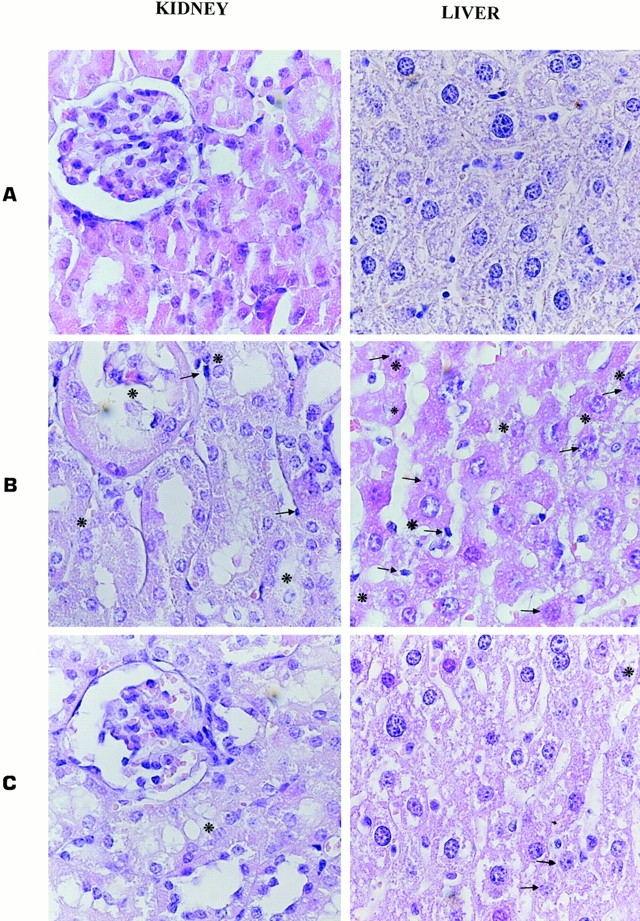
Focal necrosis/apoptosis and mononuclear cell infiltrate in the liver and kidney of untreated C57BL/6 mice (A), or C57BL/6 (B) and NKT-deficient C57BL/6 (C) mice injected and challenged with LPS (original magnification: 10 × 40). Asterisks indicate areas of necrosis/apoptosis and arrows indicate mononuclear cells infiltration.
The finding of reduced IFN-γ, but normal IL-12 levels in sera from NKT-deficient mice (Table ) suggested that defective IFN-γ production was responsible for protection from lethal shock and that NKT cells were the main source of LPS-induced IFN-γ production.
We directly analyzed this finding by intracellular staining of IFN-γ produced by NK1.1+ and NK1.1− cells sorted from the livers of IL-12–primed NKT and control C57LB/6 mice. The cell sorting analysis showed that in normal C57BL/6 mice, IL-12 treatment increased from 0.3 to 42% the numbers of IFN-γ1 cells (Fig. 2 B) at 6 h, and combined cytoplasmic and surface analysis further showed that NK1.1+ cells were also αβ+, whereas low numbers of IFN-γ–producing cells were detected within the NK1.1+αβ− population. However, as shown in Fig. 2A and Fig. B, treatment with IL-12 for 24 h induced IFN-γ staining of both NK1.1+αβ− (NK cells; Fig. 2 B) and NK1.1−αβ− cells (Fig. 2 A), whereas poor IFN-γ staining was detected within the NK1.1+αβ+ population (NKT cells; Fig. 2 B).
Figure 2.
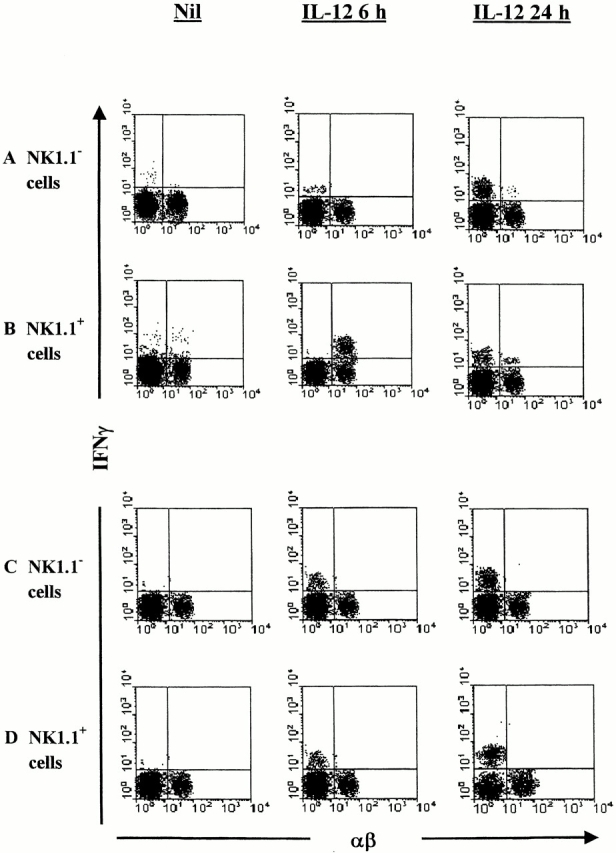
IFN-γ production by liver mononuclear cells after priming with IL-12. NK1.1+ and NK1.1− cells were sorted from the livers of C57BL/6 (A and B) and NKT-deficient (C and D) mice that had been primed 6 or 24 h previously with IL-12, or left untreated (Nil). The cells were surface stained with FITC-anti–TCR-α/β mAb and intracellularly stained with PE-anti–IFN-γ mAb, and analyzed by FACScan™. The analysis gate was set on small lymphocytes by forward and side scattering. The figure shows representative results from three different experiments, each carried out with five mice per group.
These data have been confirmed in NKT cell–deficient mice (Fig. 2C and Fig. D): IL-12 treatment for 6 h induced IFN-γ production by both NK1.1−αβ− (Fig. 2 C) and NK1.1+αβ+ (Fig. 2 D) cells, and the percentages of IFN-γ+ NK1.1−αβ− (Fig. 2 C) and NK1.1+αβ+ (Fig. 2 D) cells increased after 24 h of treatment with IL-12.
Together, the above reported results suggest that at an early time (6 h) after IL-12 injection, NKT cells are the main source of IFN-γ in the liver, whereas at a later time after IL-12 injection (24 h), NK cells 17 18 and other cells (presumably dendritic cells, as described recently 19) become the main IFN-γ–producing cells.
Of note, the absolute number of liver NKT cells was not modified 6 h after IL-12 treatment (1.2 ± 0.3 × 106/liver in IL-12 treated mice vs. 1 ± 0.4 × 106/liver in control mice), but was strongly reduced 24 h after IL-12 treatment (0.22 ± 0.08 × 106/liver), thus supporting the possibility that IL-12 causes activation-induced cell death of liver NKT cells, as demonstrated recently 20.
It was of further major importance to compare the priming effects of IL-12 and IFN-γ inoculation on the mortality of LPS-challenged NKT cell–defective mice. The results after IL-12 priming showed only very low mortality (1/15; 7%) in NKT cell–deficient mice, but high mortality (13/15; 86%) of control mice (Table , top). Accordingly, NKT cell–deficient mice had lower serum levels of TNF-α than control mice. This result suggests that the Vα14 NKT cell population was the primary functional target for IL-12 because the mutant mice exclusively lack Vα14 NKT cells while the other lymphoid populations are intact. We attribute the failure of IL-12 to prime for the Shwartzman reaction in NKT cell–deficient mice to their intrinsic defect to produce IFN-γ.
Table 2.
IFN-γ, but Not IL-12, Primes NKT Cell–deficient Mice for the Generalized Shwartzman Reaction
| Strain | Priming | Challenge | Deaths/tested | Mortality | TNF-α |
|---|---|---|---|---|---|
| n | % | ng/ml | |||
| C57BL/6 | IL-12 | LPS | 13/15 | 86 | 91 ± 11 |
| C57BL/6 NKT−/− | IL-12 | LPS | 1/15 | 7 | 0.3 ± 0.05 |
| C57BL/6 | IFN-γ | LPS | 12/15 | 80 | 65 ± 8 |
| C57BL/6 NKT−/− | IFN-γ | LPS | 10/15 | 67 | 54 ± 6 ‡ |
We further tested this hypothesis by priming the NKT cell–deficient mice with IFN-γ. As expected, the postchallenge mortality was found to be high in both NKT cell–deficient (10/15; 67%) and control (12/15; 80%) mice (Table , bottom). Both strains also had comparably elevated serum levels of TNF-α.
Previous studies have reported a role for NK cells in the response to LPS and Shwartzman reaction 10. Importantly, Caligiuri and coworkers 21 have clearly demonstrated that the Shwartzman reaction can be elicited in SCID mice, which have no NKT cells. To address the possible role of NK and NKT cells, we analyzed the Shwartzman reaction elicited by injection of different doses and preparation of LPS in SCID, NKT-deficient, and CD1-deficient and control mice.
The results obtained (Fig. 3 A) showed that normal C57BL/6 mice had very high mortality rates of 90% (9/10 mice) upon priming with 50 μg LPS, whereas all SCID, NKT-, and CD1-deficient mice survived this priming dose. However, priming with 200 μg LPS caused 60–70% mortality in SCID, NKT-deficient, and CD1-deficient mice, and all the mutant mice succumbed after priming with 500 μg LPS.
Similar results have been obtained using a different LPS preparation purified by ion-exchange chromatography (Fig. 3 B). In this experiment, 90% (9/10 mice) of normal C57BL/6 mice succumbed upon priming with 5 μg LPS, whereas all SCID, NKT-deficient, and CD1-deficient mice survived. However, priming with 50 μg LPS caused 80% mortality of SCID, NKT-, and CD1-deficient mice.
We interpret these results to indicate that NKT cells make a major contribution to the Shwartzman reaction elicited by low doses of LPS, whereas at higher doses of LPS NK cells play a prominent role. The relative contribution of NKT and NK cells might be variably dependent on the amount of IL-12 produced in response to the doses of LPS administered. It has been recently demonstrated that both NK and NKT cells constitutively express the IL-12 receptor 7, although the expression on NKT cells was higher than that on NK cells 7. Therefore, the large amount of IL-12 produced by injection of high doses of LPS in NKT-deficient mice might be sufficient to activate NK cells to overcome the impairment of NKT cells, which would be preferentially responsive to lower doses of IL-12 (and, by extension, of LPS). This possibility has been recently raised to explain controversial results on the role of NK and NKT cells in IL-12–induced immune responses in vivo, with particular regard to its antitumor effects 7 22 23.
In conclusion, a comprehensive explanation of the pathogenesis of the Shwartzman reaction emerges, whereby: LPS-induced IL-12 production by macrophages promotes IFN-γ production by NKT or NK cells (first priming step), and subsequent challenge with LPS induces massive production of inflammatory cytokines (including TNF-α) by IFN-γ–sensitized macrophages (second effector step), which then cause hepatocyte necrosis/apoptosis and death. The novel important finding here is that NKT cells as a source of IFN-γ after LPS or IL-12 priming play an essential pathogenic role in the development of generalized Shwartzman reactions.
The identification of this role of NKT cells opens new possibilities for cell-targeted intervention against diseases that may involve this pathogenesis (e.g., tuberculosis 24). One possibility could involve ligands resulting in Fas-mediated apoptosis of NKT cells 25. Finally, our data also indicate that it may be prudent to exercise caution when considering the use of IL-12 as a vaccine adjuvant 26.
Acknowledgments
We would like to thank Steve Porcelli and Marc Bonneville for helpful suggestions and criticism and for their final reviewing of the manuscript.
This work has been supported by grants from the National Research Council (Consiglio Nazionale delle Ricerche to F. Dieli) and from the Ministry for University and Scientific and Technological Research (MURST 60% to F. Dieli and A. Salerno).
References
- Ozmen L., Pericin M., Hakimi J., Chizzonite R.A., Wysocka M., Trinchieri G., Gately M., Garotta G. Interleukin 12, interferon γ, and tumor necrosis factor α are the key cytokines of the generalized Shwartzman reaction. J. Exp. Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka M., Kubin M., Vieira L.Q., Ozmen L., Garotta G., Scott P., Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- Heinzel F.P., Rerko R.M., Ling P., Hakimi J., Schoenhaut D.S. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect. Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H.R. NK1.1+ T cell receptor-α/β+ cellsnew clues to their origin, specificity, and function. J. Exp. Med. 1995;181:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O., Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Arase N., Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Takeda K., Mendiratta S.K., Kawamura H., Van Kaer L., Yagita H., Abo T., Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J. Immunol. 1998;160:16–19. [PubMed] [Google Scholar]
- Burdin N., Brossay L., Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells toward Th2 cytokine synthesis. Eur. J. Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ogasawara K., Takeda K., Hashimoto W., Sakihara H., Kumagai K., Anzai R., Satoh M., Seki S. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- Heremans H., Dillen C., van Damme J., Billiau A. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur. J. Immunol. 1994;24:1155–1160. doi: 10.1002/eji.1830240522. [DOI] [PubMed] [Google Scholar]
- Ogasawara K., Takeda K., Hashimoto W., Satoh M., Okuyama R., Yanai N., Obinata M., Kumagai K., Takada H., Hiraide H., Seki S. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J. Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- Sempowski G.D., Lee D.M., Scearce R.M., Patel D.D., Haynes B.F. Resistance of CD7-deficient mice to lipopolysaccharide-induced shock syndromes. J. Exp. Med. 1999;189:1011–1016. doi: 10.1084/jem.189.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakao Y., Takeda K., Tsutsui H., Kaisho T., Nomura F., Okamura H., Nakanishi K., Akira S. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 1999;11:471–480. doi: 10.1093/intimm/11.3.471. [DOI] [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Pied S., Roland J., Louise A., Voegtle D., Soulard V., Mazier D., Cazenave P.A. Liver CD4−CD8− NK1.1+ TCR αβ intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J. Immunol. 2000;164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- Dieli F., Taniguchi M., Asherson G.L., Sireci G., Caccamo N., Sciré E., Bonanno C.T., Salerno A. Development of hapten-induced IL-4-producing CD4+ T lymphocytes requires early IL-4 production by αβ T lymphocytes carrying invariant Vα14 TCR α chains. Int. Immunol. 1998;10:413–420. doi: 10.1093/intimm/10.4.413. [DOI] [PubMed] [Google Scholar]
- Chan S.H., Perussia B., Gupta J.W., Kobayashi M., Pospisil M., Young H.A., Wolf S.F., Young D., Clark S.C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factorcharacterization of the responder cells and sinergy with other inducers. J. Exp. Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Trinchieri G. The role of natural killer cells in host-parasite interactions. Curr. Opin. Immunol. 1995;7:34–40. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Fukao T., Suzue K., Maki C., Ito M., Nakamura M., Koyasu S. Interleukin 12–dependent interferon gamma production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G., MacDonald H.R. Rapid death and regeneration of NKT cells in anti-CD3ε or IL-1-treated micea major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- Fehniger T.A., Yu H., Cooper M.A., Suzuki K., Shah M.H., Caligiuri M.A. IL-15 costimulates the generalized Shwartzman reaction and innate immune IFN-γ production in vivo. J. Immunol. 2000;164:1643–1647. doi: 10.4049/jimmunol.164.4.1643. [DOI] [PubMed] [Google Scholar]
- Kodama T., Takeda K., Shimozato O., Hayakawa Y., Atsuta M., Kobayashi K., Ito M., Yagita H., Okumura K. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur. J. Immunol. 1999;29:1390–1396. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Smyth M.J., Thia K.Y.T., Street S.E.A., Cretney E., Trapani J.A., Taniguchi M., Kawano T., Pelikan S.B., Crowe N.Y., Godfrey D.I. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton M.J., Vermeulen M.W. Immunopathology of tuberculosisroles of macrophages and monocytes. Infect. Immun. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno T., Attinger A., Rimoldi D., Hahne M., Tschopp J., MacDonald H.R. Expression of B220 on activated T cell blasts precedes apoptosis. Eur. J. Immunol. 1998;28:540–547. doi: 10.1002/(SICI)1521-4141(199802)28:02<540::AID-IMMU540>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Gabaglia C.R., Pedersen B., Hitt M., Burdin N., Sercarz E.E., Graham F.L., Gauldie J., Braciak T.A. A single intramuscular injection with an adenovirus-expressing IL-12 protects BALB/c mice against Leishmania major infection, while treatment with an IL-4-expressing vector increases disease susceptibility in B10.D2 mice. J. Immunol. 1999;162:753–760. [PubMed] [Google Scholar]