Abstract
Lipooligosaccharide (LOS) has been implicated in the adhesion and invasion of host epithelial cells. We examined the adhesive and invasive abilities of isogenic gonococcal opacity-associated outer membrane protein–negative, pilus-positive (Opa−Pil+) Neisseria gonorrhoeae strains expressing genetically defined LOS. Strain F62 (Opa−Pil+), expressing the lacto-N-neotetraose and the galNac-lacto-N-neotetraose LOS, and its isogenic derivative that expressed only the lacto-N-neotetraose LOS (F62ΔlgtD), adhered to, and invaded, to the same extent the human cervical epidermoid carcinoma cell line, ME180. While the adhesive abilities of Opa−Pil+ isogenic strains that express LOS molecules lacking the lacto-N-neotetraose structure were similar to that seen for F62, their invasive abilities were much lower than the strains expressing lacto-N-neotetraose. Fluorescence microscopy studies showed that the adherence of F62, but not the strains lacking lacto-N-neotetraose, induced the rearrangement of actin filaments under the adherent sites. Electron microscopy studies demonstrated that F62, but not the strains lacking lacto-N-neotetraose, formed extensive and intimate associations with epithelial cell membranes. Thus, in the absence of detectable Opa protein, the lacto-N-neotetraose LOS promotes gonococcal invasion into ME180 cells. The data also suggest that LOS is involved in the mobilization of actin filaments in host cells, and in the formation of a direct interaction between the bacterial outer membrane and the plasma membrane of ME180 cells.
Keywords: pathogenesis, cell biology, immunofluorescence, outer membrane proteins, virulence factors
Introduction
In a human challenge study, Schneider et al. 1 showed that the gonococcal variant MS11mkC (gonococcal opacity-associated outer membrane protein–negative, pilus-positive [Opa−Pil+]), expressing lipooligosaccharide (LOS) containing the lacto-N-neotetraose structure, was more infectious than strain MS11mkA (Opa−Pil+), expressing lactosyl LOS. Since adherence to, and invasion of, host epithelial cells are the first steps in disease caused by the Neisseria gonorrhoeae, it is reasonable to conclude that LOS plays an important role in initiating infection in humans. However, the role of LOS in the development of gonorrhea remains to be established.
Much of our understanding of disease pathogenesis has been derived from studies using variations of the in vitro tissue culture model, originally described by Shaw and Falkow 2. Shown in both epithelial cell lines 3 4 5 6 7 8 and primary human urethral epithelial cells 9 10 11 12, the adherence of gonococci to epithelial cell membranes induces a series of events in host cells, which include the accumulation of F-actin under the adherent site and the elongation of microvilli 9 10 13 14. This leads to the engulfment of gonococci by host cells 15. Adherence and invasion are initiated by the interaction between surface molecules of N. gonorrhoeae and host epithelial cells. Pili, Opa, and LOS are the major surface molecules of N. gonorrhoeae and are all capable of undergoing phase variation 16 17 18 19 20. The role that these molecules play in infections, especially pili and Opa, has been studied intensively during the past decade. The data indicate that pili play a major role in the initial adherence 21, and Opa is involved in both adherence and invasion 22 23. Griffiss et al. 24 showed that pili and Opa are both required for inducing the elongation of microvilli during the invasion of gonococci into the human endometrial cell line, HEC-1-B. Few researchers have studied the role of LOS in the disease process.
LOS molecules consist of three oligosaccharide chains attached to a lipid A core 25. The oligosaccharide chains of LOS are similar in sequence and linkage with the oligosaccharides expressed on the surface of human cells 26. The synthesis of these compounds requires a series of glycosyl transferases 16 18 20. Besides their highly conserved core structures, the terminal oligosaccharides of LOS molecules undergo rapid phase variation 27. LOS variation is mediated by a change in the number of guanines in the middle of the coding sequences of several key enzymes, which results in alterations in the expression of these glycosyl transferases and the surface expression of various LOS isoforms 16 18 20. At any given time, several LOS structures with varying terminal oligosaccharides may be expressed on the outer membrane of N. gonorrhoeae 28. Because LOS structures can vary in strains, it has been difficult to study the structure and function of LOS. Early studies 1 29 30 that used the male challenge model indicated that LOS was involved in host cell invasion. Wang et al. 31 showed that the lacto-N-neotetraose LOS structure can inhibit the invasion of HEC-1-B cells by gonococci. Using cultured hepatoma cells as a model system, Porat et al. 32 identified the asialoglycoprotein receptor as the binding site of LOS-containing lacto-N-neotetraose, and showed that gonococci increased the expression level of the asialoglycoprotein receptor in the hepatoma cells. The asialoglycoprotein receptor is also expressed in human urethral epithelial cells 11 13.
In this work, we studied the role of LOS in the adherence and the invasion of N. gonorrhoeae into the cultured human cervical epithelial cell line, ME180, using isogenic gonococcal strains. Each of these gonococcal strains was genetically designed to express a distinct LOS structure that does not undergo phase variation. We designed these strains to express individual LOS structures that have been found in naturally occurring infections. Our studies provide strong evidence that LOS-containing lacto-N-neotetraose facilitates the invasion into but not adherence of N. gonorrhoeae to ME180 cells.
Materials and Methods
Reagents and Enzymes.
Restriction enzymes, DNA polymerases, and T4 DNA ligase were obtained from New England Biolabs. All reagents used for this study were of molecular biology grade or better. Acrylamide and bisacrylamide were purchased from Boehringer Mannheim Biochemicals. Ammonium persulfate and N,N,N′,N′-tetramethyl–ethylenediamine were obtained from Bio-Rad Laboratories. Silver nitrate, formaldehyde, and ammonium hydroxide were obtained from Fisher Scientific Co. Periodic acid was purchased from J.T. Baker, Inc. Proteinase K was from Sigma Chemical Co. mAbs 2-1-L8 and 1-17-L1 were provided by Wendell Zollinger (Walter Reed Army Institute of Research, Forest Glen Annex, MD), and mAb 1B2 was provided by J. McLeod Griffiss (University of California, San Francisco, CA). mAb 3G9 was provided by Peter Rice (Boston University, Boston, MA). The Opa-specific mAb 4B12 was obtained from Milan Blake (North American Vaccines, Inc., Beltsville, MD).
Strains and Vectors.
Gonococcal strain F62 was obtained from Dr. P. Frederick Sparling (University of North Carolina, Chapel Hill, NC), and strain PID2 was obtained from Dr. Herman Schneider (Walter Reed Army Institute for Research, Forest Glen Annex, MD). Gonococci were grown on GCK agar or in GCP broth (both made from gonococcal media base) supplemented with Kellogg's solution 33 and sodium bicarbonate (0.042%). Escherichia coli was grown in LB broth or on LB agar supplemented with kanamycin (50 mg/ml), ampicillin (30 mg/ml), and 5-bromo-4-chloro-3-indolyl-β–galactopyranoside (35 mg/ml) when appropriate 34. The cloning vector used in this study was pK18UP 35.
Genetic Techniques.
E. coli transformations were performed using cells made competent by the CaCl2 procedure 34. N. gonorrhoeae transformations were performed using the spot transformation procedure of Gunn and Stein 36. Various derivatives of strain F62 were made by amplifying the region of interest using the PCR, and cloning the resulting amplicon into the cloning vector pK18UP. All primers used in this study are presented in Table . The region encoding lgtA-lgtE was amplified using the primers JL50 and JL51, giving pK18-F62LgtA-E; the region containing lgtG was amplified using the primers 196-230F and 196-140-R, giving plasmid pK18-lgtG; and the region containing lgtF-rfaK was amplified using primers RfaK-R and RfaK-F, giving rise to plasmid pK18-lgtFrfaK. Strain F62ΔlgtA was made by deleting a 239-bp ApoI fragment of Pk18-F62LgtA-E, and then using this deletion plasmid to introduce the deletion into the chromosome of strain F62 by the spot transformation procedure. Strain F62ΔlgtD was made using the primers lgtDfixF and lgtDfixR to change the polyguanine tract contained in lgtD of plasmid pK18lgtA-E to GGGAGGCGGAGGTGG, and then using this DNA to transform F62ΔlgtA. Positive transformants were identified by the acquisition of a unique NruI site, which had been introduced adjacent to the polyguanine tract. Strain F62-L1+ was made using the same strategy as above, except primers LgtCfixF and lgtCfixR were used to change the polyguanine tract contained in lgtC, such that it was no longer able to vary. Transformants of F62ΔlgtA were identified by their acquisition to mAb 1-17-L1 reactivity, when transformants were screened by colony blot analysis. Strain F62ΔlgtA-lgtG+ was made by transforming strain F62ΔlgtA with pK18-lgtG, with selection for transformants that had acquired reactivity with the mAb 3G9. A specific deletion was introduced into the lgtF gene by digesting pk18-lgtFrfaK with BsiWI and BsrGI, and deleting a 240-bp fragment from the coding sequence of lgtF. This plasmid was used to transform F62ΔlgtA. Transformants that failed to bind the mAb 2-1-L8 were identified. The validity of each of the strain constructions was demonstrated by PCR amplification of the region of interest, with verification that the amplicon now contained the desired DNA sequence alteration.
Table 1.
Primers Used to Amplify Various Gonococcal DNA Sequences
| Primers | DNA sequence | Accession no. |
|---|---|---|
| JL50 | CTGAATTCGGCCGACATCGCGCTTTTGGGCG | U15992 |
| JL51 | ATGGATCCGGGGCGATTTTACCTAGCAGATGAA | U15992 |
| RFAK-F | GGAGGATCCATGGAAAAAGAATTCAGGATA | U39810 |
| RFAK-R | GTTGGATCCAAGGCTTTCAGACGGC | U39810 |
| LgtDfixF | GGATCGCGAGAACCGATGCCGACGATATTGCCT | U15992 |
| LgtDfixR | GGTTCTCGCGATATATTCTCCACCTCCGCCACCCGACTTTGCCATTCGTCCAGCCCGAT | U15992 |
| LgtCfixF | TTACTCCGCGGAGGTGGTAATATCCGCTTTATAGACGTA | U15992 |
| LgtCfixR | TTACCCGCGGAGATTGGCGGCAACCGCCGCCCGGTT | U15992 |
| 196-230-F | CTGTGCATGCCATGTTTTCGGAGAGGACG | AF076919 |
| 196-1140-R | CCCGAGCTCAAAGGATAAAGGCAAAATGCC | AF076910 |
Electrophoresis and Immunoblotting.
Gonococcal LOS was purified from proteinase K–treated whole bacterial cell lysates by the procedure of Hitchcock and Brown 37. Approximately 0.2 μg of purified LOS was subjected to SDS-PAGE on 16% isocratic gels in a Tris-tricine buffer (0.025 M Tris, 0.100 M tricine, 0.1% SDS, pH 8.3) at a constant current of 30 mA/gel for ∼2 h at 10°C. Gels were fixed overnight in a solution containing 40% ethanol and 5% acetic acid. LOS was visualized by silver staining 38. For colony blot analysis, overnight colonies were transferred to a nitrocellulose membrane (Schleicher and Schuell) and screened for reactivity to the appropriate mAb 39. For Opa expression analysis, cells were suspended in saline to a turbidity of 100 Klett units (Green Filter), and 1 ml of solution was collected by centrifugation. The pellet was resuspended in 50 μl of lysing buffer, boiled for 10 min, and 3 μl was loaded onto duplicate 13% acrylamide gels. After electrophoresis, one gel was stained with Coomassie blue, and the other was subjected to Western blot analysis. Opa were detected by reacting the nitrocellulose filter with mAb 4B12, and visualizing the bound antibody by reacting the blot with goat anti–mouse horseradish peroxidase–labeled IgG.
Invasion and Adhesion Assay.
ME180 cells, a human cervical epidermal carcinoma cell line (HTB-33; American Type Culture Collection), were used for all adhesion and invasion assays. Cells were maintained in RPMI 1640 medium (Sigma Chemical Co.) containing 5% fetal bovine serum. The cells were seeded in 24-well plates (FALCON) at 2 × 105 cells/well, and cultured at 37°C in 5% CO2 for 24 h. The cells were infected with 106 bacterial CFU, and further incubated at 37°C in 5% CO2 for 6 h. After incubation, the cells were washed five times with PBS to remove nonadherent bacteria. To analyze the adhesion and invasion (i.e., total number of bacteria associated with cells), the infected cells were treated with 1% saponin (Sigma Chemical Co.) solution in PBS for 5 min at 37°C to lyse the cells and release the adherent and internalized bacteria. Appropriate dilutions of the cell lysate were plated on GCK agar to determine the number of viable bacteria. To measure invasion (the number of intracellular bacteria), cells were incubated with medium containing 100 μg/ml gentamicin (Sigma Chemical Co.) at 37°C in 5% CO2 for 2 h to kill extracellular gonococci. After washing with PBS, the cells were lysed, and the number of viable bacteria was determined as described above. In the experiments where the effect of cytochalasin D (Sigma Chemical Co.) was analyzed, the ME180 cells were pretreated with cytochalasin D in the medium at 37°C for 1 h, and then incubated with bacteria at 37°C for 6 h in the presence of cytochalasin D.
Immunofluorescence Staining.
ME180 cells (2 × 105) were seeded onto coverslips 24 h before inoculation. The cells were incubated with medium containing 107 CFU/ml of gonococci at 37°C in 5% CO2 for 3 h. After washing with PBS, the infected cells were fixed for 20 min in PBS containing 4% paraformaldehyde (Sigma Chemical Co.), and permeabilized by incubation with 0.1% Triton X-100 in PBS for 5 min. The coverslips were blotted with pilin-specific mAb 894 (Chemicon International), and a rhodamine-conjugated goat anti–mouse IgG antibody (Jackson ImmunoResearch Laboratories). The coverslips were stained with FITC-labeled phalloidin (Molecular Probes). The cells were analyzed using a confocal fluorescence microscope (model MRC-1024; Bio-Rad). Optical sections where cell-associated gonococci were visible were selected. Fluorescent images from these optical sections were collected. The rearrangement of actin filaments in gonococcal associated cells was scored positive when >90% of the actin filament staining (green) colocalized with gonococci staining (red), which showed as yellow.
Electron Microscopy.
A confluent monolayer of ME180 cells in a 100-mm plate was incubated with 107 CFU/ml of gonococci at 37°C in 5% CO2 for 6 h. After washing with PBS, the cells were removed from the plate with a rubber scraper. The cells were fixed with 2% glutaraldehyde, postfixed with 1% osmium tetroxide, dehydrated, infiltrated, and embedded in epoxy resin (EM Science) for thin sections. Thin sections of the cells were contrasted with uranyl acetate and lead citrate, and examined in a Zeiss EM10CA electron microscope.
Results
Characterization of LOS Molecules Made by Isogenic Strains of N. gonorrhoeae.
To characterize the types of LOS made by strain F62 and its various isogenic derivatives, proteinase K–treated whole bacterial cell lysates were analyzed on a 16% acrylamide gel, using the Tris-tricine buffering system 40. These data, presented in Fig. 1, indicate that each isogenic derivative of F62 produces a single predominant LOS structure. Using Western blot analysis, and mAbs that reacted specifically with different terminal sugar components of LOS, we defined the LOS structure expressed in each of the isogenic strains (data not shown). Fig. 2 shows the predicted chemical structures of the LOS isoforms made by each of these strains.
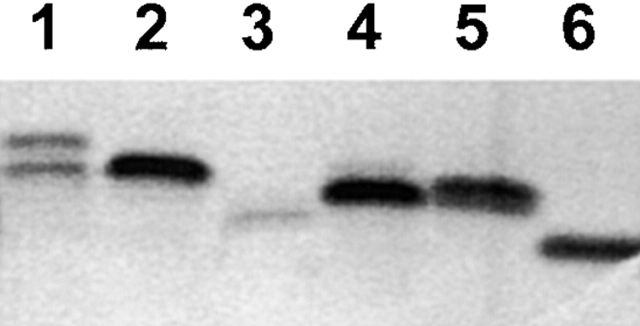
Figure 1.
SDS-PAGE analysis of LOS expressed by N. gonorrhoeae strain F62 and its derivatives. LOS was isolated by the method of Hitchcock and Brown (reference 37), and analyzed on 16% acrylamide gels using the Tris-tricine buffering system (reference 40). The lanes represent LOS isolated from strain: 1, F62; 2, F62ΔlgtD; 3, F62ΔlgtA; 4, F62ΔlgtAlgtG+; 5, F62ΔlgtAlgtC+; and 6, F62ΔlgtAΔlgtE.
Figure 2.
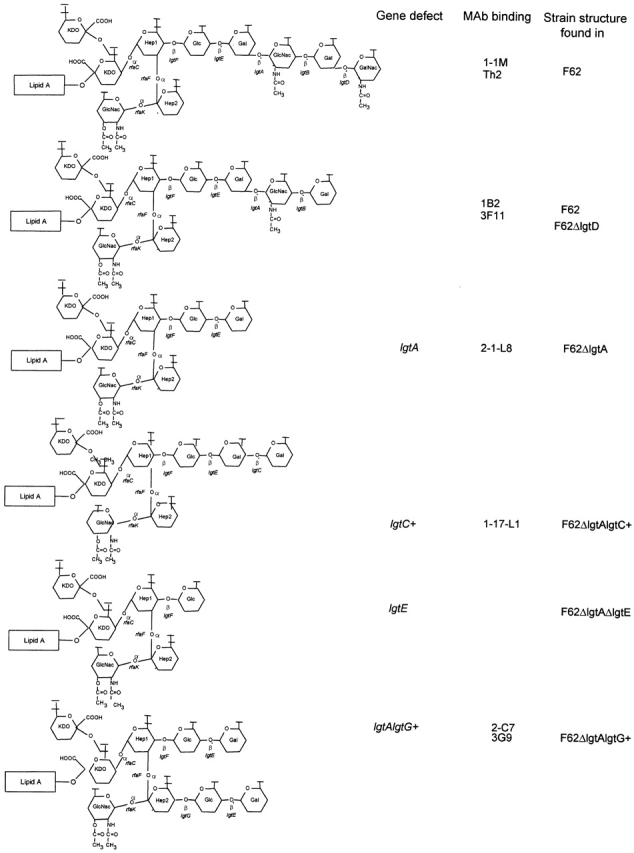
Predicted chemical structures of LOS made by strain F62 and its derivatives. Note that the four terminal sugars found on the structure shown that binds the mAb 1B2 is lacto-N-neotetraose.
Invasive Ability of Gonococcal Strains Expressing Distinct LOS Structures.
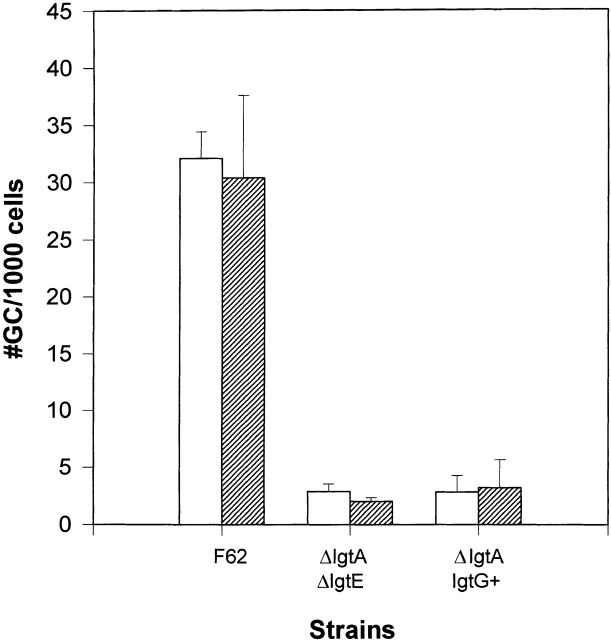
To investigate if LOS molecules are important for the infectivity of gonococci into host cells, we determined the adhesive and invasive abilities of isogenic gonococcal strains to the cultured human cervical epidermal carcinoma cell line, ME180. All gonococcal strains were derived from strain F62 and express genetically defined and invariant LOS structures on their surface. Strain F62 makes two predominant LOS components, the lacto-N-neotetraose LOS and the galNac-lacto-N-neotetraose LOS, in approximately equal abundance (25; Fig. 1). Piliated F62 cells were able to adhere to and invade into ME180 cells (Fig. 3). F62ΔlgtD, expressing only lacto-N–neotetraose LOS, was able to invade into ME180 cells at approximately the same frequency as F62 (Fig. 3 A). However, the invasive abilities of the strains whose LOS lacks the lacto-N-neotetraose structure were significantly less than that seen for F62 (Fig. 3 A). All strains expressing the variant LOS structures adhered to ME180 cells to the same extent as seen for F62 (Fig. 3 B). These results indicate that the structure of LOS expressed does not affect gonococcal adhesive capabilities, but the presence of the lacto-N-neotetraose increased gonococcal invasive abilities for ME180 cells.
Figure 3.
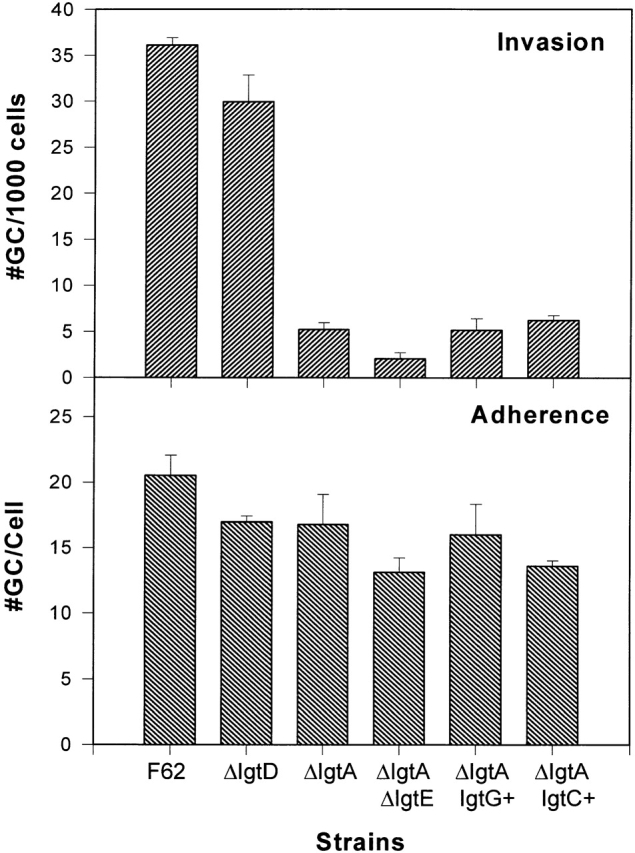
The adherence and invasion of the strain F62 and its isogenic derivatives to ME180 cells. ME180 cells (2 × 105 cells/well) were incubated with 106 CFU gonococci at 37°C for 6 h. The cells were treated or not treated with gentamicin for 2 h at 37°C. The cells were washed and lysed. Appropriate dilutions of the cell lysates were plated onto GCK agar plates. The gentamicin-resistant gonococci were counted as the internalized bacteria, and plotted as the number of gonococci (GC) per thousand cells (A). The numbers of viable gonococci from the cells untreated with gentamicin were counted as the total number of cell-associated gonococci, and plotted as the number of gonococci per cell (B). The results are the average of five independent experiments.
Opa Expression by F62 and Its Derivatives.
Previous reports 3 22 23 41 have shown that Opa expression can facilitate gonococcal invasion into host epithelial cells. It is possible that the loss of invasive capabilities of the LOS variants could be due to differences or variation in Opa expression, whereas Opa expression is enhancing and/or interfering with the invasion process. To examine this possibility, we screened our LOS variants for Opa expression using SDS-PAGE gels and Western blotting. These data, presented in Fig. 4, show the protein profile of whole bacterial cell lysates in the molecular weight region where Opa are expected to be found on an SDS-PAGE gel. The protein profiles of F62 and its derivatives did not show any significant differences (Fig. 4 A), and none of the proteins found in this region were capable of binding mAb 4B12 (Fig. 4 B), a mAb shown to react with all known Opa 42 43. This indicates that all strains used in this study do not express Opa at a detectable level, and the difference in invasive ability of the LOS variants was not caused by the differential expression of any Opa.
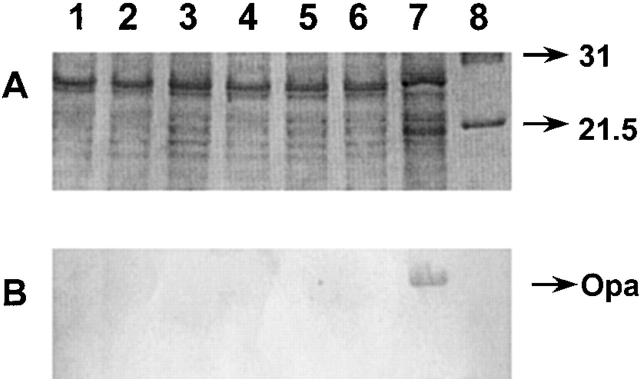
Figure 4.
SDS-PAGE analysis of Opa expression by various N. gonorrhoeae strains. Whole bacterial cell lysates were prepared by suspending bacteria in lysing buffer and boiling the extract for 10 min. An aliquot of the bacterial cell lysate was analyzed on a 13% acrylamide gel using the Tris-glycine buffering system (reference 34). (A) A photoreproduction of the Coomassie blue–stained gel; (B) a Western blot of an identical gel where the proteins were transferred to nitrocellulose and Opa was detected by incubation with the Opa-specific mAb 4B12 (references 42, 43). The lanes represent: 1, F62; 2, F62ΔlgtAlgtG+; 3, F62ΔlgtA; 4, F62ΔlgtD; 5, F62ΔlgtAΔlgtE; 6, F62ΔlgtAlgtC+; 7, PID2; and 8, molecular weight.
Effect of the Interaction of Gonococci with Epithelial Cells on Gonococcal Invasive Ability.
Because many of the surface components of N. gonorrhoeae undergo phase variation at high frequency, gonococci from the same culture preparation may express different combinations of these outer membrane surface components. It is possible that a small number of gonococci expressing the right combination of surface molecules might be preferentially internalized by host cells. As such, colonies arising from gonococci that had invaded would be enriched for these invasion-enhancing surface components. To test this hypothesis, we randomly chose several colonies, generated from the intracellular gonococci that survived the gentamicin treatment in the invasion assays, and compared their invasive abilities to the original stock of gonococci that had never had contact with the epithelial cells. In these reinvasion assays, we found that the gonococcal cells had invaded into ME180 cells and had a similar number of gentamicin-resistant bacteria as gonococcal cells that had never been incubated with ME180 cells (Fig. 5). Using Western blot, we examined the Opa expression of colonies derived from gonococcal cells that had been internalized by ME180 cells. Our results show that they remained Opa− (data not shown). This data indicates that the invasive phenotype and the Opa expression of F62 and the isogenic LOS-altered strains were not affected by their interaction with host cells. The invasive capability of gonococci reflects the invasive phenotype of the colony as a whole, and does not result from the individualities of bacteria that make up the colony.
Figure 5.
The intracellular gonococci invaded ME180 cells at a level similar to their original stock. 10 colonies were randomly selected from the gentamicin-resistant, internalized gonococci generated from the experiments described in the legend to Fig. 3. The invasive ability of these intracellular gonococci was determined in parallel with their original stock as described in the legend to Fig. 3. The gray bars are the average invasive ability of the 10 colonies derived from the intracellular gonococci, and the white bars are the average invasive ability of their original stock.
The Invasion of Gonococcal F62 Is Dependent on Pili.
To investigate the role of pili in the invasion of gonococcal F62 into host epithelial cells, piliated and nonpiliated gonococci were selected under a microscope based on the morphology of colonies. ME180 cells were incubated with piliated or nonpiliated gonococci for 6 h at 37°C, and the numbers of cell-associated bacteria and intracellular bacteria were determined. The adhesive and invasive abilities of nonpiliated F62 and its isogenic derivatives were all dramatically decreased compared with that of piliated ones (Fig. 6). Thus, losing pili reduced the adhesive ability of F62, and this loss of adherence was translated into a decrease in its invasive ability. This result indicates that the adherence and invasion of gonococcal F62 is pili dependent, and that pili play an important role in the adherence step of the invasion process.
Figure 6.
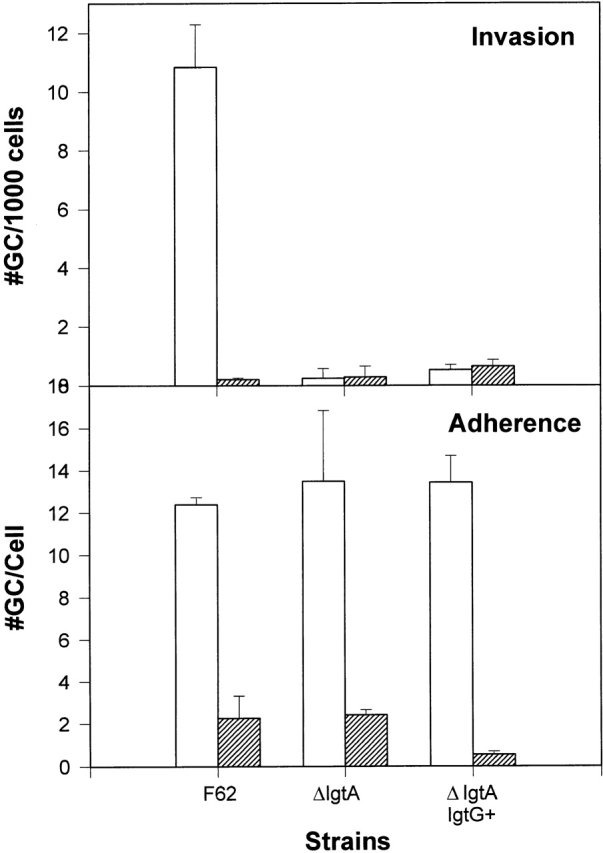
The role of pili on adherence and invasion of strain F62 and its derivatives to ME180 cells. Piliated and nonpiliated gonococci were selected under a microscope based on the morphology of colonies. The invasion assays were carried out as described in the legend to Fig. 3. White bars represent data obtained using piliated gonococci, and gray bars represent data generated using nonpiliated gonococci. Shown are a set of typical results obtained from three independent experiments.
Induction of the Rearrangement of Actin Filaments in Epithelial Cells.
The contact of gonococci with epithelial cells induces the rearrangement of actin filaments in host epithelial cells. This phenomenon has been reported in both epithelial cell lines 14 and in primary cultures of human urethral epithelium 9. Cytochalasin D, an actin filament–disrupting agent, inhibits gonococcal invasion into epithelial cells 23. To test if the internalization of gonococcal F62 also depends on actin filaments, we treated ME180 cells with different concentrations of cytochalasin D before and during the gonococcal inoculation. In the presence of cytochalasin D (at concentrations as low as 10 μM), the invasive ability of F62 was dramatically decreased (data not shown). The number of intracellular F62ΔlgtA observed remained at a low level. This indicates that efficient invasion by F62 is dependent on actin filaments in the ME180 cells.
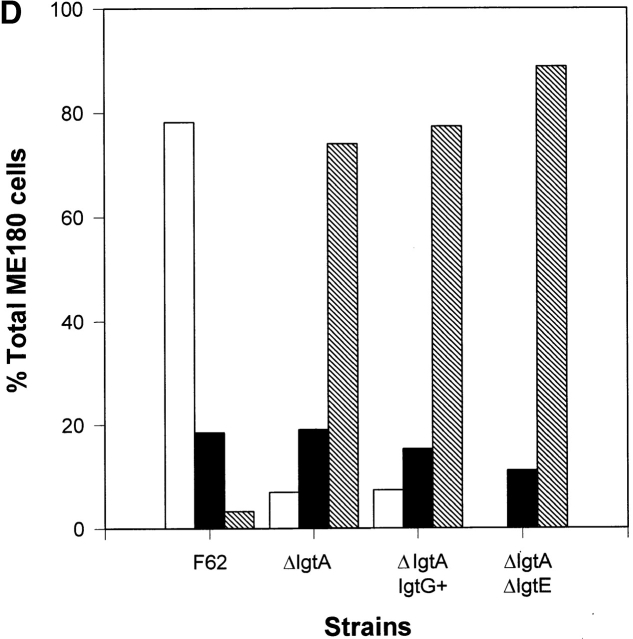
We used confocal fluorescence microscopy to analyze the intracellular distribution of actin filaments in ME180 cells infected with F62 or its isogenic derivatives. Epithelial cells were grown on glass coverslips and incubated with gonococci for 3 h. After fixation and permeabilization, gonococci were visualized with gonococcal pilin–specific mAb 894 and a rhodamine-labeled secondary antibody, and actin filaments were stained with FITC-conjugated phalloidin. In the untreated cells, cortical actin filaments were evenly distributed under the plasma membrane (Fig. 7 A). In >95% of the epithelial cells that associated with F62, the intracellular distribution of the cortical actin filaments was dramatically changed. The actin filaments (green) were no longer evenly distributed under the plasma membrane, but preferentially accumulated under the area of the plasma membrane where gonococci (red) adhered (Fig. 7B and Fig. d). The colocalization of actin filaments and gonococci was indicated as yellow. In contrast, in >80% of the cells associated with the F62 derivatives lacking the lacto-N-neotetraose LOS, the intracellular distribution of actin filaments appeared similar to the untreated cells, even though adherent bacteria on the surface of the cells were clearly visible (Fig. 7C and Fig. d). Some of these gonococci appeared to colocalize with the local actin filaments. These results indicate that LOS is involved in the induction of actin filament rearrangement, and that in the absence of Opa, the lacto-N-neotetraose LOS is essential for this induction.
Figure 7.
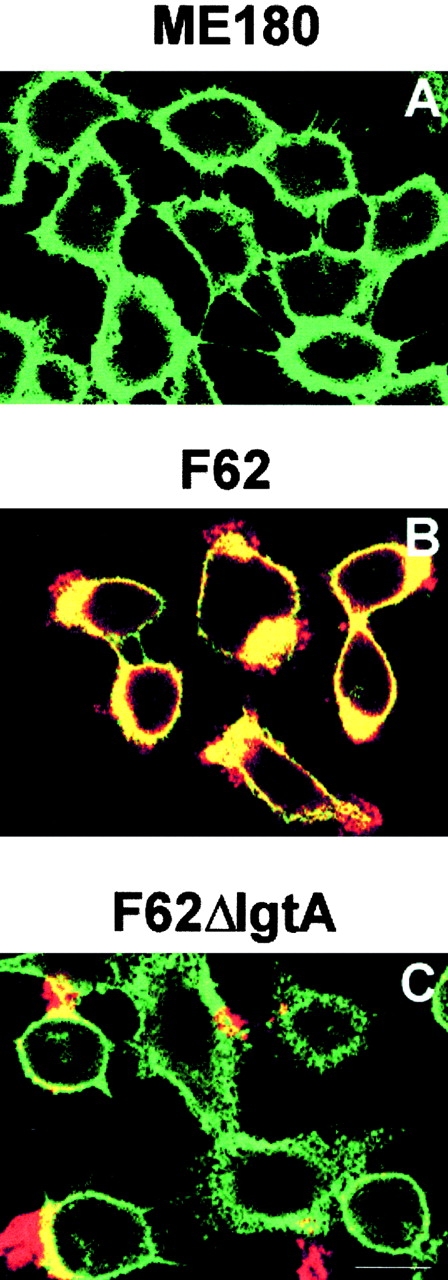
The rearrangement of actin filaments in ME180 induced by gonococci. ME180 cells, grown on coverslips, were incubated with 107 CFU/ml of gonococci at 37°C for 3 h. After washing, fixation, and permeabilization, gonococci were visualized with pilin-specific mAb and a rhodamine-conjugated goat anti–mouse IgG antibody (red). Actin filaments were stained with FITC-labeled phalloidin (green). The cells were analyzed using a confocal fluorescence microscope. The images were taken from the optical sections where epithelial cell–associated gonococci were visible. Shown are the representative images: (A) untreated cells; (B) cells inoculated with the F62; (C) cells inoculated with F62ΔlgtA. (D) The quantitative analysis of images randomly taken from the epithelial cells inoculated by gonococci. For each strain of gonococci, >10 images from 2 or 3 independent experiments were analyzed. White bars represent the cells where almost of all visible actin filaments were accumulated under gonococcal adherent sites. The colocalization of actin filaments and gonococci are shown as yellow. Black bars represent the cells where only a small amount of actin filaments was rearranged. Gray bars represent the cells where the rearrangement of actin filaments was not detected. These data were plotted as the percentage of total number of epithelial cells associated with gonococci. Bar, 12 μm.
Ultrastructural Analysis of ME180 Cells Infected with F62 and Its Isogenic LOS-altered Derivatives.
To understand why gonococcal strains lacking lacto-N-neotetraose LOS structures were unable to invade ME180 cells as effectively as those expressing this LOS, we analyzed the interaction between ME180 cells and gonococci through electron microscopy. Gonococci were incubated with ME180 cells for 6 h, washed, fixed, and processed for electron microscopy. Most (69%) of the F62 cells were found adhering to the tip of microvilli. A significant number (29%) of the organisms had built intimate contact with the plasma membrane of epithelial cells and induced dramatic morphological changes on the surface of the epithelial cells (Fig. 8A–C, and Table ). Some microvilli were elongated to extraordinary lengths to form raffle-like structures that wrapped around gonococci (Fig. 8B and Fig. C). Extensive interactions between the blebbing outer membrane of gonococci and the plasma membrane of the epithelial cells were observed (Fig. 8B and Fig. C). In each wrapping pocket, only one or a few gonococci were found (Fig. 8 C). A small number (9%) of the organisms were found inside the cells (Table ). The interaction between the outer membrane of these intracellular bacteria and host cell membrane was so tight and extensive that it was very hard to distinguish the outer membrane of bacteria from host cell membrane (Fig. 8 D).
Figure 8.
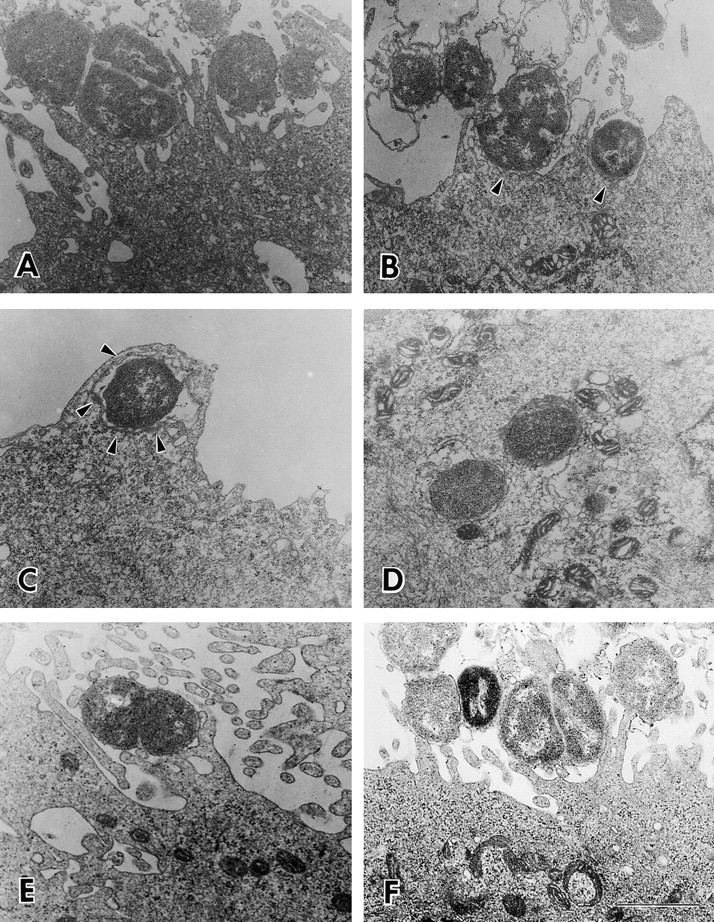
Ultrastructural analysis of ME180 cells infected with gonococci. ME180 cells were inoculated with 107 CFU/ml of gonococci at 37°C for 6 h. The cells were washed, fixed, and processed for electron microscopy. Thin sections (60 nm) were contrasted with uranyl acetate and lead citrate, then analyzed. Shown are representative images. (A–D) The cell inoculated with F62; (E) the cell inoculated with F62ΔlgtA; (F) the cell inoculated with F62ΔlgtAlgtG. Arrows indicate the intimate interaction between gonococci and the plasma membrane of epithelial cells. Bar, 0.5 μm.
Table 2.
Quantitative Analysis of Gonococcal Distribution in ME180 Cells
| Strains | F62 | F62ΔlgtA |
|---|---|---|
| Loosely associated | 69 | 73 |
| Microvilli associated | 2 | 27 |
| Intimately associated | 20 | 0 |
| Internalized | 9 | 0 |
Experiments were carried out as described in the legend to Fig. 8. Electron microscopy images were randomly taken from sections generated from two individual experiments. All gonococci visible in the images were counted and divided into four catagories as indicated. The total number of gonococci analyzed was ∼100. The data were expressed as the percentage of the total number of gonococci counted.
In contrast to what was seen with strain F62, almost all the LOS variants lacking lacto-N-neotetraose LOS adhered to the tip of the microvilli (Table and Fig. 8E and Fig. F). The microvilli contacted by the gonococci appeared to be elongated, but not in the process forming extended raffle-like structures. Occasionally, the organisms seemed to pull the microvilli to themselves. Very few of the LOS variants were found to intimately interact with ME180 cells.
Discussion
Gonococcal LOS is an important virulence factor. The gonococcus has evolved complex regulatory mechanisms that allow for the variable expression of LOS. The identification of the genes involved in its biosynthesis and the mechanisms that regulate their expression 16 18 20 28 have allowed us to genetically manipulate the expression of genes involved in LOS biosynthesis, resulting in the production of a series of isogenic strains derived from N. gonorrhoeae F62. Each of these strains expressed a distinct, invariant, genetically defined LOS structure. The LOS structures that we chose to study represent those that are expressed during natural infection and/or are seen when the gonococcus is grown under nonselective conditions in the laboratory. By using these Opa−Pil+ isogenic strains, we were able to define a role for LOS in the infection process. Our results showed that gonococcal strains expressing LOS lacking an intact lacto-N-neotetraose had significantly lower invasive ability than gonococcal strains expressing lacto-N-neotetraose LOS, even though all of the isogenic strains used in this study adhered to the epithelial cell at a level similar to F62. These data demonstrate that lacto-N-neotetraose LOS promotes gonococcal invasion, but not adherence to ME180 cells. This is consistent with the early studies using human challenge models, where Schneider et al. 1 showed that N. gonorrhoeae MS11mkC (Opa−Pil+) expressing LOS terminating with lacto-N-neotetraose was more infectious than MS11mkA (Opa−Pil+) expressing LOS lacking the lacto-N-neotetraose structure. It should be noted that while strains expressing the lacto-N-neotetraose LOS were able to promote invasion at significantly higher levels than strains that lacked this structure, the level of invasion seen is significantly less than that reported by others for Opa-mediated invasion 23 44.
For the gonococcus to enter an ME180 cell, it first adheres at the tip of microvilli, then forms an intimate association with the plasma membrane of host epithelial cells where actin filaments are reorganized and microvilli are extended to reach and wrap around gonococci. Finally, the gonococci are internalized. Each of these steps is a multifactorial process, whose efficiency can be dramatically altered depending on the type(s) of surface molecules expressed. For strain F62, the initial adherence process is mediated by pili, as nonpiliated F62 cells adhered poorly to the ME180 cells.
Several groups have demonstrated that Opa can play a major role in the formation of intimate association between the plasma membrane of the host cell and the gonococcus. These associations seem to be mediated by specific Opa–eukaryotic cell surface receptor interactions 3 4 5 6 11 12. Since LOS has been shown to be capable of binding to a specific eukaryotic cell surface receptor 32, we wished to determine if LOS could mediate the establishment of intimate associations in the absence of Opa. To understand the role of LOS in this process, we examined the behavior of ME180 cells inoculated with Opa−Pil+ gonococcal strains expressing different LOS structures. Using electron microscopy and immunofluorescence microscopy, we showed that Opa−Pil+ F62, and its LOS-altered derivatives, were able to establish the initial adherence at the tips of microvilli. For F62, the invasion process progressed where this strain then established an intimate interaction with the host cell's plasma membrane. These interactions resulted in the internalization of the bacteria. However, derivatives of F62 lacking the lacto-N-neotetraose LOS structure failed to induce the rearrangement of actin filament and the further morphological changes of the plasma membrane. Furthermore, they were unable to establish the intimate interaction with the plasma membrane of host epithelial cells, and did not enter the host cells as efficiently as strains expressing lacto-N-neotetraose LOS. These data indicate that it is possible to induce actin filament rearrangement and membrane morphological changes in host epithelial cells in the absence of Opa. Our data suggest that the lacto-N-neotetraose structure is responsible for the induction of these events.
These morphological data support our conclusion drawn from our invasion analysis that the lacto-N-neotetraose LOS promotes gonococcal invasion but not adherence to ME180 cells. In the absence of the terminal lacto-N-neotetraose structure, the invasion of Opa−Pil+ gonococci was retained at the first step of the invasion process, the initial adherence. Gonococci that could not stimulate actin filament rearrangement failed to form an intimate association and the induction of the extension of microvilli in host cells. How gonococcal LOS induces these events in host epithelial cells is still unknown. An attractive model is LOS's interactions with specific receptors on the surface of host epithelial cells to initiate these events. Using N. gonorrhoeae 1291 which expresses the lacto-N-neotetraose, Porat et al. 32 identified the asialoglycoprotein receptor and a 70-kD unknown protein as the specific binding sites on the surface of hepatocytes. The asialoglycoprotein receptor is expressed in human urethral epithelial cells 11 13. It will be interesting to see if the asialoglycoprotein receptor serves as a binding site for LOS on the surface of ME180 cells, and to determine what cellular events are initiated by the binding of LOS to these receptors. Our findings are of particular interest as they suggest that host cell invasion occurs when a gonococcal surface molecule interacts with a host cell surface receptor. This interaction is likely mediated by high-density receptor–ligand interactions, as piliated gonococci, in the absence of an additional receptor–ligand (LOS or Opa) interaction, fail to invade ME180 cells.
The induction of the morphological changes on the surface of epithelial cells to be engulfed is not unique to F62 strains. Expression of both Opa and pili were required for the activation of microvilli in HEC-1-B cells when strains FA1090 and MS11 were used 24. Our data show that in the absence of Opa, lacto-N-neotetraose LOS is not required for the initial morphological changes in host epithelial cells, but is essential to promote the further extension of the plasma membrane and to establish the intimate and stable interaction with host epithelial cells.
In this study, we did not address the role of Opa in gonococcal invasion. However, we noticed that the invasive ability of Opa−Pil+ F62 used in this study was lower than that of Opa+Pil+ F62 reported previously 45. It is not known whether the expression of an invasive Opa would increase the invasive ability of F62 and its derivatives lacking lacto-N-neotetraose LOS. However, van Putten et al. 46 showed that a galE mutation, which disrupts the addition of terminal sugars to LOS molecules, had no significant effect on the invasion of gonococci expressing invasive Opa. Several studies 9 14 have shown that Opa promotes the rearrangement of actin filaments and the morphological change of the plasma membrane of host epithelial cells, although the LOS structures of the gonococci used in these studies were unknown. The ability of Opa to promote actin rearrangement, independent of LOS, was clearly demonstrated in invasion studies where gonococcal Opa were expressed in E. coli 47. Combining all of these results, we have concluded that the invasive Opa and lacto-N-neotetraose LOS should play an additive role in the invasion process.
Recent experiments have suggested a role of pili in the invasion process distinct from their role in adhesion (for a review, see reference 48). Merz et al. 49 implicated pili in host cell invasion. However, the level of pilin-mediated invasion seen in this report was much lower than what we have shown for F62 (Opa−Pil+) and is similar to the levels we report for F62 strains lacking the lacto-N-neotetroase structure. This suggests that the role of pili in the invasion process is much more important in initiating adherence, rather than facilitating gonococcal entry into host cells.
It is not clear if Opa-mediated cell entry, LOS-mediated cell entry, and Opa- and LOS-independent cell entry occur via the same pathway. However, the following can be considered as a general model for gonococcal invasion. The presence of pili allows the gonococcus to form a casual interaction with host cells. This initial interaction overcomes the electrostatic repulsion that should occur between the two cells. The interaction of LOS and/or Opa with a specific host cell surface receptor allows the gonococcus to form intimate interactions with the host cell. This interaction results in the localized deformation of eukaryotic cell membrane, and stimulates the host cell to rearrange its intracellular actin cytoskeleton. This rearrangement generates further alterations in the host cell membrane, resulting in the internalization of gonococci. Sialylation of gonococcal LOS reduces the infectivity of a strain in vivo, and significantly lessens a strain invasive ability in vitro 50 51, because the presence of neuraminic acid on gonococcal LOS interferes with receptor-specific binding on host cell surface 32. Taken together, all of these results help to explain the variability of clinical outcomes seen in gonococcal disease. Individuals infected with organisms expressing invasion-promoting Opa and/or LOS would be at the highest risk for invasive disease. However, the expression of noninvasion-promoting Opa and/or LOS structures is likely necessary for transcytosis and/or intracellular survival. This provides a reason for the need to vary the expression of Opa and LOS.
Acknowledgments
This work was supported by grants AI24452 and AI01470 from the National Institutes of Health to D.C. Stein.
Footnotes
Abbreviations used in this paper: LOS, lipooligosaccharide; Opa, gonococcal opacity-associated outer membrane protein(s); Pil, gonococcal pilus.
References
- Schneider H., Cross A.S., Kuschner R.A., Taylor D.N., Sadoff J.C., Boslego J.W., Deal C.D. Experimental human gonococcal urethritis250 Neisseria gonorrhoeae MS11mkC are infective. J. Infect. Dis. 1995;172:180–185. doi: 10.1093/infdis/172.1.180. [DOI] [PubMed] [Google Scholar]
- Shaw J.H., Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae . Infect. Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbeser L.S., Ajioka R.S., Merz A.J., Puaoi D., Lin L., Thomas M., So M. The opaH locus of Neisseria gonorrhoeae MS11A is involved in epithelial cell invasion. Mol. Microbiol. 1994;13:919–928. doi: 10.1111/j.1365-2958.1994.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Gray-Owen S.D., Dehio C., Haude A., Grunert F., Meyer T.F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten J.P., Paul S.M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Makepeace K., Ferguson D.J., Watt S.M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic Neisseriae . Mol. Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- Metz S.A., Kowluru A., Seavey S.E. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CD42. J. Clin. Invest. 1996;98:540–555. doi: 10.1172/JCI118822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C., Eugene E., de Saint Martin L., Nassif X. Interaction of Neisseria meningitidis with a polarized monolayer of epithelial cells. Infect. Immun. 1997;65:4836–4842. doi: 10.1128/iai.65.11.4836-4842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina P.C., Williams R., Lubaroff D., Apicella M.A. Neisseria gonorrhoeae induces focal polymerization of actin in primary human urethral epithelium. Infect. Immun. 1998;66:3416–3419. doi: 10.1128/iai.66.7.3416-3419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M.E., Watt P.J. Adherence of Neisseria gonorrhoeae to urethral mucosal cellsan electron microscopic study of human gonorrhea. J. Infect. Dis. 1972;126:601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- Apicella M.A., Ketterer M., Lee F.K., Zhou D., Rice P.A., Blake M.S. The pathogenesis of gonococcal urethritis in menconfocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae . J. Infect. Dis. 1996;173:636–646. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- Mosleh I.M., Boxberger H.J., Sessler M.J., Meyer T.F. Experimental infection of native human urethral tissue with Neisseria gonorrhoeaeadhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect. Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey H.A., Ketterer M.R., Preston A., Lubaroff D., Williams R., Apicella M.A. Ultrastructural analysis of primary human urethral epithelial cultures infected with Neisseria gonorrhoeae . Infect. Immun. 1997;65:2420–2427. doi: 10.1128/iai.65.6.2420-2427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassme H.U., Ireland R.M., van Putten J.P. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect. Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.F. Pathogenic Neisseria—interplay between pro- and eukaryotic worlds. Folia Microbiol. 1998;43:311–319. doi: 10.1007/BF02818617. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Wang R., Uljon S., Rice P.A., Gotschlich E.C., Stein D.C. Identification of the gene (lgtG) encoding the lipooligosaccharide beta chain synthesizing glucosyl transferase from Neisseria gonorrhoeae . Proc. Natl. Acad. Sci. USA. 1998;95:10872–10877. doi: 10.1073/pnas.95.18.10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell T.D., Black W.J., Kawula T.H., Barritt D.S., Dempsey J.A., Kverneland K., Jr., Stephenson A., Schepart B.S., Murphy G.L., Cannon J.G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol. Microbiol. 1988;2:227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Danaher R.J., Levin J.C., Arking D., Burch C.L., Sandlin R., Stein D.C. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J. Bacteriol. 1995;177:7275–7279. doi: 10.1128/jb.177.24.7275-7279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C.P., Reimann B.Y., Schultz E., Kaufmann A., Haas R., Meyer T.F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989;338:651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- Gotschlich E.C. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z.A., Stephens D.S., Hoffman L.H., Schlech W.F., III, Horn R.G. Mechanisms of mucosal invasion by pathogenic Neisseria . Rev. Infect. Dis. 1983;5:S708–S714. doi: 10.1093/clinids/5.supplement_4.s708. [DOI] [PubMed] [Google Scholar]
- Kupsch E.M., Knepper B., Kuroki T., Heuer I., Meyer T.F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., van Putten J.P., Meyer T.F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J.M., Lammel C.J., Wang J., Dekker N.P., Brooks G.F. Neisseria gonorrhoeae coordinately uses pili and opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci. Infect. Immun. 1999;67:3469–3480. doi: 10.1128/iai.67.7.3469-3480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R., Bacon B.E., Nasholds W., Schneider H., Griffiss J.M. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods Biochemistry 30 1991. 10566 10575[published erratum at 31:316] [DOI] [PubMed] [Google Scholar]
- Mandrell R.E., Griffiss J.M., Macher B.A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes J. Exp. Med. 168 1988. 107 126[published erratum at 168:1517] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hammack C.A., Apicella M.A., Griffiss J.M. Instability of expression of lipooligosaccharides and their epitopes in Neisseria gonorrhoeae . Infect. Immun. 1988;56:942–946. doi: 10.1128/iai.56.4.942-946.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch C.L., Danaher R.J., Stein D.C. Antigenic variation in Neisseria gonorrhoeaeproduction of multiple lipooligosaccharides. J. Bacteriol. 1997;179:982–986. doi: 10.1128/jb.179.3.982-986.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J.M., Boslego J.W., Hitchcock P.J., McJunkin K.O., Apicella M.A. Expression of paragloboside-like lipooligosaccharide may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 1991;174:1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.S., Cannon J.G. Human experimentation with Neisseria gonorrhoeaeprogress and goals. J. Infect. Dis. 1999;179:S375–S379. doi: 10.1086/513847. [DOI] [PubMed] [Google Scholar]
- Wang, J.F., H. Schneider, and J.M. Griffiss. 1996. Neisseria gonorrhoeae must express the paraglobosyl LOS in order to invade human genitourinary epithelial cells. 10th International Pathogenic Neisseria Conference. Baltimore. 112–113.
- Porat N., Apicella M., Blake M.S. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect. Immun. 1995;63:1498–1506. doi: 10.1128/iai.63.4.1498-1506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.S., Peacock W.L., Deacon W.E., Brown L., Pirkle C.I. Neisseria gonorrhoeae I. Virulence genetically linked to clonal variation. J. Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Sandlin R.C., Apicella M.A., Stein D.C. Cloning of a gonococcal DNA sequence that complements the lipooligosaccharide defects of Neisseria gonorrhoeae 1291d and 1291e . Infect. Immun. 1993;61:3360–3368. doi: 10.1128/iai.61.8.3360-3368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J.S., Stein D.C. Use of a non-selectable transformation technique to construct a multiple restriction modification deficient mutant of Neisseria gonorrhoeae . Mol. Gen. Genet. 1996;251:509–517. doi: 10.1007/BF02173639. [DOI] [PubMed] [Google Scholar]
- Hitchcock P.J., Brown T.M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver stained polyacrylamide gels. J. Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.M., Frasch C.E. A sensitive silver stain for detecting lipooligosaccharide in polyacrylamide gels. Anal. Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Petricoin E.F., III, Stein D.C. Molecular analysis of lipooligosaccharide biosynthesis in Neisseria gonorrhoeae . Infect. Immun. 1989;57:2847–2852. doi: 10.1128/iai.57.9.2847-2852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Chen T., Grunert F., Medina-Marino A., Gotschlich E.C. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Neibert M., Crowe B.A., Strittmatter W., Kusecek B., Weyse E., Walsh M.J., Slawig B., Morelli G., Moll A. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J. Exp. Med. 1988;168:507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A.E., Cohen M.S., Drown P.M., Whicker L.G., Isbey S.F., Seifert H.S., Cannon J.G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Gotschlich E.C. Interactions of gonococci with HeLa cellsattachment, detachment, replication, penetration, and the role of protein II. Infect. Immun. 1986;54:154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Putten J.M., Duensing T.D., Carlson J. Gonococcal invasion of epithelial cells driven by PIAa bacterial ion channel with GTP binding properties. J. Exp. Med. 1998;188:941–952. doi: 10.1084/jem.188.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten J.P., Grassme H.U., Robertson B.D., Schwan E.T. Function of lipopolysaccharide in the invasion of Neisseria gonorrhoeae into human mucosal cells. Prog. Clin. Biol. Res. 1995;392:49–58. [PubMed] [Google Scholar]
- Simon D., Rest R.F. Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial epithelial cell lines. Proc. Natl. Acad. Sci. USA. 1992;89:5512–5516. doi: 10.1073/pnas.89.12.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X., So M. Interaction of pathogenic Neisseriae with nonphagocytic cells. Clin. Microbiol. Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz A.J., Enns C.A., So M. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 1999;32:1316–1332. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- Schneider H., Schmidt K.A., Skillman D.R., Van De Verg L., Warren R.L., Wylie H.J., Sadoff J.C., Deal C.D., Cross A.S. Sialylation lessens the infectivity of Neisseria gonorrhoeae MS11mkC. J. Infect. Dis. 1996;173:1422–1427. doi: 10.1093/infdis/173.6.1422. [DOI] [PubMed] [Google Scholar]
- van Putten J.P. Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae . EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:4043–4051. doi: 10.1002/j.1460-2075.1993.tb06088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]