Abstract
The specificity of immunoglobulins and α/β T cell receptors (TCRs) provides a framework for the molecular basis of antigen recognition. Yet, evolution has preserved a separate lineage of γ/δ antigen receptors that share characteristics of both immunoglobulins and α/β TCRs but whose antigens remain poorly understood. We now show that T cells of the major tissue γ/δ T cell subset recognize nonpolymorphic CD1c molecules. These T cells proliferated in response to CD1+ presenter cells, lysed CD1c+ targets, and released T helper type 1 (Th1) cytokines. The CD1c-reactive γ/δ T cells were cytotoxic and used both perforin- and Fas-mediated cytotoxicity. Moreover, they produced granulysin, an important antimicrobial protein. Recognition of CD1c was TCR mediated, as recognition was transferred by transfection of the γ/δ TCR. Importantly, all CD1c-reactive γ/δ T cells express Vδ1 TCRs, the TCR expressed by most tissue γ/δ T cells. Recognition by this tissue pool of γ/δ T cells provides the human immune system with the capacity to respond rapidly to nonpolymorphic molecules on professional antigen presenting cells (APCs) in the absence of foreign antigens that may activate or eliminate the APCs. The presence of bactericidal granulysin suggests these cells may directly mediate host defense even before foreign antigen-specific T cells have differentiated.
Keywords: T lymphocytes, T cell antigen receptors γ/δ, CD1, cytolysis, granulysin
Introduction
Studies in mice and humans have implicated γ/δ T cells in host defense. For example, γ/δ T cells modulate the severity of disease or mediate a component of protective immunity in murine models of listeriosis 1, tuberculosis 2 3 4, malaria 5, and HSV-1 encephalitis 6. γ/δ T cells are critically important in preventing death from airway infection with Nocardia asteroides 7. In humans, large expansions of γ/δ T cells during infections suggest their importance. γ/δ T cells increase from normal levels of 4% of all circulating T cells to a mean of 12, 14, 29, and 57% of all circulating T cells during infection with Listeria monocytogenes, Mycobacterium tuberculosis, Brucella melitensis, and Ehrlichia chaffeensis, respectively 8 9 10 11. Despite the growing list of experimental models and human diseases with γ/δ T cell expansions, only a few examples of well-characterized antigen-specific γ/δ T cells have been noted, and these do not support MHC-restricted peptide-specific recognition 12 13. Instead, in mice, γ/δ T cell recognition of an intact (unprocessed) cell surface glycoprotein of HSV was noted in one case, whereas in other cases, alloreactive γ/δ T cell recognition of TL and I-E was not dependent on bound peptides 14 15 16. These studies, together with analysis of CDR3 length distribution of the TCR δ chain 17 and the structure of a Vδ domain revealing shared features with both immunoglobulins and α/β TCRs 18, led to the suggestion that, in some cases, γ/δ recognition may be more like that of immunoglobulins than of α/β TCRs.
Major advances in the study of antigen recognition by human γ/δ T cells were made with the discovery that most circulating human γ/δ T cells that bear Vγ2/Vδ2 (Vγ2 is also termed Vγ9) TCRs could be stimulated by nonpeptide antigens (for a review, see reference 19) derived from Mycobacteria 20 21 22 23 24. These nonpeptide antigens were identified as isopentenyl pyrophosphate (IPP) and related prenyl pyrophosphate molecules 24 25 and the more recently characterized alkylamine antigens 26. Both the phosphate and the amine antigens are small molecules consisting of short (typically one to five carbons) straight or branched aliphatic chains and either a phosphate or an amine moiety. They are important products of microbes as well as self-antigens. The mechanism by which these antigens are presented is not known, but it does not involve the known MHC class I or MHC class II peptide antigen-presenting molecules 23 27 28, and we have suggested they may be recognized much as haptens are recognized by either immunoglobulins or TCRs 23 29. Namely, their requirement for an antigen-presenting element is unclear, but their recognition is critically dependent on the CDR3 sequence of the γ/δ TCR 29 30. The recognition of these small aliphatic phosphate and amine organic molecules was exclusively found among γ/δ T cells of the Vδ2 subset.
In contrast to Vγ2/Vδ2+ T cells that are the major circulating pool of γ/δ T cells, human γ/δ T cells bearing Vδ1-encoded TCRs account for the vast majority of γ/δ T cells in tissues such as intestine and spleen 31. Yet, little is known about the antigen reactivity of these tissue γ/δ T cells. Recently, examples of γ/δ T cells in this subset were found to recognize the MHC-encoded proteins, MICA and MICB 32. Recognition was through the activating NKG2D C-type lectin 33 with an unclear contribution from the γ/δ TCR. MICA and MICB class I molecules identify stressed cells and have a very restricted pattern of expression primarily limited to the intestine. MICA and MICB probably do not present peptides, as they have a limited peptide binding groove 34. Instead, these molecules may function in innate immunity as important targets for Vδ1+ γ/δ T cell killing of stressed cells 32.
Here, we provide evidence that an important TCR-mediated reactivity of tissue γ/δ T cells is against CD1 molecules. Remarkably, all of the Vδ1 cells studied were focused specifically on CD1c, one member of a family of nonpolymorphic CD1 molecules, expressed exclusively on APCs, that present lipid and glycolipid foreign antigens to T cells 35. However, the γ/δ T cell lines and clones showed highly specific, direct reactivity to CD1c proteins that was not dependent on the presence of an exogenous foreign antigen. These CD1c-specific γ/δ T cells produced inflammatory cytokines, killed CD1c-bearing targets, and contained bactericidal granulysin.
Materials and Methods
mAbs.
The following mAbs were used for flow cytometry and blocking experiments: P3 (IgG control) 22, SPV-T3b (anti-CD3) 36, anti–TCR-δ1 (pan anti-Cδ) 37, δTCS1 (anti-Vδ1/Jδ1) 38, TiγA (anti-Vγ2) 39 40, 9.3 (anti-CD28) 41, OKT4 (anti-CD4; American Type Culture Collection), OKT8 (anti-CD8α; American Type Culture Collection), DX1 (anti–NKR-P1A; provided by Dr. L. Lanier, DNAX, Palo Alto, CA), BMAO31 (pan anti–TCR-α/β; provided by Dr. R.G. Kurrle, Boehringwerke, Marburg, Germany), 7C6 (anti-CD1c) 42, F10/21A3 (anti-CD1c) 42a, BCD1b3.2 (anti-CD1b) 43, 10H3.9.3 (anti-CD1a) 44, W6/32 (anti–MHC class I; American Type Culture Collection), L243 (anti–HLA-DR; American Type Culture Collection), NS4.1 (IgM control; American Type Culture Collection), 4A11 (anti-Vγ1.4) 45, CD95 Fas ZB4 clone (anti-Fas; Immunotech), δG9 (antiperforin; Ancell), DH2 (antigranulysin) 46, and MPC11 (IgG2b control; American Type Culture Collection).
Immunofluorescence Analysis.
Cells were incubated with mouse mAbs on ice for 30 min, washed, and stained with FITC-conjugated F(ab′)2 goat anti–mouse Ig (Tago) for an additional 30 min on ice. After washing, the cells were resuspended in propidium iodide and analyzed by flow cytometry (FACSort™; Becton Dickinson). Results were expressed as percentage of positive cells compared with negative cells stained with isotype-matched control mAbs.
T Cell Lines and Clones.
Lymphocytes were isolated from the blood of random healthy donors by Ficoll-Hypaque centrifugation. γ/δ T cells were enriched by staining with the anti–TCR-α/β mAb, BMAO31, followed by depletion of α/β T cells with magnetic beads coated with goat anti–mouse IgG (Dynal). T cell lines JR.2 and XV.1 were established from two separate donors by culturing 1 × 106 freshly isolated γ/δ T cells in 1-ml culture wells with 1 × 106 irradiated (5,000 rads) autologous CD1+ monocyte-derived dendritic cells as APCs and the organic phase of a chloroform/methanol/water (2:1:1) extract of M. tuberculosis prepared as described 47 at 1:5,000 final dilution. After 2 wk of culture, viable cells were recovered and the residual α/β T cells were depleted with BMAO31 mAb and magnetic beads (Dynal). The resulting population was restimulated with heterologous CD1+ dendritic cells, an organic phase extract of M. tuberculosis, and rIL-2 (1 nM; Ajinomoto Co.). T cells were maintained by restimulation every 2 wk with irradiated heterologous CD1+ dendritic cells and rIL-2. T cell clones were derived from lines JR.2 and XV.1 by limiting dilution culture using PHA stimulation. T cells were seeded at 1 and 5 cells per well in 96-well round-bottomed plates in a volume of 0.2 ml, with 5 × 104 irradiated (4,000 rads) heterologous PBMCs and 5 × 104 irradiated (5,000 rads) EBV-transformed B lymphoblastoid cells as feeders in RPMI medium supplemented with PHA-P (1:4,000 final dilution; Difco) and IL-2 (2 nM).
APC Lines.
Monocyte-derived dendritic cells were generated from human blood monocytes that were isolated from the byproducts of platelet pheresis and induced to differentiate and express CD1a, CD1b, and CD1c by incubation with GM-CSF and IL-4 as described 48. The lymphoblastoid cell lines C1R were transfected with the expression vector pSRα-Neo into which cDNAs encoding either CD1a, CD1b, or CD1c were inserted as described previously 48. HeLa cells were either mock transfected (HeLa Mock) or CD1c transfected (HeLa CD1c) 49.
Cytolytic Assays.
Cytolytic assays were performed in a 4-h chromium-release assay 43. The targets used were CD1-expressing GM-CSF/IL-4–treated blood monocytes; C1R lymphoblastoid cells either mock transfected (C1R Mock), CD1b transfected (C1R CD1b), or CD1c transfected (C1R CD1c); and HeLa Mock or HeLa CD1c cells 49. In the mAb blocking experiments, targets were incubated with mAbs (20 μg/ml) for 30 min at room temperature before adding effector cells. In experiments where both anti-Fas mAb and strontium ions were used, the targets were incubated for 1 h with anti-Fas mAb (1 μg/ml) before adding the effectors. The effector T cells were treated by incubating with 25 mM strontium chloride hexahydrate (Aldrich Chemical Co.) at 37°C overnight, washing, and then counting after trypan blue incubation to determine their viability. For the M. tuberculosis–specific CD1b-restricted α/β T cell line DN1 47, the target C1R/CD1b was incubated overnight at 37°C with M. tuberculosis total sonicate at 1 μg/ml before T cell addition. For Vγ2/Vδ2 T cell clones specific for IPP and related compounds, the antigen monoethyl phosphate (MEP) was added together with the effectors T cells and targets (1:300) 24. Assays were performed in triplicate, and results were expressed as percent specific 51Cr release ± SEM.
Proliferation Assays.
5 × 104 T cells were plated in triplicate in 96-well flat-bottomed plates with either 5 × 104 CD1+ dendritic cells or 5 × 104 mitomycin C–treated HeLa Mock and HeLa CD1c cells as APCs. In the mAb blocking experiments, mAbs were added as ascites (final dilution of 1:200) or purified mAbs (20 μg/ml). Cultures were incubated at 37°C, pulsed with 1 μCi of [3H]thymidine (2 Ci/mmol; Amersham Pharmacia Biotech) on day 2, and harvested 16 h later using a Tomtec harvester. The filter papers were counted on a Betaplate scintillation counter (Wallac). Results were expressed as cpm ± SEM.
Cytokine Assay.
5 × 105 T cells from the JR.2 and XV.1 lines were cultured with 5 × 105 CD1+ monocyte-derived dendritic cells in the absence or presence of an anti-CD1c mAb (7C6) or an IgM mAb control (NS4.1). PHA-P (1:4,000) was added as a positive control. Supernatants were harvested after 24 and 48 h of culture. Cytokine release was determined for IL-2, IL-4, IL-10, and IFN-γ by sandwich ELISA assay 50 using antibody pairs purchased from PharMingen (IL-2, IL-4, IL-10) or from Endogen (IFN-γ). Results were expressed as ng/ml ± SEM.
Confocal Microscopy Analysis.
For confocal microscopy analysis, cells were fixed and permeabilized as described 49. For the JR.2 line, cells were incubated with either antiperforin directly conjugated with FITC (δG9 mAb, 1–4 ng/ml) or with antigranulysin (DH2 mAb [51], 2.5–8 μg/ml) followed by incubation with goat anti–mouse IgG-Cy3 (4 μg/ml; Jackson ImmunoResearch Labs), in single and double staining. Negative controls were MPC11-FITC mAb (IgG2b isotype control for δG9 mAb) together with DH2-Cy3, and P3-Cy3 mAb (IgG1 isotype control for DH2 mAb) together with δG9-FITC. For the 12G12 clone, cells were incubated either with δG9 mAb (3 μg/ml) or with DH2 mAb followed by incubation with anti–mouse Ig–Texas red (2.5–5 μg/ml; Molecular Probes) and goat anti–mouse IgG1-FITC (2.5–5 μg/ml; Southern Biotechnology Associates), respectively, in single and double staining. Controls were δG9–Texas red with P3-FITC and DH2-FITC with MPC11–Texas red, double staining. Each incubation was performed at 4°C for 1 h; in the double staining, cells were incubated for 10 min with 10% mouse serum between the first and second mAb staining.
Immunoelectron Microscopy.
For electron microscopy, T cells were harvested from culture and dead cells were removed by Ficoll-Hypaque density gradient centrifugation. 0.5–1 × 107 live T cells were fixed at room temperature with 2% glutaraldehyde (Polysciences) in 0.1 M phosphate buffer for 2 h. The fixed cells were then collected into 0.1 M phosphate buffer containing 0.2% paraformaldehyde (Electron Microscopy Sciences) and processed for ultrathin cryosectioning as described 52. Cryosections were incubated with antigranulysin mAb, DH2, for 45 min, washed, and then incubated with protein A–gold (Electron Microscopy Laboratory, Utrecht University, Utrecht, The Netherlands) for 30 min. Labeled sections were viewed with a JOEL 1010 electron microscope at 80 kV.
TCR Analysis by PCR.
Total RNA was isolated as described 53 from six JR.2 clones obtained from the JR.2 line. The γ and δ chains were amplified by PCR as described 29 using the following primers: Vδ1 chain, 5′-GGGCTCGAGCTTCAGGCAGCACAACT-3′ (5′ untranslated region [UTR]) and 5′-GGGAGATCTTGGCAGCTCTTTGATGGTGGTTGCTTTGGTTT-3′ (Cδ region); Vγ2 chain, 5′-GGGGTCGACCTGGTGAAGTCATACAGT-3′ (Vγ2 internal region) and 5′-GGGXCTAGAGTGAGGTTCTCTGTGT-3′ (Cγ 3′ UTR).
The PCR products were cloned into pBluescript II (Stratagene), and the sequences of the V-J junctional region were determined using an automated sequencer (PE Applied Biosystems).
TCR Transfection.
Full-length cDNAs encoding the JR.2 TCR were amplified by PCR using the following primers: 5′-GGGCTCGAGCTTCAGGCAGCACAACT-3′ (Vδ1 5′ UTR), 5′-GGGGGATCCGGAGTGTAGCTTCCTCAT-3′ (Vδ1 3′ UTR), 5′-GGGGGTACCTGCCCTGGCAGAAAGCA-3′ (Vγ2 5′ UTR), and 5′-GGGCTCGAGATGGCCTCCTTGTGC-3′ (Vγ2 3′ UTR).
TCR reconstitution was carried out essentially as described 54. The γ and δ cDNAs were cloned into pREP7 and pREP9 (Invitrogen), respectively. J.RT3-T3 cells, Jurkat derivatives that lack cell surface TCR expression 55, were obtained from American Type Culture Collection. J.RT3-T3.5 cells were transfected with 20 μg each of pREP7-JR.2γ and pREP-JR.2δ by electroporation at 250 V, 960 μF with a Gene Pulser (Bio-Rad). Control transfectants were electroporated with 20 μg each of the pREP7 and pREP9 vectors alone. After 48 h, transfectants were placed into medium containing 1 mg/ml geneticin (Life Technologies) and 0.5 mg/ml hygromycin B (Life Technologies). After 2 wk, transfectants were analyzed for cell surface TCR expression by flow cytometry using anti-CD3 (SPV-T3b) and anti-Cδ TCR (anti–TCR-δ1) mAbs and used as responders in T cell stimulation assays. The JR.2/J.RT3 derived in this fashion had 35% TCR-γ/δ+ cells that stained with anti–TCR-δ1 to a mean fluorescence intensity of ∼100 on flow cytometry.
T Cell Transfectant Stimulation Assay.
J.RT3-T3.5 transfectants (1 × 105/well) were cultured in 96-well flat-bottomed microtiter plates either in the presence of 10 ng/ml PMA alone, or in the presence of PMA plus CD1+ monocyte-derived dendritic cells with or without the following mAbs (at a concentration of 20 μg/ml): P3 (isotype control), 10H3.9.3 (anti-CD1a), BCD1b3.2 (anti-CD1b), or F10/21A3.1 (anti-CD1c) in a total volume of 200 μl/well. 50 μg aliquots of the culture supernatants were collected after 20–24 h culture at 37°C and diluted 1:2 with culture medium. To measure the amount of IL-2 released into the supernatants, HT-2 cells (5,000/well) were added and cultured 25–30 h at 37°C. [3H]Thymidine (1 μCi/well, 6.7 Ci/mmol; New England Nuclear) was added during the final 5–6 h of culture. Plates were harvested on a Tomtec plate harvester, and [3H]thymidine incorporation was measured in a Betaplate liquid scintillation counter (Wallac).
Results
Derivation of CD1-reactive T Cell Lines.
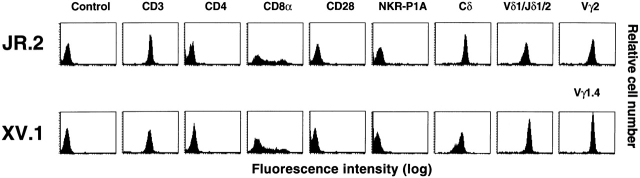
The limited germline diversity of the γ/δ TCR had led to the suggestion that these cells might recognize nonpolymorphic antigen-presenting molecules 56. Since CD1 molecules resemble MHC but lack polymorphism, we pursued efforts to isolate CD1-restricted γ/δ T cells. To derive γ/δ T cell lines, normal human PBMCs were depleted of α/β T cells by antibody staining and magnetic bead separation. The γ/δ T cell–enriched PBMCs were cultured in vitro with an organic phase extract of M. tuberculosis in the presence of CD1-expressing monocyte-derived dendritic cells (see Materials and Methods). After several restimulations and expansions, two independently derived T cell lines, JR.2 and XV.1, were obtained. Flow cytometric analyses revealed that both lines were of the Vδ1 γ/δ T cell subset. The JR.2 line expressed Vγ2 (mAb TiγA+) and Vδ1/Jδ1 (mAb δTCS1+), whereas the XV.1 line expressed Vγ1.4 (mAb 4A11+) and Vδ1/Jδ1 (mAb δTCS1+). Both cell lines lacked expression of NKR-P1A, CD28, and CD4. As is common for γ/δ T cells 57, 27% (JR.2) and 32% (XV.1) of the cells expressed the CD8 α chain but not the CD8 β chain (Fig. 1, and data not shown).
Figure 1.
Flow cytometric analysis of JR.2 and XV.1 lines. The mAbs used were P3 (IgG control), SPV-T3b (anti-CD3), OKT4 (anti-CD4), OKT8 (anti-CD8α), 9.3 (anti-CD28), DX1 (anti–NKR-P1A), anti–TCR-δ1 (pan anti-Cδ TCR), δTCS1 (anti-Vδ1/Jδ1), TiγA (anti-Vγ2), and 4A11 (anti-Vγ1.4).
γ/δ T Cells Specifically Recognize CD1c+ APCs in Proliferation and Cytolytic Assays.
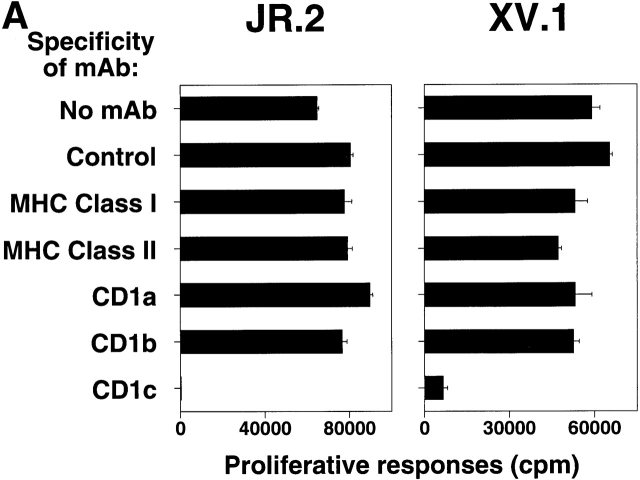
Although the cell lines were expanded in the presence of M. tuberculosis antigens, the T cells did not specifically recognize M. tuberculosis antigens. Instead, JR.2 cells proliferated in the presence of CD1+ dendritic cells (64,675 cpm), even without M. tuberculosis antigens. This response was not blocked by mAbs against CD1a or CD1b, but was blocked to 307 cpm by mAb against CD1c. Nearly identical results were obtained for XV.1 cells (Fig. 2 A). Addition of a standard M. tuberculosis lipid/lipoglycan extract 47 did not augment proliferation by either line (data not shown). To confirm the specificity suggested by mAb blocking, CD1c transfectant cells were studied. JR.2 γ/δ T cells proliferated markedly to HeLa CD1c 49 but not to HeLa Mock cells (Fig. 2 B). Note that JR.2 T cells did not appear to recognize MICA, as these T cells did not recognize the HeLa Mock cell line that expresses MICA 58.
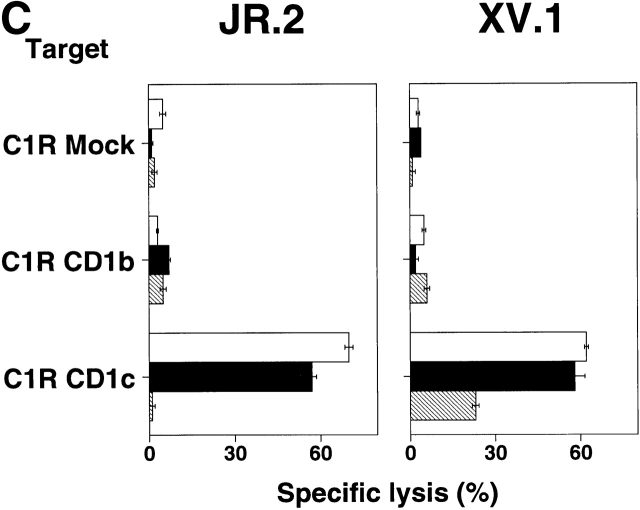
Figure 2.
CD1c-specific recognition by JR.2 and XV.1 γ/δ T cell lines. (A) The proliferative response of JR.2 and XV.1 lines to CD1c+ dendritic cells was inhibited by anti-CD1c mAb (F10/2A3) but not by anti–MHC class I, anti–MHC class II, anti-CD1a, and anti-CD1b mAbs. (B) The JR.2 T cell line proliferated in response to HeLa CD1c cells but not to HeLa Mock cells. Background proliferation of HeLa Mock and HeLa CD1c cells alone was 872 and 1,197 cpm, respectively. (C) C1R Mock, C1R CD1b, and C1R CD1c targets were 51Cr labeled and tested in cytolytic assays with JR.2 and XV.1 T cell lines at different E/T ratios (30:1 is shown) in the presence of anti-CD1b (BCD1b3.2) or anti-CD1c (F10/213) mAbs. Note that JR.2 and XV.1 lysed only C1R CD1c transfectant cells and that lysis was blocked by anti-CD1c mAb. White bars, no mAb; black bars, anti-CD1b; gray bars, anti-CD1c.
As it is known that many γ/δ cell lines are capable of cell-mediated cytolysis 13, JR.2 and XV.1 T cells were examined for their ability to lyse transfected C1R lymphoblastoid cell lines C1R CD1b, C1R CD1c, or C1R Mock 35. JR.2 and XV.1 T cells failed to lyse C1R CD1b or C1R Mock targets, but efficiently lysed C1R CD1c targets (70 and 62% specific lysis, respectively; Fig. 2 C). In each case, lysis was specifically blocked (99 and 63% inhibition) by anti-CD1c mAb. Similar results were obtained in cytolytic assays using CD1-expressing monocyte-derived dendritic cells (data not shown). Thus, JR.2 and XV.1 γ/δ T cells were CD1c specific as revealed in the proliferation and cytolytic analyses of CD1 transfectant cell lines and proliferation assays with CD1+ monocyte-derived dendritic cells. Note that these experiments were performed without addition of M. tuberculosis antigens.
CD1c-reactive γ/δ T Cells Are Th1-like.
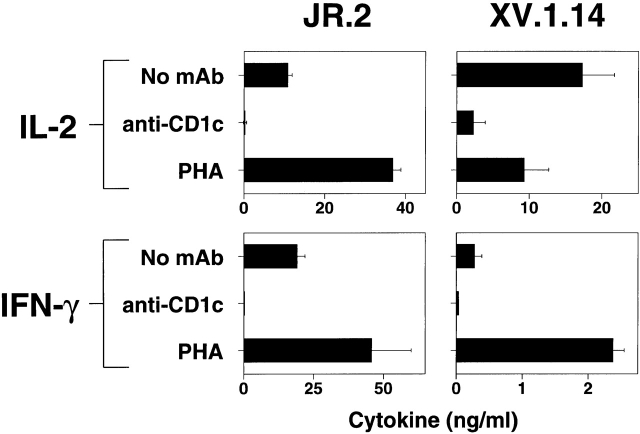
To assess the ability to produce cytokines upon exposure to CD1c, cells from the JR.2 line and the XV.1.14 clone were cocultured with CD1-expressing human dendritic cells, and cytokine levels were measured. Substantial levels of IFN-γ (10 and 0.28 ng/ml, respectively) and IL-2 (19 and 17 ng/ml, respectively) were produced after the T cell lines were exposed to CD1+ dendritic cells. Cytokines also were secreted at somewhat higher levels after stimulation with the mitogen PHA (36 and 2.4 ng/ml IFN-γ, and 45 and 9.2 ng/ml IL-2, respectively). The production of these cytokines to CD1c-expressing dendritic cells was blocked completely by the presence of an anti-CD1c mAb (Fig. 3). In contrast to these cytokines, IL-4 and IL-10 were undetectable under these conditions (detection limits were <16 pg/ml IL-4 and <24 pg/ml IL-10 for cytokine ELISA). Thus, the profile of cytokine production by CD1c-reactive γ/δ T cells was Th1-like.
Figure 3.
JR.2 and XV.1 γ/δ T cell lines show a Th1-like cytokines profile. JR.2.1 and XV.1.14 clones were incubated with CD1c+ dendritic cells for 24 and 48 h. The culture supernatants were collected and assayed for IL-2, IFN-γ, IL-4, and IL-10. Note that the JR.2.1 and XV.1.14 clones produced IFN-γ and IL-2 in response to exposure to CD1+ dendritic cells. IL-4 and IL-10 were not detected (data not shown).
γ/δ T Cells Use Perforin-dependent and Fas-dependent Cytotoxicity Pathways.
CD1-restricted, microbial-specific CD8+ α/β T cells lyse targets using perforin-mediated pathways and impart a bactericidal effect mediated by granulysin, whereas CD1-restricted CD4−CD8− α/β T cells lyse targets through Fas–FasL interaction 51 59. As γ/δ T cells typically display cytolytic potential, we examined the cellular cytotoxicity mechanisms that CD1c-reactive γ/δ T cells used. Fas-dependent lysis can be inhibited by certain anti-Fas mAbs, whereas exhaustive granule secretion induced by strontium ions renders T cells dependent on perforin and granule-related enzymes temporarily incapable of killing target cells 59. To determine the cellular cytotoxicity mechanism of γ/δ T cells, a panel of γ/δ T cells was examined including the JR.2 line and IDP2 clone that are CD1c reactive 60 and previously studied Vγ2/Vδ2-bearing clones, 12G12, DG.SF68, HD.108, and CP.1.15 25 57, that are specific for small phosphate antigens such as MEP 24. 12G12 and CP.1.15 are CD8+, whereas HD.108 and DG.SF68 are CD4−CD8−. The antigen-specific lysis of targets by all of these γ/δ T cell clones was consistently and efficiently blocked by treatment with strontium ions (60–84% average inhibition; Fig. 4). Inhibition was observed when cells were treated with anti-Fas mAb as well, but this inhibition was variable. In contrast to γ/δ T cells, the TCR-α/β–expressing CD1b-restricted CD4−CD8− T cell line, DN1 47, lysed antigen-pulsed targets in a manner that was unaffected by pretreatment with strontium ions but was efficiently blocked by mAb against Fas (Fig. 4). These data indicate that cytolysis mediated by both Vδ1+ CD1c-reactive γ/δ T cells and Vδ2+ prenyl pyrophosphate–specific γ/δ T cells can be both perforin mediated and Fas mediated, like that described for CD8+ MHC class I–restricted α/β T cells 61.
Figure 4.
Analysis of cytotoxicity mechanisms used by CD1c-reactive and prenyl pyrophosphate–specific γ/δ T cells. CD1c-reactive Vγ2/Vδ1 T cells, JR.2 and IDP2, and prenyl pyrophosphate antigen–specific Vγ2/Vδ2 T cell clones, 12G12, HD.108, CP.1.15, and DG.SF68, were used in cytolytic assays performed in the presence or absence of anti-Fas mAb to block Fas-mediated cytolysis or after treatment with strontium ions to block perforin-mediated cytolysis. The mycolic acid–specific, CD1b-restricted, CD4−CD8− α/β T cell line, DN1, is shown as a control. MEP is an alkyl phosphate analogue of the IPP antigen recognized by Vγ2/Vδ2 T cells and was used to stimulate the prenyl pyrophosphate–specific clones. The E/T ratio was 10:1. Targets for CD1c-reactive and prenyl pyrophosphate–specific γ/δ T cells were C1R CD1c cells. Targets for the CD1b-restricted clone were C1R CD1b cells that had been incubated for 16 h at 37°C with M. tuberculosis sonicate at 1 μg/ml. Note that the cytolytic activity mediated by γ/δ T cells was blocked by both treatments and thus depends on both granule secretion and Fas–FasL interactions.
γ/δ T Cells Express the Antimicrobial Protein, Granulysin.
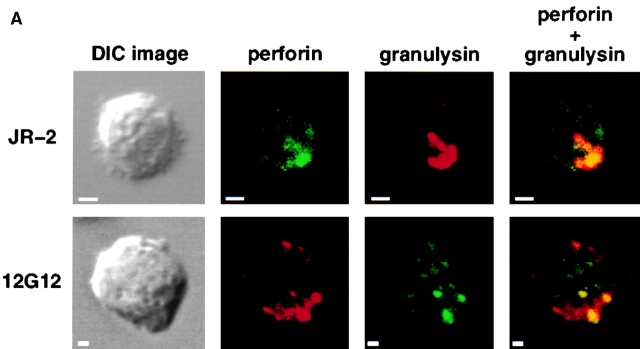
Granulysin is a protein present in the cytotoxic granules of cytolytic α/β T cells and NK cells 62 that has potent antimicrobial activity against a variety of bacteria including M. tuberculosis, Staphylococcus aureus, and Listeria monocytogenes 51. However, granulysin can kill intracellular bacteria only after the infected cells are lysed by perforin 51. To determine if CD1c-reactive γ/δ T cells express granulysin and therefore had the potential to kill intracellular bacteria, we examined the JR.2 cell line for expression of granulysin and perforin. JR.2 (CD1c-reactive) as well as 12G12 (phosphoantigen-specific) γ/δ T cell lines expressed both granulysin and perforin. These proteins were found to colocalize in cytolytic granules by confocal microscopy (Fig. 5 A). IgG control mAbs showed no significant staining regardless of isotype (data not shown). Further studies by immunoelectron microscopy confirmed the presence of granulysin in the cytolytic granules of both the JR.2 line and the 12G12 clone (Fig. 5 B) as demonstrated by the presence of immunogold particles in cytotoxic granules after staining with an antigranulysin mAb.
Figure 5.

Expression of granulysin by γ/δ T cells. (A) Colocalization of granulysin and perforin in the cytolytic granules. Differential interference contrast images of γ/δ T cells are shown on the left. Confocal microscopic analysis of fluorescent immunostaining of perforin and granulysin of the JR.2 CD1c-reactive γ/δ T cell line and 12G12 phosphoantigen–specific γ/δ T cell clone are shown as labeled. Superimposed figures are showed in the right panels. Note that the counterstaining were exchanged such that perforin is green in the top panel but red in the bottom panel, and granulysin is red in the top panel but green in the bottom panel. (B) Localization of granulysin to the cytotoxic granules of γ/δ T cells by immunoelectron microscopy. The presence of granulysin in the cytotoxic granules of JR.2 (bottom) and 12G12 (top) is demonstrated by the gold labeling (small particles) seen with antigranulysin mAb staining. c, centriole; e, endosome; P, plasma membrane; m, mitochondria; n, nucleus; G, Golgi apparatus. Bar, 200 nm.
TCR-γ/δ–mediated Recognition of CD1c.
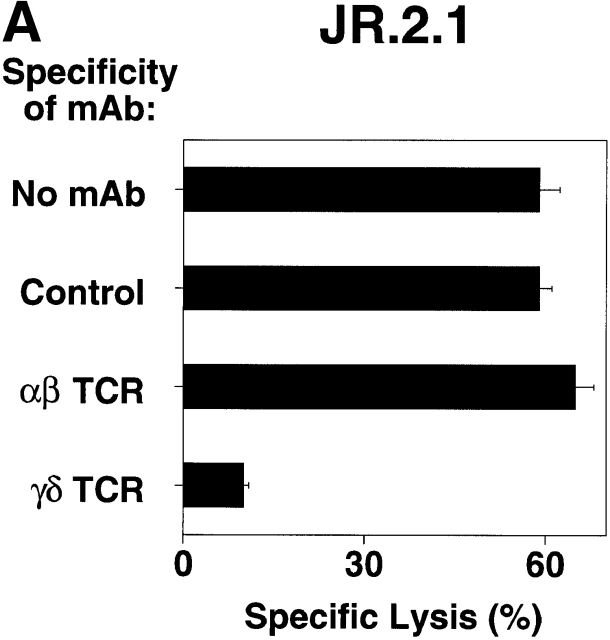
Although studies using mAb blocking and transfection of CD1c-encoding expression vectors into recipient targets showed directly that CD1c is the critical molecule recognized by Vδ1+ γ/δ T cells, we sought to determine the role of the TCR in this recognition. To demonstrate that the TCR-γ/δ mediates recognition of CD1c, a series of mAbs blocking experiments and TCR transfection studies was carried out. mAbs against TCR-α/β and TCR-γ/δ were tested for their ability to block JR.2 T cell lysis of C1R CD1c transfectant cells. Only the mAb against the TCR-γ/δ blocked killing (83% inhibition), suggesting that CD1c recognition was TCR mediated (Fig. 6 A). To formally demonstrate that the TCR plays a direct role in the recognition of CD1c, we cloned the TCR γ and δ chains from the CD1c-reactive JR.2 T cell clone by reverse transcription PCR, transfected the JR.2 Vγ2/Vδ1 TCR into the TCR-deficient recipient Jurkat T cell tumor cell line, and measured the ability of the transfectants to produce IL-2 in response to CD1+ dendritic cells. When cultured with CD1+ dendritic cells, the Vγ2/Vδ1 TCR transfectant (JR.2/J.RT3) secreted IL-2 as judged in bioassays by the proliferation of HT-2 cells (34,000 cpm; Fig. 6 B). The production of IL-2 was inhibited completely by adding anti-CD1c mAb, but not anti-CD1a or anti-CD1b mAbs. Mock transfectant J.RT3 recipient cells (Mock/J.RT3) did not produce IL-2 in response to CD1+ presenting cells. Thus, γ/δ T cell specificity for CD1c is determined by the Vδ1-containing TCR, as transfection of this TCR confers reactivity upon the recipient.
Figure 6.
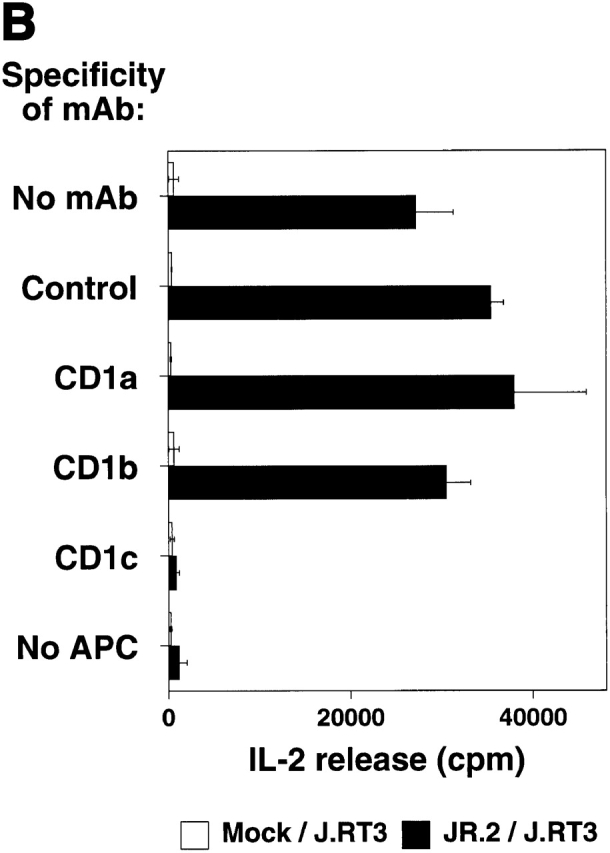
TCR-γ/δ mediates CD1c recognition. (A) JR.2.1 T cell clone lysed C1R CD1c cells. Lysis was blocked by anti-CD1c mAb (F10/2A3; not shown) as well by anti-Cδ TCR mAb (anti–TCR-δ1). E/T 30:1. (B) JR.2 Vγ2/Vδ1 transfectant recognizes CD1c. The TCR− J.RT3-T3 cell line was transfected with cDNAs encoding the Vγ2/Vδ1 TCR from the JR.2 cell line or was mock transfected. The resulting JR.2/J.RT3 or Mock/J.RT3 cell lines were cultured with CD1+ dendritic cells in the presence or absence of mAbs to CD1a, CD1b, and CD1c, and the supernatants were harvested after 24 h. IL-2 release was assessed by the proliferation of the IL-2–dependent HT-2 T cell line. Note that the JR.2/J.RT3 but not the Mock/J.RT3 cell line released IL-2 when cultured with CD1+ monocyte-derived dendritic cells. This IL-2 release was completely blocked by the addition of an anti-CD1c mAb.
CD1c-reactive γ/δ T Cells Use Diverse TCRs.
The recognition of CD1d antigen-presenting molecules by α/β T cells in the absence of exogenous foreign antigens has been observed for a unique subset of CD4+ mouse NKT cells expressing a canonical Vα14-Jα281 rearrangement 63 as well as for human T cells expressing the homologous Vα24-Jα18 rearrangement 50. The CD1c-reactive γ/δ T cell lines derived here express γ/δ TCRs encoded by Vδ1 gene segments (Fig. 1). To determine the nature of γ/δ TCR diversity involved in recognition of CD1c, we amplified and sequenced the junctional regions of the γ and δ chains of a JR.2 subclone. These sequences were compared with those of γ/δ T cell line IDP2 64 65, previously characterized as specific for CD1c 60. Both IDP2 and JR.2 rearranged Vδ1 to Dδ2, Dδ3, and Jδ1 gene segments to encode the junctional region of Vδ1 chain. However the amino acid sequences of the Vδ1 chains in the CDR3 region of the two T cells have almost no homology and are of substantially different lengths. The Vγ2 chains of the clones exhibited limited N region diversity but used different Jγ segments (Jγ1.2 for JR.2 and Jγ2.3 for IDP2; Fig. 7). Vγ usage differed in the XV.1 cell line that used the Vγ1.4 gene segment and another CD1c-reactive Vδ1+ T cell clone 66 that used either Vγ1.3 or Vγ1.4 with Jγ2.3. Thus, CD1c-reactive γ/δ T cells have divergent Vδ1 junctional regions rather than a canonical TCR as found on CD1d-reactive NKT cells and can use different Vγ gene segments. However, since only a limited number of CD1c-reactive TCRs has been analyzed, more TCRs will need to be sequenced to determine the diversity of TCR junctions used by CD1c-reactive γ/δ T cells.
Figure 7.
Diverse CDR3 junction region of two CD1c-reactive Vγ2/Vδ1 T cell clones, showing nucleotide sequence analysis of the γ and the δ gene rearrangements of JR.2 CD1c-reactive clone. Deduced amino acid sequences are in the single letter code. IDP2 sequence data are taken from Hata et al. (reference 64) and Krangel et al. (reference 65) and are included for comparison.
Discussion
We have described and functionally analyzed γ/δ T cell lines and clones derived from normal donors that directly recognize human CD1c molecules. Compared with other CD1-reactive T cells described in humans, these are remarkable because their recognition was not dependent on the presence of foreign lipid or glycolipid antigens. These CD1c-reactive T cells constitute a subset of directly reactive T cells that may be part of the innate immune system. A certain similarity exists with murine CD4+ α/β NKT cells reactive to murine CD1.1, a homologue of human CD1d 63 67. These murine α/β T cells express markers of the NK locus (NK1.1) and express nondiverse, canonical Vα14-Jα281 TCR α chains frequently paired with diverse Vβ8 chains. The production of IL-4 and IFN-γ by these NKT cells has been implicated as an early immunoregulatory response in mice 63 68 69 70, and activation of these cells in vivo by exogenous antigen directs conventional T cells to the Th2 phenotype 71 72. The secretion of large amounts of IFN-γ in response to exogenous antigen can also inhibit ongoing Th2 responses 69. In contrast, the γ/δ T cells described here are reactive to CD1c, another CD1 family member. Recognition of CD1c is through the γ/δ TCR as directly demonstrated by transfection of the TCR (Fig. 6). TCR γ and δ gene sequences from these T cells revealed the incorporation of template-independent N nucleotides resulting in diverse noncanonical V-(D)-J junctions. Moreover, CD1c-reactive γ/δ T cells lacked expression of NKR-P1A (Fig. 1), a human member of the family of NK receptors analogous to murine NK1.1 73.
It is not clear if the CD1c-reactive γ/δ T cells are responding to CD1c molecules alone or to CD1c molecules containing a self-lipid molecule. The recent findings that insect-derived murine CD1d molecules contain a bound lipid in their antigen binding pocket 74, that mammalian CD1 can bind cellular glycosylphosphatidylinositol 75, and that murine and human Vα14 invariant chain, NKR+ α/β T cells respond to autologous CD1d molecules loaded with glycosylceramides 76 77 suggest that the recognition of murine CD1d involves CD1d-bound lipid. Thus, it seems likely that the γ/δ T cells described here are recognizing a self-lipid presented by CD1c. However, such a lipid must be broadly distributed, being present in B cells, HeLa cells, and dendritic cells, as CD1c expressed on these various cell sources was recognized in each case (Fig. 2a and Fig. b).
The recognition of CD1c by human γ/δ T cells may be an important form of antigen recognition by these T cells. Recognition of CD1c by γ/δ T cells in the absence of exogenous foreign antigens could be compared with the alloreactive and autoreactive recognition of MHC class I and class II proteins by α/β T cells that are easily detected in MLRs (mixed lymphocyte reactions) and AMLRs (autologous mixed lymphocyte reactions) 78. Thus, autoreactivity to restricting elements may be a common theme between TCRs that recognize foreign antigens in the context of MHC or CD1.
The best-studied human γ/δ T cell reactivity is to small aliphatic phosphate molecules typically composed of an isoprenoid chain linked to a pyrophosphate moiety 24. Recently, we have characterized another class of aliphatic molecules typically consisting of four or five carbons chains linked to a primary amine 26. Recognition of these small aliphatic compounds may represent a form of pattern recognition by the major circulating γ/δ T cell subset defined by expression of Vδ2 TCR. The Vγ2/Vδ2 subset of γ/δ T cells accounts for the vast majority of human γ/δ T cells in the circulation.
Here, we characterized recognition by the Vδ1 subset of γ/δ T cells, the major population in tissues such as the intestine and the spleen. We suggest that this subset of γ/δ T cells focuses on recognition of nonpolymorphic cell surface molecules related in structure to classical MHC molecules. Groh and Spies have recently demonstrated that some members of this γ/δ T cell subset killed target cells expressing stress-induced MICA and MICB MHC–encoded structures 32 through reactivity with the NKG2D NK receptor 33. Although mAbs specific for the γ/δ TCR blocked γ/δ T cell killing of MICA+ targets 32, the blocking was partial and TCR gene transfer was not done. It remains to be definitely determined if Vδ1+ TCRs can recognize MICA. Here, we show that some members of this same receptor subset recognize nonpolymorphic CD1c. Two other described γ/δ T cell clones reactive with human CD1c also expressed TCRs encoded by the Vδ1 gene segment 60 66. Moreover, we have derived and partially characterized two additional Vδ1+ T cell lines that recognize CD1c (data not shown). Thus, these findings suggest that CD1c reactivity may be common among Vδ1+ T cells. However, it is clear that many Vδ1+ T cells are not CD1c reactive (data not shown), and further studies will be needed to determine the frequency of CD1c-reactive γ/δ T cells. Additionally, since the above γ/δ T cell lines were derived by in vitro culture, primary resident CD1c-reactive γ/δ T cells in situ may not be able to respond to CD1c due to anergy.
Direct recognition of CD1c by Vδ1+ T cells may represent a bridge between innate and adaptive immunity in a similar fashion to recognition of CD1d by murine and human NK+ α/β T cells 50 63. Such CD1d-reactive α/β T cells are critically required to mediate IL-12–induced tumor immunity in mice 68, can polarize other T cells to a Th2 phenotype when activated by exogenous antigen 71 72, and play regulatory roles in listerial infection 79 and Th2 responses 69. We speculate that CD1c-reactive γ/δ T cells also have unique immunological functions. Evidence from in vivo and in vitro experiments has demonstrated that γ/δ T cells play roles complementary to those of conventional α/β T cells in the host defense against infectious agents, in autoimmune diseases, and in tumor surveillance. We demonstrate here that both the CD1c- and the phosphate antigen–reactive γ/δ T cells are cytotoxic and can use the perforin-dependent cytotoxicity pathway. The perforin pathway plays an important role in antiviral immunity, resistance to intracellular bacteria, tumor surveillance, and other immune functions because it is the primary cytotoxic effector mechanism in host defense 61. Further underscoring the potential functional importance of γ/δ T cells in vivo, we show for the first time that γ/δ T cells express granulysin, a potent antimicrobial protein that in conjunction with perforin kills intracellular M. tuberculosis organisms 51. Granulysin also kills a wide variety of microbes in vitro 51 62. Moreover, the CD1c-reactive γ/δ T cells possess a Th1-like cytokine profile, producing significant amount of IFN-γ and IL-2.
CD1 molecules can be directly induced on monocytes as they differentiate into dendritic cells in response to GM-CSF or indirectly in response to agents such as bacteria or inflammatory products that induce the secretion of GM-CSF. Such de novo expression of CD1c can be seen on dendritic cells in granulomas induced by Mycobacterium leprae infection in leprosy 80. We speculate that this new expression of CD1c, or stress-induced endogenous lipid antigens presented by CD1c, could then activate tissue Vδ1+ T cells that are specific for CD1c. After their activation, the CD1c-reactive γ/δ T cells could play a role in directing conventional α/β T cells to the acquisition of a Th1 phenotype through their secretion of IFN-γ. The early secretion of cytokines by murine γ/δ T cells during responses to infectious agents provides evidence for such a role 81. These cells, then, could serve an analogous functional role to that proposed for Vα14+NK1.1+ T cells, except CD1c recognition by γ/δ T cells would lead to IFN-γ production rather than to IFN-γ and IL-4 production that is seen with most NK1.1+ T cells. As CD1c-specific γ/δ T cells are cytotoxic and express granulysin, they could also lyse infected dendritic cells via the perforin pathway and kill released intracellular bacteria by secretion of granulysin. In addition, as CD1c is expressed on some circulating B cells and on mantle zone B cells in lymph nodes (although at low levels) 82 83 84, Vδ1+ γ/δ T cells may play a role in T cell–B cell interactions.
Since various professional APCs, including B cells, tissue dendritic cells, activated monocytes, and activated macrophages in humans all express CD1c and since γ/δ T cells recognizing CD1c appear to do so in the absence of foreign antigens before foreign antigen–specific T cells differentiate and expand, upregulation of CD1c could represent an innate danger signal through the activation of CD1c-specific γ/δ T cells. Thus, such γ/δ T cells may play an early role by activating APCs via Th1 cytokines or may play a role in host defense by directly killing microbes via the combination of perforin and granulysin.
Acknowledgments
We thank Jack F. Bukowski for helpful suggestions.
This work was supported by the National Institutes of Health (M.B. Brenner, S.A. Porcelli, A.M. Krensky, and C.T. Morita), the Arthritis Foundation (S.A. Porcelli and C.T. Morita), and the American College of Rheumatology (C.T. Morita). A.M. Krensky is a Burroughs Wellcome Scholar in Experimental Therapeutics and the Shelagh Galligan Professor of Pediatrics.
Footnotes
Abbreviations used in this paper: IPP, isopentenyl pyrophosphate; MEP, monoethyl phosphate; UTR, untranslated region.
References
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J.A., Nomoto K. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel C.H., Blum C., Dreher A., Reifenberg K., Kaufmann S.H.E. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur. J. Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- Ladel C.H., Hess J., Daugelat S., Mombaerts P., Tonegawa S., Kaufmann S.H.E. Contribution of α/β and γ/δ T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guérinstudies with T cell receptor-deficient mutant mice. Eur. J. Immunol. 1995;25:838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- D'Souza C.D., Cooper A.M., Frank A.A., Mazzaccaro R.J., Bloom B.R., Orme I.M. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis . J. Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- Tsuji M., Mombaerts P., Lefrancois L., Nussenzweig R.S., Zavala F., Tonegawa S. γδ T cells contribute to immunity against the liver stages of malaria in αβ T-cell-deficient mice. Proc. Natl. Acad. Sci. USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammas R., Kodukula P., Tang Q., Hendricks R.L., Bluestone J.A. T cell receptor-γ/δ cells protect mice from herpes simplex virus type 1–induced lethal encephalitis. J. Exp. Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D.P., Hyde D.M., Jackson K.A., Novosad D.M., Ellis T.N., Putney L., Stovall M.Y., Van Winkle L.S., Beaman B.L., Ferrick D.A. Protective response to pulmonary injury requires γδ T lymphocytes. J. Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- Jouen-Beades F., Paris E., Dieulois C., Lemeland J.-F., Barre-Dezelus V., Marret S., Humbert G., Leroy J., Tron F. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect. Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi B., Valle M.T., Oddera S., Giunti D., Manca F., Rossi G.A., Allegra L. T-lymphocytes with γδ+ Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am. Rev. Respir. Dis. 1993;148:1685–1690. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- Bertotto A., Gerli R., Spinozzi F., Muscat C., Scalise F., Castellucci G., Sposito M., Candio F., Vaccaro R. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur. J. Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- Caldwell C.W., Everett E.D., McDonald G., Yesus Y.W., Roland W.E. Lymphocytosis of γ/δ T cells in human ehrlichiosis. Am. J. Clin. Pathol. 1995;103:761–766. doi: 10.1093/ajcp/103.6.761. [DOI] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu. Rev. Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M.B., Band H. Biology of the human γδ T-cell receptor. Immunol. Rev. 1991;120:137–183. doi: 10.1111/j.1600-065x.1991.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Johnson R.M., Lancki D.W., Sperling A.I., Dick R.F., Spear P.G., Fitch F.W., Bluestone J.A. A murine CD4−, CD8− T cell receptor-γδ T lymphocyte clone specific for herpes simplex virus glycoprotein I. J. Immunol. 1992;148:983–988. [PubMed] [Google Scholar]
- Schild H., Mavaddat N., Litzenberger C., Ehrich E.W., Davis M.M., Bluestone J.A., Matis L., Draper R.K., Chien Y.-h. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Crowley M.P., Reich Z., Mavaddat N., Altman J.D., Chien Y.-h. The recognition of the nonclassical major histocompatibility complex (MHC) class I molecule, T10, by the γδ T cell, G8. J. Exp. Med. 1997;185:1223–1230. doi: 10.1084/jem.185.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock E.P., Sibbald P.R., Davis M.M., Chien Y.-h. CDR3 length in antigen-specific immune receptors. J. Exp. Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lebedeva M.I., Llera A.S., Fields B.A., Brenner M.B., Mariuzza R.A. Structure of the Vδ domain of a human γδ T-cell antigen receptor. Nature. 1998;391:502–506. doi: 10.1038/35172. [DOI] [PubMed] [Google Scholar]
- Morita C.T., Lee H.K., Leslie D.S., Tanaka Y.T., Bukowski J.F., Märker-Hermann E. Recognition of nonpeptide prenyl pyrophosphate antigens by human γδ T cells. Microbes Infect. 1999;1:175–186. [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Schondelmaier S., Schoel B., Kaufmann S.H.E. A large fraction of human peripheral blood γ/δ+ T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J. Exp. Med. 1990;171:667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Gulle H., Kaufmann S.H.E., Wagner H. Primary responses of human T cells to mycobacteriaa frequent set of γ/δ T cells are stimulated by protease-resistant ligands. Eur. J. Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- Panchamoorthy G., McLean J., Modlin R.L., Morita C.T., Ishikawa S., Brenner M.B., Band H. A predominance of the T cell receptor Vγ2/Vδ2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J. Immunol. 1991;147:3360–3369. [PubMed] [Google Scholar]
- Morita C.T., Beckman E.M., Bukowski J.F., Tanaka Y., Band H., Bloom B.R., Golan D.E., Brenner M.B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Morita C.T., Tanaka Y., Nieves E., Brenner M.B., Bloom B.R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sano S., Nieves E., De Libero G., Roca D., Modlin R.L., Brenner M.B., Bloom B.R., Morita C.T. Nonpeptide ligands for human γδ T cells. Proc. Natl. Acad. Sci. USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J.F., Morita C.T., Brenner M.B. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and teaimplications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- Lang F., Peyrat M.A., Constant P., Davodeau F., David-Ameline J., Poquet Y., Vié H., Fournié J.J., Bonneville M. Early activation of human Vγ9Vδ2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J. Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- Holoshitz J., Romzek N.C., Jia Y., Wagner L., Vila L.M., Chen S.-J., Wilson J.M., Karp D.R. MHC-independent presentation of mycobacteria to human γδ T cells. Int. Immunol. 1993;5:1437–1443. doi: 10.1093/intimm/5.11.1437. [DOI] [PubMed] [Google Scholar]
- Bukowski J.F., Morita C.T., Tanaka Y., Bloom B.R., Brenner M.B., Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J. Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- Bukowski J.F., Morita C.T., Band H., Brenner M.B. Crucial role of TCRγ chain junctional region in prenyl pyrophosphate antigen recognition by γδ T cells. J. Immunol. 1998;161:286–293. [PubMed] [Google Scholar]
- Falini B., Flenghi L., Pileri S., Pelicci P., Fagioli M., Martelli M.F., Moretta L., Ciccone E. Distribution of T cells bearing different forms of the T cell receptor γ/δ in normal and pathological human tissues. J. Immunol. 1989;143:2480–2488. [PubMed] [Google Scholar]
- Groh V., Steinle A., Bauer S., Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Li P., Willie S.T., Bauer S., Morris D.L., Spies T., Strong R.K. Crystal structure of the MHC class I homolog MIC-A, a γδ T cell ligand. Immunity. 1999;10:577–584. doi: 10.1016/s1074-7613(00)80057-6. [DOI] [PubMed] [Google Scholar]
- Beckman E.M., Melian A., Behar S.M., Sieling P.A., Chatterjee D., Furlong S.T., Matsumoto R., Rosat J.P., Modlin R.L., Porcelli S.A. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J. Immunol. 1996;157:2795–2803. [PubMed] [Google Scholar]
- Spits H., Keizer G., Borst J., Terhorst C., Hekman A., de Vries J.E. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2:423–437. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M.S., Brenner M.B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor δ subunit. Science. 1987;238:682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Wu Y.J., Tian W.T., Snider R.M., Rittershaus C., Rogers P., LaManna L., Ip S.H. Signal transduction of γ/δ T cell antigen receptor with a novel mitogenic anti-δ antibody. J. Immunol. 1988;141:1476–1479. [PubMed] [Google Scholar]
- Jitsukawa S., Faure F., Lipinski M., Triebel F., Hercend T. A novel subset of human lymphocytes with a T cell receptor-γ complex. J. Exp. Med. 1987;166:1192–1197. doi: 10.1084/jem.166.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band H., Hochstenbach F., Parker C.M., McLean J., Krangel M.S., Brenner M.B. Expression of human T cell receptor-γδ structural forms. J. Immunol. 1989;142:3627–3633. [PubMed] [Google Scholar]
- Linsley P.S., Greene J.L., Tan P., Bradshaw J., Ledbetter J.R., Anasetti C., Damle N.K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.H., Calabi F., Lefebvre F.A., Bilsland C.A., Milstein C. Structure and expression of the human thymocyte antigens CD1a, CD1b, and CD1c. Proc. Natl. Acad. Sci. USA. 1987;84:9189–9193. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E.P., Degano M., Rosat J.P., Stenger S., Modlin R.L., Wilson I.A., Porcelli S.A., Brenner M.B. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 1999;189:195–205. doi: 10.1084/jem.189.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar S.M., Porcelli S.A., Beckman E.M., Brenner M.B. A pathway of costimulation that prevents anergy in CD28− T cellsB7-independent costimulation of CD1-restricted T cells. J. Exp. Med. 1995;182:2007–2018. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D., Dubreuil P., Mawas C. Two distinct TL-like molecular subsets defined by monoclonal antibodies on the surface of human thymocytes with different expression on leukemia lines. Immunogenetics. 1984;20:253–264. doi: 10.1007/BF00364207. [DOI] [PubMed] [Google Scholar]
- De Libero G., Casorati G., Giachino C., Carbonara C., Migone N., Matzinger P., Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γ/δ T cells. J. Exp. Med. 1991;173:1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D.A., Kasper A., Poulain F.R., Krensky A.M. Biosynthesis of granulysin, a novel cytolytic molecule. Mol. Immunol. 1999;36:413–422. doi: 10.1016/s0161-5890(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Beckman E.M., Porcelli S.A., Morita C.T., Behar S.M., Furlong S.T., Brenner M.B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Morita C.T., Brenner M.B. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Sugita M., Jackman R.M., van Donselaar E., Behar S.M., Rogers R.A., Peters P.J., Brenner M.B., Porcelli S.A. Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science. 1996;273:349–352. doi: 10.1126/science.273.5273.349. [DOI] [PubMed] [Google Scholar]
- Exley M., Garcia J., Balk S.P., Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J. Exp. Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger S., Hanson D.A., Teitelbaum R., Dewan P., Niazi K.R., Froelich C.J., Ganz T., Thoma-Uszynski S., Melian A., Bogdan C. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- Peters P.J., Neefjes J.J., Oorschot V., Ploegh H.L., Geuze H.J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Brawley J.V., Concannon P. Modulation of promiscuous T cell receptor recognition by mutagenesis of CDR2 residues. J. Exp. Med. 1996;183:2043–2051. doi: 10.1084/jem.183.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Stobo J.D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M.B., Strominger J.L., Krangel M.S. The γδ T cell receptor. Adv. Immunol. 1988;43:133–192. [PubMed] [Google Scholar]
- Morita C.T., Verma S., Aparicio P., Martinez-A C., Spits H., Brenner M.B. Functionally distinct subsets of human γ/δ T cells. Eur. J. Immunol. 1991;21:2999–3007. doi: 10.1002/eji.1830211215. [DOI] [PubMed] [Google Scholar]
- Zwirner N.W., Fernández-Viña M.A., Stastny P. MICA, a new polymorphic HLA-related antigen, is expressed mainly by keratinocytes, endothelial cells, and monocytes. Immunogenetics. 1998;47:139–148. doi: 10.1007/s002510050339. [DOI] [PubMed] [Google Scholar]
- Stenger S., Mazzaccaro R.J., Uyemura K., Cho S., Barnes P.F., Rosat J.P., Sette A., Brenner M.B., Porcelli S.A., Bloom B.R., Modlin R.L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M.B., Greenstein J.L., Balk S.P., Terhorst C., Bleicher P.A. Recognition of cluster of differentiation 1 antigens by human CD4−CD8− cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- Kagi D., Ledermann B., Burki K., Zinkernagel R.M., Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo . Annu. Rev. Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- Pena S.V., Hanson D.A., Carr B.A., Goralski T.J., Krensky A.M. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 1997;158:2680–2688. [PubMed] [Google Scholar]
- Bendelac A., Rivera M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Hata S., Brenner M., Krangel M. Identification of putative human T cell receptor δ complementary DNA clones. Science. 1987;238:678–682. doi: 10.1126/science.3499667. [DOI] [PubMed] [Google Scholar]
- Krangel M., Band H., Hata S., McLean J., Brenner M. Structurally divergent human T cell receptor proteins encoded by distinct Cγ genes. Science. 1987;237:64–67. doi: 10.1126/science.2955517. [DOI] [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Miossec C., Hercend T. CD1c as a target recognition structure for human T lymphocytesanalysis with peripheral blood γ/δ cells. Eur. J. Immunol. 1990;20:703–706. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- Porcelli S.A. The CD1 familya third lineage of antigen-presenting molecules. Adv. Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Cui J., Watanabe N., Kawano T., Yamashita M., Kamata T., Shimizu C., Kimura M., Shimizu E., Koike J., Koseki H. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaud C., Lee D., Donnars O., Park S.H., Beavis A., Koezuka Y., Bendelac A. Cross-talk between cells of the innate immune systemNKT cells rapidly activate NK cells. J. Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- Burdin N., Brossay L., Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Singh N., Hong S., Scherer D.C., Serizawa I., Burdin N., Kronenberg M., Koezuka Y., Van Kaer L. Activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- Lanier L.L., Chang C., Phillips J.H. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Zeng Z.-H., Castaño A.R., Segelke B.W., Stura E.A., Peterson P.A., Wilson I.A. Crystal structure of mouse CD1an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- Joyce S., Woods A.S., Yewdell J.W., Bennink J.R., De Silva A.D., Boesteanu A., Balk S.P., Cotter R.J., Brutkiewicz R.R. Natural ligand of mouse CD1d1cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Spada F.M., Koezuka Y., Porcelli S.A. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L.A., Chattopadhyay S. The molecular basis of allorecognition. Annu. Rev. Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- Szalay G., Ladel C.H., Blum C., Brossay L., Kronenberg M., Kaufmann S.H. Anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-β2 and Th1 cytokines and ameliorates listeriosis in mice. J. Immunol. 1999;162:6955–6958. [PubMed] [Google Scholar]
- Sieling P.A., Jullien D., Dahlem M., Tedder T.F., Rea T.H., Modlin R.L., Porcelli S.A. CD1 expression by dendritic cells in human leprosy lesionscorrelation with effective host immunity. J. Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- Ferrick D.A., Schrenzel M.D., Mulvania T., Hsieh B., Ferlin W.G., Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo . Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Small T.N., Knowles R.W., Keever C., Kernan N.A., Collins N., O'Reilly R.J., Dupont B., Flomenberg N. M241 (CD1) expression on B lymphocytes. J. Immunol. 1987;138:2864–2868. [PubMed] [Google Scholar]
- Small T.N., Keever C., Collins N., Dupont B., O'Reilly R.J., Flomenberg N. Characterization of B cells in severe combined immunodeficiency disease. Hum. Immunol. 1989;25:181–193. doi: 10.1016/0198-8859(89)90081-5. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Berti E., Parravicini C., Buscaglia M., Cappio F., Caputo R., Cerri A., Crosti L., Delia D., Gaiera G., Polli N. Expression of CD1 molecules on dendritic cellsontogeny, epitope analysis on normal and malignant cells, and tissue distribution. In: Knapp W., Dorken B., Gilks W.R., Rieber E.P., Schmidt R.E., Stein H., von dem Borne A.E.G.K., editors. Leukocyte Typing IV. Oxford University Press; Oxford: 1989. pp. 263–264. [Google Scholar]