Although the role of class I and class II MHC in adaptive immunity against microbial pathogens is clear, the participation of nonpolymorphic MHC molecules in host defense remains less well defined. Roles for murine class I MHC molecule Qa-2 and class Ib molecule H2-M3 have been suggested 1 2 3, and accumulating data have prompted speculation that CD1 family members may be important in immunity against pathogenic mycobacteria and parasites 4. In this issue, Spada et al. report the restriction of at least some human Vγ2/Vδ1 T cells, the most common tissue γ/δ T cells, by CD1c 5. Like other populations of CD1-restricted T cells, as discussed further below, the CD1c-restricted cells were autoreactive in vitro. These cells produced IFN-γ, but not IL-4, and displayed cytotoxicity against CD1c+ targets, leading the authors to speculate that such cells might be involved in innate host defense against prevalent pathogens. In this way, γ/δ and other CD1-restricted T cells would represent unique small populations of lymphocytes that have been evolutionarily maintained because of their capacity to react rapidly to microbes.
However, pathogens are clever, and an equally plausible hypothesis is that pathogens have exploited unusual T cell populations that exist for reasons different than immunity. Indeed, based on comparisons with other nonpolymorphic MHC molecules, we suspect that the primary role of such molecules may not entail immunity to infectious organisms, but may rather underlie a basic mechanism for the maintenance of cell and tissue homeostasis. The ancient process by which MHC molecules sample distinct cellular compartments may have been later coopted by classical MHC molecules to mediate protective immunity at the time of acquisition of bacteria-derived recombination activating gene (RAG) transposases and the establishment of a system for adaptive immunity 6. By this alternate hypothesis, mycobacteria uniquely exploit the underlying biological processes mediated by CD1.
The CD1 Family: Genomic Organization and Structure
The CD1 family comprises a heterogeneous group of β2-microglobulin (β2m)-associated transmembrane proteins that bear a strong structural resemblance to the classic MHC antigens 4. However, in contrast to the latter, CD1 genes are relatively nonpolymorphic, and are encoded by genes distant from the classic MHC loci. In humans, five CD1 genes on chromosome 1 are known to encode four proteins, designated CD1a, b, c, and d; CD1e may represent a pseudogene. The CD1 family is further subdivided into group 1, comprising CD1a, b, and c, and group 2, comprising CD1d, based on sequence and functional homology. In the mouse, an ancient translocation likely resulted in the loss of the group 1 genes 7; only CD1d1 and a duplicated gene, CD1d2, remain on the syntenic region of chromosome 3.
Crystallographic analysis of mouse CD1d1 confirmed the preservation of domain organization between this molecule and the structure of classic MHC 8. As with class I MHC, an externally disposed binding cleft formed by an eight-stranded antiparallel β-sheet floor bounded by the α1 and α2 helices provided evidence for a molecule involved in ligand display. However, in contrast to the sequential small binding pockets that accommodate individual amino acids of the peptide backbone in classic MHC, the CD1d1 cleft consisted of two large pockets lined with hydrophobic residues. Although capable of binding long, highly hydrophobic peptides 9, it is likely that the unique structure underlies the capacity of CD1 to present lipid ligands. The functionally interchangeable nature of mouse and human CD1d molecules, such that mouse CD1 can present to human CD1-restricted T cells and vice versa 10, suggests that the human CD1d structure will be similar.
Recognition of CD1 by T Cells Bearing Limited TCR Diversity
The description of CD1-restricted tumor cytotoxicity by CD4−CD8− α/β or γ/δ human T cells provided the initial hypothesis that CD1 might subserve an immune function 11 12 13. Shortly thereafter, murine NK1.1+ T cells were demonstrated to be CD1 restricted 14.
The unusual nature of the TCRs that recognize these nonpolymorphic CD1 molecules suggested limited ligand diversity. Best characterized are CD1d-restricted TCRs expressed on NK1.1 T cells from mice and humans 15. In mice, these double negative or CD4+ T cells express an invariant Vα14Jα281 TCR paired with a highly restricted set of Vβ chains, usually Vβ8, Vβ7, or Vβ2, that may reflect tissue-specific expansion or homing. Strikingly, CD1d-restricted human T cells use essentially the same TCR, the homologous Vα24JαQ/Vβ11 TCR. In mice, these cells coexpress typical NK lineage markers and undergo thymic selection by CD1-expressing, bone marrow–derived cortical thymocytes 16. A second subset of CD1d-restricted cells does not express NK lineage markers or the invariant Vα14 TCR, although the expressed TCRs reveal highly restricted VαJα usage, frequently paired with Vβ8 17.
Although α/β TCR usage by T cells restricted by CD1 antigens other than CD1d remains less well studied, emerging evidence suggests that these also will show limited diversity. In humans, such cells are typically double negative or CD8α/α+. Expressed TCRs from such cells displayed limited numbers of TCR α chains (Vα4, Vα7, Vα19, and Vα24) that were shared among individuals with no identity at classic MHC molecules 18. The most prevalent (human Vα7S2, Jα33, and Vβ2S1 or Vβ13) were restricted by CD1b, and strikingly were completely homologous to TCRs on double negative T cells from mice and cattle 19. In mice, such cells were present in CD1d-deficient but not β2m-deficient mice, and the selecting ligand remains unknown. The report by Spada et al. in this issue 5 extends the concept of limited diversity to human CD1c-restricted T cells. Although extrapolated from relatively few examples, these cells all expressed Vγ2/Vδ1 TCRs with or without CD8α/α, and reacted to CD1c presented by a variety of cell types.
CD1-restricted α/β and γ/δ T cells are present in low numbers at birth, and then rise progressively in blood and select tissues—liver, bone marrow, and spleen—to constitute from 1 to 20% of cells, a prevalence not unlike NK cells. In humans, but not mice or rats, they can express CD8α/α, which may reflect their activation status 20. These cells display a memory effector antigen profile on their surface that is consistent with their capacity to rapidly secrete large amounts of cytokines or to generate cytotoxicity after TCR ligation 4 15. Such instantaneous effector capacity distinguishes these cells compared with mainstream naive CD4 and CD8 T lymphocytes. Although the Vγ2/Vδ1 T cells described by Spada et al. 5 demonstrated cytotoxicity and a type 1 cytokine profile, such findings need to be tempered by the observation that these cells were selected by repeated incubation with mycobacterial antigens that mediate IL-12 production from APCs through Toll-like receptor interactions 21. It is likely that such cells, when derived under less biased conditions, might display a wide range of cytokine and effector potential 22.
Intracellular Trafficking of CD1 Molecules
An important property of CD1-restricted T cells is their inherent autoreactivity 4 15. Different types of CD1-restricted T cells can respond to CD1 molecules in distinct tissue compartments, suggesting that autoantigens are expressed in different organs 17 23. More intriguingly, the same CD1 molecule, at least for the group II CD1d antigen, can activate distinct classes of CD1-restricted T cells depending on its pattern of intracellular trafficking. Normally, CD1d molecules traffic from their synthesis in the Golgi complex to the cell surface. There, after ligation or antigen loading, CD1 is internalized to endosomes that subsequently acidify and eventually colocalize in the MHC class II peptide-loading compartment (MIIC 24). Internalized CD1 is then returned to the cell surface. By mutating the tyrosine-based amino acid motif in the CD1 tail such that the molecule failed to be internalized to endosomes, Bendelac and colleagues could indirectly assess the effects of endolysosomal trafficking on the capacity of CD1 to activate NK T cells 17. Unexpectedly, CD1 that had trafficked through MIIC activated NK1+ Vα14Jα281 T cells, whereas CD1 that had trafficked to the cell membrane from the Golgi activated only the non-NK, non-Vα14, CD1d-restricted T cells. Thus, these two sets of CD1d-restricted T cells respond to self-antigens differentially localized to cytosolic secretory or endosomal compartments in a manner highly reminiscent of the way in which CD8+ and CD4+ T cells respond to peptides presented by class I and II antigens. With the exception of CD1a, which does not traffic to MIIC, the other CD1 family members contain similar targeting motifs embedded within their cytoplasmic tails.
Presentation of Nonpeptide Mycobacterial Ligands by Group 1 CD1 Molecules and of Glycosylphosphatidylinositols by CD1d
Substantial invigoration of the field occurred with the isolation of human T cell clones that reacted to antigens from Mycobacterium tuberculosis and Mycobacterium leprae in a CD1b-restricted manner 25. Antigen processing required endosomal trafficking, but was transporter for antigen presentation (TAP) and HLA-DM independent. Several human double negative or CD8 lines and clones that reacted in a CD1a-, 1b-, or 1c-restricted manner to various antigens derived from mycobacterial cell walls, including mycolic acid, glucose monomycolate, and lipoarabinomannan, have been described; most generated IFN-γ or demonstrated cytotoxicity upon activation 26 27 28 29 30. Although most such lines expressed TCR-α/β, TCR-γ/δ was also seen. Analysis of the antigens revealed rigid requirements for carbohydrates and other polar moieties, with the lipid requirements being less specific. Thus, in this model, lipid components of glucose monomycolate or lipoarabinomannan would be accommodated in the large hydrophobic cleft in CD1, positioning hydrophilic structures outwards to enable recognition by the TCR 31.
The concept of presentation of lipid antigens via CD1 to T cells provoked a series of experiments to find the ligand for CD1d, the only known CD1 family member retained in the mouse. Despite the ease with which human group 1 CD1-restricted T cells reactive to mycobacterial antigens could be isolated in vitro, it seems clear that CD1d plays no role in immunity to M. tuberculosis in the mouse based on intact immunity in CD1d-deficient animals 32. Evidence has been reported supporting the role of endogenous glycosylphosphatidylinositol (GPI) anchors as a CD1d ligand 33, and of parasite-derived GPI anchors in mediating CD1d-dependent NK T cell activation for B cell help in antibody production 34. A striking observation remains the capacity of α-galactosylceramide, a glycolipid naturally present in sea sponges but undetectable in mammals, to activate Vα14- and Vα24-bearing T cells in mice and humans, respectively 10 35 36. Despite only 60% identity in the peptide-binding domains of mouse and human CD1d, either molecule could present this ligand and activate both mouse and human NK T cells 10.
The Host Defense Model of CD1 Family Members
The presence in mice and humans of nonpolymorphic MHC molecules that present nonpeptide antigens across species to T cells of limited diversity that circulate with a preprogrammed effector phenotype suggests strong evolutionary pressure for their maintenance. Although the CD1d-deficient mouse has a rather limited phenotype 37 38 39, the persistence of similar types of T cells in these mice suggests that other nonpolymorphic MHC molecules might contribute to this pool of cells 19. The ability to demonstrate CD1 group 1– and group 2–restricted reactivity to mycobacterial and parasite-derived antigens, respectively, suggests the plausible hypothesis that these lipid- and glycolipid-presenting molecules have been maintained to confer the ability to respond to conserved cell wall determinants from prevalent pathogens of substantial morbidity and mortality. Indeed, the most prevalent γ/δ T cells in human blood, Vγ2 and Vδ2, also recognize conserved nonpeptide antigens from mycobacteria, e.g., prenyl pyrophosphate 40 and phosphorylated thymidine nucleotides 41, as well as widely distributed alkylamine compounds 42. Together, these models envision marked evolutionary pressure from mycobacteria and perhaps other organisms such that substantial numbers of T cells with limited diversity exist to enhance early immune responses to these pathogens. Before considering some of the problems inherent in such a model, a review of the emerging functions of other nonpolymorphic MHC-like molecules will be considered.
Nonpolymorphic MHC Molecules Participate in Diverse Homeostatic Functions
A wealth of recent data has cast new light on the role of nonpolymorphic MHC molecules (Table ). The unexpected roles for several of these molecules were revealed in β2m-deficient mice, which are deficient not only of class I MHC, but also of several nonpolymorphic MHC molecules that rely on β2m for protein stability and expression. As reviewed below, several of these molecules demonstrate a chaperone function for ligated “cargo” and, as with CD1, serve as ligands for receptors on distinct populations of effector T cells (Table ). An emerging theme is the function of these molecules to sample cellular compartments, perhaps reflecting a role in monitoring ligands that serve as surrogate markers indicative of cellular health.
Table 1.
Proposed Homeostatic Roles of Mouse CD1 and Other Nonpolymorphic MHC-like Molecules
| MHC or MHC-like molecule | Functional binding groove | Cargo | Recognition by lymphocyte receptor | Interacting ligand and/or cell types | Proposed homeostatic function |
|---|---|---|---|---|---|
| Classical | |||||
| Class I | + | Peptide | + | CD8/TCR-α/β T cells | Host defense |
| Class II | + | Peptide | + | CD4/TCR-α/β T cells | Host defense |
| Nonpolymorphic | |||||
| CD1 | + | Lipid | + | CD4+ or DN NK, CD8α/α+, γ/δ T cells | Lipid membrane integrity? |
| H2-M3 | + | Formylated peptide | + | CD4/TCR-α/β T cells | Mitochondrial integrity? |
| HLA-E (Qa-1) | + | MHC I leader peptide | + | CD94/NKG2A, B, C NK cells, and CD8+ T cells | Class I MHC expression |
| HLA-G | + | Peptide | + | ILT-2, p49 KIR NK and T cells | Materno-fetal tolerance |
| MIC-A/MIC-B | − | Unknown | + | CD94/NKG2D NK, γ/δ T cells, CD8+ T cells | Gut epithelial integrity? |
| FcRn | − | IgG | + | IgG | Serum Ig |
| ZAG | Probable | Lipid | Unknown | Unknown | Lipid metabolism? |
| HFE | − | TfR | + | Transferrin/iron/TfR | Iron |
The gene for hereditary hemochromatosis (HFE) is a nonpolymorphic MHC molecule involved in the regulation of iron metabolism. Mutation of the gene in humans is responsible for hereditary hemochromatosis, a disorder of iron storage that affects between 1 in 200 and 1 in 400 individuals of northern European ancestry 43. Tissue iron overload can eventually lead to chronic liver failure, as well as dysfunction of other organs. The crystal structure of HFE revealed a classic MHC-like structure, although the putative peptide-binding groove was narrowed by a translation of the α1 helix that resulted in burial of potential peptide-binding pockets in the floor of the cleft 44. Biochemical and mutational studies have defined a pathway by which membrane HFE forms a ternary complex with iron-bound transferrin and the transferrin receptor that assists in the endosomal targeting of the complex (Fig. 1). A histidine patch in HFE may serve to titrate a pH-dependent dissociation of HFE from the complex within endosomes, allowing the delivery of cellular iron. The most common abnormalities leading to hemachromatosis are missense mutations that abrogate the association of HFE with β2m, thus destabilizing the protein.
Figure 1.
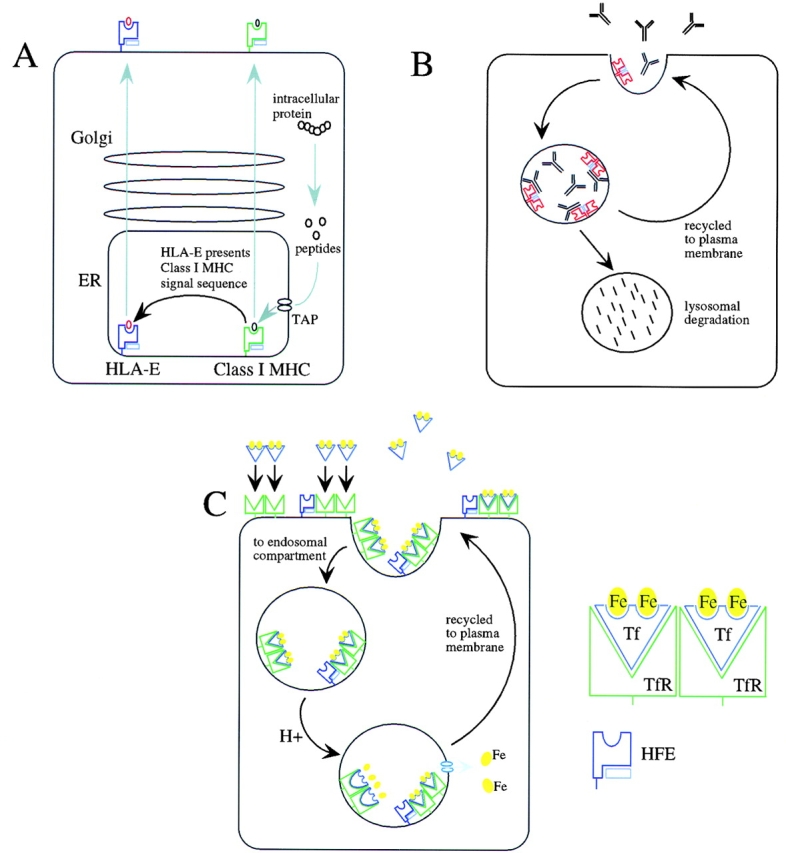
Nonpolymorphic MHC-related molecules are involved in homeostatic maintenance by surveying self-ligands from distinct intracellular compartments. (A) HLA-E presents peptides derived from the signal sequences of class I MHC molecules that are loaded in the endoplasmic reticulum (ER). (B) The neonatal Fc receptor transports IgG from neonatal gut epithelium to blood (not shown), and protects IgG catabolism in adult vascular endothelium, skin, muscle, and gut epithelium. (C) HFE binds iron-bound transferrin on the cell surface of gut epithelium and is trafficked through the endosomal compartment. HFE acts as a negative modulator of transferrin receptor (TfR)-mediated uptake of transferrin (Tf)-bound iron, and thus plays an important role in iron homeostasis.
FcRn, the neonatal Fc Ig receptor, facilitates the transport of IgG from the mother's serum across the placenta and from the mother's milk across the neonatal intestine. The receptor is widely expressed on vascular endothelium throughout life, where it participates in maintaining normal levels of serum IgG; mice deficient in β2m and hence deficient in FcRn demonstrate markedly accelerated clearance of IgG 45. The potential peptide-binding groove in FcRn is essentially closed, with a surface area too small (∼235 Å2) to accommodate peptides like MHC class I molecules, which have a pocket surface area of ∼760 Å2 46. Rather, like HFE, FcRn binds IgG along the side at the interface of the Fc CH2 and CH3 domains of Ig near a cluster of histidines that could facilitate the pH-dependent association of the molecules, analogous to the HFE pathway 47. In adults, FcRn may sequester endocytosed serum IgG, thus protecting it from lysosomal degradation (Fig. 1); saturation of FcRn may underlie the therapeutic efficacy of high-dose Ig for autoimmune disease by accelerating the degradation of autoreactive antibodies 48.
CD1, in contrast to HFE and FcRn, has a large hydrophobic pocket that does bind ligands, and CD1 presents ligands to specialized types of lymphocytes. In fact, both of these properties are shared with other nonpolymorphic MHC molecules. Of these, the molecule with a binding cleft most similar to CD1 is zinc-α2-globulin (ZAG), which circulates as a soluble serum protein. ZAG, unlike CD1, has a network of hydrogen bonds that stabilize the molecule without a requirement for β2m, but like CD1 forms a large, central hydrophobic pocket which, although smaller than CD1, contained a nonpeptide ligand in the crystal structure 49. In vitro and in vivo, ZAG promotes lipolysis and fat depletion; it was isolated as a lipid-catalyzing moiety from the urine of cancer patients with wasting 50. Although further work is required, evidence is consistent with a role for ZAG in mediating lipid homeostasis in normal or pathologic conditions. Concise mechanisms for regulating cellular membrane lipids are beginning to be elucidated and are consistent with such homeostatic processes 51.
Whereas CD1 interacts with TCR via its externally disposed binding pocket, FcRn interacts with IgG or soluble B cell receptors (BCRs) at a region distinct from its closed cleft. Several other nonpolymorphic MHC molecules, including H2-M3, HLA-E (Qa-1 in mouse), HLA-G, MIC-A, and MIC-B, can interact with immune receptors on lymphocytes (Table ). H2-M3 is a murine class I homologue that also forms a large surface area pocket of neutral and hydrophobic residues capable of accommodating peptides with N-formylated methionine termini 52. H2-M3–restricted CTLs that respond to formylated peptides from bacteria have been demonstrated 2. The ability of H2-M3 to bind endogenous formylated peptides from mitochondria would be consistent with a primitive role in monitoring mitochondrial integrity, although no evidence exists for such a function. Selection of an H2-M3–restricted TCR by one of the endogenous 13 formylated proteins of mitochondria, NADH dehydrogenase subunit 1, has been demonstrated 53. Recent evidence published in this journal suggests that preformed H2-M3 exists intracellularly and can be rapidly mobilized to the surface when provided with an appropriate formylated peptide ligand 54. After infection with Listeria, the rapid accumulation of H2-M3–restricted CD8+ T cells with specificity for Listeria-derived antigens could be detected using H2-M3 tetramer reagents 55. In contrast to conventional class I– or II–restricted T cells, such cells displayed no evidence for a memory response. Although the authors used one mitchondrial-derived control peptide to assess specificity, analysis with each of the self-peptide mitochondrial tetramers will be required to show definitively that these T cells are not reacting to self-peptides that mediate stress-induced rescue of intracellular H2-M3.
HLA-E in humans and Qa-1 in mice are homologous MHC molecules that present leader sequences acquired in the endoplasmic reticulum from MHC class I molecules in a peptide-binding cleft at the cell surface 56. Using tetramer technology, the ligand for HLA-E and its bound peptide was shown to be CD94/NKG2A, B, or C, a heterodimeric, C-type lectin superfamily receptor on NK and some T cells that regulates NK cell activation for cytotoxicity through association with the adaptor protein, DAP12 57 58. In this way, HLA-E serves to monitor the homeostatic surface expression of cell class I MHC molecules for surveillance by effector lymphocytes, and thus constitutes a mechanism for rapidly removing cells pathologically altered by several conditions (Fig. 1).
The intestinal epithelial MHC-like molecules, MIC-A and MIC-B, remarkably illustrate the preservation of this homeostatic function despite the substantial alteration of the underlying MHC domains. A disordering of the α2 helix results in complete occlusion of the putative binding cleft, and the molecules adopt an extended structure that is incompatible with binding by β2m 59. MIC-A and MIC-B are induced on intestinal cells by stress-activatible promoters, where they are recognized by Vδ1-expressing γ/δ T cells that occupy subepithelial locations in the intestine 60. Diverse nonhuman primate MIC molecules activate cytotoxicity by human Vδ1 T cell clones, consistent with recognition of a conserved surface on the side of MIC molecules near the footprint occupied by β2m in class I MHC. Despite interaction at a domain distinct from the residual binding pocket, MIC molecules, like HLA-E, interact with C-type lectin NK receptors, NKG2D expressed on Vδ1 T cells, NK cells, and some CD8+ T cells 61. Activation of the lymphocytes occurs through association of NKG2D with an activating adaptor protein, DAP10, resulting in cell death of the MIC-bearing target 62. Although its biological function remains unknown, MIC molecules likely play a role in monitoring intestinal epithelial integrity. Indeed, the turnover of epithelial cells in intestinal villi is substantially reduced in γ/δ-deficient mice 63.
A Reevaluation of CD1
As shown by the above examples, the capacity to bind ligands, including lipids, and to interact with relatively invariant lymphocyte receptors are properties of CD1 shared by other nonpolymorphic MHC molecules (Table ). Frequently, T cells restricted by nonpolymorphic MHC are localized to distinct tissue compartments. Examples include NK T cells in the liver, and MIC-A– and MIC-B–restricted T cells and (as most recently described in this journal) CD8α/α intraepithelial T cells in the intestine 64 65. The restricted expression of HLA-G in the placenta may represent yet another example suggesting a specialized homeostatic function for these invariant molecules in surveying placental integrity 66. These considerations suggest that these various properties were evolutionarily endowed upon CD1 before the appearance of mycobacterial human pathogens. The concept that CD1 remains “hard-wired” in order to confront M. tuberculosis or M. leprae is difficult to conceptualize, given the biology of these organisms. First, M. tuberculosis has probably existed as a human pathogen only since the domestication of cattle some 7,500 years ago, presumably reflecting a transspecies adaptation by Mycobacterium bovis. Second, both M. tuberculosis and M. leprae, although undeniably of immense public health concern, cause disease in only a minority of persons infected during their reproductive life spans. Third, although the group 1 CD1 proteins can present antigens from M. tuberculosis, mice, which are inherently more resistant to tuberculosis than humans, have deleted the group 1 CD1 genes, whereas guinea pigs, which have expanded numbers of these genes, are extraordinarily susceptible to tuberculosis 4. Finally, the ubiquitous environmental mycobacteria are relatively nonpathogenic organisms that require marked deficiencies in the adaptive immune system, e.g., advanced HIV infection or genetic deletions of the IL-12, IL-12R, or IFN-γR genes 67, to cause disease. The likelihood that the evolutionary impetus for CD1 derived from these organisms seems remote.
The sequencing of the M. tuberculosis genome offers extraordinary insights regarding the biology of this organism 68. Compared with Escherichia coli, which has only 50 genes devoted to fatty acid metabolism, M. tuberculosis has >250 distinct enzymes involved in the synthesis and catabolism of a diverse array of lipophilic molecules. Mycobacteria contain enzymes representing all of the classes of lipid and polyketide biosynthesis normally found in mammals, plants, and bacteria. It seems likely that mycobacteria infection would result in substantial effects on endogenous lipid metabolism of the cell. If CD1 molecules were normally loaded with an endogenous cell-derived lipid, as suggested by the autoreactive nature of NK T cells 4 15 or the Vγ2/Vδ1 T cells isolated by Spada et al. 5, it is likely that disruption of normal lipid synthetic pathways could alter the distribution or amount of the endogenous CD1 ligand. As such, CD1 function would more closely resemble that of other nonpolymorphic MHC molecules in providing information regarding deviation from normal cellular biosynthetic pathways, perhaps relating to lipid membrane homeostasis (Table ). In this model, mycobacteria have evolved to displace or induce the normal CD1-associated ligand. But why would M. tuberculosis have evolved to activate CD1-restricted T cells?
M. tuberculosis is unusual because of its airborne transmissibility. In contrast, most bacteria that cause pneumonia do so after establishing colonization in the pharynx, from which microaspiration can occur and cause disease in the appropriate host and clinical setting. Airborne transmission relies on the discharge of enormous numbers of bacilli, usually by coughing. The pathology of tuberculosis is marked by an inflammatory granulomatous response that results in sequestration of organisms within areas of liquefaction and tissue destruction, termed caseous necrosis. It is in these caseous areas that organisms remain viable for years, awaiting periods of diminished immunity that will allow reactivation of dormant bacilli. The capacity of mycobacteria to elicit strong inflammatory responses—the adjuvant in Freund's adjuvant—may be critical in inducing sufficient tissue destruction to facilitate passage of organisms to the airspaces, where triggering of cough and transmission to the environment can occur. It is likely that mycobacteria have exploited the inherent effector capacity of NK and related T cells, and perhaps γ/δ T cells, to engender the granulomatous inflammatory response that ensures both longevity in the host and eventual transmission to others. Recent experiments suggest such contributions from NK T cells 69. By this hypothesis, M. tuberculosis exploits the effector potential and ligand recognition properties of CD1, which have evolved for unrelated housekeeping functions, to ensure its survival and transmission, consistent with the capacity of this organism to have infected up to one third of the human population on earth.
On the Origin of Class I and Class II MHC
Taken together, we would speculate that it is the ability to present, either through recognition of the molecule itself or of its chaperoned cargo, information regarding the general baseline health of the cell that unites the function of these diverse MHC molecules. Frequently, this involves trafficking through distinct cellular compartments that allow dynamic interactions with their various ligands. This, of course, is exactly the function of class I and class II MHC, which display peptide ligands acquired from different cell compartments for surveillance by lymphocytes. However, for these molecules, the capture of bacterial transposases allowed the unlimited rearrangements of the scanning receptors, establishing the capacity to monitor cellular homeostasis with unprecedented precision. Of importance is the presence of these surveillance detectors on lymphocytes, cells capable of clonal expansion, thus enabling the amplification of highly specific effector programs. Again, evolutionary precedents established by the nonpolymorphic MHC molecules have identified a preexisting relationship, whereby lymphocytes survey these types of molecules to unleash effector pathways involved in monitoring tissue health and integrity. It is intriguing to speculate that some primordial footprint of the evolutionary role of class I and class II MHC might remain that is independent of the widely accepted role of these molecules in adaptive immunity.
Acknowledgments
The authors thank members of the laboratory and R. Modlin for spirited discussions, and F. Brodsky for her critical review.
This work was supported by the Howard Hughes Medical Institute and by grants from the National Institutes of Health. K. Shinkai is supported by National Institute of General Medical Sciences training grant MSTP GM07618.
References
- Kurlander R.J., Shawar S.M., Brown M.L., Rich R.R. Specialized role for a murine class 1-b MHC molecule in prokaryotic host defense. Science. 1992;257:678–679. doi: 10.1126/science.1496381. [DOI] [PubMed] [Google Scholar]
- Pamer E.G., Wang C.-R., Flaherty L., Lindahl K.F., Bevan M.J. H2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- Rotzschke O., Falk K., Stevanovic S., Grahovac B., Soloski M.J., Jung G., Rammensee H.-G. Qa-2 molecules are peptide receptors of higher stringency than ordinary class I molecules. Nature. 1993;361:642–644. doi: 10.1038/361642a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S.A., Modlin R.L. The CD1 systemantigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- Spada F.M., Grant E.P., Peters P.J., Sugita M., Melian A., Leslie D.S., Lee H.K., van Donselaar E., Hanson D.A., Krensky A.M. Self-recognition of CD1 by γ/δ T cellsimplications for innate immunity. J. Exp. Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.B. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995;3:531–539. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Balk S. MHC evolution. Nature. 1995;374:505–506. doi: 10.1038/374505b0. [DOI] [PubMed] [Google Scholar]
- Zeng Z.-H., Castano A.R., Segelke B.W., Stura E.A., Peterson P.A., Wilson I.A. Crystal structure of mouse CD1an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- Castano A.R., Tangri S., Miller J.E., Holcombe H.R., Jackson M.R., Huse W.D., Kronenberg M., Petersen P.A. Peptide binding and presentation by mouse CD1. Science. 1995;269:223–226. doi: 10.1126/science.7542403. [DOI] [PubMed] [Google Scholar]
- Brossay L., Chioda M., Burdin N., Koezuka Y., Casorati G., Dellabona P., Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S., Brenner M.B., Greenstin J.L., Balk S.P., Terhorst C., Bleicher P.A. Recognition of CD 1 antigens by human CD4−CD8− cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Miossec C., Hercend T. CD1c as a target recognition structure for human T lymphocytesanalysis with peripheral blood gamma/delta cells. Eur. J. Immunol. 1990;20:703–706. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- Balk S.P., Ebert E.C., Blumenthal R.L., McDermott F.V., Wucherpfennig K.W., Landau S.B., Blumberg R.S. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991;253:1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Lantz O., Quimby M.E., Yewdell J.W., Bennink J.R., Brutkiewicz R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Rivera M.N., Park S.-H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.-H., Jayawardena J., Weiss A., Lee D., Park S.-H., Dautry-Varsat A., Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 1999;189:103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Harrison L., Kehn P., Stevenson K., Currier J., Robinson M.A. Invariant or highly conserved TCR α are expressed on double-negative (CD3+CD4−CD8−) and CD8+ T cells. J. Immunol. 1999;163:301–311. [PubMed] [Google Scholar]
- Tilloy F., Treiner E., Park S.-H., Garcia C., Lemonnier F., de la Salle H., Bendelac A., Bonneville M., Lantz O. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class 1b–restricted α/β T cell subpopulation in mammals. J. Exp. Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., Malefijt R.W., de Vries J.E., Spits H. Interleukin-4 mediates CD8 induction on human CD4+ T cell clones. Nature. 1988;335:642–644. doi: 10.1038/335642a0. [DOI] [PubMed] [Google Scholar]
- Brightbill H.D., Libraty D.H., Krutzik S.R., Yang R.-B., Belisle J.T., Bleharski J.R., Maitland M., Norgard M.V., Plevy S.E., Smale S.T. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Ferrick D.A., Schrenzel M.D., Mulvania T., Hsieh B., Ferlin W.G., Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Eberl G., Lees R., Smiley S.T., Taniguchi M., Grusby M.J., MacDonald H.R. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- Jackman R.M., Stenger S., Lee A., Moody D.B., Rogers R.A., Niazi K.R., Sugita M., Modlin R.L., Peters P.J., Porcelli S.A. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity. 1998;8:341–351. doi: 10.1016/s1074-7613(00)80539-7. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Morita C.T., Brenner M.B. CD1b restricts the response of human CD4−CD8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Beckman E.M., Porcelli S.A., Morita C.T., Behar S.M., Furlong S.T., Brenner M.B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Beckman E.M., Melian A., Behar S.M., Sieling P.A., Chatterjee D., Furlong S.T., Matsumoto R., Rosat J.P., Modlin R.L. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J. Immunol. 1995;157:2795–2803. [PubMed] [Google Scholar]
- Dellabona P., Casorati G., Friedli B., Angman L., Sallusto F., Tunnacliffe A., Roosneek E., Lanzavecchia A. In vivo persistence of expanded clones specific for bacterial antigens within the human T cell receptor α/β CD4−CD8− subset. J. Exp. Med. 1993;177:1763–1771. doi: 10.1084/jem.177.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieling P.A., Chatterjee D., Porcelli S.A., Prigozy T.I., Mazzaccaro R.J., Soriano T., Bloom B.R., Brenner M.B., Kronenberg M., Brennan P.J., Modlin R.L. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Thomssen H., Ivanyi J., Espitia C., Arya A., Londei M. Human CD4−CD8− αβ+ T-cell receptor T cells recognize different mycobacteria strains in the context of CD1b. Immunology. 1995;85:33–40. [PMC free article] [PubMed] [Google Scholar]
- Moody D.B., Reinhold B.B., Guy M.R., Beckman E.M., Frederique D.E., Furlong S.T., Ye S., Reinhold V.N., Sieling P.A., Modlin R.L. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- Behar S.M., Dascher C.C., Grusby M.J., Wang C.-R., Brenner M.B. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis . J. Exp. Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce S., Woods A.S., Yewdell J.W., Bennink J.R., De Silva A.D., Boesteanu A., Balk S.P., Cotter R.J., Brutkiewicz R.R. Natural ligand of mouse CD1d1cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- Schofield L., McConville M.J., Hansen D., Campbell A.S., Fraser-Reid B., Grusby M.J., Tachado S.D. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuda Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramide. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Spada F.M., Koezuda Y., Porcelli S.A. CD1d-restricted recognition of synthetic glycolipid antigens by human NK T cells. J. Exp. Med. 1998;188:1529–1531. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S.T., Kaplan M.H., Grusby M.J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Chiu N.M., Mandal M., Wang N., Wang C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Morita C.T., Beckman E.M., Bukowski J.F., Tanaka Y., Band H., Bloom B.R., Golan D.E., Brenner M.B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Constant P., Davadeau F., Payrat M.A., Paquet Y., Puza G., Bonneville M., Fournie J.J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- Bukowski J.F., Morita C.T., Brenner M.B. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and teaimplications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- Bacon B.R., Olynyk J.K., Brunt E.M., Britton R.S., Wolff R.K. HFE genotype in patients with hemochromatosis and other liver diseases. Ann. Intern. Med. 1999;130:953–962. doi: 10.7326/0003-4819-130-12-199906150-00002. [DOI] [PubMed] [Google Scholar]
- Lebron J.A., Bennett M.J., Vaughn D.E., Chirino A.J., Snow P.M., Mintier G.A., Feder J.N., Bjorkman P.J. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998;93:111–123. doi: 10.1016/s0092-8674(00)81151-4. [DOI] [PubMed] [Google Scholar]
- Junghans R.P., Anderson C.L. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister W.P., Gastinel L.N., Simister N.E., Blum M.L., Bjorkman P.J. Crystal structure at 2.2Å resolution of the MHC-related neonatal Fc receptor. Nature. 1994;372:336–343. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- Burmeister W.P., Huber A.H., Bjorkman P.J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372:379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- Yu Z., Lennon V.A. Mechanisms of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N. Engl. J. Med. 1999;340:227–228. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- Sanchez L.M., Chirino A.J., Bjorkman P.J. Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Science. 1999;283:1914–1919. doi: 10.1126/science.283.5409.1914. [DOI] [PubMed] [Google Scholar]
- Hirai K., Hussey H.J., Barber M.D., Price S.A., Tisdale M.J. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res. 1998;58:2359–2365. [PubMed] [Google Scholar]
- Brown M.S., Goldstein J.L. The SREBP pathwayregulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Wang C.-R., Castano A.R., Peterson P.A., Slaughter C., Lindahl K.F., Deisenhofer J. Nonclassical binding of formylated peptide in crystal structure of the MHC class 1b molecule H2-M3. Cell. 1995;82:655–664. doi: 10.1016/0092-8674(95)90037-3. [DOI] [PubMed] [Google Scholar]
- Berg R.E., Princiotta M.F., Irion S., Moticka J.A., Dahl K.R., Staerz U.D. Positive selection of an H2-M3 restricted T cell receptor. Immunity. 1999;11:33–43. doi: 10.1016/s1074-7613(00)80079-5. [DOI] [PubMed] [Google Scholar]
- Chiu N.M., Chun T., Fay M., Mandal M., Wang C.-R. The majority of H2-M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J. Exp. Med. 1999;190:423–434. doi: 10.1084/jem.190.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerksiek K.M., Busch D.H., Pilip I.M., Allen S.E., Pamer E.G. H2-M3–restricted T cells in bacterial infectionrapid primary but diminished memory responses. J. Exp. Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L. Follow the leaderNK cell receptors for classical and nonclassical MHC class I. Cell. 1998;92:705–707. doi: 10.1016/s0092-8674(00)81398-7. [DOI] [PubMed] [Google Scholar]
- Braud V.M., Allan D.S., O'Callaghan C.A., Soderstrom K., D'Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H. HLA-E binds to natural killer cell receptors CD94/NKG2A, B, and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Corliss B., Wu J., Phillips J.H. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- Li P., Willie S.T., Bauer S., Morris D.L., Spies T., Stron R.K. Crystal structure of the MHC class I homolog MIC-A, a γδ T cell ligand. Immunity. 1999;10:577–584. doi: 10.1016/s1074-7613(00)80057-6. [DOI] [PubMed] [Google Scholar]
- Groh V., Steinle A., Bauer S., Spies T. Recognition of stress-inducible MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Wu J., Song Y., Bakker A.B., Bauer S., Spies T., Lanier L.L., Phillips J.H. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- Komano H., Fujiura T., Kawaguchi M., Matsumoto S., Hashimoto Y., Obana S., Mombaerts P., Tonegawa S., Yamamoto H., Itohara S. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Janeway C.A., Jr. Development of CD8α/α and CD8α/β T cells in major histocompatibility complex class I–deficient mice. J. Exp. Med. 1999;190:881–884. doi: 10.1084/jem.190.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-H., Guy-Grand D., Lemonnier F.A., Wang C.-R., Bendelac A., Jabri B. Selection and expansion of CD8α/α T cell receptor α/β intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J. Exp. Med. 1999;190:885–890. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosella E.D., Dausset J., Rouas-Freiss N. Immunotolerant functions of HLA-G. Cell Mol. Life Sci. 1999;55:327–333. doi: 10.1007/s000180050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F., Jauanguy E., Lamhamedi S., Doffinger R., Fisher A., Casanova J.L. Mendelian susceptibility to mycobacterial infection in man. Curr. Opin. Immunol. 1998;10:413–417. doi: 10.1016/s0952-7915(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Cole S.T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S.V., Eiglmeier K., Gas S., Barry C.E., III Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Apostolou I., Takahama Y., Belmant C., Kawano T., Huerre M., Marchal G., Cui J., Taniguchi M., Nakauchi H., Fournie J.-J. Murine natural killer cells contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl. Acad. Sci. USA. 1999;96:5141–5146. doi: 10.1073/pnas.96.9.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]


