Abstract
The initial interaction between B cells and follicular dendritic cells (FDCs) appears to be essential for germinal center (GC) formation. To identify molecules regulating this interaction, we generated FDC-staining monoclonal antibodies (mAbs) and screened them for their ability to block FDC-mediated costimulation of growth and differentiation of CD40-stimulated B cells. Using one of the inhibitory mAbs, 8D6, we expression cloned the cDNA encoding the 8D6 antigen (Ag) from a human FDC line, HK. The 8D6 Ag is a novel protein of 282 amino acids that is expressed abundantly on FDCs. Monolayers of COS cells transiently transfected with the 8D6 Ag cDNA stimulate B cell growth. The mAb 8D6 blocks the costimulatory function completely. The inhibitory activity of the mAb 8D6 was demonstrated to be due to an inhibition of cell cycle progression of CD40 ligand–stimulated GC B cells. In addition, the mAb 8D6 inhibits the growth of a lymphoma of GC origin, L3055, which depends on FDCs or HK cells for its growth. These findings suggest that the primary function of FDCs in the GC is to stimulate B cell growth. An FDC signal molecule, 8D6 Ag, may be an important molecule to mediate this function.
Keywords: follicular dendritic cell molecule, B cell proliferation, germinal center
Introduction
The germinal center (GC) of the secondary lymphoid follicles provides a microenvironment for B cells to undergo clonal expansion and selection before differentiation into memory B cells 1. The GC reaction is initiated by rapid proliferation of Ag-stimulated B cells in association with follicular dendritic cells (FDCs 2). The mechanism for this rapid growth is largely unknown. The GC B cells exhibit features distinct from naive or memory B cells in that they display a unique pattern of Ag expression on the cell surface 3, undergo Ag receptor–mediated apoptosis 4, and require essential survival signals from FDCs, as disruption of FDC–B cell clusters results in apoptosis of B cells 5 6. This in vitro observation was confirmed in vivo by demonstrating in the lymphotoxin α knockout mice that the initial interaction between FDCs and B cells is essential for GC formation 7 8. T cells expressing CD40 ligand (CD40L) at the same time play a pivotal role in the GC reaction, as evidenced in hyper-IgM patients and in mouse models that have null mutations in the CD40 9 or CD40L genes 10. However, the signals for the survival, proliferation, and differentiation of GC B cells are poorly defined, partly because of the lack of a proper in vitro model to analyze the cellular and molecular interactions between B cells and FDCs.
To overcome the practical difficulty of isolating pure FDCs and to mimic the GC reaction in vitro, we have established an FDC line, HK, from human tonsils and used it to determine molecular and cellular requirements for GC B cell differentiation 11 12. Using this in vitro model, we have identified a key FDC-signaling molecule that stimulates GC B cell proliferation.
Materials and Methods
Abs and Reagents.
Abs used in this work were anti-CD40 (G28-5; American Type Culture Collection); DRC-1 (clone R4/23; DAKO); 7D6 (a gift from Dr. Yong-Jun Liu, Laboratory for Immunological Research, Schering-Plough Corp., Dardilly, France); biotin-conjugated goat anti–mouse Ig or horseradish peroxidase–conjugated goat anti–human IgG (BioSource International); streptavidin–biotinylated horseradish peroxidase complex (Amersham Life Science); FITC-conjugated goat anti–mouse IgG (PharMingen); and rabbit anti–human IgG or alkaline phosphatase–conjugated rabbit anti–mouse IgG (ICN Biomedicals). Annexin V-FITC apoptosis detection kit was purchased from Trevigen. Cytokines used in this work were CD40L (Immunex Corp.), recombinant human (rh)IL-2 (Hoffman-La Roche), rhIL-10 (R&D Systems), rhIL-4 (a gift from Schering-Plough Corp.), and rhIL-6 (Sandoz Research Institute).
Cell Preparation and Cell Lines.
Tonsillar B cells were prepared and GC B cells were isolated by magnetic cell separation (MACS; Miltenyi Biotec) as described previously 12. Fresh FDC clusters were isolated from tonsils of 3–10-yr-old children as described elsewhere 13. The FDC line, HK, was established and maintained as described previously 11. The L3055 cell line was a gift from Dr. Christopher D. Gregory (Institute of Cell Signaling and School of Biomedical Sciences, University of Nottingham Medical School, Queen's Medical Centre, Nottingham, UK) and was cocultured with HK cells in IMDM (Irvine Scientific) supplemented with 10% FCS (Life Technologies), 2 mM glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin (Irvine Scientific).
Preparation of mAbs.
Murine mAbs that react to human tonsillar FDCs were generated using a procedure that involved tolerization before immunization 14. Tolerization was achieved by injection of newborn (within 40 h after birth) BALB/c mice with tonsillar mononuclear cells (MNCs) containing human T and B cells. At 2 mo of age, this animal was injected intraperitoneally with freshly isolated FDC clusters (2 × 104) three times in a 2-wk interval. After the third immunization, the serum from the immunized mouse showed strong reactivity to tonsillar FDCs in frozen tissue sections, but no reactivity to MNCs as assayed by the cell-based ELISA. Spleen cells from this mouse were used as fusion partners with a mouse myeloma cell line, SP 2/0, to generate mAbs. After the cell fusion, >600 hybridomas were grown, and their supernatants were screened for FDC staining as follows. The hybridoma supernatants containing >10 μg/ml of mouse Ig were subjected to test their reactivities to MNCs by ELISA. The hybridomas reacting to MNCs were discarded. The hybridomas that were negative in MNC-based ELISA were subjected to the next screening step: HK binding by ELISA and immunohistochemical staining of the frozen sections of the tonsillar tissues. These screening steps were repeated during a limiting dilution procedure to select a single mAb-producing clone.
Immunohistological Staining.
The adjacent cryostat sections of a human tonsil were fixed in cold acetone. Slides were blocked with 1% (wt/vol) BSA-PBS, then incubated with mAbs 8D6 or DRC-1 followed by biotin-conjugated goat anti–mouse Ig and streptavidin–biotinylated horseradish peroxidase complex. Labeled peroxidase activity was revealed using DAB substrate (Sigma Chemical Co.). Hematoxylin was used as counterstain. For staining FDCs, cytospin preparations were fixed and blocked as above. After incubation with mAb 8D6 or 7D6, the slides were stained with FITC-conjugated goat anti–mouse Ig, then observed under the fluorescence microscope.
Flow Cytometry.
HK cells were released with trypsin and EDTA. Single cell suspensions were stained with mAbs 8D6, 3C8, or mIgG1 followed by FITC-conjugated goat anti–mouse Ig. Flow cytometric analysis was carried out on a FACScan™ (Becton Dickinson) with CELLQuest™ software (Becton Dickinson).
Expression Cloning of 8D6 Ag cDNA.
The gene coding for the 8D6 Ag was expression cloned as described previously 15. In brief, a cDNA expression library from HK cells (average insert size ∼2 kb) was electroporated into Escherichia coli DH10B, and plasmid DNA was prepared from pools of transformants using Wizard® Maxiprep resin (Promega). DNA was transfected into COS-1 cells (clone M6) using LipofectAMINE (GIBCO BRL) according to the manufacturer's instructions. Cells in 100-mm plates were screened by in situ staining 48 h after transfection. Monolayers were washed with PBS and fixed with methanol, followed by a wash with binding buffer (1% [wt/vol] BSA and 0.2% [wt/vol] sodium azide in PBS). Primary mAb (8D6 or mouse IgG) was added at a final concentration of 5 μg/ml in binding buffer for 90 min at 4°C. After washing again with binding buffer, cells were stained with alkaline phosphatase (AP)-conjugated rabbit anti–mouse IgG (1:500 in binding buffer) for 45 min at 4°C. Before developing, AP buffer (100 mM Tris-HCl, pH 9.2, 100 mM NaCl, 50 mM MgCl2, 0.1% [vol/vol] Tween 20, 1 mM levamisole) was added to block endogenous alkaline phosphatase at room temperature for 10 min. Alkaline phosphatase activity was visualized with 0.33% (vol/vol) nitro blue tetrazolium and 0.165% (vol/vol) 5-bromo-4-chloro-3-indolyl-phosphate (Promega) in AP buffer. Once the signal had developed, the positive cells were scored by light microscopy.
Cultures of GC B Cells with HK Cells.
GC B cells (106 cells/ml) were cocultured with irradiated HK cells (2 × 104 cells per well; 5,000 rads) in 24-well plates with or without mAb 8D6 (10 μg/ml, if not indicated otherwise) in the presence of CD40L (100 ng/ml), rIL-2 (10 U/ml), and rIL-10 (20 ng/ml) for 2 d, washed, and then recultured (2 × 105 cells/well) with irradiated HK cells for another 4 d. Viable cells were counted by trypan blue exclusive assay for proliferation. For differentiation, triplicate culture supernatants were harvested and pooled, and IgG concentrations were measured by ELISA as described elsewhere 16. For cell cycle analysis, GC B cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE, 5 μM/ml in PBS; Sigma Chemical Co.) at 4°C for 10 min. After culture as indicated above, the CFSE intensity was analyzed by FACScan™.
Cultures of B Cells with COS Cells Expressing 8D6 Ag.
COS cells (2 × 105/well) in 6-well plates were transfected with 2 μg 8D6 Ag cDNA and LipofectAMINE. After 24 h, transfected COS cells were used for coculture with tonsillar B cells (106/ml) in the presence of anti-CD40 (100 ng/ml), rhIL-2 (10 U/ml), rhIL-4 (50 U/ml), rhIL-6 (20 ng/ml), and rhIL-10 (20 ng/ml) for 24 h. Activated B cells were removed from COS cells, recultured in triplicate in 96-well plates in the presence of the above cytokines, and subjected to proliferation (3 d) or differentiation (10 d) assays as described above.
Results and Discussion
Generation of mAbs Staining FDCs.
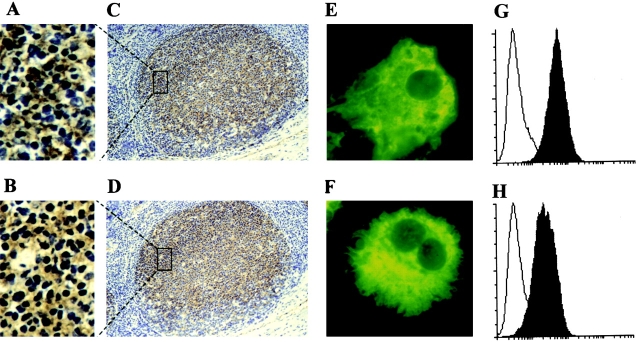
To identify FDC molecules involved in the FDC–B cell interaction, a panel of murine mAbs was raised against FDC clusters isolated from human tonsils, and the mAbs were tested for their ability to inhibit costimulation by FDCs of B cell growth and differentiation. Mice were first tolerized neonatally with human T and B cells, then immunized with FDC clusters to obtain FDC-specific mAbs. 28 hybridomas were identified that specifically stained the tonsillar GC. Of 28 hybridomas, 17 mAb clones were obtained by a limiting dilution method. 16 IgG mAbs were purified from ascites by affinity chromatography with protein A/G. One of these mAbs, an IgG1 called 8D6, recognized a molecule expressed in human tonsillar GCs (Fig. 1A and Fig. C) with an immunohistochemical staining pattern similar to that of the known FDC-specific mAb, DRC-1 (Fig. 1B and Fig. D; reference 17). The mAb 8D6 staining was restricted to the follicles (Fig. 1 C). There is a clear demarcation between the GC and the T cell–rich area outside of the GC, which is negative for all three mAbs (e.g., DRC-1, 7D6, and 8D6). The 8D6 Ag is abundantly expressed in the GC, staining the reticular network in the higher magnification (Fig. 1 A). The diffuse staining pattern of mAb 8D6 in the GC is characteristic of FDCs surrounding MNCs in the follicles. Such a staining pattern was confirmed by the simultaneous staining of the adjacent tissue sections with known FDC-specific mAbs such as DRC-1 (Fig. 1 B; reference 17) and 7D6 18. As it is virtually impossible to obtain a sufficient number of purified FDCs, without contamination with B cells, for FACS® analysis, a single cell suspension was prepared as a cytospin. The cytospin preparations were stained with FDC-staining mAbs and examined under the microscope. At the single cell level, mAbs 8D6 (Fig. 1 E) and 7D6 (Fig. 1 F), another known FDC marker 18, both stained large, cytoplasm-rich, sometimes binucleated FDCs. The isotype-matched control Abs did not stain FDCs, excluding the possibility of autofluorescence.
Figure 1.
(A–F) Immunohistological localization of 8D6 Ag expression in human FDCs. The serial cryostat sections from a normal human tonsil (C and D) or cytospin preparations of human tonsil FDC clusters (E and F) were stained with mAbs 8D6 (A, C, and E), DRC-1 (B and D), and 7D6 (F). A and B are higher power views of boxed areas in C and D, respectively, showing the network staining pattern. Original magnifications: (C and D) ×100; (E and F) ×1,000. (G and H) 8D6 Ag expression on HK cells. HK cells were stained with mAbs 8D6 (G, filled histogram), 3C8 (H, filled histogram), or isotype control mouse IgG1 (G and H, open histograms), followed by FITC-conjugated goat anti–mouse IgG. FITC intensity was analyzed by FACScan™.
Functional Blocking Activity of FDC-specific mAb 8D6 in FDC–B Cell Interaction.
To measure its ability to block FDC–B cell interaction, mAb 8D6 was used in the coculture of B cells and FDC clusters. Because Ag-activated T cells participate in GC reactions by direct cell-to-cell contact and by secreting cytokines 19, we used the defined signals of activated T cells, such as anti-CD40, IL-2, and IL-10, to characterize mAb 8D6 functions. mAb 8D6 consistently inhibited the FDC-mediated B cell IgG secretion (Fig. 2 A). The blocking activity of mAb 8D6 is specific because another FDC-specific murine IgG1, mAb 3C8, did not inhibit the FDC costimulatory activity (Fig. 2 A). mAb 3C8 was prepared by immunizing mice with HK cells, and its Ag was abundantly expressed in FDC and HK cells (Fig. 1 H; reference 12). In addition to mAb 3C8, the other 13 IgG1, FDC-specific mAbs prepared in parallel with mAb 8D6 did not show blocking activity. Furthermore, five other known human FDC-specific mAbs obtained by various investigators, namely DRC-1, 7D6, HJ-2, GP93, and Ki-M4 17 18 20 21 22, did not inhibit the FDC costimulatory activity in our assay (data not shown).
Figure 2.
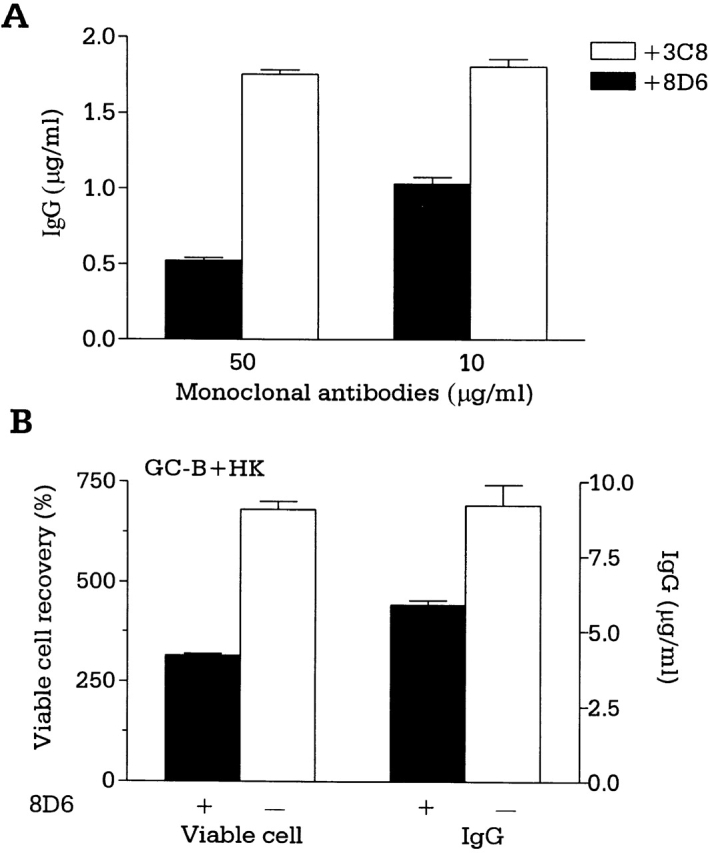
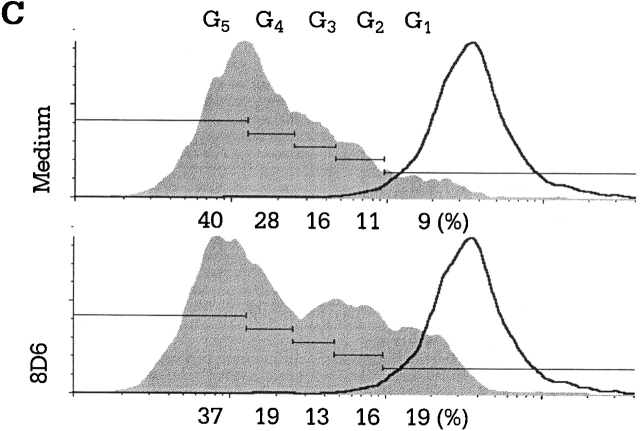
mAb 8D6 inhibits FDC–B cell interaction. (A) Tonsillar B cells were cocultured with irradiated FDC clusters (2,000 clusters per well; 5,000 rads) for 10 d in the presence of mAb 8D6 or 3C8, anti-CD40 (100 ng/ml), rhIL-2 (10 U/ml), and rhIL-10 (30 ng/ml). IgG levels in conditioned media were measured by human IgG–specific ELISA. IgG concentrations in the control cultures with B cells and B cells plus FDCs were 0.26 and 1.8 μg/ml, respectively. (B and C) GC B cells were cocultured with or without mAb 8D6 in the presence of irradiated HK cells, CD40L, rhIL-2, and rhIL-10. Results of viable cell recovery (B, left), IgG secretion (B, right), and cell cycle progression (C) are shown.
A human FDC line, HK, resembles primary FDCs in its ability to rescue GC B cells from apoptosis 11 and to support GC B cell growth and differentiation 12, suggesting that HK cells and FDCs share common signaling molecules for GC B cell differentiation. In the cocultures with HK cells, GC B cell proliferation and differentiation supported by HK cells were inhibited by the addition of mAb 8D6, but not by the isotype control mAb 3C8 (Fig. 2 B). Such inhibitory activity was also confirmed by demonstrating the inhibition of the cell cycle progression of the CFSE-labeled GC B cells after activation by soluble CD40L. When compared with the control, GC B cells were observed in twofold excess (19 vs. 9%) in the first generation and 1.5-fold (16 vs. 11%) in the second generation after HK cells were treated with mAb 8D6 in the culture with CD40L, IL-2, and IL-10 for 6 d (Fig. 2 C). This result suggests that inhibition by mAb 8D6 is due to the delay of B cell proliferation, rather than to direct cell killing.
Expression Cloning of the 8D6 Ag cDNA.
We used the HK cell line to isolate the 8D6 Ag because of the difficulty of obtaining a sufficient number of ex vivo FDCs to prepare a cDNA library, and because HK expresses the 8D6 and 3C8 Ags (Fig. 1G and Fig. H). The 8D6 Ag is expressed on the HK cell membrane surface as well as in the intracellular compartments. The cDNA encoding the 8D6 Ag was expression cloned in transfected COS cells using the HK cell cDNA library. The predicted amino acid sequence encoded by this cDNA consists of 282 residues (Fig. 3 A). Hydropathy analysis revealed a putative NH2-terminal signal sequence of 32 amino acids and a second hydrophobic region close to the COOH terminus that was likely to be a transmembrane domain followed by a short intracellular tail.
Figure 3.
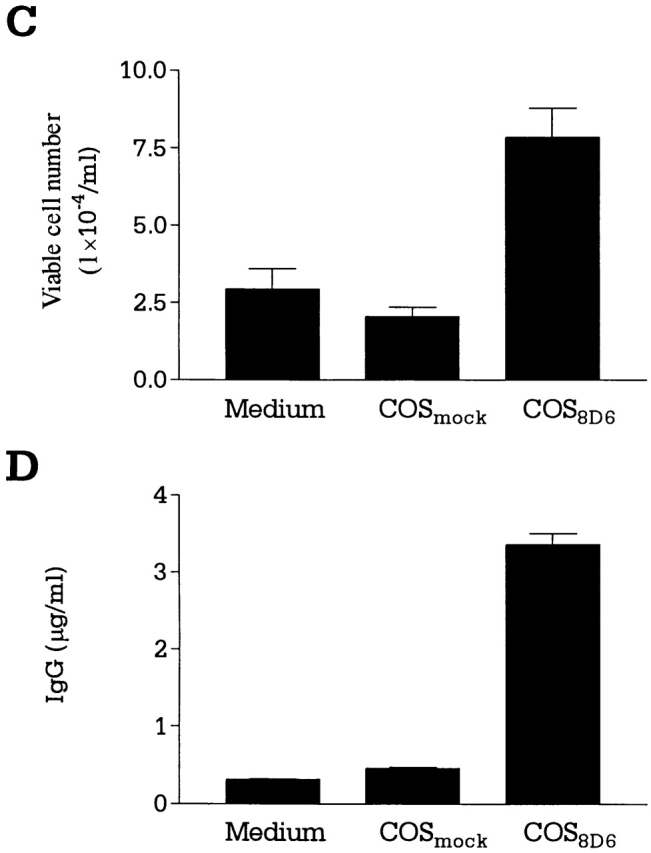
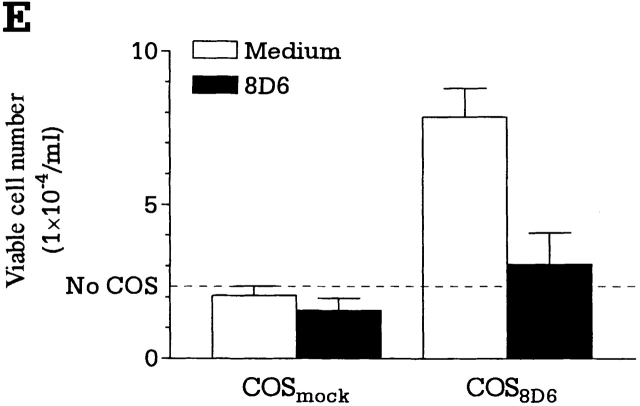
(A and B) Expression cloning of the 8D6 Ag cDNA. (A) An HK cell cDNA expression library was screened by in situ staining of transiently transfected COS cells with the mAb 8D6. A cDNA clone encoding the 8D6 Ag was isolated, and its predicted amino acid sequence is shown. The nucleotide sequence was deposited with EMBL/GenBank/DDBJ (sequence data available under accession no. AF161254). A putative signal sequence cleavage site is indicated with an arrowhead, and a predicted transmembrane domain is underlined. Three consensus sites for N-linked glycosylation are each marked with an asterisk. Each cysteine residue is highlighted with dark shading, and the two regions homologous to the LDL-R type A domain are shown in light shaded boxes. (B) The two regions homologous to the LDL-R (LDLR) type A domain, named the 8D6 Ag A (8D6-A, residues 54–89) and the 8D6 Ag B (8D6-B, residues 132–167) domains are shown aligned to the NH2-terminal type A domain of the human LDL-R and the human complement C9 protein. Identical residues are shown in dark background, and conserved residues in light background. (C–E) 8D6 Ag cDNA–transfected COS cells stimulate B cell proliferation and differentiation. Mock (COSMock)or 8D6 Ag cDNA (COS8D6)-transfected COS cell–B cell cocultures were performed. The viable cell number of B cells (C) and IgG secretion data (D) was shown. In blocking experiments, transfected COS cells were treated with mAb 8D6 for 30 min at 37°C before coculture with B cells (E).
The extracellular region contains two domains homologous to the type A cysteine-rich repeats found in the low-density lipoprotein receptor (LDL-R) family and several other proteins, including the terminal complement components such as C8 and C9 23 24. Each domain of the 8D6 Ag contains both the six cysteines and the acidic residues between the fifth and sixth cysteines that are characteristic of this domain (Fig. 3 B). The three-dimensional structure of one domain of the human LDL-R has been determined by nuclear magnetic resonance spectroscopy and has been found to consist of a β hairpin followed by a series of β turns, with the majority of the acidic residues on one side of the structure 25. The two domains of the 8D6 Ag are separated by an additional cysteine-rich region interrupted by a stretch of four proline residues. There is a consensus site for N-glycosylation in this spacer region and two additional sites located between the second LDL-R type A domain and the putative transmembrane domain.
The similarity of the 8D6 Ag cysteine-rich type A domains to homologous sequences was further examined using the GAP program with the blosum62 matrix (www.gcg.com; Fig. 3 B). The first cysteine-rich domain of the 8D6 Ag (8D6A) has 62% similarity (50% identity) to the domain of human C9, and 54% similarity (49% identity) to that of the NH2-terminal domain of the human LDL-R. The second cysteine-rich domain of the 8D6 Ag (8D6B) has 44% similarity (38% identity) to the C9 domain, and 46% similarity (43% identity) to that of the LDL-R. The homology of the 8D6 Ag with the LDL-R family was limited to these domains.
The LDL-R has been intensively investigated because its malfunction causes atherosclerosis 26. The LDL-R is essential to transport LDL out of plasma into the tissues. Recently, it has become clear that most of the LDL-R superfamily does not have a primary function in LDL import, but binds multiple ligands in motility and adhesions of neurons 27. For example, very low-density lipoprotein receptor (VLDL-R) is involved in neuron traffic in brain development, as demonstrated recently in VLDL-R knockout mice 28. It is intriguing to find that the 8D6 molecule shares a significant homology with LDL-R. Hence, it is important to investigate whether the 8D6 molecule expressed in the GC is involved in attracting Ag-activated B cells to expand in the initial interaction stage of FDC B cells. This hypothesis can be studied in the 8D6 gene-targeted mice.
Similar to the broad expression of LDL-R genes in tissues, 8D6 Ag gene is expressed in many tissues, as 8D6 mRNA was detected in most human tissues by Northern blot (data not shown). As 8D6 Ag is expressed abundantly in FDCs in the GC (Fig. 1), the mAb 8D6 may recognize the functionally important epitope of the 8D6 protein involved in FDC–B cell interaction in the GC.
Recombinant 8D6 Ag Enhances B Cell Growth.
The functional activity of 8D6 Ag in B cell costimulation was analyzed in vitro by using transfected COS cells. In the cocultures with anti-CD40–activated B cells, the 8D6 Ag–transfected COS cells enhanced B cell proliferation (Fig. 3 C) and differentiation by inducing IgG secretion (Fig. 3 D) two to seven times higher than the mock-transfected COS cells. In addition, this activity was specifically blocked by mAb 8D6 (Fig. 3 E), but not by the control Ab with the same isotype. Thus, the 8D6 Ag expressed by COS cells was able to provide a specific costimulatory signal in augmenting growth and differentiation of GC B cells.
The Growth Inhibition of Lymphoma Cell Line, L3055, by mAb 8D6.
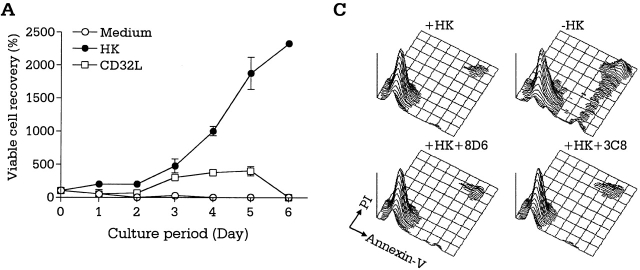
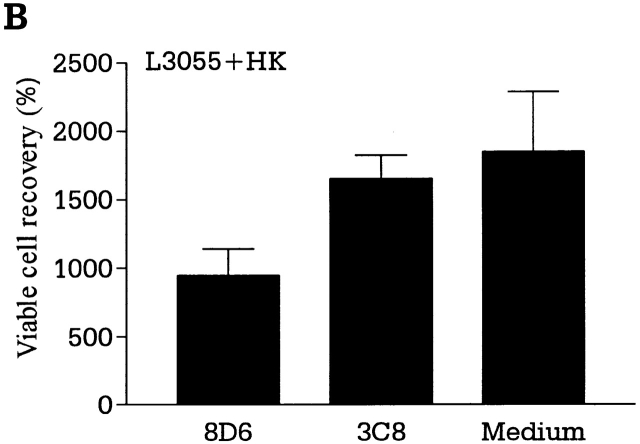
GC B cells undergo complex interactions with FDCs and T cells in the course of differentiation into memory B and plasma cells. GC B cells freshly isolated from tonsils are heterogeneous regarding the stage of differentiation, mutation frequency, and Ig class 29. They may not be ideal for characterizing the external signals operating at each stage of B cell growth and differentiation in the GC. A monoclonal population of dividing cells would be devoid of the problems of freshly isolated GC B cells. To delineate the individual roles of FDCs and T cells in GC B cell differentiation at the clonal level and to analyze the signals involved, we adopted a unique experimental model using an FDC line, HK, and a lymphoma cell line, L3055, that resembles centroblasts 13. A detailed phenotypic analysis revealed L3055 cells to be a clonal population originating from the GC. Like freshly isolated centroblasts, L3055 cells underwent spontaneous apoptosis when cultured in the absence of fresh FDCs or HK cells. L3055 cells proliferated continuously in the presence of HK cells (Fig. 4 A), whereas they differentiated into a population with the phenotype of centrocytes after stimulation with CD40L and IL-4 13. The experimental results demonstrate a distinct function of FDCs, in that FDCs provide signals for rapid proliferation of centroblasts, whereas T cells confer signals for differentiation of centroblasts into centrocytes. T cells collaborate with FDCs in the protection and expansion of the Ag-specific GC B cells. The L3055 growth-supporting activity of HK cells cannot be replaced by CD32-transfected L cells, and therefore is presumably not a nonspecific feeder cell effect (Fig. 4 A). In addition, the apparent activity of HK cells in this coculture is decreased by the addition of the mAb 8D6, but not of isotype control 3C8 (Fig. 4 B). These results suggest that 8D6 Ag may be an important factor for the growth of lymphoma cells originating in the GC. The inhibition by mAb 8D6 alone is not complete, suggesting the presence of other growth factors.
Figure 4.
mAb 8D6 inhibits HK-dependent L3055 cell proliferation. (A and B) HK-dependent L3055 cells (2 × 104 cells/ml) were cultured in the presence of irradiated HK or CD32-transfected L cells (2 × 104 cells per well; 5,000 rads) for the indicated time periods in 24-well plates (A). In a separate experiment, L3055 cells were cultured with or without mAb 8D6 or 3C8 in the presence of irradiated HK cells for 5 d (B). Viable cells were counted by trypan blue exclusion assay, and viable cell recovery percentages are shown. According to a Student's t test, the differences between the treatments with mAbs 8D6 or 3C8 were statistically significant (P < 0.05), and the differences between the treatment with mAb 3C8 and no treatment were not significant (P > 0.05). (C) L3055 cells (104 cells/ml) were cultured in the presence or absence of irradiated HK cells for 24 h. HK cells were pretreated with mAbs 8D6 or 3C8 for blocking experiments. Cultured cells were stained with FITC-labeled annexin V and propidium iodide.
The growth inhibition by mAb 8D6 could result either from the apoptosis of L3055 cells or from neutralizing a growth factor. To determine whether mAb 8D6 affected apoptosis, we analyzed the effect of mAb 8D6 on apoptosis by labeling the cells with annexin V and propidium iodide. As shown in Fig. 4 C, mAb 8D6 did not interfere with the capacity of HK cells to prevent spontaneous apoptosis. This result suggests that the 8D6 Ag molecule is not an antiapoptosis factor. Taken together, these results strongly suggest that 8D6 Ag is a novel membrane molecule of FDCs that augments proliferation of B cells in the GC.
In the early stage, follicular lymphoma, one of the most common hematological malignancies in adults, is usually indolent, regressing spontaneously and showing susceptibility to chemotherapy 30. However, this tumor usually recurs and can undergo blast transformation to an aggressive form, ultimately becoming a fatal disease. The generation and blast transformation of this tumor occurs in close association with FDCs in the GC 31. We have demonstrated that the in vitro growth of L3055 can be blocked by mAb against the 8D6 Ag. Thus, this molecule deserves consideration as a critical signal in the early stage of lymphomagenesis. If our observation in vitro extrapolates to the in vivo growth of such tumors, antagonists of the 8D6 Ag signaling pathway may be of value in countering the growth of follicular lymphomas or other tumors that metastasize to the lymphoid follicles.
Acknowledgments
We would like to thank Dr. B. Butcher, the other otolaryngologists at Ochsner Clinic for providing tonsil specimens, Dr. C.G. Figdor (University Hospital, Nijmegen, The Netherlands) for anti-CD44 Ab, Dr. R. Armitage (Immunex Corp., Seattle, WA) for CD40L, and Dr. C.D. Gregory (University of Nottingham Medical School, Nottingham, UK) for the L3055 cell line.
Footnotes
L. Li and X. Zhang contributed equally to this work.
References
- Kelsoe G. The germinal center reaction. Immunol. Today. 1995;16:324–326. doi: 10.1016/0167-5699(95)80146-4. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Barthelemy C., de Bouteiller O., Arpin C., Durand I., Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2:239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Billian G., Mondiere P., Berard M., Bella C., Defrance T. Antigen receptor-induced apoptosis of human germinal center B cells is targeted to a centrocytic subset. Eur. J. Immunol. 1997;27:405–414. doi: 10.1002/eji.1830270210. [DOI] [PubMed] [Google Scholar]
- Kosco M.H., Pflugfelder E., Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro . J. Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- Koopman G., Keehnen R.M., Lindhout E., Newman W., Shimizu Y., van Seventer G.A., de Groot C., Pals S.T. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J. Immunol. 1994;152:3760–3767. [PubMed] [Google Scholar]
- Gonzalez M., Mackay F., Browning J.L., Kosco-Vilbois M.H., Noelle R.J. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.-X., Huang G., Wang Y., Chaplin D.D. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α–dependent fashion. J. Exp. Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune responses in CD40-deficient miceimpaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Renshaw B.R., Fanslow W.C., III, Armitage R.J., Campbell K.A., Liggitt D., Wright B., Davison B.L., Maliszewski C.R. Humoral immune responses in CD40 ligand–deficient mice. J. Exp. Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-S., Zhang X., Klyushnenkova E., Choi Y.S. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J. Immunol. 1995;155:1101–1109. [PubMed] [Google Scholar]
- Choe J., Kim H.-S., Zhang X., Armitage R.J., Choi Y.S. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti-Ig down-regulates Fas expression on CD40 ligand-stimulated germinal center B cells and inhibits Fas-mediated apoptosis. J. Immunol. 1996;157:1006–1016. [PubMed] [Google Scholar]
- Choe J., Li L., Zhang X., Gregory C.D., Choi Y.S. Distinct role of follicular dendritic cells and T cells in the proliferation, differentiation, and apoptosis of a centroblast cell line, L3055. J. Immunol. 2000;164:56–63. doi: 10.4049/jimmunol.164.1.56. [DOI] [PubMed] [Google Scholar]
- Golumbeski G.S., Jr., Dimond R.L. The use of tolerization in the production of monoclonal antibodies against minor antigenic determinants. Anal. Biochem. 1986;154:373–381. doi: 10.1016/0003-2697(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Tai X.-G., Yashiro Y., Abe R., Toyooka K., Wood C.R., Morris J., Long A., Ono S., Kobayashi M., Hamaoka T. A role for CD9 molecules in T cell activation. J. Exp. Med. 1996;184:753–758. doi: 10.1084/jem.184.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Young D., Wolf S.F., Choi Y.S. Interleukin-12 stimulates B cell growth by inducing IFN-γ. Cell. Immunol. 1996;168:133–140. doi: 10.1006/cimm.1996.0059. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D.Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J. Clin. Pathol. 1983;36:167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Xu J., de Bouteiller O., Parham C.L., Grouard G., Djossou O., de Saint-Vis B., Lebecque S., Banchereau J., Moore K.W. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J. Exp. Med. 1997;185:165–170. doi: 10.1084/jem.185.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Hathcock K., Zheng B., Kepler T.B., Hodes R., Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- Butch A.W., Hug B.A., Nahm M.H. Properties of human follicular dendritic cells purified with HJ2, a new monoclonal antibody. Cell. Immunol. 1994;155:27–41. doi: 10.1006/cimm.1994.1099. [DOI] [PubMed] [Google Scholar]
- Farace F., Kieffer N., Caillou B., Vainchenker W., Tursz T., Dokhelar M.C. A 93-kDa glycoprotein expressed on human cultured monocytes and dendritic reticulum cells defined by an anti-K562 monoclonal antibody. Eur. J. Immunol. 1986;16:1521–1526. doi: 10.1002/eji.1830161209. [DOI] [PubMed] [Google Scholar]
- Parwaresch M.R., Radzun H.J., Hansmann M.L., Peters K.P. Monoclonal antibody Ki-M4 specifically recognizes human dendritic reticulum cells (follicular dendritic cells) and their possible precursor in blood. Blood. 1983;62:585–590. [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K.K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptorsmacrophage scavenger receptors and LDL receptor-related protein (LRP) Annu. Rev. Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Daly N.L., Scanlon M.J., Djordjevic J.T., Kroon P.A., Smith R. Three dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 1995;92:6334–6338. doi: 10.1073/pnas.92.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.A., Howell B.W. Lipoprotein receptorssignaling functions in the brain? Cell. 1999;97:671–674. doi: 10.1016/s0092-8674(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Bar I., Goffinet A.M. Developmental neurobiology. Decoding the Reelin signal. Nature. 1999;399:645–646. doi: 10.1038/21340. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R.E., Richardson J.A., Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Pascual V., Liu Y.J., Magalski A., de Bouteiller O., Banchereau J., Capra J.D. Analysis of somatic mutation in five B cell subsets of human tonsil. J. Exp. Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning S.J., Rosenberg S.A. The natural history of initially untreated low-grade non-Hodgkin's lymphoma. N. Engl. J. Med. 1984;311:1471–1475. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- Petrasch S., Kosco M., Perez-Alvarez C., Schmitz J., Brittinger G. Proliferation of non-Hodgkin-lymphocytes in vitro is dependent upon follicular dendritic cell interactions. Br. J. Haematol. 1992;80:21–26. doi: 10.1111/j.1365-2141.1992.tb06395.x. [DOI] [PubMed] [Google Scholar]