Abstract
Signal transduction through the B cell antigen receptor (BCR) is altered in B cells that express a receptor that recognizes self-antigen. To understand the molecular basis for the change in signaling in autoreactive B cells, a transgenic model was used to isolate a homogeneous population of tolerant B lymphocytes. These cells were compared with a similar population of naive B lymphocytes. We show that the BCR from naive B cells enters a detergent-insoluble domain of the cell within 6 s after antigen binding, before a detectable increase in BCR phosphorylation. This fraction appears to be important for signaling because it is enriched for lyn kinase but lacks CD45 tyrosine phosphatase and because the BCR that moves into this domain becomes more highly phosphorylated. Partitioning of the BCR into this fraction is unaffected by src family kinase inhibition. Tolerant B cells do not efficiently partition the BCR into the detergent-insoluble domain, providing an explanation for their reduced tyrosine kinase activation and calcium flux in response to antigen. These results identify an early, regulated step in antigen receptor signaling and self-tolerance.
Keywords: B cell antigen receptor signaling, tyrosine phosphorylation, membrane raft, self-tolerance, anergy
Introduction
Mice expressing transgenes for anti–hen egg lysozyme (HEL) IgM/IgD B cell antigen receptor (BCR) contain large homogeneous populations of B cells that are functionally naive. When anti-HEL transgenic mice are crossed to transgenic mice expressing soluble HEL, the B cells in the double-transgenic offspring are constantly exposed to low levels of antigenic stimulation and become functionally tolerant (anergic). The tolerant cells lose some responses to antigen, notably BCR-induced proliferation, but retain other responses, including BCR-induced apoptosis, changes in follicular homing, and inhibition of plasmacyte differentiation 1 2 3. Binding of HEL to the BCR on these cells results in only a subset of the biochemical responses that are triggered by HEL in naive anti-HEL transgenic B cells. For example, the BCR on tolerant B cells continually activates low intracellular calcium oscillations and extracellular signal–regulated kinase (ERK), and nuclear factor of activated T cells (NFAT)c pathways but does not produce high calcium elevations or activate the c-Jun NH2-terminal kinase (JNK) and nuclear factor (NF)-κB pathways 4.
How the BCR is selectively uncoupled from some signaling pathways but not others is unclear. Antigen-induced protein tyrosine phosphorylation of the BCR and other molecules is greatly reduced in HEL-tolerant B cells 5. However, tolerant B cells express at normal levels and with normal basal activity proteins important for BCR-induced signal transduction, such as syk, lyn, CD45, and Src homology 2 domain–containing protein tyrosine phosphatase (SHP)-1.
Here we present evidence for an early, regulated step in BCR signaling that may resolve this paradox. We find that in naive cells, the stimulated BCR becomes associated with a detergent-insoluble fraction containing lyn, and that in tolerant cells, the BCR does not efficiently associate with this fraction. Partitioning of the naive BCR occurs in less than 6 s and does not require src family tyrosine kinase activation. This suggests a novel mechanism of maintaining tolerance by physically segregating the BCR from downstream signaling components.
Materials and Methods
Mice.
Mice transgenic for the MD4 anti-HEL–specific BCR were bred either to B6 mice or to mice expressing the soluble HEL ML5 transgene 1. All mice used were between 8 and 16 wk of age.
Stimulation.
Spleens from transgenic animals were harvested and washed in room temperature RPMI media containing 10 mM Hepes, pH 7.6, and 1% FCS. Red blood cells were lysed at room temperature with Tris ammonium chloride. In experiments using pure B cells, spleen cells were incubated with 10 μl anti-B220 microbeads (Miltenyi Biotec) per 107 cells and purified on a miniMACS™ column (Miltenyi Biotec) to purity of >95% B220+. Cells were resuspended at 107 cells per 200-μl sample in RPMI, 10 mM Hepes. Cells were warmed to 37°C for 3 min and then stimulated as indicated. In some experiments, PP1 6 was added to cells for 10 min before stimulation at the indicated concentration (see Fig. 4).
Figure 4.
BCR partitioning to the detergent-insoluble fraction is independent of src family kinase activity. Spleen cells from anti-HEL BCR transgenic mice were stimulated in the presence of varying concentrations of the src family–specific kinase inhibitor PP1. Tyrosine phosphorylation of Igα and Igβ in unstimulated and stimulated cells was measured by antiphosphotyrosine Western blotting (bottom panel). Partitioning of the BCR to the salt extract fraction was measured by probing the same blots for Igα and Igβ (top panel).
Fractionation.
Cells were fractionated to isolate signaling domains essentially as described by Marano et al. 7. Cells were centrifuged for 3 min at 800 g after stimulation and resuspended at 107 cells per 200 μl of 4°C lysis buffer (0.5% Triton X-100, 20 mM Hepes, pH 7.6, 10.3% sucrose, 20 mM NaCl, 5 mM MgCl2, 0.1 mg/ml BSA, 2 mM sodium orthovanadate, 2.5 mM PMSF, 500 μg/ml aprotinin, 500 μg/ml leupeptin, 100 μM phenylarsine oxide, 5 μM PP1). In time course experiments, 1.3× lysis buffer containing no NaCl was added directly to the stimulated cells instead of centrifuging and resuspending. Lysed cells were centrifuged at 400 g for 10 min at 4°C, and the ‘detergent-soluble’ fraction was removed from the pellet. The pellet was washed in the same buffer and then resuspended in the same buffer containing 150 mM NaCl instead of 20 mM and centrifuged again. The supernatant was removed and labeled as the detergent-insoluble ‘salt extract.’
Western Blotting.
Samples were added to 5× sample buffer, boiled, and resolved on 10% SDS-PAGE gels. In all cases, three times as many cell equivalents of salt extract were loaded as compared with corresponding lanes of the detergent-soluble fraction. Gels were transferred to polyvinylidene difluoride nylon membranes and immunoblotted with antiphosphotyrosine mAb 4G10 (Upstate Biotechnology, Inc.), rabbit anti-syk (a gift from J. Richards and A. DeFranco, University of California San Francisco, San Francisco, CA), rabbit anti-CD22 (gift from P. Crocker, Oxford University, Oxford, UK), rabbit anti-CD45, or with a mixture of anti-lyn, anti-Igα, anti-Igβ 8, and anti-κ L chain (Southern Biotechnology Associates, Inc.). Cholera toxin–reactive GM1 sphingolipids were detected on the dye front using cholera toxin–horseradish peroxidase (Sigma Chemical Co.). For quantitation, blots were developed with Protein A–125I (ICN), imaged on a Molecular Dynamics PhosphorImager, and quantitated with ImageQuant software (Molecular Dynamics). For Fig. 1 E, images of chemiluminescent blots were scanned and analyzed with QuantityOne (Bio-Rad Labs.). Statistical analysis was performed by first standardizing the densitometric values of Igα phosphorylation in each experiment by dividing them by the value for the extract fraction in that experiment and expressing them as percent of phosphorylation in extract (i.e., extract phosphorylation = 100%). The values for relative phosphorylation induced by antigen in the supernatant were then analyzed by one-tailed t test, with H0: μ = 100% and H1: μ < 100%.
Figure 1.
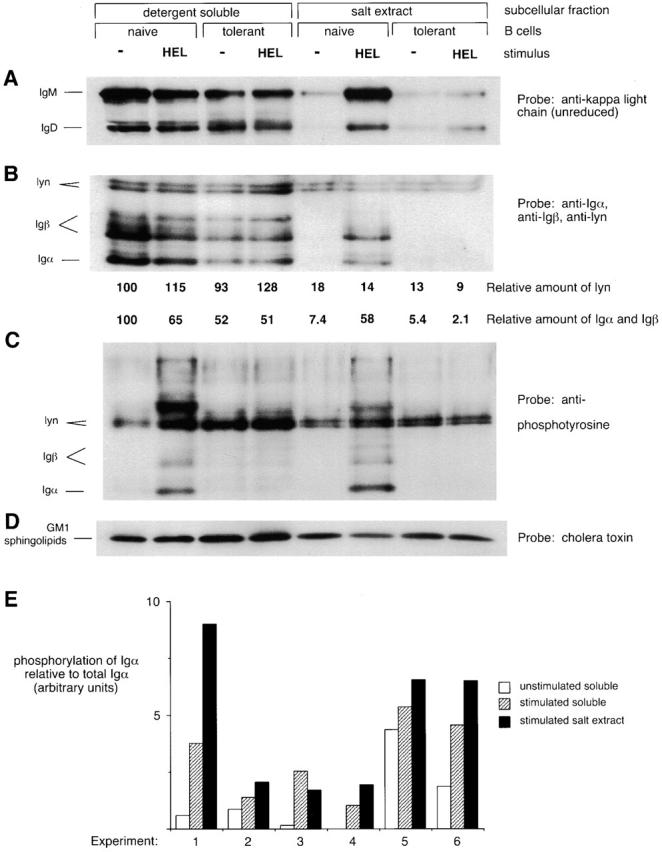
BCRs accumulate in a subcellular compartment that is detergent insoluble and salt extractable after antigen stimulation of naive but not tolerant B cells. Spleen cells from anti-HEL BCR transgenic (naive B cells) or anti-HEL/soluble HEL double-transgenic mice (tolerant B cells) were mock stimulated or stimulated with 10 μg/ml HEL for 3 min and then fractionated to obtain the detergent-soluble supernatant and the detergent-insoluble salt extract. The salt extract lanes represent three times as many cell equivalents as the detergent-soluble lanes. (A) Unreduced SDS-PAGE Western blots were probed with anti–κ L chain to resolve IgM and IgD H chain–L chain BCR complexes. (B–D) Reduced SDS-PAGE Western blots were probed (B) with a mixture of anti-Igα, anti-Igβ, and anti-lyn; (C) with 4G10 antiphosphotyrosine; (D) with cholera toxin, which binds to GM1 sphingolipids running with the dye front. In B, numbers below each lane are the amount of lyn immunoreactivity, corrected for loading, and the amount of Igα and Igβ immunoreactivity normalized to the average amount of lyn in each fraction. Quantitation was determined by PhosphorImager analysis of 125I–Protein A-developed blots. (E) Data from six independent experiments show that Igα in the salt extract fraction is more highly phosphorylated than Igα in the soluble fraction after stimulation of naive B cells. The degree of phosphorylation of Igα was obtained by dividing the intensity of the Igα-sized band on the antiphosphotyrosine blot by the intensity of the Igα band on the same blot reprobed with anti-Igα. The degree of Igα phosphorylation induced in the extract was significantly different from that induced in the supernatant to P < 0.1.
Results
To track the subcellular location of BCR and signaling molecules on naive and tolerant B cells, we adapted a fractionation technique described by Marano et al. 7. Spleen B cells lysed in 0.5% Triton X-100, sucrose, and low salt buffer at 4°C release most of their BCR into the detergent-soluble supernatant. However, after stimulation with HEL antigen, a portion of the BCR from anti-HEL transgenic B cells becomes insoluble in this buffer but can be released by increasing the NaCl concentration to 150 mM. IgM and IgD BCR complexes containing H chain, L chain, Igα, and Igβ become detergent insoluble and salt extractable after stimulation (Fig. 1). While antigen stimulated an increased tyrosine phosphorylation of Igα in both the detergent-soluble and the salt extract fractions, Igα in the salt extract fraction was more highly phosphorylated (Fig. 1C and Fig. E).
The salt extract fraction of unstimulated and stimulated cells contains 10–30% of cellular lyn (15% in the experiment shown in Fig. 1 B). A similar fraction of GM1 sphingolipids in the cell, detected by cholera toxin Western blotting, was also present in the salt-extracted fraction. The compartmentalization of the BCR to the detergent-insoluble fraction is consistent with the idea that to generate antigen-induced signals, kinase activity must be separated from phosphatase activity 9. To determine whether there was segregation of the BCR from other membrane proteins, other membrane-associated molecules were examined by Western blotting. The salt extract fraction contains <0.3% of cellular CD45 and no detectable CD22 (Fig. 2). Also, only 2.5% of cellular Igα and Igβ is present in the salt extract fraction before stimulation (Fig. 1 B). These data show that the salt extract fraction is enriched for certain positive signaling molecules and depleted for certain negative signaling molecules. The exclusion of CD45 from the detergent-insoluble fraction is particularly significant, as this transmembrane tyrosine phosphatase represents most of the phosphatase activity at the membrane and could serve to inhibit signal transduction 9.
Figure 2.
The salt extract fraction contains lyn and stimulated BCR but not syk, CD22, or CD45. Spleen cells from anti-HEL BCR transgenic mice were stimulated and separated as in Fig. 1 and probed with a mixture of anti-Igα, anti-Igβ, and anti-lyn or with anti-syk, anti-CD22, or anti-CD45. Three times as many cell equivalents were loaded in the salt extract fraction lanes as in the soluble fraction lanes. The CD45 blot is from a different experiment than the other blots, but BCR partitioning was similar in that experiment. 125I quantitation shows >300-fold enrichment of CD45 in the soluble fraction.
As early signaling events are inhibited in tolerant B cells, we analyzed whether or not BCRs on tolerant cells could partition into the salt extract fraction after stimulation. The salt extract fraction of tolerant B cells appeared similar to unstimulated naive cells in that it contained similar amounts of lyn, GM1 sphingolipids, and basal amounts of the BCR. However, only a small increase in the amount of BCR in the salt extract was detected after stimulation of tolerant B cells (Fig. 1). Quantitation of 125I-developed Western blots showed a sevenfold increase in the amount of the Igαβ complex in the salt extract of naive cells after stimulation, whereas no increase was detectable in the amount of the Igαβ complex in the salt extract of tolerant cells after stimulation (Fig. 1 B). Spleen B cells from some tolerant mice do exhibit a slight increase (less than twofold) in the amount of Igαβ in the salt extract after stimulation, consistent with weak residual signals seen in tolerant B cells from some double-transgenic mice 5. This experiment was done seven times with similar results, and experiments using pure B cells (>95% B220+) showed the same results.
Because inducibly phosphorylated BCR was preferentially associated with the salt extract fraction, we wanted to determine whether partitioning into this domain was an early step after antigen binding. In particular, Does partitioning precede or follow an increase in Igα phosphorylation by src family kinases? Both partitioning and increase in Igα phosphorylation in response to antigen binding occur within 6 s of antigen binding at 37°C (Fig. 3). However, when stimulation is performed at 4°C, partitioning still occurs within 6 s, whereas little tyrosine phosphorylation of Igα is induced until 30 s. Some increase in phosphorylation of Igα and Igβ is detected in the salt extract fraction at 6 s, but this may be due to the increased amount of Igα and Igβ in the fraction rather than any increase in phosphorylation levels. The phosphorylation of this material increases between 6 and 30 s, with no additional increase in amount of Igα and Igβ.
Figure 3.
BCR becomes detergent insoluble before Igα and Igβ phosphorylation increases. Spleen cells from anti-HEL BCR transgenic mice were stimulated for the indicated time at either 4 or 37°C. Detergent insolubility of BCR was monitored by following appearance of Igα and Igβ in the salt extract (top panels), whereas tyrosine phosphorylation was monitored by probing the same blots with antiphosphotyrosine (bottom panels).
To examine the role of src kinases in partitioning of the BCR into detergent-insoluble domains, src family kinase activity was blocked using the kinase inhibitor PP1 10. PP1 eliminated both the basal and induced tyrosine phosphorylation of most detergent-soluble cellular substrates, including Igα and Igβ receptor subunits, but did not inhibit partitioning of BCR into the detergent-insoluble salt extract fraction (Fig. 4). These experiments show that partitioning into the salt extract does not require src family kinase activation and may precede the activation of these kinases.
Discussion
The importance of spatial localization of both receptors and signaling molecules into subcellular structures is becoming appreciated in many biological systems. For example, proteins containing PDZ domains concentrate ion channels with downstream effector molecules 11. In lymphocytes, the adapter proteins LAT (linker for activation of T cells; reference 12) and BLNK (B cell linker protein; reference 13, 14) are necessary to bring together molecules such as grb2 and phospholipase C γ. On the surfaces of activated T cells, the TCR and certain signaling molecules form an ‘immunological synapse’ structure surrounded by adhesion molecules 15 16. The data presented here suggest that the localization of antigen receptors on B lymphocytes into signaling domains is an important step during lymphocyte activation. The block in partitioning of tolerant cells' BCRs may underlie the changes in tolerant BCR signaling that lead to different biological responses from naive B cells.
Many studies have documented BCR association with the cytoskeleton or reorganization of the cytoskeleton after B cell stimulation 17 18 19 20. The cytoskeleton is an important part of lymphocyte activation and is clearly necessary for the formation of the ordered structure of the TCR described above 15 21 22. Partitioning of BCR into signaling domains shown here may be an early step in the association with the cytoskeleton, as the buffer used stabilizes the cytoskeleton of the lysed cells 23.
The detergent-insoluble salt extracts may correspond to membrane rafts, glycolipid- and GPI-linked, protein-rich domains of the cell surface also known as detergent-resistant membranes, detergent-insoluble glycolipid domains, or glycolipid-enriched membranes 24. Consistent with this hypothesis, the salt extracts are enriched for lyn, a doubly acylated src kinase, and GM1 sphingolipids, both known to be raft components 24. In leukocytes, the FcεR on mast cells was the first receptor shown to enter membrane rafts after stimulation 25 26. Recent experiments show that the TCR also enters membrane rafts 27 28. In addition to src kinases, rafts also contain the T cell adapter protein LAT 29, consistent with the hypothesis that rafts concentrate signaling molecules together with antigen receptors.
An intriguing possibility is that rafts and the cytoskeleton are interconnected. In rat basophilic leukemia mast cells and Jurkat T cells, clustering of raft components into patches reveals high concentrations of actin microfilaments underlying the patch 30 31. Partitioning of antigen receptors into membrane rafts could represent the first step in their cytoskeletal association and in the formation of more ordered structures.
Partitioning of BCRs into the detergent-insoluble signaling domains described here may represent the first step in BCR signaling, allowing efficient phosphorylation of the BCR by src family kinases. Concentration of src kinases and antigen receptors, along with exclusion of tyrosine phosphatases from the same sites, may be necessary to allow efficient phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM)-containing receptor subunits. CD45 is required to dephosphorylate the inhibitory site in src family kinases, a necessary step in antigen receptor signaling. However, CD45 can also inhibit src family kinases, and it may therefore be necessary to sequester kinase activity from phosphatase activity to propagate signals through the BCR 9. The failure to sequester kinase activity from phosphatase activity in anergic B cells may explain their inability to generate signals efficiently through the BCR.
The diminished partitioning of BCRs into signaling domains in tolerant B cells may explain their ability to generate only a subset of the normal signals in response to antigen. Signaling events that are blocked in tolerant cells, such as protein tyrosine phosphorylation, high intracellular calcium flux, NF-κB translocation, and JNK activation 4 5, may depend on efficient partitioning into signaling domains. By contrast, activation of the ERK cascade and the low calcium oscillations responsible for continued NFATc shuttling to the nucleus are not blocked in tolerant cells 4. It will be important to distinguish whether the latter signaling events can be activated without entry of the BCR into signaling domains, or whether the small amount of BCR that is found in signaling domains in tolerant B cells is sufficient to activate these pathways.
In summary, we have shown that the BCR becomes associated with a fraction of the cell enriched for signaling molecules such as lyn and depleted for molecules such as CD45. Partitioning of the BCR occurs within seconds of antigen binding and appears to precede tyrosine kinase activation. Partitioning is blocked in tolerant B cells, suggesting that BCR partitioning is an early, regulated step in antigen receptor signaling. Future work will need to determine what mediates the partitioning of the BCR into detergent-insoluble domains. Since this event appears to precede src kinase activity, it may result from another enzyme or from a physical change in the BCR that changes its affinity for lipid or protein membrane constituents.
Acknowledgments
This paper is dedicated to Matt Thomas, who not only contributed essential scientific insights, but also unwavering excitement for this project. Special thanks to David Holowka, Erin Sheets, and Barbara Baird for their help in understanding membrane rafts. We thank Lauren Wilson and Aisling Murtagh for PCR typing of transgenic mice, Christine White for technical assistance, and Steven Martin, Julie Blasioli, Jane Rayner, Sarah Townsend, and Richard Glynne for critical review of this manuscript.
This work was supported by grants from the Damon Runyon-Walter Winchell Cancer Research Fund (DRG-1409) to B.C. Weintraub and the Human Frontiers Science Program to M.L. Thomas, K. Rajewsky, and C.C. Goodnow and National Institutes of Health grant R01 AI44009-01 to K.M. Shokat.
References
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., Brink R., Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Hartley S.B., Goodnow C.C. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- Healy J.I., Dolmetsch R.E., Timmerman L.A., Cyster J.G., Thomas M.L., Crabtree G.R., Lewis R.S., Goodnow C.C. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- Cooke M.P., Heath A.W., Shokat K.M., Zeng Y., Finkelman F.D., Linsley P.S., Howard M., Goodnow C.C. Immunoglobulin signal transduction guides the specificity of B cell–T cell interactions and is blocked in tolerant self-reactive B cells. J. Exp. Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A.C., Shah K., Liu Y., Witucki L., Kung C., Shokat K.M. Design of allele-specific inhibitors to probe protein kinase signaling. Curr. Biol. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- Marano N., Crawford M., Govindan B. Characterization of the detergent insolubility of the T cell receptor for antigen. Mol. Immunol. 1997;34:967–976. doi: 10.1016/s0161-5890(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Bell S.E., Goodnow C.C. A selective defect in IgM antigen receptor synthesis and transport causes loss of cell surface IgM expression on tolerant B lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:816–826. doi: 10.1002/j.1460-2075.1994.tb06324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.L. The regulation of antigen-receptor signaling by protein tyrosine phosphatasesa hole in the story. Curr. Opin. Immunol. 1999;11:270–276. doi: 10.1016/s0952-7915(99)80044-2. [DOI] [PubMed] [Google Scholar]
- Hanke J.H., Gardner J.P., Dow R.L., Changelian P.S., Brissette W.H., Weringer E.J., Pollok B.A., Connelly P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Tsunoda S., Sierralta J., Sun Y., Bodner R., Suzuki E., Becker A., Socolich M., Zuker C.S. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Fu C., Turck C.W., Kurosaki T., Chan A.C. BLNKa central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- Wienands J., Schweikert J., Wollscheid B., Jumaa H., Nielsen P.J., Reth M. SLP-65a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J. Exp. Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks C.R., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. The immunological synapsea molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Braun J., Hochman P.S., Unanue E.R. Ligand-induced association of surface immunoglobulin with the detergent-insoluble cytoskeletal matrix of the B lymphocyte. J. Immunol. 1982;128:1198–1204. [PubMed] [Google Scholar]
- Gupta S.K., Woda B.A. Ligand-induced association of surface immunoglobulin with the detergent insoluble cytoskeleton may involve alpha-actinin. J. Immunol. 1988;140:176–182. [PubMed] [Google Scholar]
- Albrecht D.L., Noelle R.J. Membrane Ig-cytoskeletal interactions. I. Flow cytofluorometric and biochemical analysis of membrane IgM-cytoskeletal interactions. J. Immunol. 1988;141:3915–3922. [PubMed] [Google Scholar]
- Jugloff L.S., Jongstra-Bilen J. Cross-linking of the IgM receptor induces rapid translocation of IgM-associated Igα, Lyn, and Syk tyrosine kinases to the membrane skeleton. J. Immunol. 1997;159:1096–1106. [PubMed] [Google Scholar]
- Wulfing C., Davis M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- Penninger J.M., Crabtree G.R. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9–12. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977;10:67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. FcεRI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc. Natl. Acad. Sci. USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Montixi C., Langlet C., Bernard A.M., Wurbel M.A., Chauvin J.P., Pierres M., He H.T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Holowka D., Hine C., Baird B. Alterations in cellular lipid composition affect the interactions of aggregated FcεRI with p53-56-lyn and the microfilament cytoskeleton. FASEB J. 1996;10:A1214. [Google Scholar]
- Harder T., Simons K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cellsaccumulation of actin regulated by local tyrosine phosphorylation. Eur. J. Immunol. 1999;29:556–562. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]





