Abstract
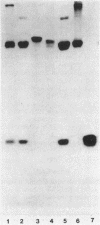
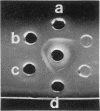
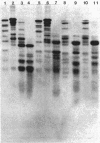
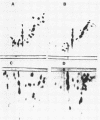
There are significant differences in the large subunits of form I and form II ribulose 1,5-bisphosphate carboxylase/oxygenase isolated from Rhodopseudomonas sphaeroides. Two-dimensional peptide mapping of carboxymethylated large subunits clearly indicates that there are differences in the primary structure of the two proteins. These results are supported by limited proteolysis with three different proteases and by subsequent analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These data, in conjunction with immunological studies and investigations on the regulation of the two enzymes, support the conclusion that the large subunits of form I and form II ribulose 1,5-bisphosphate carboxylase/oxygenase may be different gene products.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Coen D. M., Beaton A. R., Bogorad L., Rich A. Location of the single gene for the large subunit of ribulosebisphosphate carboxylase on the maize chloroplast chromosome. J Biol Chem. 1979 Feb 10;254(3):905–910. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Multiple methylation in processing of sensory signals during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1980 May;77(5):2429–2433. doi: 10.1073/pnas.77.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Activation of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides: probable role of the small subunit. J Bacteriol. 1979 Dec;140(3):1023–1027. doi: 10.1128/jb.140.3.1023-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Characterization of antiserum directed against form II ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Sep;131(3):1020–1022. doi: 10.1128/jb.131.3.1020-1022.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Isolation and preliminary characterization of two forms of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas capsulata. J Bacteriol. 1977 Dec;132(3):818–823. doi: 10.1128/jb.132.3.818-823.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kung S. D., Wildman S. G. Polypeptide chains of the large and small subunits of fraction I protein from tobacco. Arch Biochem Biophys. 1978 Jan 15;185(1):272–281. doi: 10.1016/0003-9861(78)90167-4. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M. Abnormal human haemoglobins. I. The comparison of normal human and sickle-cell haemoglobins by fingerprinting. Biochim Biophys Acta. 1958 Jun;28(3):539–545. doi: 10.1016/0006-3002(58)90516-x. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Muller E. D., Chory J., Kaplan S. Cloning and characterization of the gene product of the form II ribulose-1,5-bisphosphate carboxylase gene of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):469–472. doi: 10.1128/jb.161.1.469-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Quivey R. G., Jr, Tabita F. R. Cloning and expression in Escherichia coli of the form II ribulose 1,5-bisphosphate carboxylase/oxygenase gene from Rhodopseudomonas sphaeroides. Gene. 1984 Nov;31(1-3):91–101. doi: 10.1016/0378-1119(84)90198-7. [DOI] [PubMed] [Google Scholar]
- Robison P. D., Tabita F. R. Modification of ribulose bisphosphate carboxylase from Rhodospirillum rubrum with tetranitromethane. Biochem Biophys Res Commun. 1979 May 14;88(1):85–91. doi: 10.1016/0006-291x(79)91699-1. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Phares E. F., Long M. V., Norton I. L., Stringer C. D., Hartman F. C. Isolation, characterization, and crystallization of ribulosebisphosphate carboxylase from autotrophically grown Rhodospirillum rubrum. J Bacteriol. 1979 Jan;137(1):490–501. doi: 10.1128/jb.137.1.490-501.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Davidson E., Marrs B. L. Depression of the synthesis of the intermediate and large forms of ribulose-1,5-bisphosphate carboxylase/oxygenase in Rhodopseudomonas capsulata. Arch Microbiol. 1984 Jul;138(3):233–236. doi: 10.1007/BF00402127. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem. 1974 Jun 10;249(11):3459–3464. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Tabita F. R. Isolation and partial characterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983 Nov;156(2):507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zakin M. M., Garel J. R., Dautry-Varsat A., Cohen G. N., Boulot G. Detection of the homology among proteins by immunochemical cross-reactivity between denatured antigens. Application to the threonine and methionine regulated aspartokinases-homoserine dehydrogenases from Escherichia coli K 12. Biochemistry. 1978 Oct 3;17(20):4318–4323. doi: 10.1021/bi00613a032. [DOI] [PubMed] [Google Scholar]