Abstract
The pathogenesis of filarial disease is characterized by acute and chronic inflammation. Inflammatory responses are thought to be generated by either the parasite, the immune response, or opportunistic infection. We show that soluble extracts of the human filarial parasite Brugia malayi can induce potent inflammatory responses, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and nitric oxide (NO) from macrophages. The active component is heat stable, reacts positively in the Limulus amebocyte lysate assay, and can be inhibited by polymyxin B. TNF-α, IL-1β, and NO responses were not induced in macrophages from lipopolysaccharide (LPS)-nonresponsive C3H/HeJ mice. The production of TNF-α after chemotherapy of microfilariae was also only detected in LPS-responsive C3H/HeN mice, suggesting that signaling through the Toll-like receptor 4 (TLR4) is necessary for these responses. We also show that CD14 is required for optimal TNF-α responses at low concentrations. Together, these results suggest that extracts of B. malayi contain bacterial LPS. Extracts from the rodent filaria, Acanthocheilonema viteae, which is not infected with the endosymbiotic Wolbachia bacteria found in the majority of filarial parasites, failed to induce any inflammatory responses from macrophages, suggesting that the source of bacterial LPS in extracts of B. malayi is the Wolbachia endosymbiont. Wolbachia extracts derived from a mosquito cell line induced similar LPS-dependent TNF-α and NO responses from C3H/HeN macrophages, which were eliminated after tetracycline treatment of the bacteria. Thus, Wolbachia LPS may be one of the major mediators of inflammatory pathogenesis in filarial nematode disease.
Keywords: filariasis, endotoxin, pathogenesis, symbiosis, lipopolysaccharide
Introduction
Filariasis is a major cause of morbidity throughout the tropics. More than 140 million people are infected with the filarial nematodes Wuchereria bancrofti, Brugia malayi, and Onchocerca volvulus, which are responsible for the majority of human filarial disease 1 2. Pathology of filariasis is associated with a diverse range of inflammatory conditions. In lymphatic filariasis, inflammatory pathology can present as acute inflammation, characterized by recurrent attacks of adenolymphangitis with associated systemic febrile responses, or chronic inflammation, associated with hydrocele, lymphedema, and elephantiasis 1. In onchocerciasis, pathogenesis is principally associated with the death of microfilariae and subsequent inflammation in the skin and eye 2 3. Inflammatory responses are also a feature of the adverse reaction to filarial chemotherapy that is thought to occur after the release of large amounts of parasite material after treatment 4. The severity of adverse reaction to chemotherapy is related to the intensity of parasite burden and presents clinically as fever with local or systemic inflammation 4.
Several factors are thought to contribute to the inflammatory pathogenesis of filariasis, including the parasite, the immune response, and opportunistic infection 1 2 3 4 5 6. Infection of immunodeficient mice with Brugia species results in the development of lymphedema in the absence of T cells and opportunistic infection 7, and is associated with the local production of proinflammatory cytokines including IL-1, IL-6, TNF-α, and GM-CSF in parasitized lymphatics 8. Together with the development of inflammation after death of filarial parasites, this prompted us to investigate the role of the parasite in the induction of inflammatory responses.
The induction and regulation of inflammatory responses has been shown to be under the control of key proinflammatory cytokines including IL-1β and TNF-α 9. These cytokines are produced predominantly by macrophages and result in a cascade of inflammatory mediators and physiological responses that serve to amplify and regulate innate immunity 10. We have investigated the induction of these proinflammatory cytokines and nitric oxide (NO) from mouse macrophages by soluble extracts of B. malayi in order to characterize parasite derived inflammatory stimuli.
Materials and Methods
Parasites
The human filarial parasite B. malayi was obtained from TRS Laboratories and maintained in the peritoneal cavity of Mongolian jirds (Meriones unguiculatus). Adult male and female worms and microfilariae were collected under aseptic conditions. Microfilariae were passed through Sephadex PD-10 columns (Amersham Pharmacia Biotech) to remove host cells and serum proteins, and together with adult parasites were thoroughly washed (four times) in RPMI 1640 containing 5% FCS and 1% penicillin/streptomycin (GIBCO BRL). Parasites were cultured at 37°C in 5% CO2 for 5 d to ensure the absence of contaminating microorganisms and to select viable motile parasites. Adult parasites were separated into male and female worms under sterile conditions and washed (four times) in sterile PBS. Extracts were prepared by finely mincing the worms followed by homogenization on ice. Microfilarial extracts were prepared after disruption by sonication on ice. All procedures were carried out under stringent sterile conditions. Adult Acanthocheilonema viteae were obtained from subcutaneous tissues of Mongolian jirds and prepared as for B. malayi adult worms. Extracts were centrifuged at 20,000 g for 30 min, and the supernatant was collected and stored at −20°C until required. Protein concentration of parasite extracts was determined by the Coomassie protein assay (Pierce Chemical Co.).
Wolbachia Mosquito Cell Line
The Asian tiger mosquito, Aedes albopictus, cell line Aa23, which is naturally infected with Wolbachia, was cultured as described 11. Soluble extracts of the cell line and bacteria were prepared by sonication after washing of cultured cells in sterile PBS. Similar extracts were prepared from cells cultured for 2 wk in tetracycline (10 μg/ml), which resulted in clearance of the bacteria as judged by microscopy.
Macrophage Cultures
Primary murine macrophage cultures were derived from peritoneal exudate cells 4 d after administration of 1% thioglycollate (Difco) as described previously 12. Female CD-1 (Charles River), C3H/HeN, and C3H/HeJ (The Jackson Laboratory) mice aged 6–10 wk were used. 2.5 × 105 cells were placed in flat-bottomed 96-well tissue culture plates (Nunc), and macrophages were isolated by adherence. Cells were maintained in RPMI 1640 containing 5% FCS, 1% penicillin/streptomycin, 1% gentamycin, 1% l-glutamine, and 1% nonessential amino acids at 37°C in 5% CO2. The macrophage cell line J774.1 and the mutant J7.DEF.3 14, which does not express membrane CD14 15, were cultured under similar conditions at a concentration of 1 × 105 cells/well. Cells were stimulated with optimal concentrations (1 μg/ml) or serial dilutions of Escherichia coli LPS (026:B6; Sigma Chemical Co.) and 50 U/ml IFN-γ (Genzyme), either singly or in combination, and soluble extracts from filarial parasites for 24 h. Culture supernatants were collected for analysis of cytokines and nitrite.
Analysis of Cytokines and NO
IL-1β and TNF-α were detected in culture supernatants or plasma by capture ELISA using paired antibodies supplied by Genzyme or Biosource. NO production was analyzed by the detection of nitrite using the Griess reaction as described previously 12.
Limulus Amebocyte Lysate Test
The E-TOXATE® kit (Sigma Chemical Co.) was used according to the manufacturer's instructions.
PCR Detection of Bacteria
Eubacterial 16S rDNA PCR.
B. malayi and A. viteae were screened for bacterial 16S rDNA. 1 μl of DNA was amplified in a mixture that contained 1.25 U HotStarTaq DNA polymerase (Qiagen), 1× Qiagen PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.2 μM of each primer 13, and water to a final volume of 50 μl. Temperature cycling conditions were as follows: 15 min at 95°C, followed by 1.5 min at 94°C, 1 min at the annealing temperature (60, 55, and 50°C for 5 cycles each, then 45°C), extension at 72°C for 2.5 min (total of 35 cycles), and a final extension of 8 min. PCR products were cloned and sequenced by standard procedures.
Nested PCR.
To reevaluate the distribution of Wolbachia in a population of 50 male and 50 female B. malayi 13, a nested PCR was developed. After amplification with eubacterial primers, 1 μl of product was amplified with 3.0 mM MgCl2 and 0.5 μM of each Brugia Wolbachia–specific primer 13 with temperature cycling conditions of 95°C for 15 min, followed by 25 cycles of 94°C for 1 min, 45°C for 1 min, 72°C for 1 min, and a final extension of 8 min at 72°C.
LPS-induced TNF-α Responses after Chemotherapy
C3H/HeN and C3H/HeJ mice were infected with 350,000 B. malayi microfilariae injected intravenously. 24 h later, animals were treated intraperitoneally with a combination of ivermectin phosphate (1 mg/kg, Merck Research Laboratories) and d-galactosamine (d-gal, 100 mg/kg; Sigma Chemical Co.). Blood samples were collected from the tail vein before treatment and at 3 and 6 h after treatment, and plasma was analyzed for TNF-α by ELISA. Control uninfected animals were treated in a similar fashion.
Statistics
Student's t test was used to compare mean values with a P < 0.05 being taken as significant.
Results and Discussion
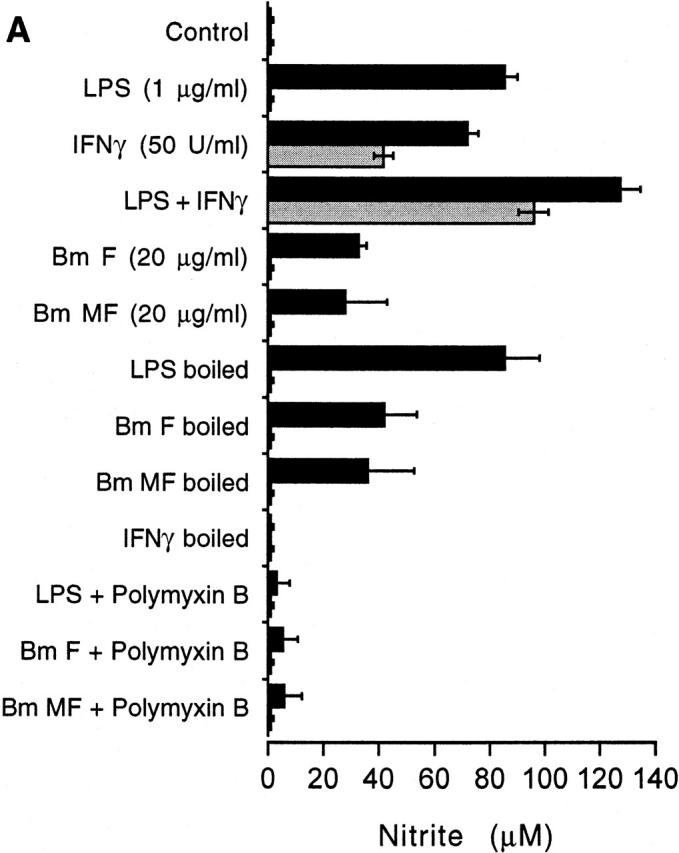
To determine the ability of parasites to directly induce inflammatory responses, we prepared soluble extracts from male and female adult worms and microfilariae of the human lymphatic filarial parasite, B. malayi, which were used to stimulate murine macrophages. Extracts from all developmental stages induced the production of IL-1β, TNF-α, and NO in a dose-dependent fashion (Fig. 1). Treatment of soluble extracts by boiling (100°C, 5 min) had no effect on the induction of inflammatory responses, whereas incubation of extracts with polymyxin B inhibited these responses (Fig. 1 C). Extracts were also shown to react positively in the Limulus amebocyte lysate (LAL) test, in which the lysate coagulates in the presence of bacterial endotoxin. Together these results suggest that the inflammatory stimulus in parasite extracts has similarities to bacterial endotoxin or LPS.
Figure 1.

Production of TNF-α, NO, and IL-1β from CD-1 adherent peritoneal exudate cells after exposure to soluble extracts of B. malayi (Bm) adults and microfilariae for 24 h (gray bars). Bm F, adult female worms; Bm M, adult male worms; Bm MF, microfilariae. Black bars represent control cultures incorporating E. coli LPS and IFN-γ, singly or in combination. (A) TNF-α; (B) nitrite; and (C) IL-1β. Concentration of filarial extract refers to the total protein concentration of the extract. Values represent the mean of three replicate wells (±SD). *Statistical significance of P < 0.05. Results are representative of at least three similar experiments.
Next we tested whether the presence of the LPS receptor, CD14, was required for the induction of inflammatory responses. Using the cell line J7.DEF.3, which has been selected from a parent J774.1 macrophage cell line to be free of surface CD14 14 15, we show that at low concentrations of parasite extract and LPS, CD14 is required for TNF-α responses (Fig. 2). At higher concentrations, CD14-independent stimulation of TNF-α occurs (Fig. 2), in accordance with previous studies on LPS 10. To confirm that the mutation in J7.DEF.3 cells is restricted to CD14 expression, we attempted to restore activity by the addition of soluble CD14 (sCD14), which was produced as described previously 16. Suboptimal concentrations of LPS and B. malayi microfilarial extract were incubated on J7.DEF.3 cells in the presence of 1–10 μg/ml of sCD14. The addition of sCD14 to cultures restored the ability of J7.DEF.3 cells to produce TNF-α, in response to both LPS and parasite extracts (Fig. 2 B). The restoration of responses to parasite extracts was equivalent to that observed from parent J774.1 cells (Fig. 2 A).
Figure 2.

The requirement for CD14 for TNF-α production from macrophages stimulated with B. malayi microfilarial extract or LPS. (A) J774.1 macrophages (black bars) or J7.DEF.3 mutant cells (gray bars, deficient in surface CD14) were stimulated with LPS or B. malayi microfilarial extract (Bm MF) for 24 h. (B) Suboptimal concentrations of LPS or B. malayi microfilarial extract (Bm MF) were cultured on J7.DEF.3 cells in the presence of sCD14 leading to a restoration of TNF-α responses. Concentration of filarial extract refers to the total protein concentration of the extract. Values represent the mean of three replicate wells (±SD). Results are representative of three similar experiments.
Recently, LPS has been shown to induce signal transduction across the plasma membrane via Toll-like receptors (TLRs, 17 18 19 20 21 22 23). A mutation in the lps gene, which renders C3H/HeJ mice unresponsive to LPS, has been mapped to a mutation in TLR4 17 18 19 20. Therefore, we tested whether TLR4 is required for the induction of inflammatory responses from B. malayi extracts. Macrophages derived from C3H/HeJ mice failed to produce IL-1β, TNF-α, and NO in response to adult female and microfilarial B. malayi extracts or LPS, but were able to respond to IFN-γ (Fig. 3 A). This suggests that these inflammatory responses induced by B. malayi extracts are dependent on signaling through the TLR4 LPS receptors.
Figure 3.
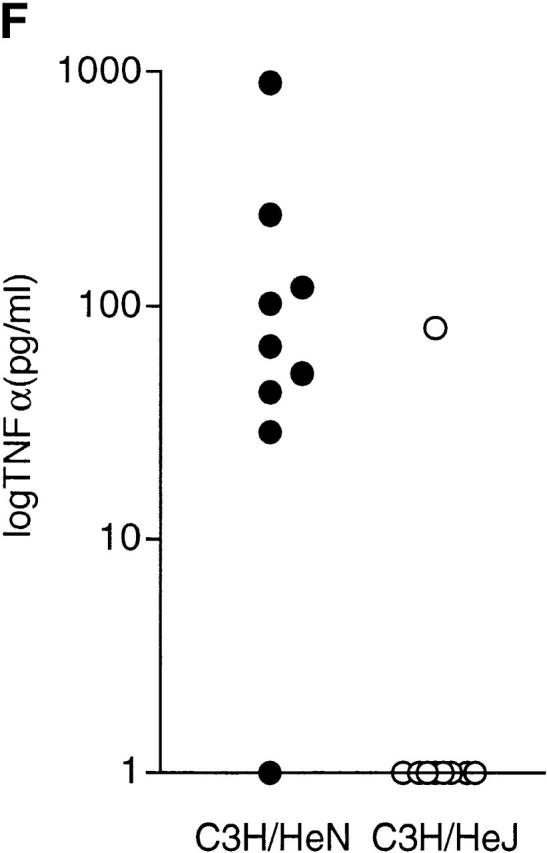
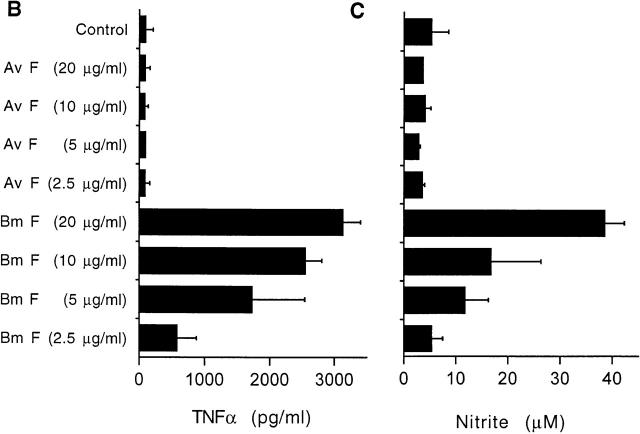
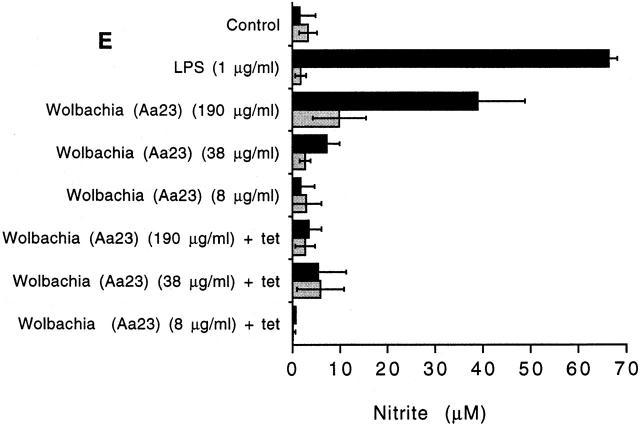
Induction of NO and TNF-α in C3H/HeN (LPS-responsive) and C3H/HeJ (LPS-nonresponsive) mice. (A) Production of NO from adherent peritoneal exudate cells derived from C3H/HeN (black bars) or C3H/HeJ mice (gray bars) after exposure to soluble extracts of B. malayi for 24 h. Extracts of B. malayi adult female worms (Bm F) or microfilaria (Bm MF) and E. coli LPS induce NO from macrophages from C3H/HeN mice but not C3H/HeJ mice. The induction of NO is unaffected by boiling the filarial extract but can be inhibited with polymyxin B. IFN-γ responses are induced from both strains of mice and are destroyed by boiling. (B) Production of TNF-α and (C) NO from C3H/HeN adherent peritoneal exudate cells after exposure to soluble extracts of B. malayi adult female (Bm F) and A. viteae adult female (Av F) extracts for 24 h. TNF-α and NO are produced only in response to B. malayi. (D) Production of TNF-α and (E) NO from C3H/HeN (black bars) and C3H/HeJ (gray bars), induced by soluble extracts derived from a Wolbachia-infected mosquito cell line (Aa23) or after tetracycline (tet) treatment of the cell line. Concentration of the extract refers to the total protein concentration. Values represent the mean of three replicate wells (±SD). Results are representative of three to six similar experiments. (F) Plasma TNF-α levels 6 h after treatment of C3H/HeN (filled circles) and C3H/HeJ mice (open circles) infected with B. malayi microfilariae and treated with ivermectin (1 mg/kg) and d-gal (100 mg/kg) (n = 9 per group). Representative of two repeat experiments.
We and others have shown that the majority of filarial parasites are infected with symbiotic intracellular bacteria belonging to the Wolbachia complex 13 24 25 26. One exception to this is the rodent filaria, A. viteae, which are free of bacteria as judged by electron microscopy, PCR, and lack of sensitivity to tetracycline therapy 24 26 27 28. Therefore, we compared the ability of extracts derived from filaria with (B. malayi) and without (A. viteae) Wolbachia infection to induce inflammatory responses from macrophages. Fig. 3B and Fig. C, shows that inflammatory responses are only induced from B. malayi. Extracts of adult A. viteae parasites failed to induce any inflammatory response from macrophages. In addition, extracts of A. viteae did not react positively with the LAL assay, supporting the notion that the endosymbiotic bacteria of B. malayi are the source of LPS. To determine whether A. viteae parasites contained endogenous inhibitors of LPS-induced inflammatory responses, we cocultured extracts of A. viteae and B. malayi. No evidence for the presence of inhibitory activity was detected in A. viteae (data not shown).
Attempts to “cure” filarial nematodes of Wolbachia with antibiotics and retain viability have so far proved impossible, suggesting a mutualistic association between nematodes and their symbionts 24. In contrast, arthropod Wolbachia can be eliminated from hosts using tetracycline. Therefore, we used a mosquito cell line–derived Wolbachia to determine LPS activity of the bacteria in the absence of nematode tissue and after antibiotic treatment. Fig. 3d and Fig. e, shows that extracts derived from Wolbachia-infected mosquito cells induced TNF-α and NO from C3H/HeN (LPS-responsive), but not C3H/HeJ (LPS-nonresponsive) macrophages. Mosquito cell lines treated with tetracycline, which eliminated Wolbachia infection, failed to demonstrate LPS-like activity (Fig. 3d and Fig. e). These results suggest that Wolbachia bacteria from both arthropods and nematodes contain LPS in accordance with other related members of the Rickettsiaceae.
PCR analysis with primers for eubacterial 16S rDNA confirmed that A. viteae was free of symbiotic bacteria, and that Wolbachia was the only bacterial sequence obtained from B. malayi. Although this does not rule out the possibility of other symbiotic bacterial species in B. malayi, it does suggest that Wolbachia are the most abundant. Remarkably high levels of infection can occur in adult parasites and microfilariae, with numerous bacteria almost entirely filling their intracellular environment 24. Previously, we have reported that in a population of B. malayi adult worms, although all female worms were infected, only ∼25% of male worms appeared to be infected when screened with Wolbachia-specific primers 13. We reanalyzed these samples using a nested PCR incorporating eubacterial primers and Wolbachia-specific primers and found that all male worms are infected, although many at a lower intensity than that observed in female worms.
We next investigated whether or not LPS was released by living parasites during in vitro culture. Previously, we have reported that 4-d cultures of B. malayi microfilariae in the presence of macrophages did not generate any detectable NO 12. Similar experiments on living adult worms and microfilariae cultured in the presence of macrophages, or the addition of spent culture medium incubated on fresh macrophages, failed to induce detectable IL-1β, TNF-α, or NO responses (data not shown). This suggests that living parasites do not release LPS in sufficient amounts to activate macrophages under the conditions tested. Alternatively, living parasites may secrete products that are inhibitory to LPS-induced responses. Furthermore, the vigorous motility of filarial parasites is also not sufficient to induce inflammatory responses from macrophages.
Therefore, the release of LPS by parasites in vivo is likely to occur after death of the parasite through natural attrition, after immunologically mediated clearance or pharmacological intervention and the subsequent release of Wolbachia bacteria. Such a situation is consistent with the inflammatory nodules produced after death of filarial parasites, and the systemic and localized responses that accompany the adverse reaction to chemotherapy 1 2 3 4. Indeed, a study using d-gal sensitization of rodents, which renders animals susceptible to TNF-α–induced hepatotoxiticity, showed that chemotherapy of B. malayi in d-gal–sensitized animals resulted in shock-like lethal side effects 29. These adverse reactions could be prevented by inhibitors of TNF-α and NO and would be consistent with the release of LPS after treatment of filarial parasites. Therefore, we tested whether or not LPS-induced proinflammatory responses occurred after chemotherapy. C3H/HeN and C3H/HeJ mice were infected with B. malayi microfilariae and treated with ivermectin and d-gal. Analysis of plasma TNF-α showed that at 6 h after treatment, TNF-α could be detected in eight out of nine LPS-responsive C3H/HeN mice (mean 175 pg/ml, range 0–915 pg/ml), whereas only one out of nine LPS-hyporesponsive C3H/HeJ mice showed elevated TNF-α (mean 9 pg/ml, range 0–81 pg/ml, P < 0.05; Fig. 3 F). TNF-α was not detected in plasma samples before treatment, at 3 h after treatment, or in control uninfected animals receiving ivermectin and d-gal. The production of TNF-α in C3H/HeN mice at 6 h after treatment coincided with a 89–100% reduction in parasites from the peripheral circulation. A repeat experiment showed similar results. These data support the idea that proinflammatory responses induced after treatment are predominantly caused by LPS.
The release of proinflammatory cytokines and inflammatory mediators including IL-1, IL-6, IFN-γ, TNF-α, and NO after filarial chemotherapy has been reported 30 31 32 33 and shown to correlate with the severity of adverse reaction 32. The severity and presentation of fever associated with acute lymphatic filarial disease also correlate with elevated levels of TNF-α 34. Together with reports of the local production of proinflammatory cytokines in pathologies of animal models 8, these studies suggest a role for these proinflammatory responses in the pathogenesis of filarial disease. Products from endosymbiotic Wolbachia bacteria may also contribute to the recruitment and activation of granulomatous inflammatory responses that occur early during Brugia infection in animal models 35, in response to O. volvulus in vitro 36, and on death of parasites following chemotherapy 3 4.
The presence of symbiotic Wolbachia in all of the major pathogenic filariae of humans 24 and the apparently exclusive induction of proinflammatory responses by bacterial LPS in B. malayi extracts suggest that Wolbachia LPS may be one of the major mediators of inflammatory pathogenesis in filarial nematode disease. With the current studies in our laboratory, we hope to further clarify the contribution of this fascinating symbiont to human filarial disease.
Acknowledgments
We thank Dr. Teruo Kirikae (Jichi Medical School, Japan) for providing the J7.DEF.3 cell line and Prof. Scott O'Neill (Yale University School of Medicine) for the A. albopictus Aa23 cell line.
This work was supported by Wellcome Trust Career Development Fellowship in Basic Medical Science Grant 047176 (to M.J. Taylor).
References
- Ottesen E.A. Infection and disease in lymphatic filariasisan immunological perspective. Parasitology. 1992;104:S71–S79. doi: 10.1017/s0031182000075259. [DOI] [PubMed] [Google Scholar]
- Ottesen E.A. Immune responsiveness and the pathogenesis of human onchocerciasis. J. Infect. Dis. 1995;171:659–671. doi: 10.1093/infdis/171.3.659. [DOI] [PubMed] [Google Scholar]
- Hall L.R., Pearlman E. Pathogenesis of onchocercal keratitis (River blindness) Clin. Microbiol. Rev. 1999;12:445–453. doi: 10.1128/cmr.12.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen E.A. Description, mechanisms and control of reactions to treatment in the human filariases. Ciba Found. Symp. 1987;127:265–283. doi: 10.1002/9780470513446.ch18. [DOI] [PubMed] [Google Scholar]
- Freedman D.O. Immune dynamics in the pathogenesis of human lymphatic filariasis. Parasitol. Today. 1998;14:229–234. doi: 10.1016/s0169-4758(98)01244-7. [DOI] [PubMed] [Google Scholar]
- Olszewski W.L., Jamal S., Manokaran G., Pani S., Kumaraswami V., Kubicka U., Lukomska B., Dworczynski A., Swoboda E., Meisel-Mikolajczyk F. Bacteriologic studies of skin, tissue fluid, lymph, and lymph nodes in patients with filarial lymphedema. Am. J. Trop. Med. Hyg. 1997;57:7–15. doi: 10.4269/ajtmh.1997.57.7. [DOI] [PubMed] [Google Scholar]
- Vincent A.L., Vickery A.C., Lotz M.J., Desai U. The lymphatic pathology of Brugia pahangi in nude (athymic) and thymic mice C3H/HeN. J. Parasitol. 1984;70:48–56. [PubMed] [Google Scholar]
- Rao U.R., Vickery A.C., Kwa B.H., Nayar J.K. Regulatory cytokines in the lymphatic pathology of athymic mice infected with Brugia malayi . Int. J. Parasitol. 1996;26:561–565. doi: 10.1016/0020-7519(96)00036-7. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Role of pro- and anti-inflammatory cytokines during inflammationexperimental and clinical findings. J. Biol. Regul. Homeost. Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- Ulevitch R.J., Tobias P.S. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- O'Neill S.L., Pettigrew M.M., Sinkins S.P., Braig H.R, Andreadis T.G., Tesh R.B. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 1997;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Cross H.F., Mohammed A.A., Trees A.J., Bianco A.E. Susceptibility of Brugia malayi and Onchocerca lienalis microfilariae to nitric oxide and hydrogen peroxide in cell-free culture and from IFNγ-activated macrophages. Parasitology. 1996;112:315–322. doi: 10.1017/s0031182000065835. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Bilo K., Cross H.F., Archer J.P., Underwood A.P. 16S rDNA phylogeny and ultrastructural characterization of Wolbachia intracellular bacteria of the filarial nematodes Brugia malayi, B. pahangi and Wuchereria bancrofti . Exp. Parasitol. 1999;91:356–361. doi: 10.1006/expr.1998.4383. [DOI] [PubMed] [Google Scholar]
- Kirikae T., Schade F.U., Kirikae F., Rietschel E.T., Morrison D.C. Isolation of a macrophage-like cell line defective in binding of lipopolysaccharide. Influence of serum and lipopolysaccharide chain length on macrophage activation. J. Immunol. 1993;151:2742–2752. [PubMed] [Google Scholar]
- Onozuka K., Kirikae T., Kirikae F., Suda Y., Kusumoto S., Yamamoto S., Shimamura T., Nakano M. Participation of CD14 in the phagocytosis of smooth-type Salmonella typhimurium by the macrophage-like cell line, J774.1. Microbiol. Immunol. 1997;41:765–772. doi: 10.1111/j.1348-0421.1997.tb01924.x. [DOI] [PubMed] [Google Scholar]
- McGinley M.D., Narhi L.O., Kelley M.J., Davy E., Robinson J., Rohde M.F., Wright S.D., Lichenstein H.S. CD14physical properties and identification of an exposed site that is protected by lipopolysaccharide. J. Biol. Chem. 1995;270:5213–5218. doi: 10.1074/jbc.270.10.5213. [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M.-Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr micemutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi S.T., Gros P., Malo D. Host resistance to infectiongenetic control of lipopolysaccharide responsiveness by TOLL-like receptor genes. Trends Genet. 1999;15:291–294. doi: 10.1016/s0168-9525(99)01782-5. [DOI] [PubMed] [Google Scholar]
- Poltorak A., Smirnova I., He X., Liu M.-Y., Van Huffel C., Birdwell D., Alejos E., Silva M., Du X., Thompson P. Genetic and physical mapping of the lps locusidentification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 1998;24:340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- Qureshi S.T., Lariviere L., Leveque G., Clermont S., Moore K.J., Gros P., Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4. J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. Cutting edgeToll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitol. Today. 1999;15:437–442. doi: 10.1016/s0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- Henkle-Dührsen K., Eckelt V., Wildenburg G., Blaxter M., Walter R.D. Gene structure, activity and localization of a catalase from intracellular bacteria in Onchocerca volvulus . Mol. Biochem. Parasitol. 1998;96:69–81. doi: 10.1016/s0166-6851(98)00109-1. [DOI] [PubMed] [Google Scholar]
- Bandi C., Anderson T.J.C., Genchi C., Blaxter M.L. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. B. Biol. Sci. 1998;265:2407–2413. doi: 10.1098/rspb.1998.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A., Nissen-Pahle K., Schmetz C., Henkle-Dührsen K., Blaxter M.L., Büttner D.W., Gallin M.Y., Al-Qaoud K.M., Lucius R., Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall J.W., Jun J.J., Bandi C. Wolbachia and the antifilarial properties of tetracycline. An untold story. Ital. J. Zool. 1999;66:7–10. [Google Scholar]
- Zahner H. Induction and prevention of shock-like lethal side effects after microfilaricidal treatment in filariae infected rodents. Trop. Med. Parasitol. 1995;46:221–229. [PubMed] [Google Scholar]
- Zheng H.J., Tao Z.H., Cheng W.F., Wang S.H., Cheng S.H., Ye Y.M., Luo L.F., Chen X.R., Gan G.B., Piessens W.F. Efficacy of ivermectin for control of microfilaremia recurring after treatment with diethylcarbamazine. II. Immunologic changes following treatment. Am. J. Trop. Med. Hyg. 1991;45:175–181. [PubMed] [Google Scholar]
- Yazdanbakhsh M., Duym L., Aarden L., Partono F. Serum interleukin-6 levels and adverse reactions to diethylcarbamazine in lymphatic filariasis. J. Infect. Dis. 1992;166:453–454. doi: 10.1093/infdis/166.2.453. [DOI] [PubMed] [Google Scholar]
- Turner P.F., Rockett K.A., Ottesen E.A., Francis H., Awadzi K., Clark I.A. Interleukin-6 and tumor necrosis factor in the pathogenesis of adverse reactions after treatment of lymphatic filariasis and onchocerciasis. J. Infect. Dis. 1994;169:1071–1075. doi: 10.1093/infdis/169.5.1071. [DOI] [PubMed] [Google Scholar]
- Winkler S., El Menyawi I., Linnau K.F., Graninger W. Total serum levels of the nitric oxide derivatives nitrite/nitrate during microfilarial clearance in human filarial disease. Am. J. Trop. Med. Hyg. 1998;59:523–525. doi: 10.4269/ajtmh.1998.59.523. [DOI] [PubMed] [Google Scholar]
- Das B.K., Sahoo P.K., Ravindran B. A role for tumour necrosis factor-α in acute lymphatic filariasis. Parasite Immunology. 1996;18:421–424. doi: 10.1046/j.1365-3024.1996.d01-126.x. [DOI] [PubMed] [Google Scholar]
- Rao U.R., Nasarre C., Coleman S.U., Horohov D.W., Klei T.R. Granulomatous inflammatory response to recombinant filarial proteins of Brugia species. Am. J. Trop. Med. Hyg. 1999;60:251–254. doi: 10.4269/ajtmh.1999.60.251. [DOI] [PubMed] [Google Scholar]
- Rubio de Kromer M.T., Kromer M., Luersen K., Brattig N.W. Detection of a chemotactic factor for neutrophils in extracts of female Onchocerca volvulus . Acta Trop. 1998;71:45–56. doi: 10.1016/s0001-706x(98)00044-8. [DOI] [PubMed] [Google Scholar]