Abstract
Recent work has revealed correlations between bacterial or viral infections and atherosclerotic disease. One particular bacterium, Chlamydia pneumoniae, has been observed at high frequency in human atherosclerotic lesions, prompting the hypothesis that infectious agents may be necessary for the initiation or progression of atherosclerosis. To determine if responses to gram-negative bacteria are necessary for atherogenesis, we first bred atherosclerosis-prone apolipoprotein (apo) E−/− (deficient) mice with animals incapable of responding to bacterial lipopolysaccharide. Atherogenesis was unaffected in doubly deficient animals. We further tested the role of infectious agents by creating a colony of germ-free apo E−/− mice. These animals are free of all microbial agents (bacterial, viral, and fungal). Atherosclerosis in germ-free animals was not measurably different from that in animals raised with ambient levels of microbial challenge. These studies show that infection is not necessary for murine atherosclerosis and that, unlike peptic ulcer, Koch's postulates cannot be fulfilled for any infectious agent in atherosclerosis.
Keywords: atherosclerosis, apolipoprotein E knockout, germ-free animal, Toll, cholesterol
Introduction
Several types of studies have suggested that infectious agents may play a role in atherosclerosis and coronary heart disease (CHD). Atherosclerosis in chickens is exacerbated by infection with Marek's disease virus, a member of the herpes virus family 1 2, and numerous studies have suggested an association of viral pathogens such as CMV and bacterial pathogens such as Helicobacter and Chlamydia with human atherosclerosis 3 4 5. Recent data has drawn special attention to the association between Chlamydia pneumonia and CHD. Most 6 7 8 though not all 9 10 studies have found that patients with CHD are more likely to carry anti-Chlamydia antibodies than healthy subjects, and several studies have demonstrated that Chlamydia can be detected immunohistologically in >70% of atherosclerotic lesions, whereas it is virtually undetectable in undiseased arteries 5 6 7 8. C. pneuomonia is a gram-negative bacteria that may produce persistent infection through intracellular growth in macrophages 11, a cell type that plays a dominant and necessary role in atherosclerotic lesions 12 13. These observations have led to the hypothesis that Chlamydia may play a causal role in atherosclerosis and CHD 14 15 16 17 18. This hypothesis is made plausible by studies on the association of Helicobacter pylori and duodenal ulcer 19 20. This condition was originally thought to be caused by excess gastric acid and was treated by reducing or neutralizing acid. It is now clear that ulcers are most commonly caused by infection with H. pylori, and the most effective treatment involves antibiotics. In the same way, atherosclerosis is currently thought to be caused by excess plasma cholesterol and is treated by agents that reduce cholesterol levels. If Chlamydia or any other pathogen causes atherosclerosis, then a more effective treatment may be an antibiotic or vaccine.
We have sought evidence for a causal role of infectious agents in atherosclerosis using the murine apolipoprotein (apo) E−/− (deficient) model. Because of the deficiency of apo E, these animals have high levels of plasma cholesterol and develop atherosclerosis with a reproducible time course 21 22 23. In a first experiment, we bred the apo E−/− with a strain of mouse that is unable to respond to bacterial LPS, but found no alteration in the rate or extent of atherogenesis. In a second study, we developed a colony of germ-free apo E−/− animals. We observed no effect of gnotobiosis on the progress of atherosclerosis. We conclude that neither the response to infectious agents nor the presence of infectious agents themselves is absolutely necessary for atherogenesis.
Materials and Methods
Mice with the lpsd gene crossed onto a C57BL background (C57BL/10ScN) were the gift of Dr. Steven K. Chapes (Kansas State University, Manhattan, KS). These mice exhibited minimal increases in serum levels of TNF-α and IL-6 in response to intraperitoneal injection of 50 μg of LPS, whereas C57BL and apo E−/− animals exhibited >100-fold elevations in both (not shown). C57BL/10ScN mice were crossed with apo E−/− mice on a C57Bl/6 background (Jackson Laboratories). Apo E−/−/lpsdanimals were identified in the F2 progeny by the presence of plasma cholesterol levels >500 mg/dl and the absence of plasma elevations of TNF-α 90 min after injection of 50 μg LPS.
Germ-free and control apo E−/− mice were generated and maintained at Taconic Farms. Sterility was verified by established procedures (standard operating procedure [SOP] no. G-LB-300.04).
For all mice in this study, offspring were weaned at 4 wk of age onto a high-fat Western-type diet containing 21.22% (g/100 g) fat, 17.01% protein, 48.48% carbohydrate, and 0.15% cholesterol (TD88137; Harlan Teklad) and maintained on the diet for the remainder of the study. Autoclaved food and water were provided ad libitum. The Institutional Animal Care and Use Committee of Merck Research Laboratories approved the animal use for experimentation, and all animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (1996, National Research Council).
To measure aortic cholesterol and cholesteryl ester, mice were killed and gently perfused through the left ventricle with cold PBS with 5 mM EDTA. All branches and any adipose tissue connected to the aorta were removed, and each aorta was carefully excised from the aortic root to the right renal artery. The aortas were blotted dry, minced, and extracted with chloroform/methanol (2:1) according to the method used by Folch et al. 24. Total and free cholesterol in the aortic extracts were determined using an enzymatic fluorometric assay modified from previously described methods 25 26, and data are expressed on a per aorta basis. Plasma cholesterol and triglyceride levels were determined using standard enzymatic kits (Sigma Chemical Co.).
For histology, mouse hearts were perfused in situ with PBS and removed with 1 mm of proximal aorta attached. The top half of the heart was embedded in O.C.T. embedding medium (Fisher Scientific) and prepared for cryosectioning. 6-μm sections were collected of the aortic root area, defined as having aortic valve leaflets present, and mounted on 10-well masked slides. Sections were stained with hematoxylin-phyloxine-saffron for morphology. Additional sections were immunolabeled with mAb L3T4 for CD4 or M1/70 for CD11b (PharMingen) using the horseradish peroxidase method with 3-3′-diamino benzadine as substrate.
Results
The time course of atherosclerogenesis in apo E−/− mice was followed by measuring aortic cholesteryl ester (Fig. 1), a parameter that tracks with histological progression of atherosclerosis (23; data not shown). To determine a role for gram-negative bacteria such as Chlamydia, we first used lpsd mice 27, animals with a congenital deficiency in responses to the principal inflammatory component of these bacteria, LPS (endotoxin). LPS is known to stimulate expression of cytokines (TNF, monocyte chemotactic protein 1 [MCP-1], and others), adhesion molecules (vascular cell adhesion molecule 1 [VCAM-1], intercellular adhesion molecule 1 [ICAM-1], β2 integrins), and enzymes (inducible nitric oxide synthetase [iNOS], matrix metalloproteinase 9 [MMP9]) observed in atherosclerotic lesions 28 and is a prime candidate to mediate possible proatherogenic effects of Chlamydia. lpsd mice fail to respond to LPS because of a deficiency in Toll-like receptor 4 (TLR-4 [29, 30]), a transmembrane protein with homology to the IL-1 receptor. lpsd animals were crossed with apo E−/− animals to yield doubly deficient animals. The inability of these animals to respond to LPS was verified by measurements of plasma TNF in response to LPS challenge, and their deficiency in apo E was verified by measurements of plasma cholesterol (described in Materials and Methods). We observed that development of atherosclerotic lesions in mice fed a high-fat Western-type diet was not altered by the deletion of TLR-4 (Fig. 1). Further studies showed that plasma cholesterol was also not affected by the absence of TLR-4 (data not shown). This result suggests that responses to gram-negative bacterial products are not critical for murine atherogenesis.
Figure 1.
Accumulation of cholesterol ester in the aortas of apo E−/− and apo E−/−/lpsdmice fed a high-fat diet. Animals were weaned onto a high-fat diet, and at the indicated times, aortic cholesterol measurements were performed as described in Materials and Methods. Each data point represents the average value from 7–10 animals, with error bars indicating the SE.
To more thoroughly ascertain the role of infectious agents in atherogenesis, we generated a germ-free colony of apo E−/− mice. Founder germ-free apo E−/− pups were delivered by Caesarian section and reared by germ-free foster mothers. The colony resulting from these founders was maintained germ free: weekly tests showed that no bacteria could be grown from feces, bedding, or swabs of the isolator. In addition, animals chosen at random showed no antibodies against any of several viral pathogens. The absence of intestinal bacteria is known to cause enlargement of the caecum 31, and dramatically enlarged caeca were observed in all of the animals from the colony.
The progress of hyperlipidemia and atherogenesis in germ-free animals was compared with that of a second set of animals taken from the germ-free colony and reared with ambient pathogens. As expected, control apo E−/− animals exhibited extremely high levels of plasma cholesterol and moderately high triglyceride at both 22 and 32 wk of age (Fig. 2A and Fig. B). Germ-free animals showed an essentially identical plasma lipid profile, and lipid changes are thus unlikely to obscure effects of gnotobiosis on atherogenesis.
Figure 2.
Plasma lipid profiles and aortic cholesterol levels of germ-free (white bars) and control (black bars) apo E−/− mice. Apo E−/− mice were reared germ free or in the presence of ambient pathogens. At 22 or 32 wk of age, animals were killed, blood was collected for analysis of plasma cholesterol and triglyceride, and aortas were harvested and extracted for determination of cholesterol content. (A and B) Comparisons of plasma cholesterol and triglyceride levels in control and germ free yield nearly identical values in male (A) and female (B) apo E−/− mice at both 22 and 32 wk. None of the small differences reached statistical significance, with the exception of decreased triglyceride levels for the male germ-free animals at 22 wk (*P < 0.005) and increased triglyceride levels for the female germ-free animals at 32 wk (**P = 0.03). (C and D) Comparisons of aortic free cholesterol and cholesteryl ester levels in male (C) and female (D) apo E−/− mice at 22 and 32 wk show a trend toward lower levels in the germ-free animals at both time points, although statistical significance was achieved only for the cholesteryl ester in germ-free males at 22 wk (*P < 0.005) and for the germ-free cholesterol in germ-free females at 22 wk († P = 0.05). However, data shown are expressed on a per aorta basis, and after correction for the decreased body weight of the germ-free mice, none of the differences attained statistical significance. For each time point and sex, group size was ≥10 animals, with the exception of the 32-wk male germ-free mice, which were reduced to 3 by mortality caused by aggressive behavior.
In both male and female animals, we observed that the exclusion of infectious agents caused no consistent differences in the rate or extent of free cholesterol or cholesteryl ester accumulation in the aorta (Fig. 2C and Fig. D). We did observe slightly reduced aortic cholesterol ester in the germ-free animals that attained statistical significance at 22 wk of age. However, these small differences disappeared upon correction for reduced body weight observed in the germ-free animals at the 22-wk time point (28 ± 0.6 g for germ-free males vs. 34 ± 0.8 g for control males, and 22 ± .02 g for germ-free females vs. 24 ± 0.6 g for controls; P = 0.001). The values for aortic cholesteryl ester in germ-free mice were also similar to those seen in a control colony that had never been made germ free (values in Fig. 1).
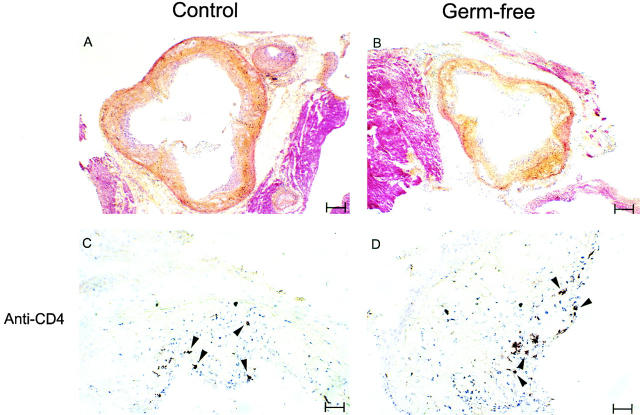
The similarity of atherogenesis in control and germ-free animals was confirmed by histologic examination of the aortic root in animals taken at the 22-wk time point. Well-developed lesions with necrotic cores, large foam cells, and fibrous caps were observed in both control and germ-free specimens (Fig. 3A and Fig. B), and neither quantitative nor qualitative differences could be detected in observations of either male or female lesions. A difference in aortic size reflecting the smaller body size of the germ-free animals 31 was readily detected histologically. T cells are found in human and murine atherosclerotic lesions 12 23, suggesting activity of the adaptive immune system. We identified T cells by immunohistochemistry in both control and germ-free animals (Fig. 3C and Fig. D), indicating that pathogens are not required to drive the influx of T cells into atherosclerotic lesions.
Figure 3.
Histology of aortic root lesions in germ-free and control apo E−/− mice. (A and B) Cryosections of the aortic root area in mice at 22 wk of age were stained with hematoxylin-phyloxine-saffron. Lesions in both types of animal have fibrous components (indicated by yellow staining) and large areas that are rich in foam cells (indicated by light purple staining). White rice grain–shaped spaces indicative of the extracellular deposition of cholesterol are also visible in lesions from control and germ-free mice. The tissue staining red is surrounding muscle. Bar = 156 μm. (C and D) These sections are stained brown for the T lymphocyte marker CD4. Arrowheads indicate the location of T lymphocytes, present in both control and germ-free animals. Bar = 31 μm. Hearts from five mice of each type for both males and females were examined histologically, and the sections shown are representative of the results from all of the mice. The same morphology and cellular composition was observed in both female and male mice. Histology of hearts from two mice of each type at 32 wk of age similarly revealed no differences between germ-free apo E−/− and control apo E−/− mice (not shown). Additionally, staining for the macrophage marker CD11b revealed comparably stained areas in control and germ-free animals (not shown).
Discussion
The observations presented here indicate that infectious agents, whether bacterial, viral, or fungal, are not necessary for murine atherogenesis. Infectious agents do not appear necessary either for initiation or progression of atherosclerosis, nor does the presence of infectious agents alter the morphology or cellular composition of the atherosclerotic lesions. Finally, the presence of infectious agents does not appear to alter the pattern of distribution of lesions along the aorta (not shown). Rather, it appears that the high-circulating cholesterol levels in the apo E−/− animals are sufficient to initiate and drive atherogenesis. We conclude that Koch's postulates cannot be fulfilled for any infectious agent in murine atherosclerosis. Atherosclerosis in apo E−/− mice closely resembles that in humans with respect to histology, progression, and dependence on circulating cholesterol 23, and we may thus speculate that infectious agents may not be necessary for the development of human atherosclerosis.
We wish to emphasize that the observations reported here relate to atherogenesis, not to plaque rupture, thrombus formation, or myocardial infarction. It is possible that infectious agents play an important role in one or more of these acute processes. Several current studies are seeking to define a role for bacterial infection in human CHD by studying endpoints such as myocardial infarction in patients randomized to placebo or antibiotic therapy of varying duration. The largest of these studies to report results thus far has failed to demonstrate a decline in cardiovascular events in the 6 mo after treatment with antibiotic 32. However, definitive results must await completion of larger, more extended trials.
Although our results suggest that pathogens do not serve as etiologic agents for atherosclerosis, they may still have a role in exacerbating the disease. Recent reports suggest that inoculation of atherosclerosis-prone mice with high doses of Chlamydia may cause an approximately twofold increase in the size of atherosclerotic lesions 33 34. It is important to note that stresses such as infection provoke a set of physiological responses that are uniformly proatherogenic 35 36 37. These include insulin resistance and hyperglycemia, elevation of plasma triglycerides, elevated white blood cell counts, elevated acute phase reactants (e.g., C-reactive protein and fibrinogen), and depression of high density lipoprotein (HDL) cholesterol levels. It is thus possible that infection may exacerbate atherosclerogenesis, and antibiotics may ameliorate atherogenesis not through the action of an etiologic agent, but through indirect effects on metabolism and known risk factors.
Acknowledgments
We thank Dr. Stephanie Vogel for advice in breeding and typing lpsd animals, Dr. Stephen Chapes for the gift of C57BL/10ScN animals, and the staff of Taconic Farms for excellent animal husbandry.
References
- Fabricant C.G., Fabricant J., Litrenta M.M., Minick C.R. Virus-induced atherosclerosis. J. Exp. Med. 1978;148:335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C.G., Fabricant J., Minick C.R., Litrenta M.M. Herpesvirus-induced atherosclerosis in chickens. Fed. Proc. 1983;42:2476–2479. [PubMed] [Google Scholar]
- Epstein S.E., Speir E., Zhou Y.F., Guetta E., Leon M., Finkel T. The role of infection in restenosis and atherosclerosisfocus on cytomegalovirus Lancet 348Suppl.1996. 13 17 [DOI] [PubMed] [Google Scholar]
- Patel P., Mendell M.A., Carrington D., Strachan D.P., Leatham E., Molineaux N., Levy J., Blakeston C., Seymour C.A., Camm A.J. Heart disease and cardiovascular risk factors. BMJ. 1997;311:711. doi: 10.1136/bmj.311.7007.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J., Collins R., Peto R. Chronic infections and coronary heart diseaseis there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- Kuo C.C., Grayston J.T., Campbell L.A., Goo A., Wissler R.W., Benditt E.P. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15-34 years old) Proc. Natl. Acad. Sci. USA. 1995;92:6911–6914. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M., Kuo C.C., Middaugh J.P., Campbell L.A., Wang S.P., Newman W.P., III, Finley J.C., Grayston J.T. Confirmed previous infection with Chlamydia pneumoniae (TWAR) and its presence in early coronary atherosclerosis. Circulation. 1998;98:628–633. doi: 10.1161/01.cir.98.7.628. [DOI] [PubMed] [Google Scholar]
- Campbell L.A., O'Brien E.R., Capuccio A.L., Kuo C.C., Wang S.P., Stewart D., Patton D.L., Cummings P.I., Grayston J.T. Detection of Chlamydia pneuomoniae TWAR in human coronary atherectomy tissues. J. Infect. Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- Coles K.A., Plant A.J., Riley T.V., Smith D.W., McQuillan B.M., Thompson P.L. Lack of association between seropositivity to Chlamydia pneumoniae and carotid atherosclerosis. Am. J. Cardiol. 1999;84:825–828. doi: 10.1016/s0002-9149(99)00445-2. [DOI] [PubMed] [Google Scholar]
- Daus H., Ozbek C., Saage D., Scheller B., Schiefffer H., Pfreundschuh M., Grause A. Lack of evidence for a pathogenic role of Chlamydia pneumoniae and cytomegalovirus infection in coronary atheroma formation. Cardiology. 1998;90:83–88. doi: 10.1159/000006824. [DOI] [PubMed] [Google Scholar]
- Moazed T.C., Kuo C.C., Grayston J.T., Campbell L.A. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J. Infect. Dis. 1998;177:1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Smith J.D., Trogan E., Ginsberg M., Grigaux C., Tian J., Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J.T. Does Chlamydia pneumoniae cause atherosclerosis? Arch. Surg. 1999;134:930–934. doi: 10.1001/archsurg.134.9.930. [DOI] [PubMed] [Google Scholar]
- Campbell L.A., Kuo C.C., Grayston J.T. Chlamydia pneumoniae and cardiovascular disease. Emerg. Infect. Dis. 1998;4:571–579. doi: 10.3201/eid0404.980407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll G. Pathogenesis of atherosclerosisa possible relation to infection Atherosclerosis. 140Suppl.1998. 3 9 [DOI] [PubMed] [Google Scholar]
- Gupta S., Camm A.J. Is there an infective aetiology to atherosclerosis? Drugs Aging. 1998;13:1–7. doi: 10.2165/00002512-199813010-00001. [DOI] [PubMed] [Google Scholar]
- Mehta J.L., Saldeen T.G., Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J. Am. Coll. Cardiol. 1998;31:1217–1225. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- Taylor D.N., Blaser M.J. The epidemiology of Helicobacter pylori infection. Epidemiol. Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. 1994. NIH Consensus Conference. Helicobacter pylori in Peptic Ulcer Disease. JAMA. 272:65–69. [PubMed]
- Plump A.S., Smith J.D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J.G., Rubin E.M., Breslow J.L. Sever hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Piedrahita J.A., Zhang S.H., Hagaman J.R., Oliver P.M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Plump A.S., Raines E.W., Breslow J.L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.A. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gamble W., Vaughn M., Kruth H.S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J. Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- Heider J.G., Boyett R.L. The picomole determination of free and total cholesterol in cells in culture. J. Lipid Res. 1978;19:514–518. [PubMed] [Google Scholar]
- Vogel S. Insights into the genetic and molecular basis of LPS responsiveness and macrophage differentiation. In: Beutler B., editor. Tumor Necrosis Factorsthe Molecules and Their Emerging Role in Medicine. Raven Press, Ltd.; New York: 1992. pp. 485–513. [Google Scholar]
- Wright S.D. Innate recognition of microbial lipids. In: Gallin J.I., Snyderman R., editors. InflammationBasic Principles and Clinical Correlates. 3rd ed. Lippincott Williams and Wilkins; Philadelphia: 1999. pp. 525–535. [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M.Y., Huffel C.V., Du X., Birdwell D., Alejos E., Silva M., Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr micemutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Wright S.D. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1984. The germ-free animal in biomedical research. In Laboratory Animal Handbooks 9. M.E. Coates and B.E. Gustafsson, editors. Laboratory Animals, Ltd., London. 100–108.
- Anderson J.L., Muhlestein M.B., Carlquist J., Allen A., Trehan S., Nielson C., Hall S., Brady J., Egger M., Horne B., Lim T. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection. Circulation. 1999;99:1540–1547. doi: 10.1161/01.cir.99.12.1540. [DOI] [PubMed] [Google Scholar]
- Hu H., Pierce G.N., Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumonia . J. Clin. Invest. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed T.C., Campbell A., Rosenfeld M.E., Grayston J.T., Kuo C.-C. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J. Infect. Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- Danesh J., Collins R., Appleby P., Peto R. Association of fibrogen, C-reactive protein, albumin, or leukocyte count with coronary heart diseasemeta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- Pickup J.C., Matock M.B., Chusney G.D., Burt D. NIDDM as a disease of the innate immune systemassociation of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- Ingenbleek Y., Bernstein L. The stressful condition as a nutritionally dependent adaptive dichotomy. Nutrition. 1999;15:305–320. doi: 10.1016/s0899-9007(99)00009-x. [DOI] [PubMed] [Google Scholar]